Theory and Practice of Glucocorticoids in COVID-19: Getting to the Heart of the Matter—A Critical Review and Viewpoints
Abstract
:1. Introduction
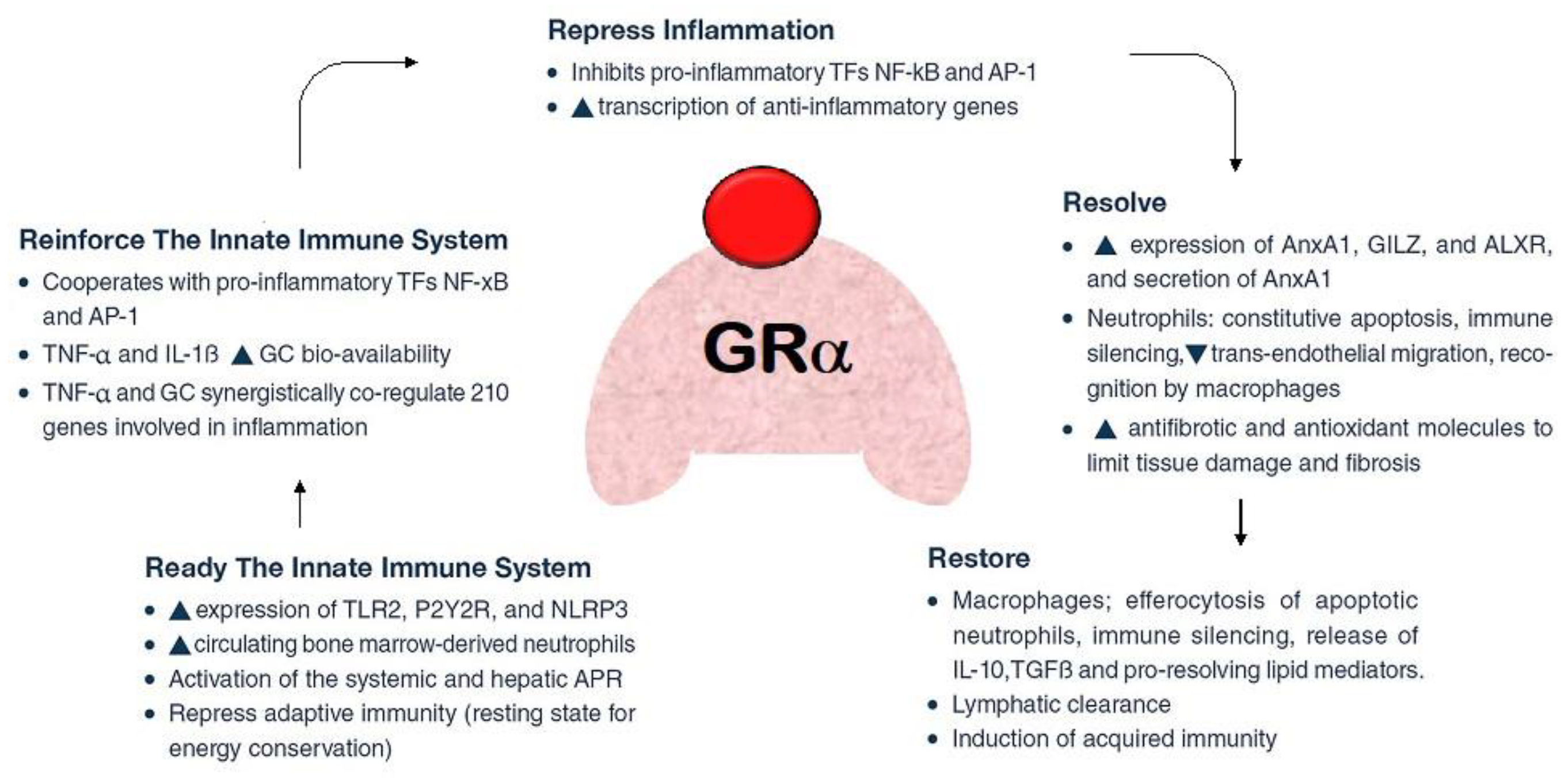
2. The Role of Glucocorticoids in COVID-19
3. Glucocorticoid Administration: Principles and Evidence
3.1. Glucocorticoid Molecules
3.2. Timing of Administration and Initial Dose
3.3. Administration Modality
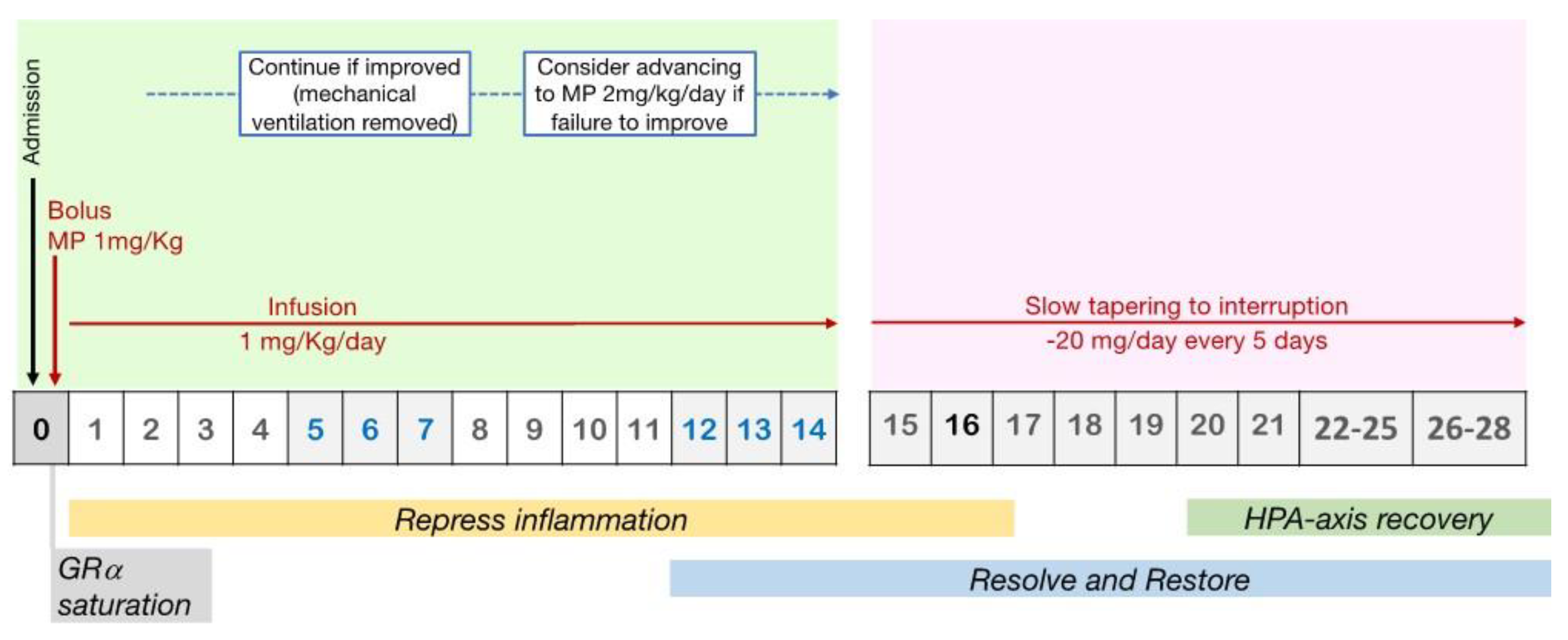
3.4. Timing of GCs Administration and Tapering
3.5. Infection Surveillance and Clinical Monitoring during Mechanical Ventilation
3.6. Post-Extubation Monitoring and Treatment Adjustment
3.7. Independent Factors Affecting Response to GCs Treatment
4. Complications and Adverse Events
5. Advantages and Disadvantages of Other Standardized Protocols Using GC in COVID-19
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rhen, T.; Cidlowski, J.A. Antiinflammatory Action of Glucocorticoids—New Mechanisms for Old Drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [Green Version]
- Bruscoli, S.; Puzzovio, P.G.; Zaimi, M.; Tiligada, K.; Levi-Schaffer, F.; Riccardi, C. Glucocorticoids and COVID-19. Pharmacol. Res. 2022, 185, 106511. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Pastores, S.M.; Rochwerg, B.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; et al. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I). Crit. Care Med. 2017, 45, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients with COVID-19. JAMA 2020, 324, 1330. [Google Scholar] [CrossRef]
- Siemieniuk, R.A.C.; Meade, M.O.; Alonso-Coello, P.; Briel, M.; Evaniew, N.; Prasad, M.; Alexander, P.E.; Fei, Y.; Vandvik, P.O.; Loeb, M.; et al. Corticosteroid Therapy for Patients Hospitalized with Community-Acquired Pneumonia. Ann. Intern. Med. 2015, 163, 519–528. [Google Scholar] [CrossRef]
- Meduri, G.U.; Annane, D.; Confalonieri, M.; Chrousos, G.P.; Rochwerg, B.; Busby, A.; Ruaro, B.; Meibohm, B. Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. Intensive Care Med. 2020, 46, 2284–2296. [Google Scholar] [CrossRef] [PubMed]
- Pastores, S.M.; Annane, D.; Rochwerg, B. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part II): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM). Intensive Care Med. 2018, 44, 474–477. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.; Griesel, M.; Mikolajewska, A.; Mueller, A.; Nothacker, M.; Kley, K.; Metzendorf, M.I.; Fischer, A.L.; Kopp, M.; Stegemann, M.; et al. Systemic corticosteroids for the treatment of COVID. Cochrane Database Syst. Rev. 2021, 2021, CD014963. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Zhou, Y.; Zhao, X.; Zhao, Q.; Liu, J. The effect of corticosteroid treatment on patients with coronavirus infection: A systematic review and meta-analysis. J. Infect. 2020, 81, e13–e20. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Almekhlafi, G.A.; Hussein, M.A.; Jose, J.; Pinto, R.; Al-Omari, A.; Kharaba, A.; et al. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 757–767. [Google Scholar] [CrossRef]
- Ogimi, C.; Greninger, A.L.; Waghmare, A.A.; Kuypers, J.M.; Shean, R.C.; Xie, H.; Leisenring, W.M.; Stevens-Ayers, T.L.; Jerome, K.R.; Englund, J.A.; et al. Prolonged Shedding of Human Coronavirus in Hematopoietic Cell Transplant Recipients: Risk Factors and Viral Genome Evolution. J. Infect. Dis. 2017, 216, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Gao, H.; Zhou, B.; Deng, X.; Hu, C.; Deng, C.; Lu, H.; Li, Y.; Gan, J.; Liu, J.; et al. Adjuvant Corticosteroid Treatment in Adults with Influenza A (H7N9) Viral Pneumonia. Crit. Care Med. 2016, 44, e318–e328. [Google Scholar] [CrossRef] [PubMed]
- Boudreault, A.A.; Xie, H.; Leisenring, W.; Englund, J.; Corey, L.; Boeckh, M. Impact of Corticosteroid Treatment and Antiviral Therapy on Clinical Outcomes in Hematopoietic Cell Transplant Patients Infected with Influenza Virus. Biol. Blood Marrow Transplant. 2011, 17, 979–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamontagne, F.; Agarwal, A.; Rochwerg, B.; Siemieniuk, R.A.; Agoritsas, T.; Askie, L.; Lytvyn, L.; Leo, Y.S.; Macdonald, H.; Zeng, L.; et al. A living WHO guideline on drugs for COVID-19. BMJ 2020, 4, m3379. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Fisher, C.J.; Clemmer, T.P.; Slotman, G.J.; Metz, C.A.; Balk, R.A. A Controlled Clinical Trial of High-Dose Methylprednisolone in the Treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 1987, 317, 653–658. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Madokoro, S.; Kondo, Y.; Okamoto, K.; Tanaka, H. Corticosteroid treatment for early acute respiratory distress syndrome: A systematic review and meta-analysis of randomized trials. J. Intensive Care 2020, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Derde, L.; Al-Beidh, F.; Annane, D.; Arabi, Y.; Beane, A.; van Bentum-Puijk, W.; Berry, L.; Bhimani, Z.; Bonten, M.; et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients with Severe COVID-19. JAMA 2020, 324, 1317. [Google Scholar] [CrossRef] [PubMed]
