Comparison of Bevacizumab and Aflibercept for Suppression of Angiogenesis in Human Retinal Microvascular Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. Effects on Tube Formation Capacity
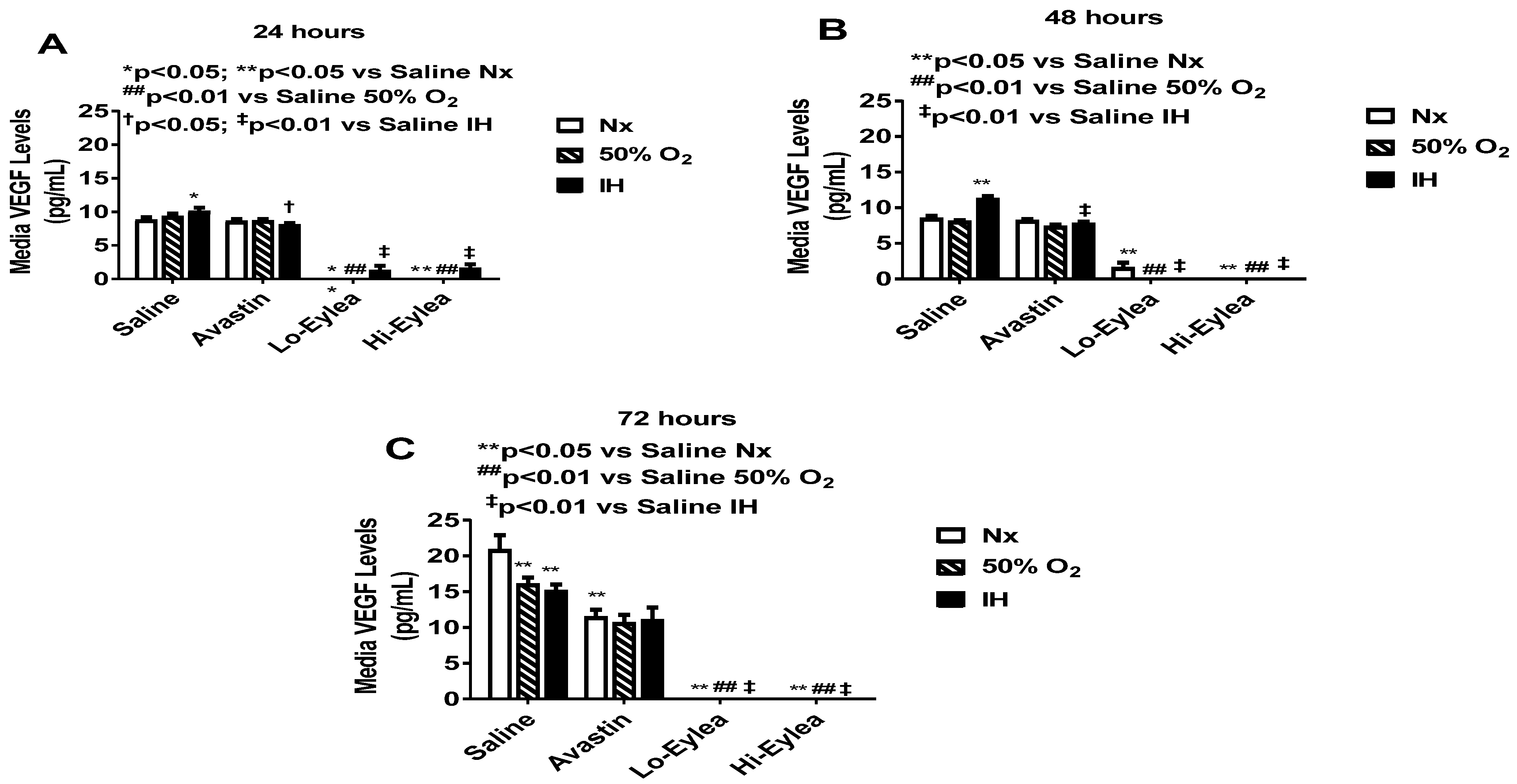
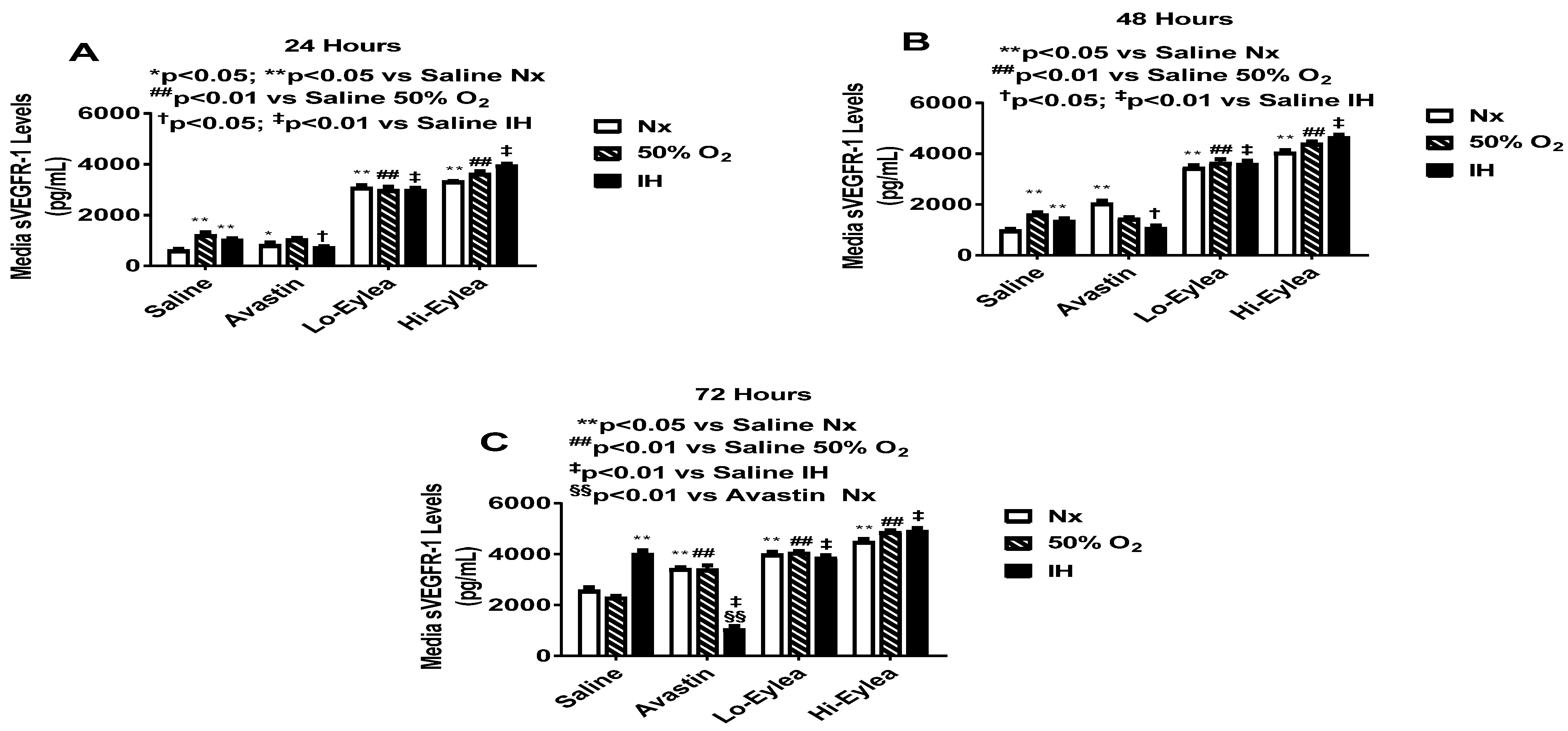
2.2. Effect on VEGF and sVEGFR-1 Media Levels
2.3. Effect on IGF-I Media Levels
2.4. Effect on GSH/GSSG
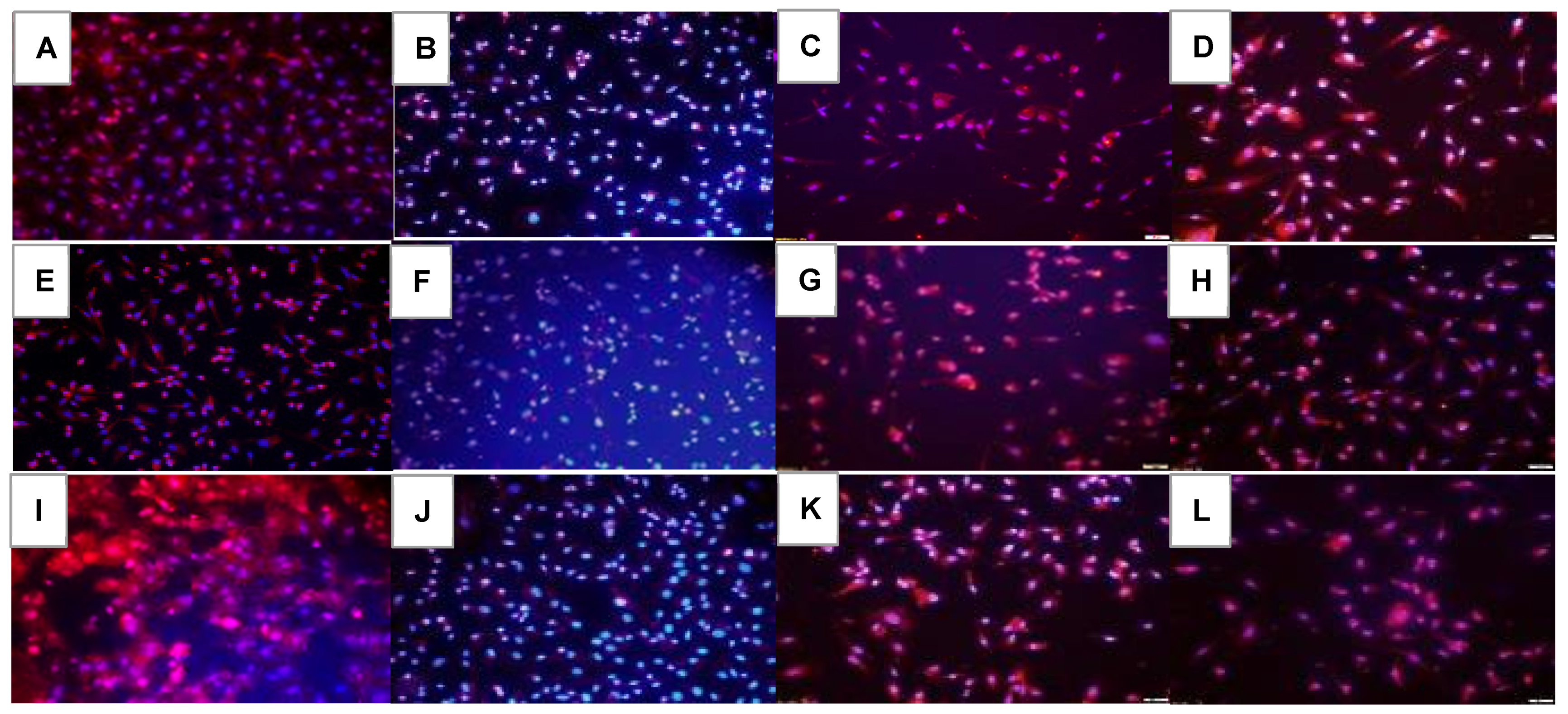
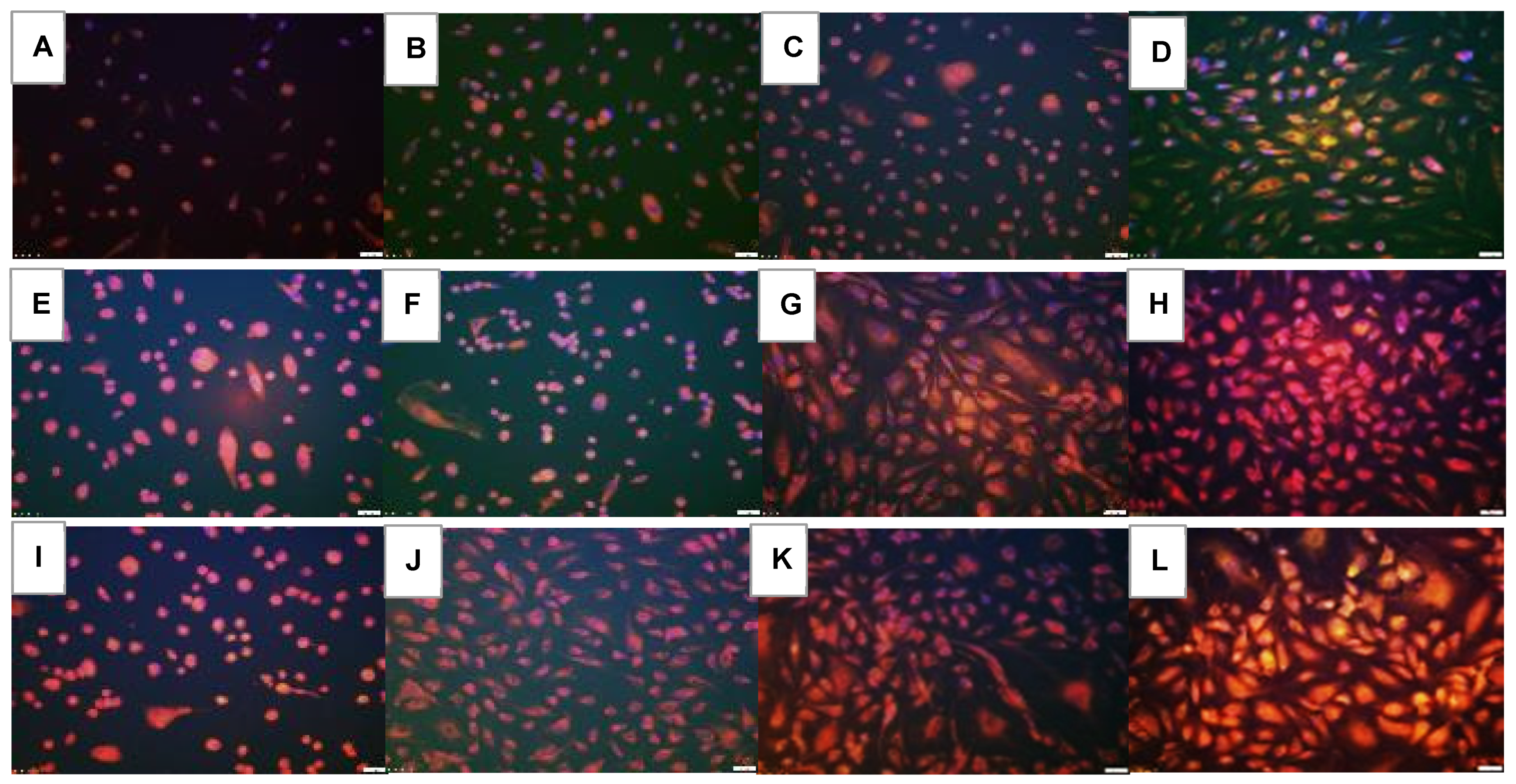
2.5. Effect on HIF1α
2.6. Effect on VEGF Signaling
2.7. Effect on Notch Signaling
2.8. Effects on Lipid Peroxidation
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Experimental Design
4.3. Hx and Intermittent Hypoxia Profiles
4.4. Tube Formation Assay
4.5. VEGF, sVEGFR-1 & IGF-I Assays
4.6. GSH/GSSG Ratios
4.7. Immunofluorescence
4.8. Lipid Peroxidation
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Cell Physiol. 2001, 280, C1358–C1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, N. VEGF: An update on biological and therapeutic aspects. Curr. Opin. Biotechnol. 2000, 11, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef]
- Robinson, C.J.; Stringer, S.E. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 2001, 114, 853–865. [Google Scholar] [CrossRef]
- Tah, V.; Orlans, H.O.; Hyer, J.; Casswell, E.; Din, N.; Sri Shanmuganathan, V.; Ramskold, L.; Pasu, S. Anti-VEGF Therapy and the Retina: An Update. J. Ophthalmol. 2015, 2015, 627674. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.K.; Narendran, V.; Tawansy, K.A.; Raghuram, A.; Narendran, K. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian J. Ophthalmol. 2007, 55, 75–76. [Google Scholar] [CrossRef]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z.; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Beharry, K.D.; Valencia, G.B.; Lazzaro, D.R.; Aranda, J.V. Pharmacologic interventions for the prevention and treatment of retinopathy of prematurity. Semin. Perinatol. 