An In Silico Study Investigating Camptothecin-Analog Interaction with Human Protein Tyrosine Phosphatase, SHP2 (PTPN11)
Abstract
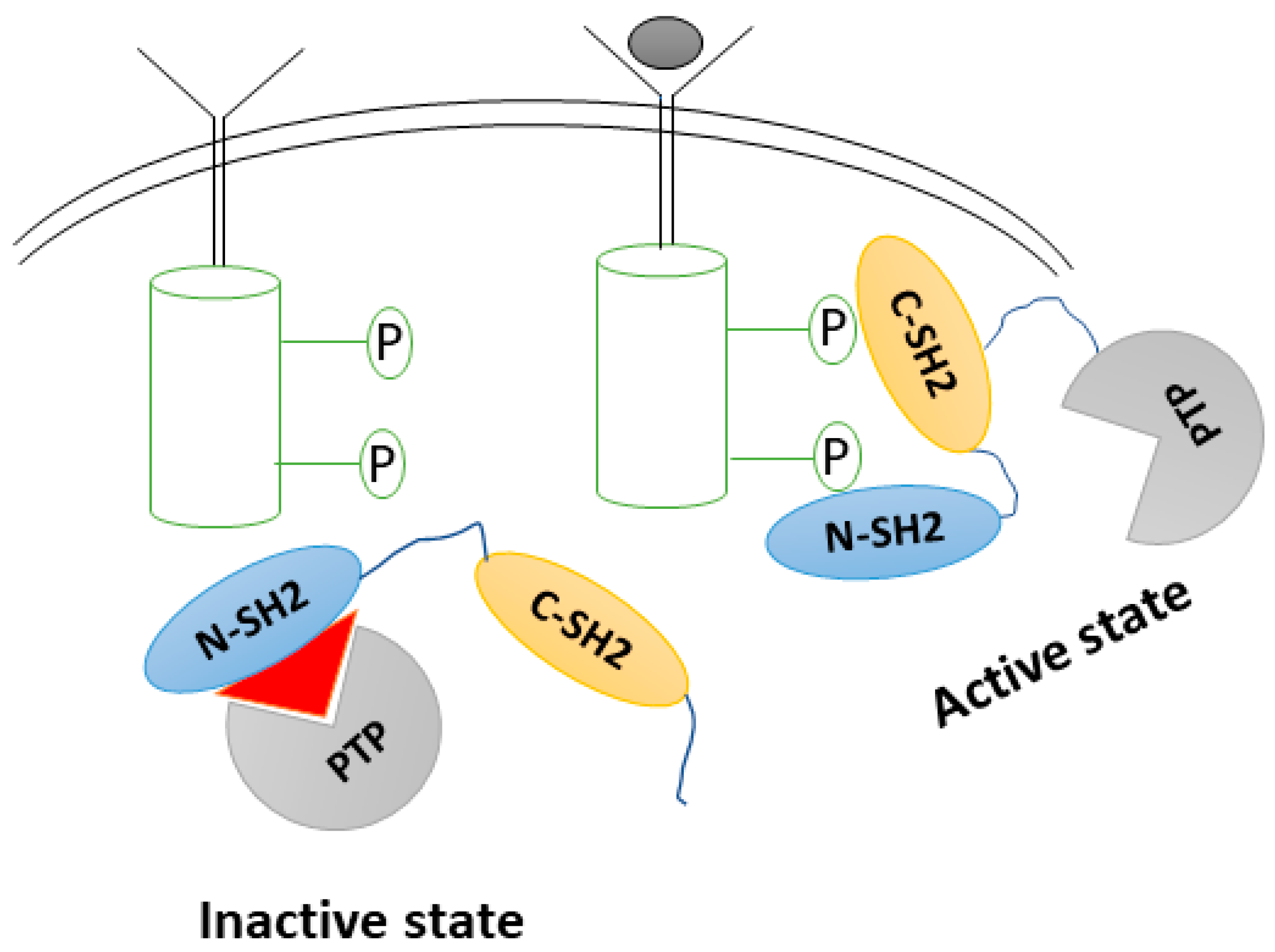
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterisation of Ligand
2.2. Prediction of Structure–Activity Relationship: ADMET of Compounds, FL118, and Irinotecan
2.3. Mutagenesis In Silico
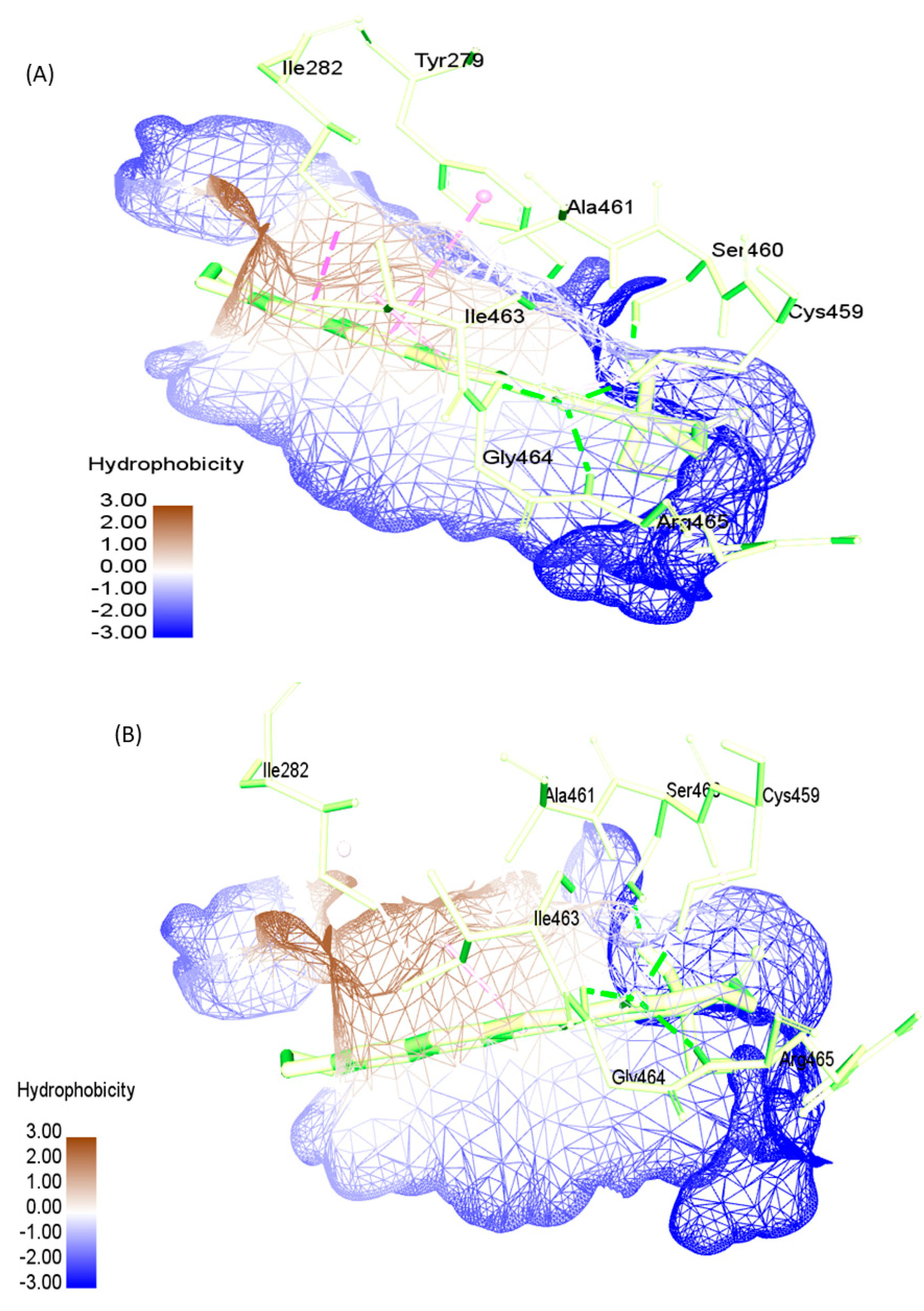
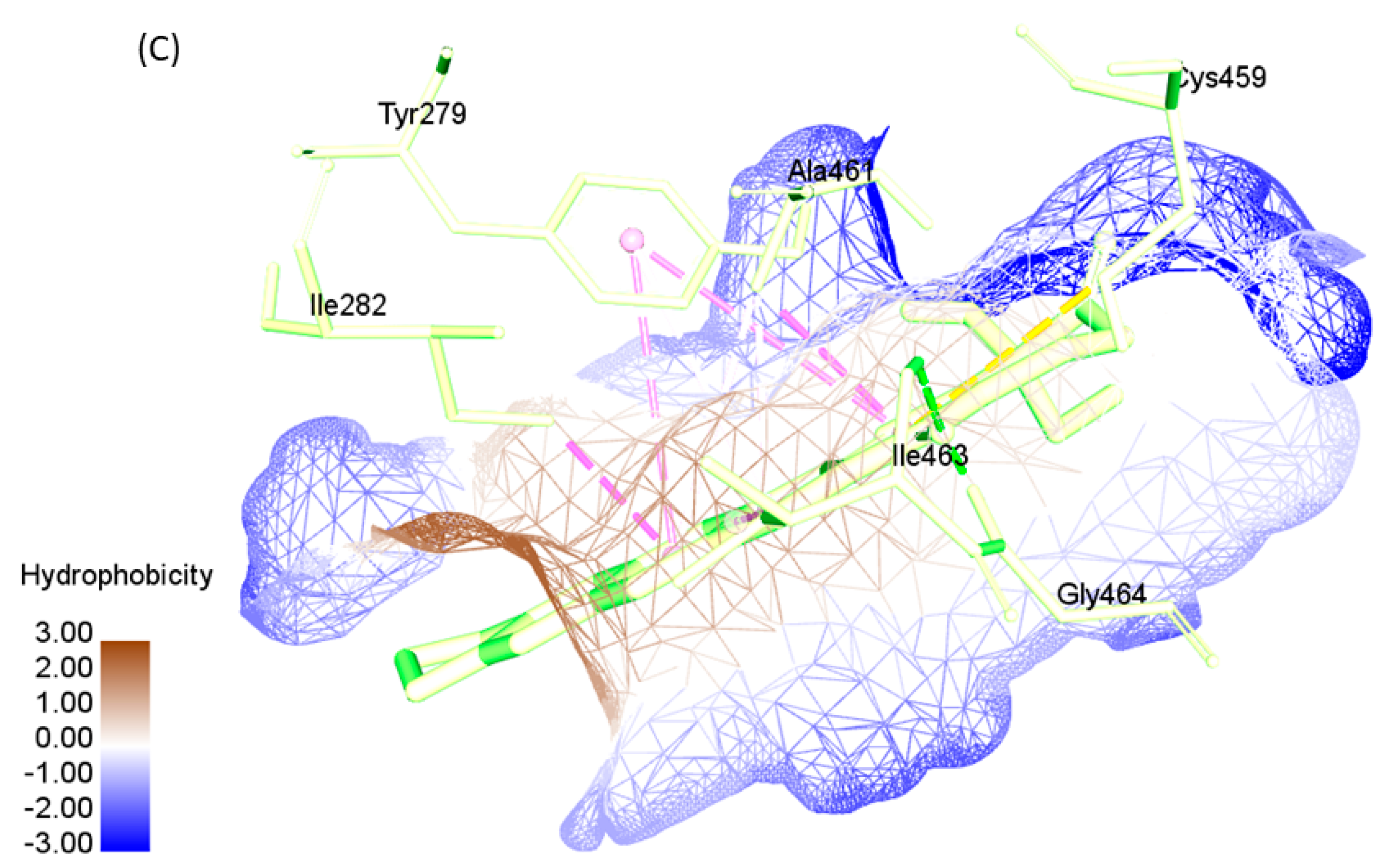
2.4. Active Site Recognition in Structures
2.5. Physicochemical Characterisation of SHP2-WT, SHP2-Y279C and SHP2-R465G Mutant Structures
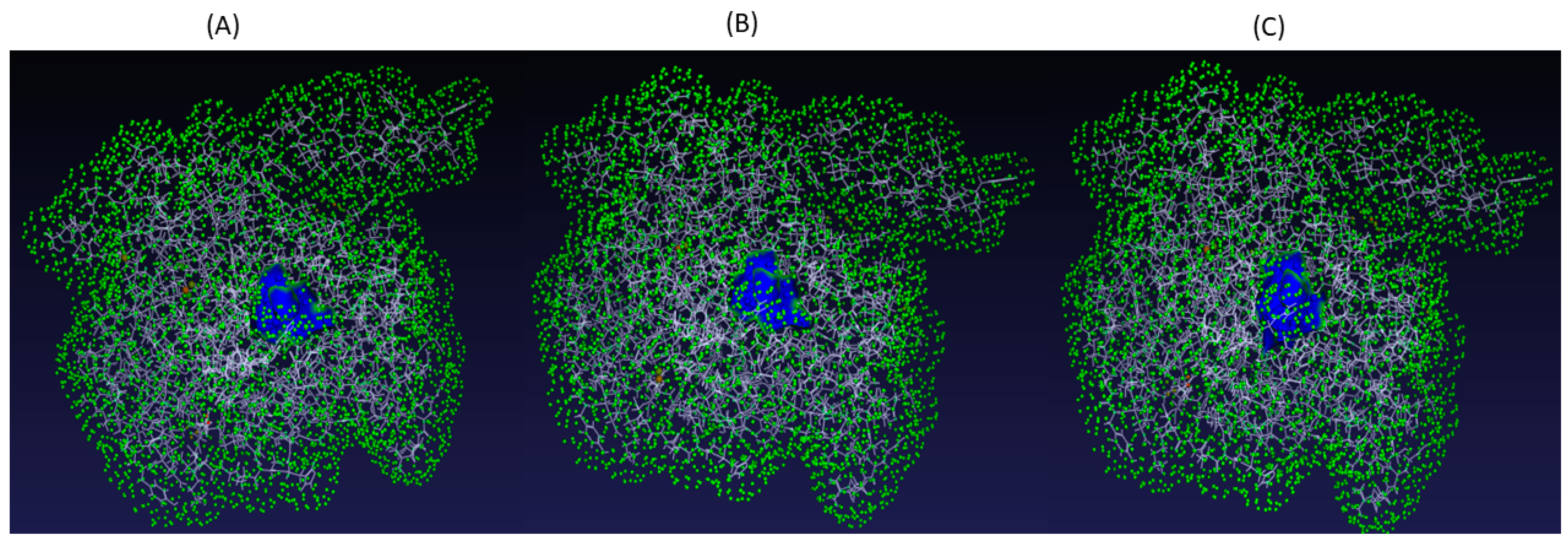
2.6. Modelling and Assessment of Target Protein Structures
2.7. Molecular Docking of FL118 and Irinotecan to PTPc-SHP2-Wildtype (SHP2-WT); and FL118 to Mutant SHP2 Structures
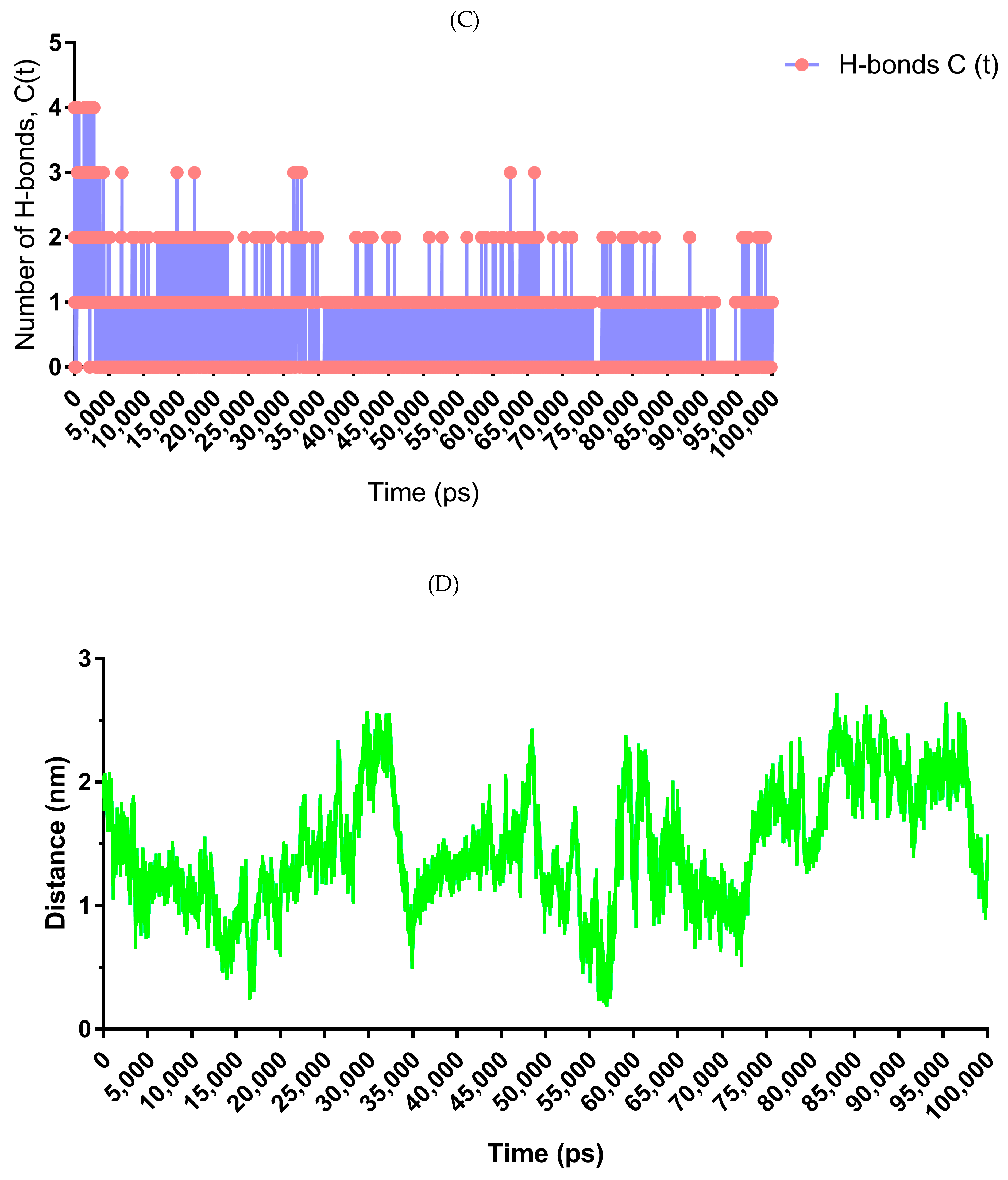
2.8. Model Simulation and Evaluation of FL118-SHP2 Wildtype Complex
3. Materials and Methods
3.1. Ligand Structure and Characterization
3.2. ADMET Profiling of Both Compounds Was Performed by Employing SwissAdme and Pro Tox-II Webservers
3.3. Target Structures
3.4. In Silico Mutagenesis
3.5. Active Site RECOGNITION
3.6. Model Simulation and Assessment
3.7. Molecular Docking
3.8. Docking Analysis of Ligand-Protein Complex
3.9. Molecular Dynamics Simulation for LIGAND–Protein Complex
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCR-ABL | Breakpoint cluster region-Abelson gene |
| CPT | Camptothecin |
| CSF1 | Colony stimulating factor 1 |
| CTLA | Cytotoxic T-lymphocyte-associated protein 4 |
| DUSP6 | Dual specificity protein phosphatase 6 |
| GAB | GRB2-associated binding protein |
| GPCR | G-protein-coupled receptor |
| GRAVY | Grand Average of Hydropathicity |
| GRB2 | Growth factor receptor-bound protein 2 |
| H-bonds | Hydrogen bond interactions |
| ITIM | Immunoreceptor tyrosine-based inhibitory motif |
| JAK | Janus kinase |
| KI | Inhibitor constant |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| MAPK/ERK | Mitogen-activated protein kinase/Extracellular signal-regulated kinase 1/2 |
| MD | Molecular dynamics |
| mTOR | mammalian target of rapamycin |
| MW | Molecular weight |
| MW | Molecular weight |
| nOHNH | number of OH-NH bonds |
| nON | number of O-N bonds |
| nrotb | Number of rotational bonds |
| P13K/AKT | Phosphatidylinositol-3-kinase (PI3K)/Ak strain transforming |
| PDB | Protein data bank |
| pI | Isolectric point |
| PTK | Protein tyrosine kinase |
| PTP | Protein tyrosine phosphatase |
| PTPc-N11/PTPN11 | Protein tyrosine phosphatase catalytic-Non receptor type 11 |
| RMSD | Root mean spuare deviation |
| RMSF | Root mean spuare fluctuation |
| RS | Right/left |
| SHP2 | Src tyrosine phosphatase protein |
| STAT3 | Signal transducer and activator of transcription 3 |