- Dequin, P.F.; Heming, N.; Meziani, F.; Plantefève, G.; Voiriot, G.; Badié, J.; François, B.; Aubron, C.; Ricard, J.D.; Ehrmann, S.; et al. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients with COVID-19. JAMA 2020, 324, 1298. [Google Scholar] [CrossRef]
- Taboada, M.; Rodríguez, N.; Varela, P.M.; Rodríguez, M.T.; Abelleira, R.; González, A.; Casal, A.; Díaz Peromingo, J.A.; Lama, A.; Domínguez, M.J.; et al. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: An open-label, randomised clinical trial. Eur. Respir. J. 2022, 60, 2102518. [Google Scholar] [CrossRef] [PubMed]
- Maskin, L.P.; Bonelli, I.; Olarte, G.L.; Palizas, F.; Velo, A.E.; Lurbet, M.F.; Lovazzano, P.; Kotsias, S.; Attie, S.; Lopez Saubidet, I.; et al. High- Versus Low-Dose Dexamethasone for the Treatment of COVID-19-Related Acute Respiratory Distress Syndrome: A Multicenter, Randomized Open-Label Clinical Trial. J. Intensive Care Med. 2022, 37, 491–499. [Google Scholar] [CrossRef]
- Saeed, M.A.M.; Mohamed, A.H.; Owaynat, A.H. Comparison between methylprednisolone infusion and dexamethasone in COVID-19 ARDS mechanically ventilated patients. Egypt. J. Intern. Med. 2022, 34, 19. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, K.; Moghadami, M.; Mirahmadizadeh, A.; Fallahi, M.J.; Khaloo, V.; Shahriarirad, R.; Erfani, A.; Khodamoradi, Z.; Gholampoor Saadi, M.H. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: A triple-blinded randomized controlled trial. BMC Infect. Dis. 2021, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Chang, H.T.; Wang, P.H.; Chang, M.Y.; Hsu, H.S. Mortality in patients with COVID-19 versus non-COVID-19- related acute respiratory distress syndrome: A single center retrospective observational cohort study. PLoS ONE 2023, 18, e0286564. [Google Scholar] [CrossRef]
- Salton, F.; Confalonieri, P.; Centanni, S.; Mondoni, M.; Petrosillo, N.; Bonfanti, P.; Lapadula, G.; Lacedonia, D.; Voza, A.; Carpenè, N.; et al. Prolonged higher dose methylprednisolone versus conventional dexamethasone in COVID-19 pneumonia: A randomised controlled trial (MEDEAS). Eur. Respir. J. 2023, 61, 2201514. [Google Scholar] [CrossRef]
- Salton, F.; Confalonieri, P.; Meduri, G.U.; Santus, P.; Harari, S.; Scala, R.; Lanini, S.; Vertui, V.; Oggionni, T.; Caminati, A.; et al. Prolonged Low-Dose Methylprednisolone in Patients with Severe COVID-19 Pneumonia. Open Forum Infect. Dis. 2020, 7, ofaa421. [Google Scholar] [CrossRef]
- Maamar, M.; Artime, A.; Pariente, E.; Fierro, P.; Ruiz, Y.; Gutiérrez, S.; Tobalina, M.; Díaz-Salazar, S.; Ramos, C.; Olmos, J.M.; et al. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: A cross-sectional study. Curr. Med. Res. Opin. 2022, 38, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef]
- Meduri, G.U.; Bridges, L.; Shih, M.C.; Marik, P.E.; Siemieniuk, R.A.C.; Kocak, M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: Analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016, 42, 829–840. [Google Scholar] [CrossRef]
- Schreiber, S.N.; Emter, R.; Hock, M.B.; Knutti, D.; Cardenas, J.; Podvinec, M.; Oakeley, E.J.; Kralli, A. The estrogen-related receptor α (ERRα) functions in PPARγ coactivator 1α (PGC-1α)-induced mitochondrial biogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 6472–6477. [Google Scholar] [CrossRef] [Green Version]
- Diederich, S.; Eigendorff, E.; Burkhardt, P.; Quinkler, M.; Bumke-Vogt, C.; Rochel, M.; Seidelmann, D.; Esperling, P.; Oelkers, W.; Bähr, V. 11β-Hydroxysteroid Dehydrogenase Types 1 and 2: An Important Pharmacokinetic Determinant for the Activity of Synthetic Mineralo- and Glucocorticoids. J. Clin. Endocrinol. Metab. 2002, 87, 5695–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Tsubochi, H.; Ishibashi, H.; Suzuki, T.; Kondo, T.; Sasano, H. Increased expression of 11beta-hydroxysteroid dehydrogenase type 2 in the lungs of patients with acute respiratory distress syndrome. Pathol. Int. 2003, 53, 751–756. [Google Scholar] [CrossRef]
- Draghici, S.; Nguyen, T.M.; Sonna, L.A.; Ziraldo, C.; Vanciu, R.; Fadel, R.; Morrison, A.; Kenney, R.M.; Alangaden, G.; Ramesh, M.; et al. COVID-19: Disease pathways and gene expression changes predict methylprednisolone can improve outcome in severe cases. Bioinformatics 2021, 37, 2691–2698. [Google Scholar] [CrossRef]
- Kino, T.; Su, Y.A.; Chrousos, G.P. Human glucocorticoid receptor isoform β: Recent understanding of its potential implications in physiology and pathophysiology. Cell. Mol. Life Sci. 2009, 66, 3435–3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiyama, K.; Matsuda, N.; Yamamoto, S.; Takano, K.; Takano, Y.; Yamazaki, H.; Kageyama, S.; Yokoo, H.; Nagata, T.; Hatakeyama, N.; et al. Modulation of glucocorticoid receptor expression, inflammation, and cell apoptosis in septic guinea pig lungs using methylprednisolone. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L998–L1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Q.; Zhou, X.; Zhou, Y.; Rong, L.; Gao, L.; Xu, W. Low-dose dexamethasone alleviates lipopolysaccharide-induced acute lung injury in rats and upregulates pulmonary glucocorticoid receptors. Respirology 2008, 13, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Fruchter, O.; Kino, T.; Zoumakis, E.; Alesci, S.; De Martino, M.; Chrousos, G.; Hochberg, Z. The Human Glucocorticoid Receptor (GR) Isoform β Differentially Suppresses GRα-Induced Transactivation Stimulated by Synthetic Glucocorticoids. J. Clin. Endocrinol. Metab. 2005, 90, 3505–3509. [Google Scholar] [CrossRef]