2016, 40, 189–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Blair, M.P.; Shapiro, M.J.; Lichtenstein, S.J.; Galasso, J.M.; Kapur, R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch. Ophthalmol. 2012, 130, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Henaine-Berra, A.; Garcia-Aguirre, G.; Quiroz-Mercado, H.; Martinez-Castellanos, M.A. Retinal fluorescein angiographic changes following intravitreal anti-VEGF therapy. J. AAPOS 2014, 18, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Ittiara, S.; Blair, M.P.; Shapiro, M.J.; Lichtenstein, S.J. Exudative retinopathy and detachment: A late reactivation of retinopathy of prematurity after intravitreal bevacizumab. J. AAPOS 2013, 17, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Balakrishnan, D.; Zeynalova, Z.; Padhi, T.R.; Rani, P.K. Serious adverse events and visual outcomes of rescue therapy using adjunct bevacizumab to laser and surgery for retinopathy of prematurity. The Indian Twin Cities Retinopathy of Prematurity Screening database Report number 5. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 98, F327–F333. [Google Scholar] [CrossRef]
- Karaca, C.; Oner, A.O.; Mirza, E.; Polat, O.A.; Sahiner, M. Bilateral effect of unilateral bevacizumab injection in retinopathy of prematurity. JAMA Ophthalmol. 2013, 131, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castellanos, M.A.; Schwartz, S.; Hernández-Rojas, M.L.; Kon-Jara, V.A.; García-Aguirre, G.; Guerrero-Naranjo, J.L.; Chan, R.V.; Quiroz-Mercado, H. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina 2013, 33, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chablani, J.; Rani, P.K.; Balakrishnan, D.; Jalali, S. Unusual Adverse Choroidal Reaction to Intravitreal Bevacizumab in Aggressive Posterior Retinopathy of Prematurity: The Indian Twin Cities ROP Screening (ITCROPS) Data Base Report Number 7. Semin. Ophthalmol. 2014, 29, 222–225. [Google Scholar] [CrossRef]
