Probiotic Formulations Containing Fixed and Essential Oils Ameliorates SIBO-Induced Gut Dysbiosis in Rats
Abstract
:1. Introduction
2. Results
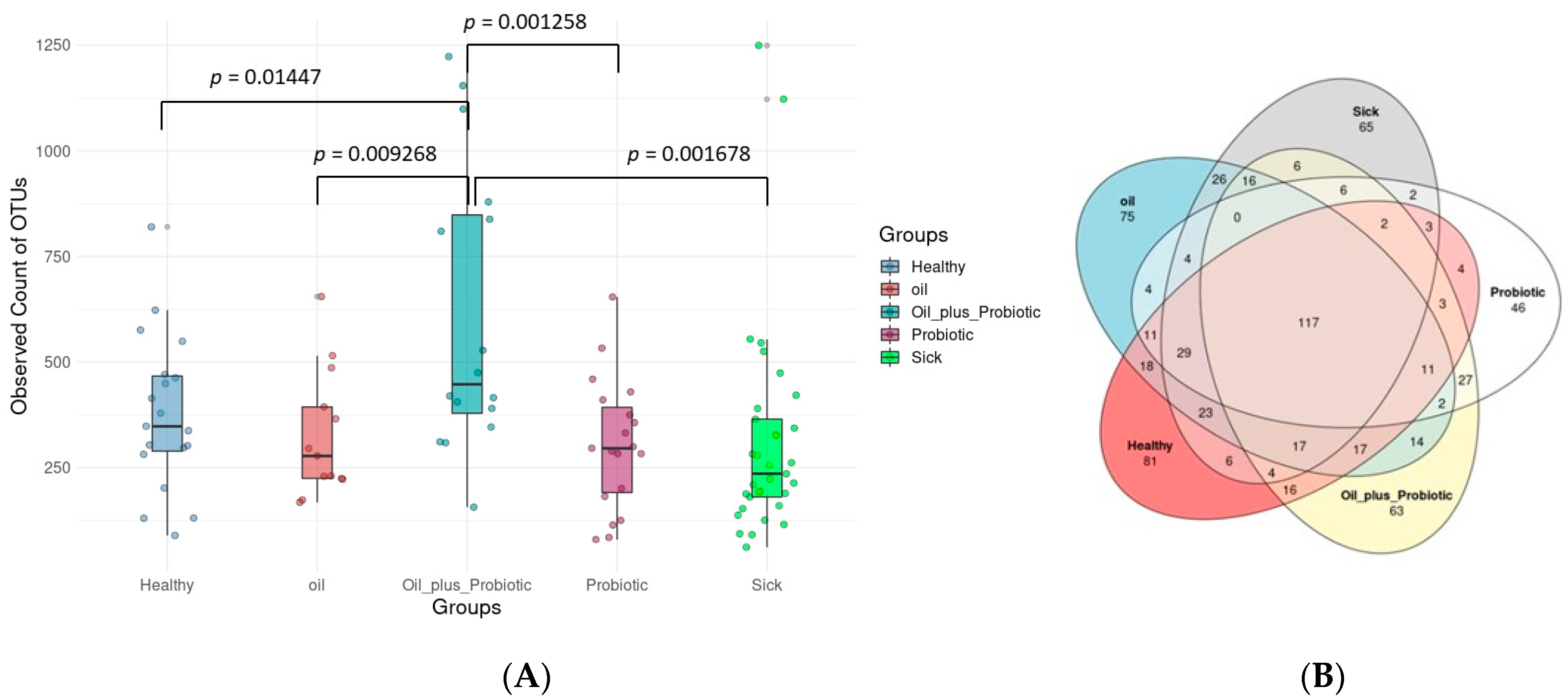
2.1. Microbiome Analysis
2.2. Biochemical Analysis Results
2.3. Histopathological Analysis
3. Discussion
4. Materials and Methods
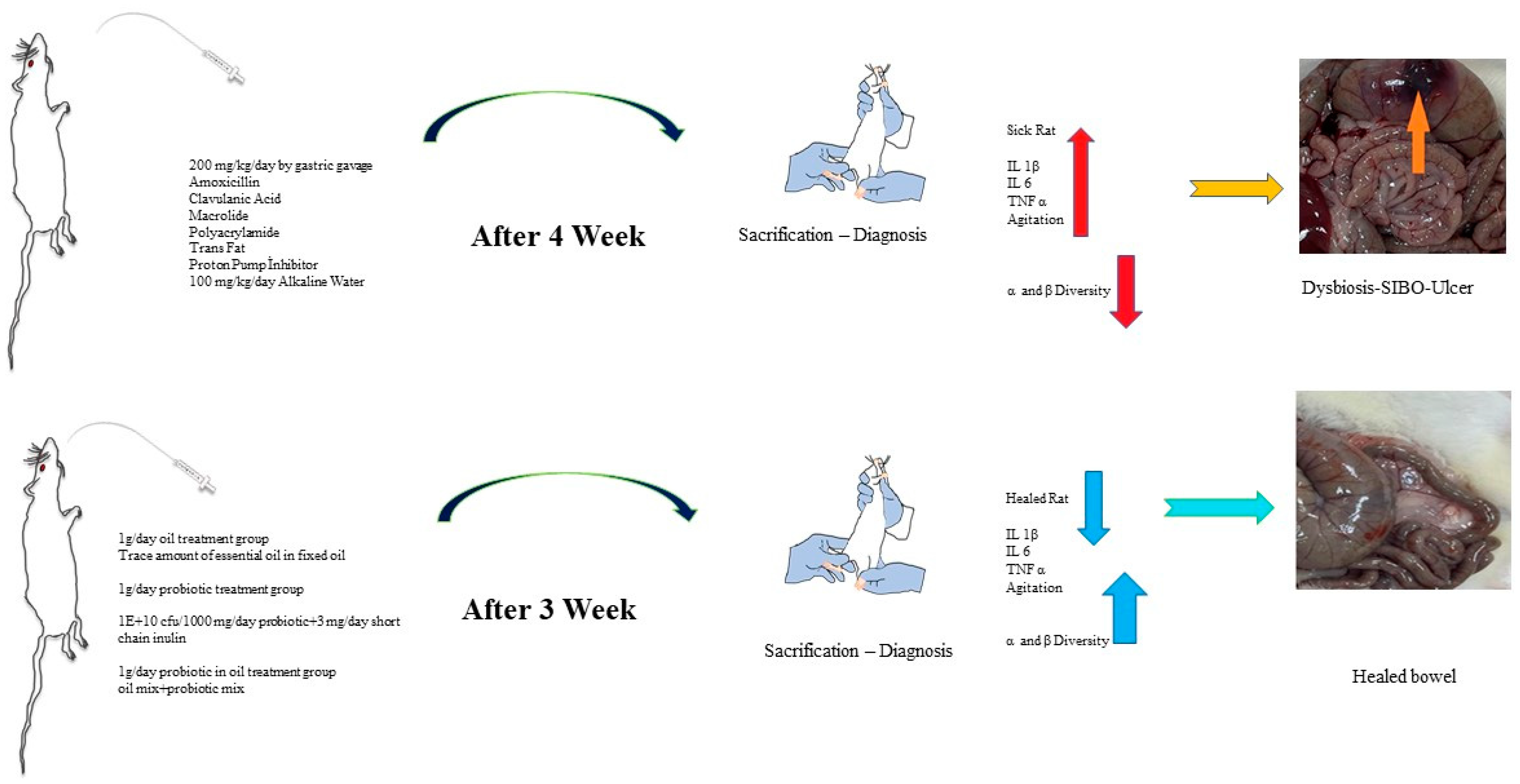
4.1. Test Animals
4.2. Experimental Design
4.3. Rat Study Groups
4.4. Application Protocol
4.5. Collection and Storage of Samples
4.6. Diagnostic Analysis Steps
4.7. Fecal Gene Extraction
4.8. High-Throughput 16S rRNA Gene Sequence and Data Analysis
4.9. Biochemical Analysis
4.10. Histopathological Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.; Morrison, M.; Burger, D.; Martin, N.; Rich, J.; Jones, M.; Koloski, N.; Walker, M.M.; Talley, N.J.; Holtmann, G.J. Systematic review with meta-analysis: The prevalence of small intestinal bacterial overgrowth in inflammatory bowel disease. Aliment Pharmacol. Ther. 2019, 49, 624–635. [Google Scholar] [CrossRef]
- Dukowicz, A.C.; Lacy, B.E.; Levine, G.M. Small intestinal bacterial overgrowth: A comprehensive review. Gastroenterol. Hepatol. 2007, 3, 112–122. [Google Scholar]
- Leite, G.; Morales, W.; Weitsman, S.; Celly, S.; Parodi, G.; Mathur, R.; Barlow, G.M.; Sedighi, R.; Millan, M.J.V.; Rezaie, A.; et al. The duodenal microbiome is altered in small intestinal bacterial overgrowth. PLoS ONE 2020, 15, e0234906. [Google Scholar] [CrossRef] [PubMed]
- Leventogiannis, K.; Gkolfakis, P.; Spithakis, G.; Tsatali, A.; Pistiki, A.; Sioulas, A.; Giamarellos-Bourboulis, E.J.; Triantafyllou, K. Effect of a Preparation of Four Probiotics on Symptoms of Patients with Irritable Bowel Syndrome: Association with Intestinal Bacterial Overgrowth. Probiotics Antimicrob. Proteins 2019, 11, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Zhang, L.; Hong, G.; Li, Y.; Li, G.; Qian, W.; Xiong, H.; Bai, T.; Song, J.; Hou, X. Duodenal and rectal mucosal microbiota related to small intestinal bacterial overgrowth in diarrhea-predominant irritable bowel syndrome. J. Gastroenterol. Hepatol. 2020, 35, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Shukla, R.; Ghoshal, U. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome: A Bridge between Functional Organic Dichotomy. Gut Liver 2017, 11, 196–208. [Google Scholar] [CrossRef]