- Vichyanond, P.; Irvin, C.; Larsen, G.; Szefler, S.; Hill, M. Penetration of corticosteroids into the lung: Evidence for a difference between methylprednisolone and prednisolone. J. Allergy Clin. Immunol. 1989, 84, 867–873. [Google Scholar] [CrossRef]
- Greos, L.S.; Vichyanond, P.; Bloedow, D.C.; Irvin, C.G.; Larsen, G.L.; Szefler, S.J.; Hill, M.R. Methylprednisolone Achieves Greater Concentrations in the Lung Than Prednisolone: A Pharmacokinetic Analysis. Am. Rev. Respir. Dis. 1991, 144, 586–592. [Google Scholar] [CrossRef]
- Meduri, G.U.; Chrousos, G.P. General Adaptation in Critical Illness: Glucocorticoid Receptor-alpha Master Regulator of Homeostatic Corrections. Front. Endocrinol. 2020, 11, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combes, A.; Costa, M.A.; Trouillet, J.L.; Robinson, C.; Sganzerla, D.; Loss, S.H.; Lora, P.; Micheletti, V.D. Morbidity, mortality, and quality-of-life outcomes of patients requiring ≥14 days of mechanical ventilation. Crit. Care Med. 2003, 31, 1373–1381. [Google Scholar] [CrossRef]
- Wilson, M.E.; Barwise, A.; Heise, K.J.; Loftsgard, T.O.; Dziadzko, M.; Cheville, A.; Majzoub, A.; Novotny, P.J.; Gajic, O.; Biehl, M. Long-Term Return to Functional Baseline After Mechanical Ventilation in the ICU. Crit. Care Med. 2018, 46, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Nathanson, B.H.; Ways, J.; Shorr, A.F. Characteristics, Hospital Course, and Outcomes of Patients Requiring Prolonged Acute Versus Short-Term Mechanical Ventilation in the United States, 2014–2018. Crit. Care Med. 2020, 48, 1587–1594. [Google Scholar] [CrossRef]
- Giordano, G.; Pugliese, F.; Bilotta, F. Mechanical ventilation and long-term neurocognitive impairment after acute respiratory distress syndrome. Crit. Care 2020, 24, 30. [Google Scholar] [CrossRef] [Green Version]
- Straub, R.H.; Schradin, C. Chronic inflammatory systemic diseases—An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. Public Health 2016, 2016, 35–37. [Google Scholar] [CrossRef] [Green Version]
- Buttgereit, F. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: Current questions and tentative answers in rheumatology. Ann. Rheum. Dis. 2002, 61, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
- Jantz, M.A.; Sahn, S.A. Corticosteroids in Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 1999, 160, 1079–1100. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Kanangat, S.; Bronze, M.; Patterson, D.R.; Meduri, C.U.; Pak, C.; Tolley, E.A.; Schaberg, D.R. Effects of Methylprednisolone on Intracellular Bacterial Growth. Clin. Diagn. Lab. Immunol. 2001, 8, 1156–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, C.R.; Vysokanov, A.; Mukherjee, A.; Ludden, T.M.; Tolley, E.; Meduri, G.U.; Dalton, J.T. Time-Variant Increase in Methylprednisolone Clearance in Patients with Acute Respiratory Distress Syndrome: A Population Pharmacokinetic Study. J. Clin. Pharmacol. 2001, 41, 415–424. [Google Scholar] [CrossRef]
- Ibarra-Estrada, M.A.; Chávez-Peña, Q.; Reynoso-Estrella, C.I.; Rios-Zermeño, J.; Aguilera-González, P.E.; García-Soto, M.A.; Aguirre-Avalos, G. Timing, method and discontinuation of hydrocortisone administration for septic shock patients. World J. Crit. Care Med. 2017, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, M.; Urbino, R.; Potena, A.; Piattella, M.; Parigi, P.; Puccio, G.; Della Porta, R.; Giorgio, C.; Blasi, F.; Umberger, R.; et al. Hydrocortisone Infusion for Severe Community-acquired Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Abdelsalam Rezk, N.; Mohamed Ibrahim, A. Effects of methyl prednisolone in early ARDS. Egypt. J. Chest Dis. Tuberc. 2013, 62, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Weber-Carstens, S.; Keh, D. Bolus or continuous hydrocortisone—That is the question. Crit. Care 2007, 11, 113. [Google Scholar] [CrossRef] [Green Version]
- Loisa, P.; Parviainen, I.; Tenhunen, J.; Hovilehto, S.; Ruokonen, E. Effect of mode of hydrocortisone administration on glycemic control in patients with septic shock: A prospective randomized trial. Crit. Care 2007, 11, R21. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.S.; Osuchowski, M.F.; Payen, D.; Torres, A.; Dickel, S.; Skirecki, T. Renaissance of glucocorticoids in critical care in the era of COVID-19: Ten urging questions. Crit. Care 2022, 26, 308. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS). Clinical Trials Network. Efficacy and Safety of Corticosteroids for Persistent Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2006, 354, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Bridges, L.; Siemieniuk, R.A.C.; Kocak, M. An Exploratory Reanalysis of the Randomized Trial on Efficacy of Corticosteroids as Rescue Therapy for the Late Phase of Acute Respiratory Distress Syndrome*. Crit. Care Med. 2018, 46, 884–891. [Google Scholar] [CrossRef]