- Tahija, S.G.; Hersetyati, R.; Lam, G.C.; Kusaka, S.; McMenamin, P.G. Fluorescein angiographic observations of peripheral retinal vessel growth in infants after intravitreal injection of bevacizumab as sole therapy for zone I and posterior zone II retinopathy of prematurity. Br. J. Ophthalmol. 2014, 98, 507–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Wada, K.; Arahori, H.; Kuno, N.; Imoto, K.; Iwahashi-Shima, C.; Kusaka, S. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am. J. Ophthalmol. 2012, 153, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.R.; Kim, Y.H.; Kim, S.Y.; Nam, G.Y.; Cheon, H.J.; Lee, S.J. Plasma concentrations of vascular endothelial growth factor in retinopathy of prematurity after intravitreal bevacizumab injection. Retina 2015, 35, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Lien, R.; Liao, P.J.; Wang, N.K.; Chen, Y.P.; Chao, A.N.; Chen, K.J.; Chen, T.L.; Hwang, Y.S.; Lai, C.C. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol. 2015, 133, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.; Bhatt, A.R.; Demny, A.B.; Coats, D.K.; Li, A.; Rahman, E.Z.; Smith, O.E.; Steinkuller, P.G. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 2015, 56, 956–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieh, C.; Agostini, H.; Buschbeck, C.; Krüger, M.; Schulte-Mönting, J.; Zirrgiebel, U.; Drevs, J.; Lagrèze, W.A. VEGF-A, VEGFR-1, VEGFR-2 and Tie2 levels in plasma of premature infants: Relationship to retinopathy of prematurity. Br. J. Ophthalmol. 2008, 92, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Morin, J.; Luu, T.M.; Superstein, R.; Ospina, L.H.; Lefebvre, F.; Simard, M.N.; Shah, V.; Shah, P.S.; Kelly, E.N. Canadian Neonatal Network and the Canadian Neonatal Follow-Up Network Investigators. Neurodevelopmental Outcomes Following Bevacizumab Injections for Retinopathy of Prematurity. Pediatrics 2016, 137, e20153218. [Google Scholar] [CrossRef] [Green Version]
- Aflibercept: AVE 0005, AVE 005, AVE0005, VEGF Trap–Regeneron, VEGF Trap (R1R2), VEGF Trap-Eye. Drugs R&D 2008, 9, 261–269.