| TIGIT | T-cell immunoreceptor with immunoglobulin and ITIM domains |
| WT | Wildtype |
References
- Frankson, R.; Yu, Z.-H.; Bai, Y.; Li, Q.; Zhang, R.-Y.; Zhang, Z.-Y. Therapeutic Targeting of Oncogenic Tyrosine Phosphatases. Cancer Res. 2017, 77, 5701–5705. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, L.; Jia, K.; Chen, C.; Li, Z.; Zhao, J.; Hu, J.; Zhang, H.; Fan, Q.; Huang, C.; Xie, H.; et al. DYRK1B-STAT3 Drives Cardiac Hypertrophy and Heart Failure by Impairing Mitochondrial Bioenergetics. Circulation 2022, 145, 829–846. [Google Scholar] [CrossRef]
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef]
- Barykin, E.P.; Yanvarev, D.V.; Strelkova, M.A.; Valuev-Elliston, V.T.; Varshavskaya, K.B.; Mitkevich, V.A.; Makarov, A.A. Phosphorylation and Dephosphorylation of Beta-Amyloid Peptide in Model Cell Cultures: The Role of Cellular Protein Kinases and Phosphatases. Life 2023, 13, 147. [Google Scholar] [CrossRef]
- Chou, Y.-T.; Bivona, T.G. Inhibition of SHP2 as an approach to block RAS-driven cancers. Adv. Cancer Res. 2022, 153, 205–236. [Google Scholar] [CrossRef]
- Gurzov, E.N.; Stanley, W.J.; Brodnicki, T.C.; Thomas, H.E. Protein tyrosine phosphatases: Molecular switches in metabolism and diabetes. Trends Endocrinol. Metab. 2015, 26, 30–39. [Google Scholar] [CrossRef]
- Hunter, T. Tyrosine phosphorylation: Thirty years and counting. Curr. Opin. Cell Biol. 2009, 21, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, F.M.; Gray, N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug Discov. 2018, 17, 353–377. [Google Scholar] [CrossRef]
- Bentires-Alj, M.; Paez, J.G.; David, F.S.; Keilhack, H.; Halmos, B.; Naoki, K.; Maris, J.M.; Richardson, A.; Bardelli, A.; Sugarbaker, D.J.; et al. Activating Mutations of the Noonan Syndrome-Associated SHP2/PTPN11 Gene in Human Solid Tumors and Adult Acute Myelogenous Leukemia. Cancer Res. 2004, 64, 8816–8820. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-P.; Chiu, H.-Y.; Hsiao, T.-L.; Hsiao, C.-H.; Lin, C.-C.; Liao, Y.-H. Scalp melanoma in a woman with LEOPARD syndrome: Possible implication of PTPN11 signaling in melanoma pathogenesis. J. Am. Acad. Dermatol. 2013, 69, e186–e187. [Google Scholar] [CrossRef]
- Hof, P.; Pluskey, S.; Dhe-Paganon, S.; Eck, M.J.; Shoelson, S.E. Crystal Structure of the Tyrosine Phosphatase SHP-2. Cell 1998, 92, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Marasco, M.; Berteotti, A.; Weyershaeuser, J.; Thorausch, N.; Sikorska, J.; Krausze, J.; Brandt, H.J.; Kirkpatrick, J.; Rios, P.; Schamel, W.W.; et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci. Adv. 2020, 6, eaay4458. [Google Scholar] [CrossRef] [Green Version]
- Neel, B.G.; Gu, H.; Pao, L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003, 28, 284–293. [Google Scholar] [CrossRef]