- Chen, P.; Cheng, C.; Li, L.; Yu, C. Pneumonia rebound after stopping steroid in a patient with COVID-19: A case report. Respirol. Case Rep. 2021, 9, e0869. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Ho, L. Disease progression after discontinuation of corticosteroid treatment in a COVID-19 patient with ARDS. Asian Pac. J. Trop. Med. 2022, 15, 47. [Google Scholar] [CrossRef]
- Murray, J.F.; Matthay, M.A.; Luce, J.M.; Flick, M.R. An Expanded Definition of the Adult Respiratory Distress Syndrome. Am. Rev. Respir. Dis. 1988, 138, 720–723. [Google Scholar] [CrossRef]
- Meduri, G.U.; Headley, S.; Kohler, G.; Stentz, F.; Tolley, E.; Umberger, R.; Leeper, K. Persistent Elevation of Inflammatory Cytokines Predicts a Poor Outcome in ARDS. Chest 1995, 107, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Tolley, E.A.; Chrousos, G.P.; Stentz, F. Prolonged Methylprednisolone Treatment Suppresses Systemic Inflammation in Patients with Unresolving Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2002, 165, 983–991. [Google Scholar] [CrossRef] [Green Version]
- Salton, F.; Confalonieri, P.; Torregiani, C.; Ruaro, B.; Confalonieri, M. Higher, but Not Too High, Dose Is Only One Determinant of Corticosteroids Treatment Success in Severe COVID-19. Ann. Am. Thorac. Soc. 2023; epub ahead of print. [Google Scholar] [CrossRef]
- Meduri, G.U.; Headley, S.; Tolley, E.; Shelby, M.; Stentz, F.; Postlethwaite, A. Plasma and BAL Cytokine Response to Corticosteroid Rescue Treatment in Late ARDS. Chest 1995, 108, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Chriguer, R.S.; Elias, L.L.K.; da Silva, I.M.; Vieira, J.G.H.; Moreira, A.C.; de Castro, M. Glucocorticoid Sensitivity in Young Healthy Individuals: In Vitro and in Vivo Studies. J. Clin. Endocrinol. Metab. 2005, 90, 5978–5984. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.; Pretorius, C.J.; Ungerer, J.P.J.; Cardinal, J.; Blumenthal, A.; Presneill, J.; Gatica-Andrades, M.; Jarrett, P.; Lassig-Smith, M.; Stuart, J.; et al. Glucocorticoid Sensitivity Is Highly Variable in Critically Ill Patients with Septic Shock and Is Associated with Disease Severity. Crit. Care Med. 2016, 44, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Golden, E.; Freire, A.X.; Taylor, E.; Zaman, M.; Carson, S.J.; Gibson, M.; Umberger, R. Methylprednisolone Infusion in Early Severe ARDS. Chest 2007, 131, 954–963. [Google Scholar] [CrossRef] [Green Version]
- Van den Akker, E.L.T.; Koper, J.W.; Joosten, K.; de Jong, F.H.; Hazelzet, J.A.; Lamberts, S.W.; Hokken-Koelega, A.C. Glucocorticoid receptor mRNA levels are selectively decreased in neutrophils of children with sepsis. Intensive Care Med. 2009, 35, 1247–1254. [Google Scholar] [CrossRef] [Green Version]
- Müller, B.; Peri, G.; Doni, A.; Landmann, R.; Pasqualini, F.; Mantovani, A. High circulating levels of the IL-1 type II decoy receptor in critically ill patients with sepsis: Association of high decoy receptor levels with glucocorticoid administration. J. Leukoc. Biol. 2002, 72, 643–649. [Google Scholar] [CrossRef] [PubMed]
- De Kruif, M.D.; Lemaire, L.C.; Giebelen, I.A.; Struck, J.; Morgenthaler, N.G.; Papassotiriou, J.; Elliott, P.J.; van der Poll, T. The influence of corticosteroids on the release of novel biomarkers in human endotoxemia. Intensive Care Med. 2008, 34, 518–522. [Google Scholar] [CrossRef]
- Perren, A.; Cerutti, B.; Lepori, M.; Senn, V.; Capelli, B.; Duchini, F.; Domenighetti, G. Influence of Steroids on Procalcitonin and C-reactive Protein in Patients with COPD and Community-acquired Pneumonia. Infection 2008, 36, 163–166. [Google Scholar] [CrossRef]
- Rinaldi, S.; Adembri, C.; Grechi, S.; De Gaudio, A.R. Low-dose hydrocortisone during severe sepsis: Effects on microalbuminuria. Crit. Care Med. 2006, 34, 2334–2339. [Google Scholar] [CrossRef]
- Wilkinson, L.; Verhoog, N.J.D.; Louw, A. Disease- and treatment-associated acquired glucocorticoid resistance. Endocr. Connect. 2018, 7, R328–R349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, J.M.; Monsalves-Alvarez, M.; Henriquez, S.; Llanos, M.N.; Troncoso, R. Glucocorticoid resistance in chronic diseases. Steroids 2016, 115, 182192. [Google Scholar] [CrossRef]
- Meduri, G.U.; Yates, C.R. Systemic Inflammation-Associated Glucocorticoid Resistance and Outcome of ARDS. Ann. N. Y. Acad. Sci. 2004, 1024, 24–53. [Google Scholar] [CrossRef]
- Koper, J.W.; van Rossum, E.F.C.; van den Akker, E.L.T. Glucocorticoid receptor polymorphisms and haplotypes and their expression in health and disease. Steroids 2014, 92, 62–73. [Google Scholar] [CrossRef]