- Dixon, J.A.; Oliver, S.C.; Olson, J.L.; Mandava, N. VEGF trap-eye for the treatment of neovascular age-related macular degeneration. Expert. Opin. Investig. Drugs 2009, 18, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Sukgen, E.A.; Söker, G.; Koçluk, Y.; Gülek, B. Effect of Intravitreal Aflibercept on Central Retinal Arterial Blood Flow in Type 1 Retinopathy of Prematurity. Eur. J. Ophthalmol. 2017, 27, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.G.; Said, A.M. Structural, visual and refractive outcomes of intravitreal aflibercept injection in high-risk prethreshold type 1 retinopathy of prematurity. Ophthalmic Res. 2015, 53, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Yeh, P.T.; Chen, S.N.; Yang, C.M.; Lai, C.C.; Kuo, H.K. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: A multicenter study in Taiwan. Ophthalmology 2011, 118, 176–183. [Google Scholar] [CrossRef]
- Jang, S.Y.; Choi, K.S.; Lee, S.J. Delayed-onset retinal detachment after an intravitreal injection of ranibizumab for zone 1 plus retinopathy of prematurity. J. AAPOS 2010, 14, 457–459. [Google Scholar] [CrossRef]
- Suk, K.K.; Berrocal, A.M.; Murray, T.G.; Rich, R.; Major, J.C.; Hess, D.; Johnson, R.A. Retinal detachment despite aggressive management of aggressive posterior retinopathy of prematurity. J. Pediatr. Ophthalmol. Strabismus 2010, 47, e1–e4. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Smith, L.E. Retinopathy of prematurity. Angiogenesis 2007, 10, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Di Fiore, J.M.; MacFarlane, P.M.; Martin, R.J. Intermittent Hypoxemia in Preterm Infants. Clin. Perinatol. 2019, 46, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, J.M.; Bloom, J.N.; Orge, F.; Schutt, A.; Schluchter, M.; Cheruvu, V.K.; Walsh, M.; Finer, N.; Martin, R.J. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 2010, 157, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Fiore, J.M.; Vento, M. Intermittent hypoxemia and oxidative stress in preterm infants. Respir. Physiol. Neurobiol. 2019, 266, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Vento, M.; Asensi, M.; Sastre, J.; García-Sala, F.; Pallardó, F.V.; Viña, J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 2001, 107, 642–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.J.; Cai, C.L.; Shrier, E.M.; McNally, L.; Lazzaro, D.R.; Aranda, J.V.; Beharry, K.D. Ocular Adverse Effects of Intravitreal Bevacizumab Are Potentiated by Intermittent Hypoxia in a Rat Model of Oxygen-Induced Retinopathy. J. Ophthalmol. 2017, 2017, 4353129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valencia, A.M.; Cai, C.L.; Tan, J.; Duggan, T.J.; Valencia, G.B.; Aranda, J.V.; Beharry, K.D. Intravitreal bevacizumab alters type IV collagenases and exacerbates arrested alveologenesis in the neonatal rat lungs. Exp. Lung Res. 2017, 43, 120–133. [Google Scholar] [CrossRef]