- Xu, D. Protein tyrosine phosphatases in the JAK/STAT pathway. Front. Biosci. 2008, 13, 4925–4932. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zheng, H.; Li, X.; Wang, S.; Meyerson, H.J.; Yang, W.; Neel, B.G.; Qu, C.-K. Gain-of-function mutations of Ptpn11 (Shp2) cause aberrant mitosis and increase susceptibility to DNA damage-induced malignancies. Proc. Natl. Acad. Sci. USA 2016, 113, 984–989. [Google Scholar] [CrossRef] [Green Version]
- Bajia, D.; Bottani, E.; Derwich, K. Effects of Noonan Syndrome-Germline Mutations on Mitochondria and Energy Metabolism. Cells 2022, 11, 3099. [Google Scholar] [CrossRef]
- Tartaglia, M.; Martinelli, S.; Stella, L.; Bocchinfuso, G.; Flex, E.; Cordeddu, V.; Zampino, G.; van der Burgt, I.; Palleschi, A.; Petrucci, T.C.; et al. Diversity and Functional Consequences of Germline and Somatic PTPN11 Mutations in Human Disease. Am. J. Hum. Genet. 2006, 78, 279–290. [Google Scholar] [CrossRef]
- Tajan, M.; Batut, A.; Cadoudal, T.; Deleruyelle, S.; Le Gonidec, S.; Saint Laurent, C.; Vomscheid, M.; Wanecq, E.; Tréguer, K.; De Rocca Serra-Nédélec, A.; et al. LEOPARD syndrome-associated SHP2 mutation confers leanness and protection from diet-induced obesity. Proc. Natl. Acad. Sci. USA 2014, 111, E4494–E4503. [Google Scholar] [CrossRef] [Green Version]
- Marin, T.M.; Keith, K.; Davies, B.; Conner, D.A.; Guha, P.; Kalaitzidis, D.; Wu, X.; Lauriol, J.; Wang, B.; Bauer, M.; et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome–associated PTPN11 mutation. J. Clin. Investig. 2011, 121, 1026–1043. [Google Scholar] [CrossRef] [Green Version]
- Mohi, M.G.; Neel, B.G. The role of Shp2 (PTPN11) in cancer. Curr. Opin. Genet. Dev. 2007, 17, 23–30. [Google Scholar] [CrossRef]
- Liu, Q.; Qu, J.; Zhao, M.; Xu, Q.; Sun, Y. Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharm. Res. 2020, 152, 104595. [Google Scholar] [CrossRef]
- Grossmann, K.S.; Rosário, M.; Birchmeier, C.; Birchmeier, W. The Tyrosine Phosphatase Shp2 in Development and Cancer. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 106, pp. 53–89. ISBN 978-0-12-374771-6. [Google Scholar]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Hellmuth, K.; Grosskopf, S.; Lum, C.T.; Würtele, M.; Röder, N.; von Kries, J.P.; Rosario, M.; Rademann, J.; Birchmeier, W. Specific inhibitors of the protein tyrosine phosphatase Shp2 identified by high-throughput docking. Proc. Natl. Acad. Sci. USA 2008, 105, 7275–7280. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Sung, S.-S.; Yip, M.L.R.; Lawrence, H.R.; Ren, Y.; Guida, W.C.; Sebti, S.M.; Lawrence, N.J.; Wu, J. Discovery of a Novel Shp2 Protein Tyrosine Phosphatase Inhibitor. Mol. Pharm. 2006, 70, 562–570. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Zeng, L.-F.; He, Y.; Zhang, S.; Zhang, Z.-Y. Small molecule tools for functional interrogation of protein tyrosine phosphatases: Small molecule probes for the PTPs. FEBS J. 2013, 280, 731–750. [Google Scholar] [CrossRef]