- Bergquist, M.; Nurkkala, M.; Rylander, C.; Kristiansson, E.; Hedenstierna, G.; Lindholm, C. Expression of the glucocorticoid receptor is decreased in experimental Staphylococcus aureus sepsis. J. Infect. 2013, 67, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Indyk, J.A.; Candido-Vitto, C.; Wolf, I.M.; Venkataraman, S.; Munoz, R.; Saladino, R.A.; Witchel, S.F.; Defranco, D.B. Reduced Glucocorticoid Receptor Protein Expression in Children with Critical Illness. Horm. Res. Paediatr. 2013, 79, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Floros, G.; Jahaj, E.; Stamogiannos, G.; Gennimata, S.; Vassiliadi, D.A.; Tsagarakis, S.; Tzanela, M.; Ilias, I.; Orfanos, S.E.; et al. Decreased glucocorticoid receptor expression during critical illness. Eur. J. Clin. Investig. 2019, 49, e13073. [Google Scholar] [CrossRef]
- Guerrero, J.; Gatica, H.A.; Rodríguez, M.; Estay, R.; Goecke, I. Septic serum induces glucocorticoid resistance and modifies the expression of glucocorticoid isoforms receptors: A prospective cohort study and in vitro experimental assay. Crit. Care 2013, 17, R107. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lee, H.K. Re-analysis of Single Cell Transcriptome Reveals That the NR3C1-CXCL8-Neutrophil Axis Determines the Severity of COVID-19. Front. Immunol. 2020, 11, 02145. [Google Scholar] [CrossRef] [PubMed]
- Van der Voort, P.H.J.; Gerritsen, R.T.; Bakker, A.J.; Boerma, E.C.; Kuiper, M.A.; de Heide, L. HDL-cholesterol level and cortisol response to synacthen in critically ill patients. Intensive Care Med. 2003, 29, 2199–2203. [Google Scholar] [CrossRef]
- Yates, C.R.; Chang, C.; Kearbey, J.D.; Yasuda, K.; Schuetz, E.G.; Miller, D.D.; Dalton, J.T.; Swaan, P.W. Structural Determinants of P-Glycoprotein-Mediated Transport of Glucocorticoids. Pharm. Res. 2003, 20, 1794–1803. [Google Scholar] [CrossRef]
- Okamoto, K.; Tanaka, H.; Makino, Y.; Makino, I. Restoration of the Glucocorticoid Receptor Function by the Phosphodiester Compound of Vitamins C and E, EPC-K1 (l-Ascorbic Acid 2-[3, 4-Dihydro-2, 5, 7, 8-tetramethyl-2-(4, 8, 12-trimethyltridecyl)-2H-1-benzopyran-6-yl Hydrogen Phosphate] Potassium Salt), via a Redox-dependent Mechanism. Biochem. Pharmacol. 1998, 56, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Tanaka, H.; Ogawa, H.; Makino, Y.; Eguchi, H.; Hayashi, S.; Yoshikawa, N.; Poellinger, L.; Umesono, K.; Makino, I. Redox-dependent Regulation of Nuclear Import of the Glucocorticoid Receptor. J. Biol. Chem. 1999, 274, 10363–10371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, D.S. Are statins analogues of vitamin D? Lancet 2006, 368, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Hoepner, R.; Bagnoud, M.; Pistor, M.; Salmen, A.; Briner, M.; Synn, H.; Schrewe, L.; Guse, K.; Ahmadi, F.; Demir, S.; et al. Vitamin D increases glucocorticoid efficacy via inhibition of mTORC1 in experimental models of multiple sclerosis. Acta Neuropathol. 2019, 138, 443–456. [Google Scholar] [CrossRef] [Green Version]
- Ojaimi, S.; Skinner, N.A.; Strauss, B.J.; Sundararajan, V.; Woolley, I.; Visvanathan, K. Vitamin d deficiency impacts on expression of toll-like receptor-2 and cytokine profile: A pilot study. J. Transl. Med. 2013, 11, 176. [Google Scholar] [CrossRef] [Green Version]
- Padayatty, S.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [Green Version]
- Patak, P.; Willenberg, H.S.; Bornstein, S.R. Vitamin C Is an Important Cofactor for Both Adrenal Cortex and Adrenal Medulla. Endocr. Res. 2004, 30, 871–875. [Google Scholar] [CrossRef]
- Björkhem, I.; Kallner, A.; Karlmar, K.E. Effects of ascorbic acid deficiency on adrenal mitochondrial hydroxylations in guinea pigs. J. Lipid. Res. 1978, 19, 695–704. [Google Scholar] [CrossRef]
- Pariante, C.M.; Pearce, B.D.; Pisell, T.L.; Sanchez, C.I.; Po, C.; Su, C.; Miller, A.H. The Proinflammatory Cytokine, Interleukin-1α, Reduces Glucocorticoid Receptor Translocation and Function. Endocrinology 1999, 140, 4359–4366. [Google Scholar] [CrossRef]
- Meduri, G.U.; Muthiah, M.P.; Carratù, P.; Eltorky, M.; Chrousos, G.P. Nuclear Factor-ĸB- and Glucocorticoid Receptor α- Mediated Mechanisms in the Regulation of Systemic and Pulmonary Inflammation during Sepsis and Acute Respiratory Distress Syndrome. Neuroimmunomodulation 2005, 12, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Bantel, H.; Schmitz, M.L.; Raible, A.; Gregor, M.; Schulze-Osthoff, K. Critical role of nuclear factor-κB and stress-activated protein kinases in steroid unresponsiveness. FASEB J. 2002, 16, 1–19. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, L.J.; Zhou, Q.G. Neuronal nitric oxide synthase is an endogenous negative regulator of glucocorticoid receptor in the hippocampus. Neurol. Sci. 2013, 34, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, K.A.; Matić, G.; Meshinchi, S.; Bresnick, E.H.; Pratt, W.B. Redox manipulation of DNA binding activity and BuGR epitope reactivity of the glucocorticoid receptor. J. Biol. Chem. 1991, 266, 10505–10509. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Okamoto, K.; Yoshikawa, N.; Aoshima, M.; Hirota, K.; Yodoi, J.; Umesono, K.; Makino, I.; Tanaka, H. Thioredoxin: A redox-regulating cellular cofactor for glucocorticoid hormone action. Cross talk between endocrine control of stress response and cellular antioxidant defense system. J. Clin. Investig. 1996, 98, 2469–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duma, D.; Silva-Santos, J.E.; Assreuy, J. Inhibition of glucocorticoid receptor binding by nitric oxide in endotoxemic rats. Crit. Care Med. 2004, 32, 2304–2310. [Google Scholar] [CrossRef]