- Duggan, T.J.; Cai, C.L.; Aranda, J.V.; Beharry, K.D. Acute and chronic effects of intravitreal bevacizumab on lung biomarkers of angiogenesis in the rat exposed to neonatal intermittent hypoxia. Exp. Lung Res. 2021, 47, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Hornig, C.; Barleon, B.; Ahmad, S.; Vuorela, P.; Ahmed, A.; Weich, H.A. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab. Investig. 2000, 80, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Kearney, J.B.; Kappas, N.C.; Ellerstrom, C.; DiPaola, F.W.; Bautch, V.L. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood 2004, 103, 4527–4535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappell, J.C.; Taylor, S.M.; Ferrara, N.; Bautch, V.L. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev. Cell 2009, 17, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmos, A.; Díaz, L.; Avila, E.; Barrera, D.; López-Marure, R.; Biruete, B.; Larrea, F.; Halhali, A. Associations between insulin-like growth factor I, vascular endothelial growth factor and its soluble receptor 1 in umbilical serum and endothelial cells obtained from normotensive and preeclamptic pregnancies. Growth Factors 2013, 31, 123–129. [Google Scholar] [CrossRef]
- Lakatos, D.; Somfai, E.; Méhes, E.; Czirók, A. Soluble VEGFR1 signaling guides vascular patterns into dense branching morphologies. J. Theor. Biol. 2018, 456, 261–278. [Google Scholar] [CrossRef]
- Arjamaa, O.; Nikinmaa, M. Oxygen-dependent diseases in the retina: Role of hypoxia inducible factors. Exp. Eye Res. 2006, 83, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Retinal oxygen: Fundamental and clinical aspects. Arch. Ophthalmol. 2003, 121, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Cringle, S.J.; Yu, D.Y. Oxygen supply and consumption in the retina: Implications for studies of retinopathy of prematurity. Doc. Ophthalmol. 2010, 120, 99–109. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.A.; Martin, J.; Muthusamy, K.K.; Luo, J.K.; Pyles, E.; Rafique, A.; Huang, T.; Potocky, T.; Liu, Y.; Cao, J.; et al. Aflibercept exhibits VEGF binding stoichiometry distinct from bevacizumab and does not support formation of immune-like complexes. Angiogenesis 2016, 19, 389–406. [Google Scholar] [CrossRef] [Green Version]
- Schlenska-Lange, A.; Knüpfer, H.; Lange, T.J.; Kiess, W.; Knüpfer, M. Cell proliferation and migration in glioblastoma multiforme cell lines are influenced by insulin-like growth factor I in vitro. Anticancer Res. 2008, 28, 1055–1060. [Google Scholar]
- Moriarty, P.; Boulton, M.; Dickson, A.; McLeod, D. Production of IGF-I and IGF binding proteins by retinal cells in vitro. Br. J. Ophthalmol. 1994, 78, 638–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Takeda, N.; Amiya, E.; Nakao, T.; Abe, H.; Semba, H.; Soma, K.; Koyama, K.; Hosoya, Y.; Imai, Y.; et al. VEGF-A induces its negative regulator, soluble form of VEGFR-1, by modulating its alternative splicing. FEBS Lett. 2013, 587, 2179–2185. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.M.; Kearney, J.B.; Johnson, J.H.; Rosenberg, M.P.; Kumar, R.; Bautch, B.L. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am. J. Pathol. 2004, 164, 1531–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappas, N.C.; Zeng, G.; Chappell, J.C.; Kearney, J.B.; Hazarika, S.; Kallianos, K.G.; Patterson, C.; Annex, B.H.; Bautch, V.L. The VEGF receptor Flt-1 spatially modulates Flk-1 signaling and blood vessel branching. J. Cell Biol. 2008, 181, 847–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappell, J.C.; Mouillesseaux, K.P.; Bautch, V.L. Flt-1 (vascular endothelial growth factor receptor-1) is essential for the vascular endothelial growth factor-Notch feedback loop during angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1952–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinovsky, E.D.; Draghia-Akli, R. Insulin-like growth factor I plasmid therapy promotes in vivo angiogenesis. Mol. Ther. 2004, 9, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Miele, C.; Rochford, J.J.; Filippa, N.; Giorgetti-Peraldi, S.; Van Obberghen, E. Insulin and insulin-like growth factor-I induce vascular endothelial growth factor mRNA expression via different signaling pathways. J. Biol. Chem. 2000, 275, 21695–21702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasioudis, D.; Minis, E.; Irani, M.; Kreines, F.; Witkin, S.S.; Spandorfer, S.D. Insulin-like growth factor-1 and soluble FMS-like tyrosine kinase-1 prospectively predict cancelled IVF cycles. J. Assist. Reprod. Genet. 2019, 36, 2485–2491. [Google Scholar] [CrossRef]
- Jakobsson, L.; Franco, C.A.; Bentley, K.; Collins, R.T.; Ponsioen, B.; Aspalter, I.M.; Rosewell, I.; Busse, M.; Thurston, G.; Medvinsky, A.; et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010, 12, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Siemerink, M.J.; Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Endothelial tip cells in ocular angiogenesis: Potential target for anti-angiogenesis therapy. J. Histochem. Cytochem. 2013, 61, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phng, L.K.; Gerhardt, H. Angiogenesis: A team effort coordinated by notch. Dev. Cell 2009, 16, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Benedito, R.; Roca, C.; Sörensen, I.; Adams, S.; Gossler, A.; Fruttiger, M.; Adams, R.H. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 2009, 137, 1124–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellstrom, M.; Phng, L.K.; Hofmann, J.J.; Wallgard, E.; Coultas, L.; Lindblom, P.; Alva, J.; Nilsson, A.K.; Karlsson, L.; Gaiano, N.; et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007, 445, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Beharry, K.D.; Cai, C.L.; Sharma, P.; Bronshtein, V.; Valencia, G.B.; Lazzaro, D.R.; Aranda, J.V. Hydrogen Peroxide Accumulation in the Choroid During Intermittent Hypoxia Increases Risk of Severe Oxygen-Induced Retinopathy in Neonatal Rats. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7644–7657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penn, J.S.; Henry, M.M.; Wall, P.T.; Tolman, B.L. The range of PaO2 variation determines the severity of oxygen-induced retinopathy in newborn rats. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2063–2070. [Google Scholar]
- Martin, R.J.; Di Fiore, J.M.; Macfarlane, P.M.; Wilson, C.G. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv. Exp. Med. Biol. 2012, 758, 351–358. [Google Scholar]
- Martin, R.J.; Wang, K.; Köroğlu, O.; Di Fiore, J.; Kc, P. Intermittent hypoxic episodes in preterm infants: Do they matter? Neonatology 2011, 100, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Upton, C.J.; Milner, A.D.; Stokes, G.M. Apnoea, bradycardia, and oxygen saturation in preterm infants. Arch. Dis. Child. 1991, 66, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Poggi, C.; Dani, C. Antioxidant strategies and respiratory disease of the preterm newborn: An update. Oxid. Med. Cell Longev. 2014, 2014, 721043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inayat, M.; Bany-Mohammed, F.; Valencia, A.; Tay, C.; Jacinto, J.; Aranda, J.V.; Beharry, K.D. Antioxidants and Biomarkers of Oxidative Stress in Preterm Infants with Symptomatic Patent Ductus Arteriosus. Am. J. Perinatol. 