- Chen, Y.-N.P.; LaMarche, M.J.; Chan, H.M.; Fekkes, P.; Garcia-Fortanet, J.; Acker, M.G.; Antonakos, B.; Chen, C.H.-T.; Chen, Z.; Cooke, V.G.; et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 2016, 535, 148–152. [Google Scholar] [CrossRef]
- Fodor, M.; Price, E.; Wang, P.; Lu, H.; Argintaru, A.; Chen, Z.; Glick, M.; Hao, H.-X.; Kato, M.; Koenig, R.; et al. Dual Allosteric Inhibition of SHP2 Phosphatase. ACS Chem. Biol. 2018, 13, 647–656. [Google Scholar] [CrossRef]
- Gottlieb, J.A.; Luce, J.K. Treatment of malignant melanoma with camptothecin (NSC-100880). Cancer Chemother. Rep. 1972, 56, 103–105. [Google Scholar]
- Muggia, F.M.; Creaven, P.J.; Hansen, H.H.; Cohen, M.H.; Selawry, O.S. Phase I clinical trial of weekly and daily treatment with camptothecin (NSC-100880): Correlation with preclinical studies. Cancer Chemother. Rep. 1972, 56, 515–521. [Google Scholar]
- Moertel, C.G.; Schutt, A.J.; Reitemeier, R.J.; Hahn, R.G. Phase II study of camptothecin (NSC-100880) in the treatment of advanced gastrointestinal cancer. Cancer Chemother. Rep. 1972, 56, 95–101. [Google Scholar]
- Li, F.; Jiang, T.; Li, Q.; Ling, X. Camptothecin (CPT) and its derivatives are known to target topoisomerase I (Top1) as their mechanism of action: Did we miss something in CPT analogue molecular targets for treating human disease such as cancer? Am. J. Cancer Res. 2017, 7, 2350–2394. [Google Scholar]
- Li, F. Compositions and Methods for Identifying agents that Alter Expression of Surviving. U.S. Patent 7,569,221, 4 August 2009. [Google Scholar]
- Li, F.; Ambrosini, G.; Chu, E.Y.; Plescia, J.; Tognin, S.; Marchisio, P.C.; Altieri, D.C. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998, 396, 580–584. [Google Scholar] [CrossRef]
- Li, F.; Ackermann, E.J.; Bennett, C.F.; Rothermel, A.L.; Plescia, J.; Tognin, S.; Villa, A.; Marchisio, P.C.; Altieri, D.C. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat. Cell Biol. 1999, 1, 461–466. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings 1PII of original article: S0169-409X(96)00423-1. The article was originally published in Advanced Drug Delivery Reviews 23 (1997) 3–25. 1. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Stromgaard, K.; Krogsgaard-Larsen, P.; Madsen, U. (Eds.) Textbook of Drug Design and Discovery; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-0279-9. [Google Scholar]
- Gore, M.; Jagtap, U.B. (Eds.) Computational Drug Discovery and Design; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1762, ISBN 978-1-4939-7755-0. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. In silico prediction of aqueous solubility using simple QSPR models: The importance of phenol and phenol-like moieties. J. Chem. Inf. Model. 2012, 52, 2950–2957. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [Green Version]