- Mohsin, M.; Zhang, Z.; Yin, G. Effect of Probiotics on the Performance and Intestinal Health of Broiler Chickens Infected with Eimeria tenella. Vaccines 2022, 10, 97. [Google Scholar] [CrossRef]
- Shah, S.C.; Day, L.W.; Somsouk, M.; Sewell, J.L. Meta-analysis: Antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol. Ther. 2013, 38, 925–934. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Qu, C.; Wang, B.; Liang, S.; Zeng, B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth: A Meta-Analysis and Systematic Review of Current Evidence. J. Clin. Gastroenterol. 2017, 51, 300–311. [Google Scholar] [CrossRef]
- Quigley, E.M.M. The Spectrum of Small Intestinal Bacterial Overgrowth (SIBO). Curr. Gastroenterol. Rep. 2019, 21, 3. [Google Scholar] [CrossRef]
- Wagner, N.R.F.; Ramos, M.R.Z.; de Oliveira Carlos, L.; da Cruz, M.R.R.; Taconeli, C.A.; Filho, A.J.B.; Nassif, L.S.; Schieferdecker, M.E.M.; Campos, A.C.L. Effects of Probiotics Supplementation on Gastrointestinal Symptoms and SIBO after Roux-en-Y Gastric Bypass: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Obes. Surg. 2021, 31, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Chatellier, K.; Stem, L.; Klatt, P.; Haugh, A.; Shelesky, G. Do probiotics provide symptom relief in patients with SIBO compared with placebo or no treatment? Evid. -Based Pract. 2021, 24, 16–17. [Google Scholar] [CrossRef]
- Djurasevic, S.; Bojic, S.; Nikolic, B.; Dimkic, I.; Todorovic, Z.; Djordjevic, J.; Mitic-Culafic, D. Beneficial Effect of Virgin Coconut Oil on Alloxan-Induced Diabetes and Microbiota Composition in Rats. Plant Foods Hum. Nutr. 2018, 73, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Mittal, P.; Clavaud, C.; Dhakan, D.B.; Roy, N.; Breton, L.; Misra, N.; Sharma, V.K. Longitudinal study of the scalp microbiome suggests coconut oil to enrich healthy scalp commensals. Sci. Rep. 2021, 11, 7220. [Google Scholar] [CrossRef]
- Matsue, M.; Mori, Y.; Nagase, S.; Sugiyama, Y.; Hirano, R.; Ogai, K.; Ogura, K.; Kurihara, S.; Okamoto, S. Measuring the Antimicrobial Activity of Lauric Acid against Various Bacteria in Human Gut Microbiota Using a New Method. Cell 2019, 28, 1528–1541. [Google Scholar] [CrossRef] [Green Version]
