Analysis of Corticosteroid-Induced Glaucoma Using the Japanese Adverse Drug Event Reporting Database
Abstract
1. Introduction
2. Results
2.1. Preparing Data Tables for Analysis
2.2. Relevance of Glaucoma to Patient Characteristics
2.3. Relationship between Glaucoma and Drugs
2.4. Hierarchical Clustering of Glaucoma-Inducing Corticosteroids
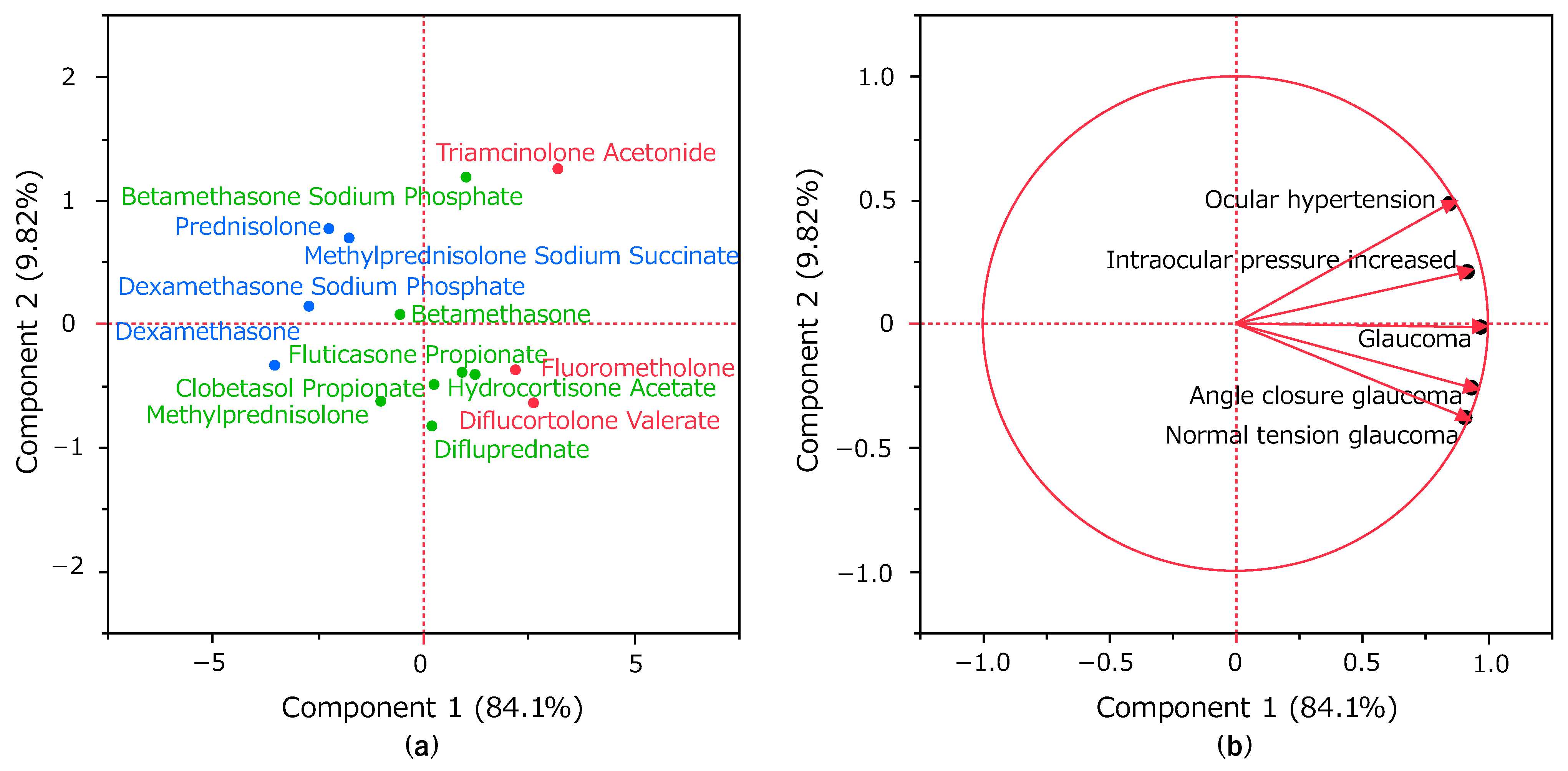
2.5. Principal Component Analysis of Glaucoma-Induced Corticosteroids
3. Discussion
3.1. Glaucoma and Characteristics of the Patient
3.2. Glaucoma and Corticosteroids
3.3. Limitations
4. Materials and Methods
4.1. Database (JADER)
4.2. Drugs to Be Analyzed and Adverse Event Terms
4.3. Creation of a Data Table for Analysis
4.4. Glaucoma and Characteristics of the Patients
4.5. Glaucoma and Corticosteroids
4.6. Hierarchical Clustering
4.7. Principal Component Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morizane, Y.; Morimoto, N.; Fujiwara, A.; Kawasaki, R.; Yamashita, H.; Ogura, Y.; Shiraga, F. Incidence and causes of visual impairment in Japan: The first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn. J. Ophthalmol. 2019, 63, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Suzuki, Y.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G.; et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi Study. Ophthalmology 2004, 111, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iwase, A.; Araie, M.; Suzuki, Y.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G.; et al. The Tajimi Study Group, Japan Glaucoma Society. The Tajimi Study report 2: Prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology 2005, 112, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.C.; Tripathi, B.J.; Haggerty, C. Drug-induced glaucomas: Mechanism and management. Drug. Saf. 2003, 26, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Razeghinejad, M.R.; Katz, L.J. Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 2012, 47, 66–80. [Google Scholar] [CrossRef]