- Barr, A.J.; Ugochukwu, E.; Lee, W.H.; King, O.N.F.; Filippakopoulos, P.; Alfano, I.; Savitsky, P.; Burgess-Brown, N.A.; Müller, S.; Knapp, S. Large-Scale Structural Analysis of the Classical Human Protein Tyrosine Phosphatome. Cell 2009, 136, 352–363. [Google Scholar] [CrossRef] [Green Version]
- Inhibitory Activity of Iron Chelators ATA and DFO on MCF-7 Breast Cancer Cells and Phosphatases PTP1B and SHP2. Anticancer. Res. 2017, 37, 4799–4806. [CrossRef] [Green Version]
- Bjørbæk, C.; Buchholz, R.M.; Davis, S.M.; Bates, S.H.; Pierroz, D.D.; Gu, H.; Neel, B.G.; Myers, M.G.; Flier, J.S. Divergent Roles of SHP-2 in ERK Activation by Leptin Receptors. J. Biol. Chem. 2001, 276, 4747–4755. [Google Scholar] [CrossRef] [Green Version]
- Tong, J.; Killeen, M.; Steven, R.; Binns, K.L.; Culotti, J.; Pawson, T. Netrin Stimulates Tyrosine Phosphorylation of the UNC-5 Family of Netrin Receptors and Induces Shp2 Binding to the RCM Cytodomain. J. Biol. Chem. 2001, 276, 40917–40925. [Google Scholar] [CrossRef] [Green Version]
- de Vet, E.C.J.M.; Aguado, B.; Campbell, R.D. G6b, a Novel Immunoglobulin Superfamily Member Encoded in the Human Major Histocompatibility Complex, Interacts with SHP-1 and SHP-2. J. Biol. Chem. 2001, 276, 42070–42076. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. ISBN 978-1-58829-343-5. [Google Scholar]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Bowie, J.U.; Lüthy, R.; Eisenberg, D. A Method to Identify Protein Sequences That Fold into a Known Three-Dimensional Structure. Science 1991, 253, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Darras, F.H.; Pang, Y.-P. On the use of the experimentally determined enzyme inhibition constant as a measure of absolute binding affinity. Biochem. Biophys. Res. Commun. 2017, 489, 451–454. [Google Scholar] [CrossRef]
- Raffa, R.B. Drug Disposition and Response. In Handbook of Drug-Nutrient Interactions; Boullata, J.I., Armenti, V.T., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 27–42. ISBN 978-1-4757-5359-2. [Google Scholar]
- Yung-Chi, C.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- Steiner, T.; Koellner, G. Hydrogen bonds with π-acceptors in proteins: Frequencies and role in stabilizing local 3D structures11Edited by R. Huber. J. Mol. Biol. 2001, 305, 535–557. [Google Scholar] [CrossRef]
- Doss, C.G.P.; NagaSundaram, N. Investigating the Structural Impacts of I64T and P311S Mutations in APE1-DNA Complex: A Molecular Dynamics Approach. PLoS ONE 2012, 7, e31677. [Google Scholar] [CrossRef] [Green Version]