- Hakim, A.; Barnes, P.J.; Adcock, I.M.; Usmani, O.S. Importin-7 mediates glucocorticoid receptor nuclear import and is impaired by oxidative stress, leading to glucocorticoid insensitivity. FASEB J. 2013, 27, 4510–4519. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.; Kelleher, D. Glucocorticoid resistance in inflammatory bowel disease. J. Endocrinol. 2003, 178, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Hearing, S.D.; Norman, M.; Smyth, C.; Foy, C.; Dayan, C.M. Wide Variation in Lymphocyte Steroid Sensitivity Among Healthy Human Volunteers. J. Clin. Endocrinol. Metab. 1999, 84, 4149–4154. [Google Scholar] [CrossRef]
- Smit, P.; Russcher, H.; de Jong, F.H.; Brinkmann, A.O.; Lamberts, S.W.J.; Koper, J.W. Differential Regulation of Synthetic Glucocorticoids on Gene Expression Levels of Glucocorticoid-Induced Leucine Zipper and Interleukin. J. Clin. Endocrinol. Metab. 2005, 90, 2994–3000. [Google Scholar] [CrossRef] [Green Version]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef]
- Yuanjing, C.; Li, K.; Pu, H.; Wu, T. Corticosteroids for pneumonia. Cochrane Database Syst. Rev. 2009, CD007720. [Google Scholar] [CrossRef]
- Edalatifard, M.; Akhtari, M.; Salehi, M.; Naderi, Z.; Jamshidi, A.; Mostafaei, S.; Najafizadeh, S.R.; Farhadi, E.; Jalili, N.; Esfahani, M.; et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. Eur. Respir. J. 2020, 56, 2002808. [Google Scholar] [CrossRef]
- Tang, X.; Feng, Y.M.; Ni, J.X.; Zhang, J.Y.; Liu, L.M.; Hu, K.; Wu, X.Z.; Zhang, J.X.; Chen, J.W.; Zhang, J.C.; et al. Early Use of Corticosteroid May Prolong SARS-CoV-2 Shedding in Non-Intensive Care Unit Patients with COVID-19 Pneumonia: A Multicenter, Single-Blind, Randomized Control Trial. Respiration 2021, 100, 116–126. [Google Scholar] [CrossRef]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19. JAMA 2020, 324, 1307. [Google Scholar] [CrossRef] [PubMed]
- Corral-Gudino, L.; Bahamonde, A.; Arnaiz-Revillas, F.; Gómez-Barquero, J.; Abadía-Otero, J.; García-Ibarbia, C.; Mora, V.; Cerezo-Hernández, A.; Hernández, J.L.; López-Muñíz, G.; et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia. Wien. Klin. Wochenschr. 2021, 133, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Leistner, R.; Schroeter, L.; Adam, T.; Poddubnyy, D.; Stegemann, M.; Siegmund, B.; Maechler, F.; Geffers, C.; Schwab, F.; Gastmeier, P.; et al. Corticosteroids as risk factor for COVID-19-associated pulmonary aspergillosis in intensive care patients. Crit. Care 2022, 26, 30. [Google Scholar] [CrossRef] [PubMed]
- Jeronimo, C.M.P.; Farias, M.E.L.; Val, F.F.A.; Sampaio, V.S.; Alexandre, M.A.A.; Melo, G.C.; Safe, I.P.; Borba, M.G.S.; Netto, R.L.A.; Maciel, A.B.S.; et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized with Coronavirus Disease 2019 (COVID-19; Metcovid): A Randomized, Double-blind, Phase IIb, Placebo-controlled Trial. Clin. Infect. Dis. 2021, 72, e373–e381. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Bancos, I. Glucocorticoid induced adrenal insufficiency. BMJ 2021, 12, 1380. [Google Scholar] [CrossRef]
- Nawab, Q.U.A.; Golden, E.; Confalonieri, M.; Umberger, R.; Meduri, U.G. Corticosteroid treatment in severe community-acquired pneumonia: Duration of treatment affects control of systemic inflammation and clinical improvement. Intensive Care Med. 2011, 37, 1553–1554. [Google Scholar] [CrossRef] [PubMed]
- Mondini, L.; Salton, F.; Trotta, L.; Bozzi, C.; Pozzan, R.; Barbieri, M.; Tavano, S.; Lerda, S.; Hughes, M.; Confalonieri, M.; et al. Host-Based Treatments for Severe COVID-19. Curr. Issues Mol. Biol. 2023, 45, 3102–3121. [Google Scholar] [CrossRef] [PubMed]
- WHO. Therapeutics and COVID-19: Living Guideline, 13 January 2023 (WHO/2019-NCoV/Therapeutics/2023.1); World Health Organization: Geneva, Switzerland, 2023.
- Salton, F.; Confalonieri, P.; Campisciano, G.; Cifaldi, R.; Rizzardi, C.; Generali, D.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lapadula, G.; et al. Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS. J. Clin. Med. 2022, 11, 2951. [Google Scholar] [CrossRef] [PubMed]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Tocilizumab in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus (COVID-19)|Drugs. Available online: https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (accessed on 13 February 2023).
- COVID-19 Treatments. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-treatments (accessed on 13 April 2023).