- Malclès, A.; Dot, C.; Voirin, N.; Vié, A.L.; Agard, É.; Bellocq, D.; Denis, P.; Kodjikian, L. Safety of intravitreal dexamethasone implant (OZURDEX): The SAFODEX study. Incidence and risk factors of ocular hypertension. Retina 2017, 37, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Hulsman, C.A.; Westendorp, I.C.; Ramrattan, R.S.; Wolfs, R.C.; Witteman, J.C.; Vingerling, J.R.; Hofman, A.; de Jong, P.T. Is open-angle glaucoma associated with early menopause? The Rotterdam study. Am. J. Epidemiol. 2001, 154, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Park, S.E.; Kang, H.G.; Byeon, S.H.; Kim, S.S.; Koh, H.J.; Lee, S.; Seong, G.J.; Kim, C.Y.; Kim, M. Intraocular pressure change after injection of intravitreal dexamethasone (Ozurdex) implant in Korean patients. Br. J. Ophthalmol. 2019, 103, 1380–1387. [Google Scholar] [CrossRef]
- Parekh, A.; Srivastava, S.; Bena, J.; Albini, T.; Nguyen, Q.D.; Goldstein, D.A. Risk factors associated with intraocular pressure increase in patients with uveitis treated with the fluocinolone acetonide implant. JAMA Ophthalmol. 2015, 133, 568–573. [Google Scholar] [CrossRef]
- Garbe, E.; LeLorier, J.; Boivin, J.F.; Suissa, S. Risk of ocular hypertension or open-angle glaucoma in elderly patients on oral glucocorticoids. Lancet 1997, 350, 979–982. [Google Scholar] [CrossRef]
- Kersey, J.P.; Broadway, D.C. Corticosteroid-induced glaucoma: A review of the literature. Eye 2006, 20, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Kazuyuki, S.; Shinichi, K.; Koji, I.; Yasushi, I. Today’s Drug Therapy in 2022, 44th ed.; Nankodo: Tokyo, Japan, 2022; ISBN 978-4-524-23211-6. [Google Scholar]
- Clark, A.F.; Wilson, K.; de Kater, A.W.; Allingham, R.R.; McCartney, M.D. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Investig. Ophthalmol. Vis. Sci. 1995, 36, 478–489. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95442-4. [Google Scholar]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L.; Pasquale, L.R. Genetics of glaucoma. Hum. Mol. Genet. 2017, 26, R21–R27. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Roth, D.B.; Verma, V.; Realini, T.; Prenner, J.L.; Feuer, W.J.; Fechtner, R.D. Long-term incidence and timing of intraocular hypertension after intravitreal triamcinolone acetonide injection. Ophthalmology 2009, 116, 455–460. [Google Scholar] [CrossRef]