- Holthof, L.C.; Van Der Horst, H.J.; Van Hal-van Veen, S.E.; Ruiter, R.W.J.; Li, F.; Buijze, M.; Andersen, M.N.; Yuan, H.; De Bruijn, J.; Van De Donk, N.W.C.J.; et al. Preclinical evidence for an effective therapeutic activity of FL118, a novel survivin inhibitor, in patients with relapsed/refractory multiple myeloma. Haematologica 2020, 105, e80–e83. [Google Scholar] [CrossRef]
- Ling, X.; Cao, S.; Cheng, Q.; Keefe, J.T.; Rustum, Y.M.; Li, F. A Novel Small Molecule FL118 That Selectively Inhibits Survivin, Mcl-1, XIAP and cIAP2 in a p53-Independent Manner, Shows Superior Antitumor Activity. PLoS ONE 2012, 7, e45571. [Google Scholar] [CrossRef] [Green Version]
- Li, F. Discovery of Survivin Inhibitors and Beyond. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 305, pp. 217–252. ISBN 978-0-12-407695-2. [Google Scholar]
- Wang, J.; Liu, Z.; Zhang, D.; Liu, R.; Lin, Q.; Liu, J.; Yang, Z.; Ma, Q.; Sun, D.; Zhou, X.; et al. FL118, a novel survivin inhibitor, wins the battle against drug-resistant and metastatic lung cancers through inhibition of cancer stem cell-like properties. Am. J. Transl. Res. 2017, 9, 3676–3686. [Google Scholar]
- Zhao, H.; Wang, D.; Yang, Z.; Ji, L.; Liu, Z.; Yang, Y.; Ma, Q.; Lin, X.; Jiang, G. FL118, a novel anticancer compound, inhibits proliferation and migration of ovarian cancer cells via up-regulation of cytoglobin in vivo and in vitro. Transl. Cancer Res. 2017, 6, 1294–1304. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Ferenczy, G.G.; Kellermayer, M. Contribution of hydrophobic interactions to protein mechanical stability. Comput. Struct. Biotechnol. J. 2022, 20, 1946–1956. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [Green Version]
- Weininger, D. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Model. 1988, 28, 31–36. [Google Scholar] [CrossRef]
- Bitencourt-Ferreira, G.; de Azevedo, W.F. Molegro Virtual Docker for Docking. In Docking Screens for Drug Discovery; de Azevedo, W.F., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 2053, pp. 149–167. ISBN 978-1-4939-9751-0. [Google Scholar]
- Biovia, D.S. Discovery studio modeling environment; Release, 2017. J. Food Nutr. Res. 2021, 9, 114–123. [Google Scholar]
- Jakubec, D.; Skoda, P.; Krivak, R.; Novotny, M.; Hoksza, D. PrankWeb 3: Accelerated ligand-binding site predictions for experimental and modelled protein structures. Nucleic Acids Res. 2022, 50, W593–W597. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Stierand, K.; Maaß, P.C.; Rarey, M. Molecular complexes at a glance: Automated generation of two-dimensional complex diagrams. Bioinformatics 2006, 22, 1710–1716. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam: A fast force field generation tool for small organic molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Elengoe, A.; Naser, M.; Hamdan, S. Modeling and Docking Studies on Novel Mutants (K71L and T204V) of the ATPase Domain of Human Heat Shock 70 kDa Protein 1. Int. J. Mol. Sci. 2014, 15, 6797–6814. [Google Scholar] [CrossRef] [Green Version]