- Kyriazopoulou, E.; Huet, T.; Cavalli, G.; Gori, A.; Kyprianou, M.; Pickkers, P.; Eugen-Olsen, J.; Clerici, M.; Veas, F.; Chatellier, G.; et al. Effect of Anakinra on Mortality in Patients with COVID-19: A Systematic Review and Patient-Level Meta-Analysis. Lancet Rheumatol. 2021, 3, e690–e697. [Google Scholar] [CrossRef]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, J.; Abbas, K.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abbott, A.; et al. Baricitinib in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial and Updated Meta-Analysis. Lancet 2022, 400, 359–368. [Google Scholar] [CrossRef]
- Grundeis, F.; Ansems, K.; Dahms, K.; Thieme, V.; Metzendorf, M.I.; Skoetz, N.; Benstoem, C.; Mikolajewska, A.; Griesel, M.; Fichtner, F.; et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst. Rev. 2023, 1, CD014962. [Google Scholar] [CrossRef]
- Mastruzzo, C.; Commodari, E.; Grasso, U.; La Rosa, V.L.; Balsamo, D.; Circo, C.; Oliveri, R. Early Stage Combination Treatment with Methylprednisolone Pulse and Remdesivir for Severe COVID-19 Pneumonia. Int. J. Environ. Res. Public Health 2023, 20, 1081. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Y.; Wang, T.; Xie, F. Innate and adaptive immune response in SARS-CoV-2 infection—Current perspectives. Front. Immunol. 2022, 13, 1053437. [Google Scholar] [CrossRef] [PubMed]
- Gressens, S.B.; Esnault, V.; De Castro, N.; Sellier, P.; Sene, D.; Chantelot, L.; Hervier, B.; Delaugerre, C.; Chevret, S.; Molina, J.M.; et al. Remdesivir in combination with dexamethasone for patients hospitalized with COVID-19: A retrospective multicenter study. PLoS ONE 2022, 17, e0262564. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, S.; Ohshimo, S.; Takeuchi, M.; Yasuda, H.; Ichikado, K.; Tsushima, K.; Egi, M.; Hashimoto, S.; Shime, N.; Saito, O.; et al. ARDS Clinical Practice Guideline 2021. J. Intensive Care 2022, 10, 32. [Google Scholar] [CrossRef]
- Apostolo, D.; D’Onghia, D.; Tonello, S.; Minisini, R.; Baricich, A.; Gramaglia, C.; Patrucco, F.; Zeppegno, P.; Acquaviva, A.; Balbo, P.E.; et al. Decreased Gas6 and sAxl Plasma Levels Are Associated with Hair Loss in COVID-19 Survivors. Int. J. Mol. Sci. 2023, 24, 6257. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.Y.; Lin, H.H.; Huang, C.T.; Kuo, P.H.; Wu, H.D.; Yu, C.J. Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: A systematic review and meta-analysis. Crit. Care 2014, 18, R63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dequin, P.F.; Meziani, F.; Quenot, J.P.; Kamel, T.; Ricard, J.D.; Badie, J.; Reignier, J.; Heming, N.; Plantefève, G.; Souweine, B.; et al. Hydrocortisone in Severe Community-Acquired Pneumonia. N. Engl. J. Med. 2023, 388, 1931–1941. [Google Scholar] [CrossRef]
- Blum, C.A.; Nigro, N.; Briel, M.; Schuetz, P.; Ullmer, E.; Suter-Widmer, I.; Winzeler, B.; Bingisser, R.; Elsaesser, H.; Drozdov, D.; et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015, 385, 1511–1518. [Google Scholar] [CrossRef]
- Ruaro, B.; Confalonieri, P.; Pozzan, R.; Tavano, S.; Mondini, L.; Baratella, E.; Pagnin, A.; Lerda, S.; Geri, P.; Biolo, M.; et al. Severe COVID-19 ARDS Treated by Bronchoalveolar Lavage with Diluted Exogenous Pulmonary Surfactant as Salvage Therapy: In Pursuit of the Holy Grail? J. Clin. Med. 2022, 11, 3577. [Google Scholar] [CrossRef]
- Russo, A.; Davoli, C.; Borrazzo, C.; Olivadese, V.; Ceccarelli, G.; Fusco, P.; Lazzaro, A.; Lionello, R.; Ricchio, M.; Serapide, F.; et al. Clinical Characteristics and Outcome of Hospitalized COVID-19 Patients Treated with Standard Dose of Dexamethasone or High Dose of Methylprednisolone. Biomedicines 2022, 10, 1548. [Google Scholar] [CrossRef]
- Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022, 11, 1704. [Google Scholar] [CrossRef]
- Baratella, E.; Bussani, R.; Zanconati, F.; Marrocchio, C.; Fabiola, G.; Braga, L.; Maiocchi, S.; Berlot, G.; Volpe, M.C.; Moro, E.; et al. Radiological-pathological signatures of patients with COVID-19-related pneumomediastinum: Is there a role for the Sonic hedgehog and Wnt5a pathways? ERJ Open Res. 2021, 7, 00346-2021. [Google Scholar] [CrossRef] [PubMed]

| Authors and Year of Publication | Title | Design | Drugs | Examined Patients | Results |
|---|---|---|---|---|---|
| The RECOVERY Collaborative Group (2021) [16] | Dexamethasone in hospitalized patients with COVID-19 | Prospective randomised trial compared with placebo | Dexamethasone 6 mg vs. placebo | 4321 placebo patients vs. 2104 treated, hospitalised with COVID-19 confirmed | Only patients needing oxygen support showed lower mortality |
| Wagner C, Griesel M, Mikolajewska A, et al. (2021) [8] | Systemic corticosteroids for the treatment of COVID-19. | Living systematic review | A total of 3072 participants were randomised to corticosteroid arms and the majority received dexamethasone (n = 2322). | 11 RCTs in 8075 participants, of whom 7041 (87%) originated from high-income countries. | 11 studies with 8075 people. About 3000 people received corticosteroids, mostly dexamethasone (2322 people). Most studies took place in high-income countries. There were also found 42 ongoing studies, and 16 completed studies that have not yet published their results. |
| The COVID STEROID 2 Trial Group (2021) [21] | Effect of 12 mg vs. 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: The COVID STEROID 2 randomized trial | Multicentre, randomised clinical trial | IV Dexamethasone 12 mg vs. IV Dexamethasone 6 mg IV | 982 adult patients with COVID-19 needing at least 10 L/min of oxygen or MV (mechanical ventilation) | No statistically significant difference on ventilator-free days over 28 days |
| The Writing Committee for the REMAP-CAP Investigators, Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, et al. (2020) [18] | Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: The REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial | Bayesian randomised clinical trial | No hydrocortisone vs. hydrocortisone (50 mg or 100 mg every 6 h or shock-dependent dosage) | 108 non-treated vs. 295 treated adult patients in ICU with severe SARS-CoV-2 pneumonia | Patients treated with hydrocortisone showed improvement in organ support-free days within 21 days |
| Dequin P-F, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. (2020) [19] | Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: A randomized clinical trial | Multicentre randomised double-blind sequential trial | Hydrocortisone 200 mg/die tapered to 100 mg and then 50 mg vs. placebo | 73 placebo vs. 76 treated, patients admitted to ICU with ARDS secondary to COVID-19 infection | The study was stopped early due to no significant reduction in treatment failure in hydrocortisone group |
| Sterne JAC, Murthy S, Diaz J V., et al. (2020) [4] | Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19. | Prospective meta-analysis | Systemic hydrocortisone, dexamethasone, or methylprednisolone (678 patients) or usual care or placebo (1025 patients). | 1703 patients with critical COVID-19 from 7 RCTs | Administration of systemic corticosteroids, compared with usual care or placebo, was associated with lower 28-day all-cause mortality |