- Sihota, R.; Konkal, V.L.; Dada, T.; Agarwal, H.C.; Singh, R. Prospective, long-term evaluation of steroid-induced glaucoma. Eye 2008, 22, 26–30. [Google Scholar] [CrossRef]
- Roberti, G.; Oddone, F.; Agnifili, L.; Katsanos, A.; Michelessi, M.; Mastropasqua, L.; Quaranta, L.; Riva, I.; Tanga, L.; Manni, G. Steroid-induced glaucoma: Epidemiology, pathophysiology, and clinical management. Surv. Ophthalmol. 2020, 65, 458–472. [Google Scholar] [CrossRef]
- Maeda, R. JADER from pharmacovigilance point of view. Jpn. J. Pharmacoepidemiol. 2014, 19, 51–56. [Google Scholar] [CrossRef]
- Pharmaceutical and Medical Devices Agency. Available online: https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0005.html (accessed on 12 April 2023).
- MedDRA Japanese Maintenance Organization. Available online: https://www.jmo.pmrj.jp/ (accessed on 12 April 2023).
- Greenland, S.; Schwartzbaum, J.A.; Finkle, W.D. Problems due to small samples and sparse data in conditional logistic regression analysis. Am. J. Epidemiol. 2000, 151, 531–539. [Google Scholar] [CrossRef]
- Van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.M.; Lindquist, M.; Orre, R.; Egberts, A.C. A Comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug. Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Wang, S.J.; Tsai, C.A.; Lin, C.J. Selection of differentially expressed genes in microarray data analysis. Pharm. J. 2007, 7, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Okunaka, M.; Kano, D.; Matsui, R.; Kawasaki, T.; Uesawa, Y. Comprehensive analysis of chemotherapeutic agents that induce infectious neutropenia. Pharmaceuticals 2021, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-470-74991-3. [Google Scholar]
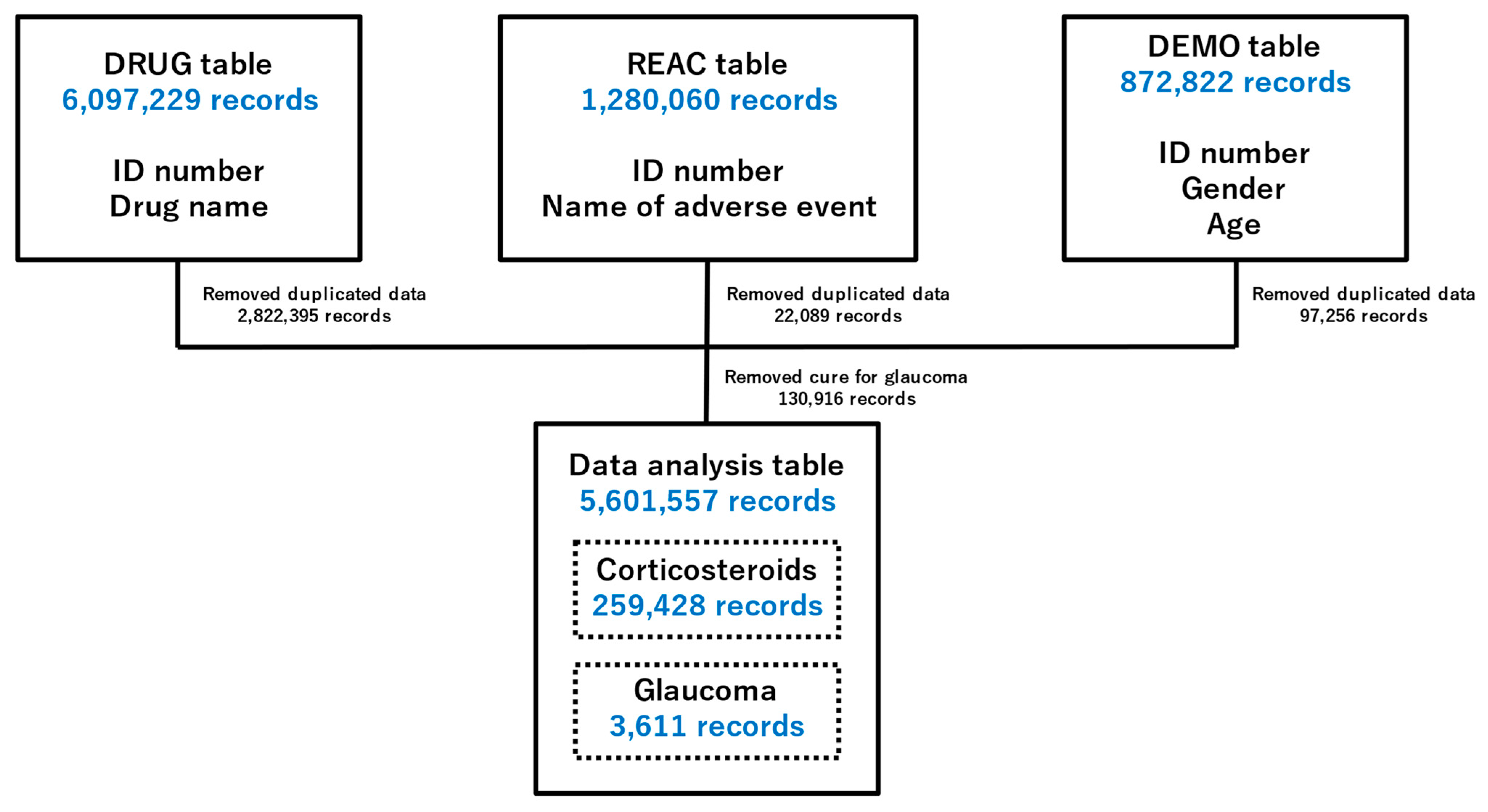


| Patient Background | Glaucoma (1405) | Nonglaucoma (1,288,906) | p-Value (Fisher’s Exact Test) | |
|---|---|---|---|---|
| Sex | Male | 653/1347 | 599,030/1,209,010 | 0.445 |
| Female | 694/1347 | 609,980/1,209,010 | ||
| Age | ≥70 years old | 436/1145 | 468,717/1,170,976 | 0.184 |
| <70 years old | 709/1145 | 702,259/1,170,976 | ||
| ≥40 years old | 949/1145 | 960,658/1,170,976 | 0.488 | |
| <40 years old | 196/1145 | 210,318/1,170,976 |