- Shipradeep; Karmakar, S.; Sahay Khare, R.; Ojha, S.; Kundu, K.; Kundu, S. Development of probiotic candidate in combination with essential oils from medicinal plant and their effect on enteric pathogens: A review. Gastroenterol. Res. Pract. 2012, 2012, 457150. [Google Scholar] [CrossRef]
- Hausmann, M.; Obermeier, F.; Paper, D.H.; Balan, K.; Dunger, N.; Menzel, K.; Falk, W.; Schoelmerich, J.; Herfarth, H.; Rogler, G. In vivo treatment with the herbal phenylethanoid acteoside ameliorates intestinal inflammation in dextran sulphate sodium-induced colitis. Clin. Exp. Immunol. 2007, 148, 373–381. [Google Scholar] [CrossRef]
- Xue, B.; Hausmann, M.; Müller, M.H.; Pesch, T.; Karpitschka, M.; Kasparek, M.S.; Hu, W.C.; Sibaev, A.; Rogler, G.; Kreis, M.E. Afferent nerve sensitivity is decreased by an iNOS-dependent mechanism during indomethacin-induced inflammation in the murine jejunum in vitro. Neurogastroenterol. Motil. 2009, 21, 322–334. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Baeza, Y.; Hyde, E.R.; Suchodolski, J.S.; Knight, R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 2016, 1, 16177. [Google Scholar] [CrossRef]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- McCarthy, J.; O’Mahony, L.; O’Callaghan, L.; Sheil, B.; Vaughan, E.E.; Fitzsimons, N.; Fitzgibbon, J.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut 2003, 52, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, T.; Palmieri, B. Therapeutical use of probiotic formulations in clinical practice. Clin. Nutr. 2010, 29, 701–725. [Google Scholar] [CrossRef]
- Takakura, W.; Pimentel, M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome—An Update. Front. Psychiatry 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; D’Angelo, G.; Di Rienzo, T.; Scarpellini, E.; Ojetti, V. Diagnosis of small intestinal bacterial overgrowth in the clinical practice. Eur. Rev. Med. Pharmacol. Sci. 2013, 17 (Suppl. S2), 30–35. [Google Scholar] [PubMed]
- Mochiki, E.; Asao, T.; Kuwano, H. Gastrointestinal Motility After Digestive Surgery. Surg. Today 2007, 37, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Cortez, M.; Carmo, L.S.; Rogero, M.M.; Borelli, P.; Fock, R.A. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation 2013, 36, 379–386. [Google Scholar] [CrossRef]