| Salton F, Confalonieri P, Meduri GU, Santus P, Harari S, Scala R, et al. (2020) [26] | Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia | Multicentre, observational, longitudinal study | Methylprednisolone 80 mg IV, followed by an infusion of 80 mg/d in 240 mL of normal saline at 10 mL/h for at least 8 days, until achieving either a PaO2:FiO2 > 350 mmHg or a CRP < 20 mg/L; then MP 16 mg o.so or 20 mg IV twice daily until CRP reached < 20% of the normal range or a PaO2:FiO2 > 400 (alternative SatO2 ≥ 95% in room air) | 83 treated patients vs. 90 control patients with severe COVID-19 pneumonia | Early administration of prolonged, low dose MP treatment was associated with a significantly lower hazard of death and decreased ventilator dependence |
| Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, et al. (2021) [23] | Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: A triple-blinded randomized controlled trial | Prospective triple-blinded randomised controlled trial | Methylprednisolone (2 mg/kg/day) or dexamethasone (6 mg/day) | 47 patients on MP vs. 46 patients on DM hospitalised with COVID-19 pneumonia | Superiority of methylprednisolone over dexamethasone |
| Saeed MAM, Mohamed AH, Owaynat AH. (2022) [22] | Comparison between methylprednisolone infusion and dexamethasone in COVID-19 ARDS mechanically ventilated patients | Prospective cohort study | Dexamethasone 6 mg/day vs methylprednisolone 2 mg/kg/day IV | 192 patients treated with dexamethasone vs. 222 patients treated with MP admitted in ICU with SARS-CoV-2 pneumonia (confirmed) | The methylprednisolone group showed an improvement of the inflammatory markers for cytokine storm in comparison to the patients on dexamethasone |
| Salton F, Confalonieri P, Centanni S, Mondoni M, Petrosillo N, Bonfanti P, et al. (2022) [25] | Prolonged higher dose methylprednisolone vs. conventional dexamethasone in COVID-19 pneumonia: A randomised controlled trial (MEDEAS) | Multicentre, open-label randomised clinical trial | Methylprednisolone 80 mg IV in continuous daily infusion for 8 days followed by slow tapering vs. dexamethasone 6 mg daily | 337 patients treated with methylprednisolone vs. 340 in dexamethasone group with COVID-19 pneumonia requiring oxygen or non-invasive respiratory support | No significant differences in mortality between the two groups |
| Taboada M, Rodríguez N, Varela PM, et al. (2022) [20] | Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial. | Randomised, open-label, controlled trial | Patients were randomly assigned in a 1:1 ratio to receive low-dose dexamethasone (6 mg once daily for 10 days) or high-dose dexamethasone (20 mg once daily for 5 days, followed by 10 mg once daily for an additional 5 days). | 200 hospitalised patients with confirmed COVID-19 pneumonia needing oxygen therapy | Among hospitalised COVID-19 patients needing oxygen therapy, high dose of dexamethasone reduced clinical worsening within 11 days after randomisation, compared with low dose. |
| Lamontagne F, Agarwal A, Rochwerg B, et al. (2020) [14] | A living WHO guideline on drugs for COVID-19 | Living guideline based on living systematic review and network analysis | Remdesivir 200 mg IV on the first day then 100 mg IV/day for 5–10 days and corticosteroids (dexamethasone 6 mg oral or IV once daily for 7–10 days, hydrocortisone 50 mg IV every 8 h for 7–10 days, methylprednisolone 10 mg IV every 6 h for 7–10 days, prednisone 40 mg oral daily for 7–10 days) | For remdesivir 4 studies with a total of 7347 patients ranging from non-severe to critical; for corticosteroids 8 RCTs with a total of 7184 patients ranging from non-severe to critical | Recommendation against remdesivir (weak); Recommendation in favour of corticosteroids (suggested regimen dexamethasone 6 mg oral or IV once daily for 7–10 days) for patients with severe and critical COVID-19 (strong), recommendation against corticosteroids in patients with non-severe COVID-19 (weak) |
| Hirano Y, Madokoro S, Kondo Y, Okamoto K, Tanaka H [17]. | Corticosteroid treatment for early acute respiratory distress syndrome: a systematic review and meta-analysis of randomized trials | Systematic review and meta-analysis of randomized controlled trials (RCTs) | RCTs analysing the efficacy of prolonged corticosteroid (methylprednisolone, dexamethasone) therapy in early ARDS | 4 RCTs, 385 patients in the corticosteroid group, 357 in the control group | Prolonged corticosteroid treatment in early ARDS improved the survival outcomes. |
| Disease-Related Factors | GR Number and Type (α vs. β) | |
| GR Binding Capacity (Bmax) | ▼ by Inflammatory State | |
| ▼ by Oxidative Stress | ||
| GR Function | ▼ by Hypovitaminosis *, Micronutrient Deficiencies | |
| Molecule-related factors | Binding affinity to GR (potency) | Increasing the dosage of GC can compensate for a reduced binding affinity |
| Treatment-related factors | initiation | GR saturation (dose-dependent) |
| Timing of initiation (early vs. late) | ||
| Dose-adjustments based on initial severity ‡ | ||
| over time | Optimal magnitude of exposure to GR → Bolus followed by continuous infusion | |
| Lung penetration and tissue concentration | ||
| Dose-adjustments based on clinical response § | ||
| Duration of treatment including tapering | ||
| potential complications | Hypothalamic-pituitary-axis suppression → Can be offset with slow tapering | |
| Failure to recognize infections in absence of fever → Can be offset with infection surveillance | ||
| Patients-related factors | Comorbidities associated with GC resistance † | |
| Interpersonal wide variability in achieved GC plasma concentration | ||
| Interpersonal wide variability in cellular sensitivity to exposure of similar GC concentration | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salton, F.; Confalonieri, P.; Meduri, G.U.; Mondini, L.; Trotta, L.; Barbieri, M.; Bozzi, C.; Torregiani, C.; Lerda, S.; Bellan, M.; et al. Theory and Practice of Glucocorticoids in COVID-19: Getting to the Heart of the Matter—A Critical Review and Viewpoints. Pharmaceuticals 2023, 16, 924. https://doi.org/10.3390/ph16070924
Salton F, Confalonieri P, Meduri GU, Mondini L, Trotta L, Barbieri M, Bozzi C, Torregiani C, Lerda S, Bellan M, et al. Theory and Practice of Glucocorticoids in COVID-19: Getting to the Heart of the Matter—A Critical Review and Viewpoints. Pharmaceuticals. 2023; 16(7):924. https://doi.org/10.3390/ph16070924
Chicago/Turabian StyleSalton, Francesco, Paola Confalonieri, Gianfranco Umberto Meduri, Lucrezia Mondini, Liliana Trotta, Mariangela Barbieri, Chiara Bozzi, Chiara Torregiani, Selene Lerda, Mattia Bellan, and et al. 2023. "Theory and Practice of Glucocorticoids in COVID-19: Getting to the Heart of the Matter—A Critical Review and Viewpoints" Pharmaceuticals 16, no. 7: 924. https://doi.org/10.3390/ph16070924
APA StyleSalton, F., Confalonieri, P., Meduri, G. U., Mondini, L., Trotta, L., Barbieri, M., Bozzi, C., Torregiani, C., Lerda, S., Bellan, M., Confalonieri, M., Ruaro, B., Tavano, S., & Pozzan, R. (2023). Theory and Practice of Glucocorticoids in COVID-19: Getting to the Heart of the Matter—A Critical Review and Viewpoints. Pharmaceuticals, 16(7), 924. https://doi.org/10.3390/ph16070924