| Corticosteroids | Reporting Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Alclometasone dipropionate | 2.10 | 0.13–33.65 | 1.000 |
| Amcinonide | 43.99 | 2.65–731.68 | 1.000 |
| Beclometasone dipropionate | 13.90 | 7.07–27.33 | <0.001 |
| Betamethasone | 3.53 | 2.02–6.15 | <0.001 |
| Betamethasone butyrate propionate | 4.12 | 2.10–8.08 | 0.001 |
| Betamethasone dipropionate | 36.99 | 14.52–94.28 | <0.001 |
| Betamethasone sodium phosphate | 12.41 | 8.95–17.22 | <0.001 |
| Betamethasone valerate | 7.41 | 2.93–18.73 | 0.004 |
| Budesonide | 4.63 | 1.84–11.70 | 0.017 |
| Ciclesonide | 1.40 | 0.09–22.39 | 1.000 |
| Clobetasol propionate | 5.89 | 3.30–10.52 | <0.001 |
| Clobetasone butyrate | 11.97 | 4.18–34.29 | 0.003 |
| Cortisone acetate | 12.32 | 0.76–199.16 | 1.000 |
| Deprodone propionate | 9.56 | 0.59–154.25 | 1.000 |
| Dexamethasone | 0.67 | 0.41–1.09 | 0.085 |
| Dexamethasone cipecilate | 50.77 | 9.98–258.32 | 0.030 |
| Dexamethasone dipropionate | 12.40 | 3.57–43.08 | 0.018 |
| Dexamethasone palmitate | 3.64 | 0.73–18.08 | 0.338 |
| Dexamethasone sodium metasulfobenzoate | 98.96 | 33.57–291.78 | <0.001 |
| Dexamethasone sodium phosphate | 1.03 | 0.67–1.60 | 0.910 |
| Dexamethasone valerate | 25.74 | 8.95–74.07 | <0.001 |
| Diflorasone diacetate | 10.71 | 3.74–30.66 | 0.005 |
| Diflucortolone valerate | 21.37 | 11.61–39.33 | <0.001 |
| Difluprednate | 6.55 | 3.57–12.01 | <0.001 |
| Fludrocortisone acetate | 2.04 | 0.13–32.67 | 1.000 |
| Fludroxycortide | 4.49 | 0.28–72.07 | 1.000 |
| Fluocinolone acetonide | 9.93 | 0.62–160.27 | 1.000 |
| Fluocinonide | 2.71 | 0.17–43.38 | 1.000 |
| Fluorometholone | 27.09 | 18.00–40.79 | <0.001 |
| Fluticasone furoate | 15.03 | 7.32–30.88 | <0.001 |
| Fluticasone propionate | 11.95 | 7.64–18.68 | <0.001 |
| Hydrocortisone acetate | 9.95 | 5.57–17.79 | <0.001 |
| Hydrocortisone probutate | 49.06 | 13.92–172.9 | 0.001 |
| Hydrocortisone sodium phosphate | 3.10 | 1.09–8.86 | 0.106 |
| Hydrocortisone sodium succinate | 0.84 | 0.36–1.93 | 0.696 |
| Methylprednisolone | 2.12 | 1.34–3.35 | 0.006 |
| Methylprednisolone acetate | 1.72 | 0.11–27.61 | 1.000 |
| Methylprednisolone sodium succinate | 2.80 | 1.97–3.98 | <0.001 |