2015, 32, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Auten, R.L. Maturation of the antioxidant system and the effects on preterm birth. Semin. Fetal Neonatal Med. 2010, 15, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ames, A., 3rd; Li, Y.Y.; Heher, E.C.; Kimble, C.R. Energy metabolism of rabbit retina as related to function: High cost of Na+ transport. J. Neurosci. 1992, 12, 840–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, B., Jr.; Saltzman, H.A. Retinal oxygen utilization measured by hyperbaric blackout. Arch. Ophthalmol. 1964, 72, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef] [PubMed]
- Kusmartsev, S.; Eruslanov, E.; Kübler, H.; Tseng, T.; Sakai, Y.; Su, Z.; Kaliberov, S.; Heiser, A.; Rosser, C.; Dahm, P.; et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: Link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 2008, 181, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Bridges, J.P.; Gilbert, J.S.; Colson, D.; Gilbert, S.A.; Dukes, M.P.; Ryan, M.J.; Granger, J.P. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am. J. Hypertens. 2009, 22, 564–568. [Google Scholar] [CrossRef] [Green Version]
- Luthra, S.; Sharma, A.; Dong, J.; Neekhra, A.; Gramajo, A.L.; Seigel, G.M.; Kenney, M.C.; Kuppermann, B.D. Effect of bevacizumab (Avastin (TM) on mitochondrial function of in vitro retinal pigment epithelial, neurosensory retinal and microvascular endothelial cells. Indian J. Ophthalmol. 2013, 61, 705–710. [Google Scholar]
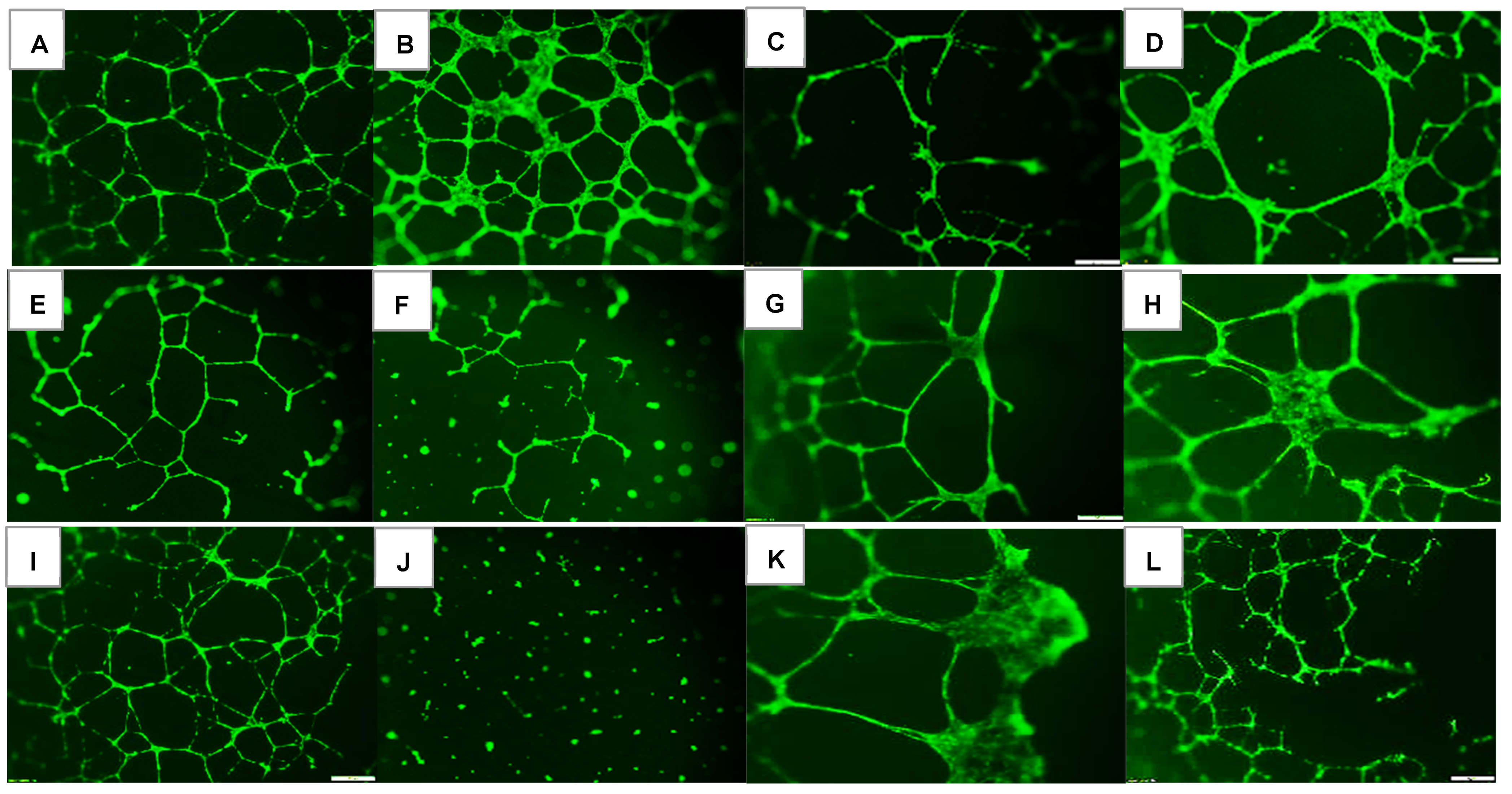


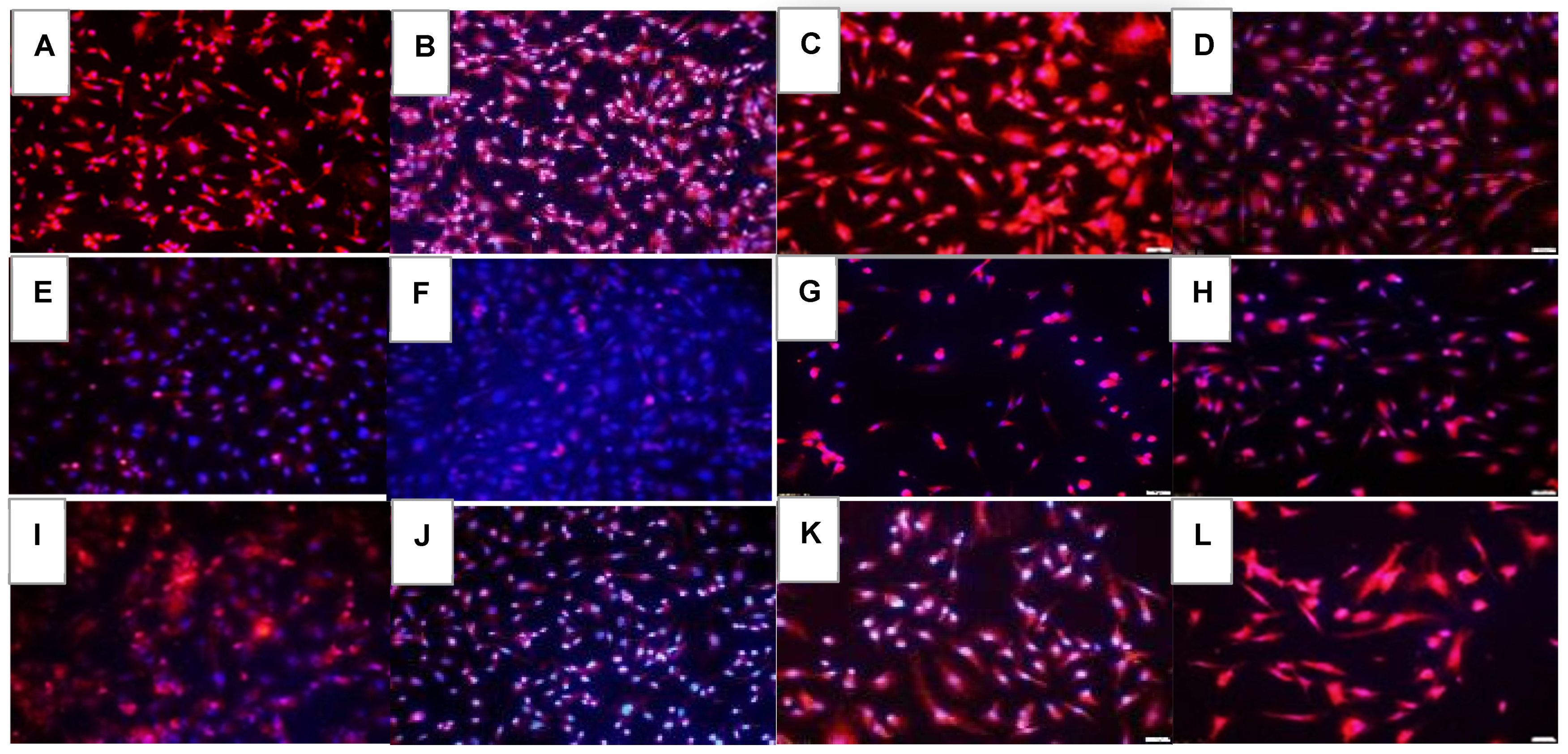

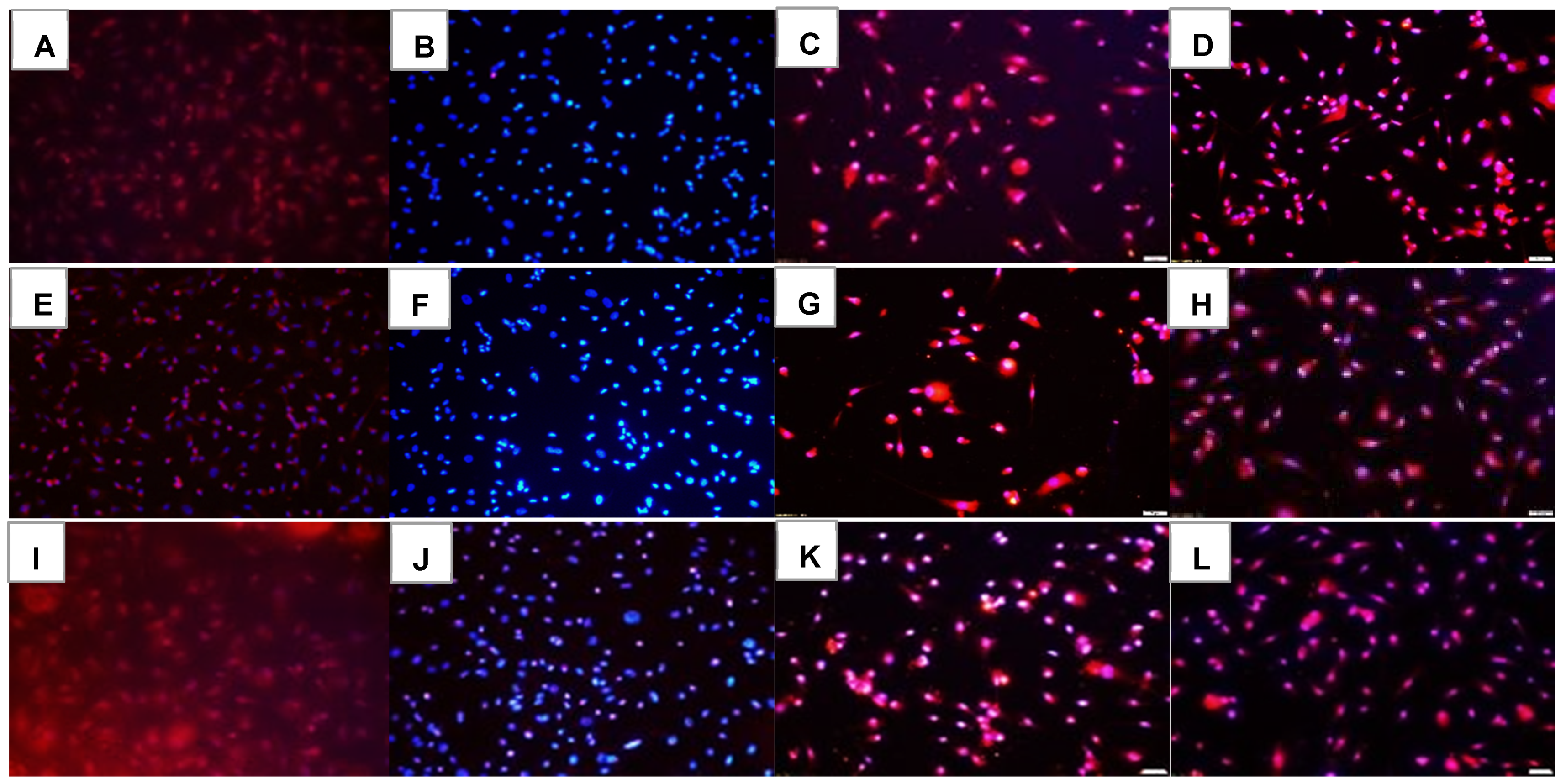




| Groups | Number Tubes | Tube Length (μm) | Number Branching Points | Thickness of Tube Sides (μm) | Central Vacuole Diameter (μm2) | Central Vacuole Perimeter (μm) |
|---|---|---|---|---|---|---|
| Nx: | ||||||
| Saline | 50.3 ± 0.13 | 222.7 ± 0.33 | 47.0 ± 0.1 | 26.0 ± 0.14 | 40,742.8 ± 6.2 | 794.6 ± 0.59 |
| Avastin | 55.6 ± 0.55 ** | 200.3 ± 0.43 ** | 58.9 ± 0.3 ** | 45.1 ± 0.22 ** | 20,156.9 ± 10.3 ** | 556.3 ± 1.2 ** |
| Lo-Eylea | 10.0 ± 0.1 ** | 126.3 ± 0.3 ** | 29.0 ± 0.5 ** | 11.5 ± 0.07 ** | 23,213.7 ± 4.7 ** | 645.3 ± 0.56 ** |
| Hi-Eylea | 31.0 ± 0.2 ** | 181.9 ± 0.32 ** | 28.0 ± 0.2 ** | 20.5 ± 0.13 ** | 51,106.8 ± 8.2 ** | 882.9 ± 0.73 ** |
| 50% O2: | ||||||
| Saline | 13.0 ± 0.1 ## | 132.1 ± 0.29 ## | 30.0 ± 0.3 ## | 7.9 ± 0.05 ## | 15,422.7 ± 4.6 ## | 492.3 ± 0.56 ## |
| Avastin | 12.0 ± 0.1 **## | 118.3 ± 0.28 **## | 37.8 ± 0.6 **## | 9.2 ± 0.07 **## | 25,789.8 ± 4.9 **## | 641.2 ± 0.54 **## |
| Lo-Eylea | 11.0 ± 0.2 **## | 215.2 ± 0.42 **## | 24.4 ± 0.1 **## | 26.4 ± 0.16 **## | 69,664.4 ± 9.5 **## | 1073.6 ± 0.78 **## |
| Hi-Eylea | 32.0 ± 0.15 **## | 213.9 ± 0.37 **## | 43.3 ± 0.6 **## | 29.0 ± 0.17 **## | 55,495.1 ± 7.3 **## | 959.6 ± 0.66 **## |
| Neonatal IH: | ||||||
| Saline | 75.9 ± 0.35 ## | 149.8 ± 0.29 ## | 83.2 ± 0.9 ## | 16.2 ± 0.12 ## | 35,960.2 ± 6.2 ## | 755.9 ± 0.6 |
| Avastin | 0 ± 0 **## | 0 ± 0 **## | 0 ± 0 **## | 0 ± 0 **## | 0 ± 0 **## | 0 ± 0 **## |
| Lo-Eylea | 10.0 ± 0.1 ** | 223.8 ± 0.43 **## | 20.3 ± 0.1 **## | 36.7 ± 0.25 **## | 915,28.4 ± 10.1 **## | 1210.1 ± 0.82 **## |