| Ligands | Physical Properties | Predicted Bioactivity | ||||||
|---|---|---|---|---|---|---|---|---|
| miLogP | MW/gmol−1 | Natoms | nON/nOHNH | Nrotb | Vol Cubic/Å | Bioactivity | Scores | |
| FL118 [10,11-Methylenedioxy-20(RS)-camptothecin] | 1.89 | 392.36 | 29 | 8/1 | 1 | 321.34 | Ion channel modulator | −0.20 |
| Enzyme inhibitor | 0.99 | |||||||
| GPCR ligand | 0.41 | |||||||
| Kinase inhibitor | 0.26 | |||||||
| Irinotecan | 4.10 | 586.68 | 43 | 10/1 | 5 | 530.67 | Ion channel modulator | −0.45 |
| Enzyme inhibitor | 0.54 | |||||||
| GPCR ligand | 0.33 | |||||||
| (A) | ||||||
| Water Solubility LogS (SILICOS-IT) | Pharmacokinetics | Druglikeness (Lipinski’s) | Lipophilicity (Log Po/w) | |||
| GI Absorption | BBB | LogKp (Skin Permeation) cm/s | ||||
| FL118 | −5.5 (moderatly soluble) | High | No | −7.59 | Yes, 0 violation | 2.06 |
| Irinotecan | −7.28 (Poorly soluble) | High | No | −7.22 | Yes, 1 violation: MW > 500 | 3.73 |
| (B) | ||||||
| Classification | Target | Prediction | Probability | |||
| FL118 | Irinotecan | FL118 | Irinotecan | |||
| Organ toxicity | Hepatotoxicity | Inactive | Inactive | 0.82 | 0.67 | |
| Stress response pathways | Nuclear factor (erythroid-derived 2)-like 2/antioxidant responsive element (nrf2/ARE) | Inactive | Inactive | 0.70 | 0.94 | |
| Heat shock factor response element (HSE) | Inactive | Inactive | 0.70 | 0.94 | ||
| Mitochondrial Membrane Potential (MMP) | Inactive | Active | 0.65 | 0.76 | ||
| Phosphoprotein (Tumor Supressor) p53 | Active | Active | 0.51 | 0.60 | ||
| ATPase family AAA domain-containing protein 5 (ATAD5) | Inactive | Inactive | 0.98 | 0.95 | ||
| Toxicity end points | Carcinogenicity | Inactive | Inactive | 0.54 | 0.61 | |
| Immunotoxicity | Active | Active | 0.99 | 0.99 | ||
| Mutagenicity | Inactive | Inactive | 0.54 | 0.67 | ||
| Cytotoxicity | Active | Active | 0.98 | 0.79 | ||
| Protein | Probability Score | Residues Forming the Binding Pocket |
|---|---|---|
| SHP2-WT | 0.776 | TYR279, ILE282, THR357, GLU361, ARG362, LYS366, TRP423, PRO424, ASP425, GLY427, VAL428, CYS459, SER460, ALA461, ILE463, GLY464, ARG465,GLN506, THR507, ALA509, GLN510 |
| SHP2-R465G | 0.893 | TYR279, ILE282, THR356, THR357, GLU361, ARG362, LYS366, TRP423, PRO424, ASP425, GLY427, VAL428, CYS459, SER460, ALA461, ILE463, GLY464, GLY465, PHE469, GLN506, THR507, ALA509, GLN510 |
| SHP2-Y279C | 0.782 | ARG278, CYS279, ILE282, THR357, GLU361, ARG362, LYS364, LYS366, TRP423, PRO424, ASP425, GLY427, VAL428, CYS459, SER460, ALA461, ILE463, GLY464, ARG465, GLN506, THR507, ALA509, GLN510 |