| Mometasone furoate | 8.74 | 2.52–30.32 | 0.034 |
| Mometasone furoate monohydrate | 9.98 | 3.49–28.56 | 0.006 |
| Prednisolone | 1.93 | 1.62–2.29 | <0.001 |
| Prednisolone acetate | 1.46 | 0.63–3.38 | 0.435 |
| Prednisolone sodium phosphate | 4.17 | 0.26–66.97 | 1.000 |
| Prednisolone sodium succinate | 1.15 | 0.40–3.29 | 1.000 |
| Prednisolone valeroacetate | 22.78 | 12.00–43.27 | <0.001 |
| Triamcinolone | 11.64 | 2.33–58.03 | 0.122 |
| Triamcinolone acetonide | 57.41 | 46.63–70.67 | <0.001 |
| Corticosteroids | Number of Times Reported |
|---|---|
| Clobetasol propionate | 3023 |
| Diflucortolone Valerate | 768 |
| Difluprednate | 2485 |
| Dexamethasone | 37,816 |
| Dexamethasone Sodium Phosphate | 30,523 |
| Triamcinolone Acetonide | 2693 |
| Hydrocortisone Butyrate | 1794 |
| Fluorometholone | 1366 |
| Fluticasone Propionate | 2543 |
| prednisolone | 107,943 |
| betamethasone | 5483 |
| Betamethasone Sodium Phosphate | 4604 |
| Methylprednisolone Sodium Succinate | 17,429 |
| Corticosteroids | |
|---|---|
| Alclometasone Dipropionate | Amcinonide |
| Beclometasone Dipropionate | Betamethasone |
| Betamethasone Butyrate Propionate | Betamethasone Dipropionate |
| Betamethasone Sodium Phosphate | Betamethasone Valerate |
| Budesonide | Ciclesonide |
| Clobetasol Propionate | Clobetasone Butyrate |
| Cortisone Acetate | Deprodone Propionate |
| Dexamethasone | Dexamethasone cipecilate |
| Dexamethasone Dipropionate | Dexamethasone Palmitate |
| Dexamethasone Sodium Metasulfobenzoate | Dexamethasone Sodium Phosphate |
| Dexamethasone Valerate | Diflorasone Diacetate |
| Diflucortolone Valerate | Difluprednate |
| Fludrocortisone Acetate | Fludroxycortide |
| Fluocinolone Acetonide | Fluocinonide |
| Fluorometholone | Fluticasone Furoate |
| Fluticasone Propionate | Hydrocortisone Acetate |
| Hydrocortisone Probutat | Hydrocortisone Sodium Phosphate |
| Hydrocortisone Sodium Succinate | Methylprednisolone |
| Methylprednisolone Acetate | Methylprednisolone Sodium Succinate |
| Mometasone Furoate | Mometasone Furoate Monohydrate |
| Prednisolone | Prednisolone Acetate |
| Prednisolone Sodium Phosphate | Prednisolone Sodium Succinate |