| Group | Healthy Rats Score Average (n = 10) | Sick Rats Score Average (n = 10) | Only Oil Mix Given Rats Score Average (n = 10) | Only Probiotic Mix Given Rats Score Average (n = 10) | Oil + Probiotic Mix Given Rats Score Average (n = 8) | |
|---|---|---|---|---|---|---|
| Epithelium Morphology | Normal morphology (0) | 0 | 0 | 0 | 0 | 0 |
| Loss of goblet cells (Low) (1) | 1 | 1 | 1 | 1 | 1 | |
| Loss of goblet cells in large areas (Strong) (2) | 0 | 2 | 0 | 0 | 0 | |
| Loss of crypts (Low) (3) | 0 | 3 | 3 | 3 | 0 | |
| Loss of crypts in large areas (Strong) (4) | 0 | 4 | 4 | 4 | 0 | |
| Inflammation | No infiltrates (0) | 0 | 0 | 0 | 0 | 0 |
| Infiltrate around crypt basis (1) | 1 | 0 | 1 | 1 | 1 | |
| Infiltrate reaching to Lamina muscularis mucosae (2) | 0 | 0 | 2 | 2 | 0 | |
| Extensive infiltration reaching the Lamina muscularis mucosae and thickening of mucosa with edema (3) | 0 | 3 | 0 | 0 | 0 | |
| Infiltration of the Lamina Submucosa (4) | 0 | 4 | 0 | 0 | 0 | |
| Mean Value | IL-1β (pg/mL) | IL-6 (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|
| Healthy rats | 20.87 | 6.13 | 35.73 |
| Oil mix + Probiotic mix given rats | 22.84 | 4.82 | 37.47 |
| Only oil mix given rats | 37.30 | 5.84 | 73.55 |
| Only Probiotic mix given rats | 21.57 | 4.92 | 39.25 |
| Sick rats | 34.30 | 7.56 | 847.54 |
| Component Name | Amount of Mg |
|---|---|
| Coconut oil | 985 mg/mL |
| Medicinal peppermint oil | 5 mg/mL |
| Patchouli oil | 5 mg/mL |
| Lemon oil | 5 mg/mL |
| Total | 1000 mg/mL |
| Component Name | Amount of Mg | Amount of CFU |
|---|---|---|
| Pure water | 967 mg/mL | - |
| Inulin | 3 mg/mL | - |
| Lactobacillus rhamnosus ATA-LRS1902 | 5 mg/mL | 2.00 × 109 CFU/mL |
| Lactobacillus gastricus ATA-LRG01101 | 5 mg/mL | 1.00 × 109 CFU/mL |
| Lactobacillus acidophilus ATA-LAP1201 | 4 mg/mL | 1.00 × 109 CFU/mL |
| Bifidobacterium longum ATA-BSP1908 | 4 mg/mL | 1.00 × 109 CFU/mL |
| Bifidobacterium bifidum ATA-BSP1709 | 3 mg/mL | 1.00 × 109 CFU/mL |
| Bifidobacterium animalis ATA-BSLA0310 | 3 mg/mL | 1.00 × 109 CFU/mL |
| Lactobacillus plantarum ATA-LPC98052 | 3 mg/mL | 1.00 × 109 CFU/mL |
| Bacillus clausii LTCS-BSSL01140 | 1 mg/mL | 1.00 × 109 CFU/mL |