| Protein | Length | Mol.Weight (Daltons) | pI | −R | +R | Extinction Coefficient | Instability Index | Aliphatic Index | GRAVY |
|---|---|---|---|---|---|---|---|---|---|
| SHP2-WT | 272 | 31,748.06 | 6.55 | 38 | 36 | 45,630 | 46.95 | 76.91 | −0.609 |
| SHP2-Y279C | 272 | 31,688.02 | 6.55 | 38 | 36 | 44,265 | 47.23 | 76.91 | −0.595 |
| SHP2-R465G | 272 | 31,648.92 | 6.34 | 38 | 35 | 45,630 | 47.09 | 76.91 | −0.594 |
| Secondary Structure | Random Coil (Cc/%) | Alpha Helix (Hh/%) | Extended Strand (Ee/%) | Beta Turn(Tt/%) |
|---|---|---|---|---|
| SHP2-WT | 43.75 | 33.09 | 23.16 | 0 |
| SHP2-Y279C | 44.85 | 32.72 | 22.43 | 0 |
| SHP2-R465G | 43.38 | 28.31 | 28.31 | 0 |
| Ligands | FL118 | Irinotecan | ||
|---|---|---|---|---|
| SHP2-WT | SHP2-Y279C | SHP2-R465G | SHP2-WT | |
| Affinity energy (binding affinity) (kcal/mol) | −7.54 | −6.94 | −6.66 | −6.85 |
| Ligand efficiency | −0.26 | −0.24 | −0.23 | −0.16 |
| Inhibition constant, Ki/µM | 3.00 | 8.14 | 13.12 | 9.53 |
| Intermolecular energy (kcal/mol) | −8.13 | −7.54 | −7.26 | −8.64 |
| Internal energy (kcal/mol) | −0.18 | −0.17 | −0.15 | −1.59 |
| Torsion energy (kcal/mol) | 0.60 | 0.60 | 0.60 | 1.79 |
| Unbounded Extended energy (kcal/mol) | −0.18 | −0.17 | −0.15 | −1.59 |
| Reference RMS (Å) | 67.11 | 67.0 | 66.57 | 65.24 |
| Ligand | Protein | Donor Atom | Acceptor Atom | Distance (Å) |
|---|---|---|---|---|
| FL118 | SHP2-WT | ASN281:HD | FL118:O20 | 2.93 |
| LYS366:HZ | FL118:O4 | 2.80 | ||
| FL118:H35 | SER460:OG | 1.90 | ||
| GLY464:HN | FL118:O29 | 2.14 | ||
| ARG465:HN | FL118:O29 | 2.20 | ||
| CYS459:HG | FL118:O29 | 1.78 | ||
| SHP2-Y279C | LYS366:HZ | FL118:O4 | 2.81 | |
| FL118:H35 | SER460:OG | 1.86 | ||
| GLY464:HN | FL118:O29 | 2.08 | ||
| ARG465:HN | FL118:O29 | 2.17 | ||
| CYS459:HG | FL118:O29 | 1.92 | ||
| SHP2-R465G | FL118:H35 | TYR279:OH | 3.11 | |
| LYS366 | FL118:O4 | 2.74 | ||
| SER460 | FL118:O4 | 2.79 | ||
| GLY464 | FL118:O29 | 1.91 | ||
| Irinotecan | SHP2-WT | Irinotecan:H54 | ASN281:OD1 | 1.86 |
| ARG465:HN | Irinotecan:O29 | 2.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajia, D.; Derwich, K. An In Silico Study Investigating Camptothecin-Analog Interaction with Human Protein Tyrosine Phosphatase, SHP2 (PTPN11). Pharmaceuticals 2023, 16, 926. https://doi.org/10.3390/ph16070926
Bajia D, Derwich K. An In Silico Study Investigating Camptothecin-Analog Interaction with Human Protein Tyrosine Phosphatase, SHP2 (PTPN11). Pharmaceuticals. 2023; 16(7):926. https://doi.org/10.3390/ph16070926
Chicago/Turabian StyleBajia, Donald, and Katarzyna Derwich. 2023. "An In Silico Study Investigating Camptothecin-Analog Interaction with Human Protein Tyrosine Phosphatase, SHP2 (PTPN11)" Pharmaceuticals 16, no. 7: 926. https://doi.org/10.3390/ph16070926