| Prednisolone Valeroacetate | Triamcinolone |
| Triamcinolone Acetonide | |
| Preferred Terms | Number of Reports |
|---|---|
| Acute myopia | 0 |
| Angle-closure glaucoma | 507 |
| Aphakic glaucoma | 0 |
| Borderline glaucoma | 0 |
| Diabetic glaucoma | 0 |
| Exfoliation glaucoma | 1 |
| Fundoscopy abnormal | 21 |
| Glaucoma | 1790 |
| Glaucoma drug therapy | 0 |
| Glaucomatocyclitic crises | 16 |
| Glaucomatous optic disc atrophy | 1 |
| Gonioscopy abnormal | 0 |
| Halo vision | 0 |
| Intraocular pressure fluctuation | 2 |
| Intraocular pressure increased | 838 |
| Intraocular pressure test abnormal | 1 |
| Loss of visual contrast sensitivity | 1 |
| Malignant glaucoma | 4 |
| Normal tension glaucoma | 52 |
| Ocular hypertension | 272 |
| Open-angle glaucoma | 41 |
| Optic discs blurred | 1 |
| The optical nerve cup/disc ratio increased | 0 |
| Optic nerve cupping | 40 |
| Phacolytic glaucoma | 0 |
| Pigmentary glaucoma | 0 |
| Pseudophakic glaucoma | 0 |
| Pupillary light reflex tests abnormal | 7 |
| Slit-lamp tests abnormal | 0 |
| Uveitic glaucoma | 1 |
| Uveitis-glaucoma-hyphaema syndrome | 0 |
| The visualVisual field tests abnormal | 15 |
| Glaucoma | Nonglaucoma | |
|---|---|---|
| Reports with the suspected medicine | a | b |
| All other reports | c | d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawabe, A.; Uesawa, Y. Analysis of Corticosteroid-Induced Glaucoma Using the Japanese Adverse Drug Event Reporting Database. Pharmaceuticals 2023, 16, 948. https://doi.org/10.3390/ph16070948
Kawabe A, Uesawa Y. Analysis of Corticosteroid-Induced Glaucoma Using the Japanese Adverse Drug Event Reporting Database. Pharmaceuticals. 2023; 16(7):948. https://doi.org/10.3390/ph16070948
Chicago/Turabian StyleKawabe, Ayano, and Yoshihiro Uesawa. 2023. "Analysis of Corticosteroid-Induced Glaucoma Using the Japanese Adverse Drug Event Reporting Database" Pharmaceuticals 16, no. 7: 948. https://doi.org/10.3390/ph16070948
APA StyleKawabe, A., & Uesawa, Y. (2023). Analysis of Corticosteroid-Induced Glaucoma Using the Japanese Adverse Drug Event Reporting Database. Pharmaceuticals, 16(7), 948. https://doi.org/10.3390/ph16070948