| Akkermansia muciniphila ATA-MDA013081 | 1 mg/mL | 1.00 × 108 CFU/mL |
| Bacillus subtilis LTCS-BSP031101 | 1 mg/mL | 1.00 × 108 CFU/mL |
| Total | 1000 mg/mL | 9.20 × 109 CFU/mL |
| Component Name | Amount of Mg | Amount of CFU |
|---|---|---|
| Coconut oil | 955 mg/mL | - |
| Medicinal Peppermint oil | 5 mg/mL | - |
| Patchouli oil | 5 mg/mL | - |
| Lemon oil | 5 mg/mL | - |
| Lactobacillus rhamnosus ATA-LRS1902 | 5 mg/mL | 2.00 × 109 CFU/mL |
| Lactobacillus gastricus ATA-LRG01101 | 5 mg/mL | 1.00 × 109 CFU/mL |
| Lactobacillus acidophilus ATA-LAP1201 | 4 mg/mL | 1.00 × 109 CFU/mL |
| Bifidobacterium longum ATA-BSP1908 | 4 mg/mL | 1.00 × 109 CFU/mL |
| Bifidobacterium bifidum ATA-BSP1709 | 3 mg/mL | 1.00 × 109 CFU/mL |
| Bifidobacterium animalis ATA-BSLA0310 | 3 mg/mL | 1.00 × 109 CFU/mL |
| Lactobacillus plantarum ATA-LPC98052 | 3 mg/mL | 1.00 × 109 CFU/mL |
| Bacillus clausii LTCS-BSSL01140 | 1 mg/mL | 1.00 × 109 CFU/mL |
| Akkermansia muciniphila ATA-MDA013081 | 1 mg/mL | 1.00 × 108 CFU/mL |
| Bacillus subtilis LTCS-BSP031101 | 1 mg/mL | 1.00 × 108 CFU/mL |
| Total | 1000 mg/mL | 9.20 × 109 CFU/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslan, I.; Tarhan Celebi, L.; Kayhan, H.; Kizilay, E.; Gulbahar, M.Y.; Kurt, H.; Cakici, B. Probiotic Formulations Containing Fixed and Essential Oils Ameliorates SIBO-Induced Gut Dysbiosis in Rats. Pharmaceuticals 2023, 16, 1041. https://doi.org/10.3390/ph16071041
Aslan I, Tarhan Celebi L, Kayhan H, Kizilay E, Gulbahar MY, Kurt H, Cakici B. Probiotic Formulations Containing Fixed and Essential Oils Ameliorates SIBO-Induced Gut Dysbiosis in Rats. Pharmaceuticals. 2023; 16(7):1041. https://doi.org/10.3390/ph16071041
Chicago/Turabian StyleAslan, Ismail, Leyla Tarhan Celebi, Hulya Kayhan, Emine Kizilay, Mustafa Yavuz Gulbahar, Halil Kurt, and Bekir Cakici. 2023. "Probiotic Formulations Containing Fixed and Essential Oils Ameliorates SIBO-Induced Gut Dysbiosis in Rats" Pharmaceuticals 16, no. 7: 1041. https://doi.org/10.3390/ph16071041
APA StyleAslan, I., Tarhan Celebi, L., Kayhan, H., Kizilay, E., Gulbahar, M. Y., Kurt, H., & Cakici, B. (2023). Probiotic Formulations Containing Fixed and Essential Oils Ameliorates SIBO-Induced Gut Dysbiosis in Rats. Pharmaceuticals, 16(7), 1041. https://doi.org/10.3390/ph16071041