| Hi-Eylea | 31.0 ± 0.3 ** | 145.2 ± 0.21 **## | 62.3 ± 0.4 **## | 15.9 ± 0.08 ## | 32,956.5 ± 7.5 **## | 699.0 ± 0.76 **## |
| Groups | HIF1α | VEGF-A | VEGFR-1 | VEGFR-2 | Notch-1 | Notch-4 | DLL-4 | Jagged-1 | Lipid Peroxidation |
|---|---|---|---|---|---|---|---|---|---|
| Nx: | |||||||||
| Saline | 276.3 ± 0.5 | 591.8 ± 0.7 | 160.7 ± 0.5 | 371.0 ± 0.2 | 184.0 ± 0.5 | 211.7 ± 0.5 | 419.0 ± 0.3 | 657.7 ± 0.7 | 126.6 ± 0.2 |
| Avastin | 243.0 ± 0.4 ** | 449.5 ± 0.5 ** | 145.0 ± 0.7 ** | 106.0 ± 0.4 ** | 400.0 ± 0.2 ** | 253.3 ± 0.3 ** | 104.5 ± 0.3 ** | 270.0 ± 0.5 ** | 158.2 ± 0.3 ** |
| Lo-Eylea | 171.3 ± 0.4 ** | 440.7 ± 0.5 ** | 223.7 ± 0.5 ** | 267.3 ± 0.4 ** | 330.3 ± 0.5 ** | 133.0 ± 0.7 ** | 484.7 ± 1.0 ** | 495.3 ± 0.6 ** | 167.7 ± 0.3 ** |
| Hi-Eylea | 290.7 ± 0.1 ** | 369.3 ± 0.5 ** | 245.0 ± 0.7 ** | 322.0 ± 0.5 ** | 428.3 ± 0.7 ** | 122.3 ± 0.3 ** | 327.8 ± 0.7 ** | 459.0 ± 1.1 ** | 185.6 ± 0.2 ** |
| 50% O2: | |||||||||
| Saline | 232.3 ± 0.2 | 196.3 ± 0.3 | 392.3 ± 0.4 | 145.3 ± 0.1 | 167.7 ± 0.9 | 166.7 ± 0.2 | 252.3 ± 0.3 | 351.7 ± 0.6 | 132.6 ± 0.2 |
| Avastin | 109.3 ± 0.4 ** | 146.3 ± 0.3 ** | 144.0 ± 0.5 ** | 97.7 ± 0.3 ** | 466.0 ± 0.4 ** | 491.7 ± 0.4 ** | 202.0 ± 0.3 ** | 233.3 ± 0.5 ** | 192.5 ± 0.3 ** |
| Lo-Eylea | 184.0 ± 0.2 ** | 170.7 ± 0.2 ** | 147.3 ± 0.2 ** | 201.3 ± 0.4 ** | 166.3 ± 0.3 ** | 154.3 ± 0.2 ** | 305.0 ± 0.6 *# | 376.7 ± 0.3 ** | 247.0 ± 0.2 ** |
| Hi-Eylea | 181.3 ± 03 ** | 219.7 ± 0.5 ** | 154.0 ± 0.3 ** | 197.7 ± 0.4 ** | 208.7 ± 0.4 ** | 182.0 ± 0.2 ** | 328.3 ± 0.4 ** | 352.3 ± 0.5 ** | 325.0 ± 0.5 ** |
| Neonatal IH: | |||||||||
| Saline | 528.0 ± 0.6 | 661.3 ± 0.6 | 676.0 ± 0.6 | 958.7 ± 1.0 | 211.3 ± 1.1 | 237.3 ± 0.6 | 563.0 ± 0.7 | 490.3 ± 0.4 | 230.0 ± 0.2 |
| Avastin | 282.0 ± 0.1 ** | 363.5 ± 0.3 ** | 165.5 ± 0.3 ** | 102.5 ± 0.2 ** | 384.5 ± 0.2 ** | 404.0 ± 0.4 ** | 528.5 ± 0.6 ** | 451.0 ± 0.3 ** | 201.5 ± 0.2 ** |
| Lo-Eylea | 251.3 ± 0.3 ** | 282.7 ± 0.5 ** | 127.3 ± 0.2 ** | 312.7 ± 0.5 ** | 365.3 ± 0.7 ** | 265.0 ± 0.4 ** | 590.7 ± 0.8 ** | 326.3 ± 0.5 | 293.0 ± 0.2 ** |
| Hi-Eylea | 164.0 ± 0.2 ** | 332.0 ± 0.2 ** | 154.7 ± 0.2 ** | 275.7 ± 0.3 ** | 319.3 ± 0.3 ** | 183.0 ± 0.4 ** | 499.3 ± 0.5 ** | 389.7 ± 0.3 ** | 400.8 ± 0.5 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdanyar, A.; Cai, C.L.; Aranda, J.V.; Shrier, E.; Beharry, K.D. Comparison of Bevacizumab and Aflibercept for Suppression of Angiogenesis in Human Retinal Microvascular Endothelial Cells. Pharmaceuticals 2023, 16, 939. https://doi.org/10.3390/ph16070939
Yazdanyar A, Cai CL, Aranda JV, Shrier E, Beharry KD. Comparison of Bevacizumab and Aflibercept for Suppression of Angiogenesis in Human Retinal Microvascular Endothelial Cells. Pharmaceuticals. 2023; 16(7):939. https://doi.org/10.3390/ph16070939
Chicago/Turabian StyleYazdanyar, Amirfarbod, Charles L. Cai, Jacob V. Aranda, Eric Shrier, and Kay D. Beharry. 2023. "Comparison of Bevacizumab and Aflibercept for Suppression of Angiogenesis in Human Retinal Microvascular Endothelial Cells" Pharmaceuticals 16, no. 7: 939. https://doi.org/10.3390/ph16070939
APA StyleYazdanyar, A., Cai, C. L., Aranda, J. V., Shrier, E., & Beharry, K. D. (2023). Comparison of Bevacizumab and Aflibercept for Suppression of Angiogenesis in Human Retinal Microvascular Endothelial Cells. Pharmaceuticals, 16(7), 939. https://doi.org/10.3390/ph16070939






