Dulaglutide Ameliorates Intrauterine Adhesion by Suppressing Inflammation and Epithelial–Mesenchymal Transition via Inhibiting the TGF-β/Smad2 Signaling Pathway
Abstract
:1. Introduction
2. Results
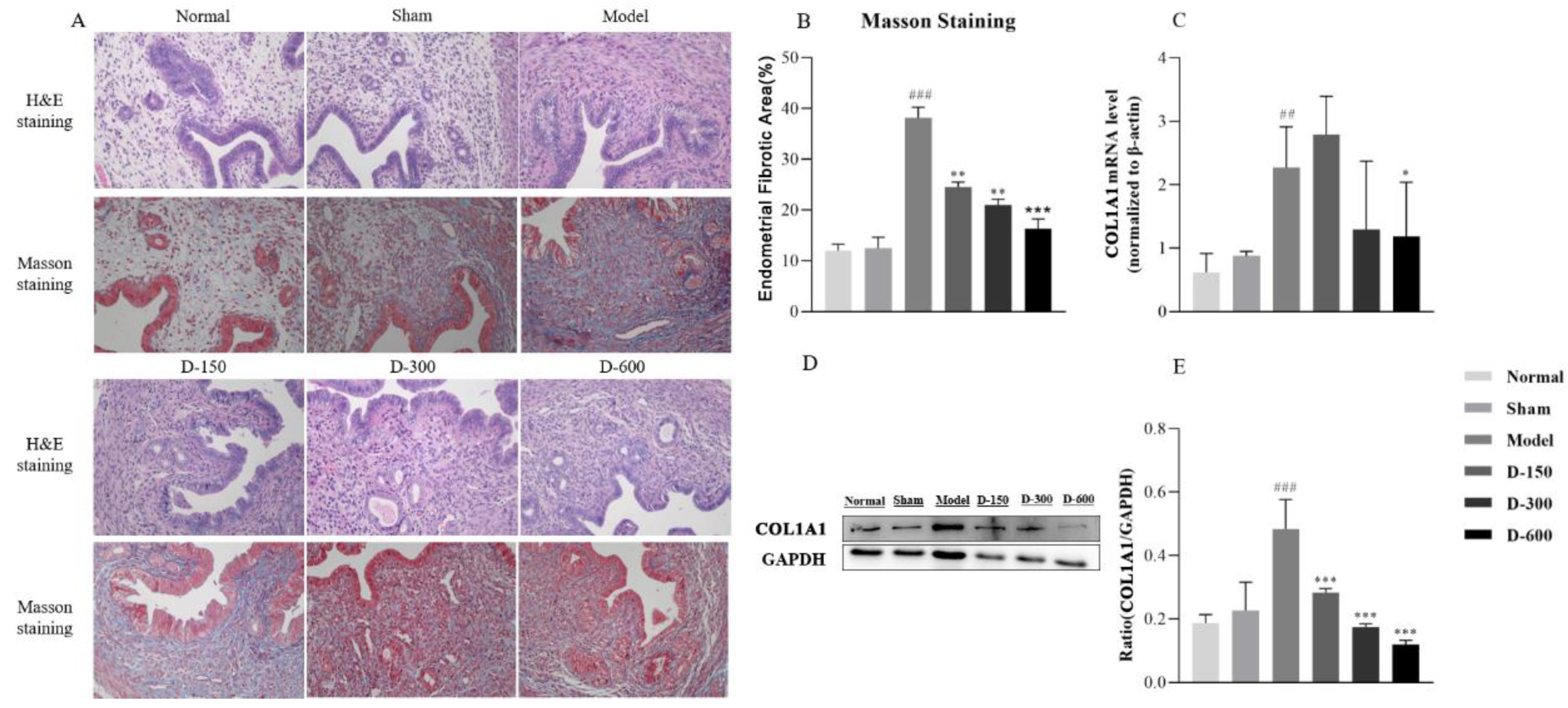
2.1. Dulaglutide Improves Endometrial Morphology and Reduces Collagen Fiber Deposition
2.2. Dulaglutide May Reduce Inflammatory Responses by Inhibiting M1 Macrophage Polarization and the Release of Inflammatory Factors
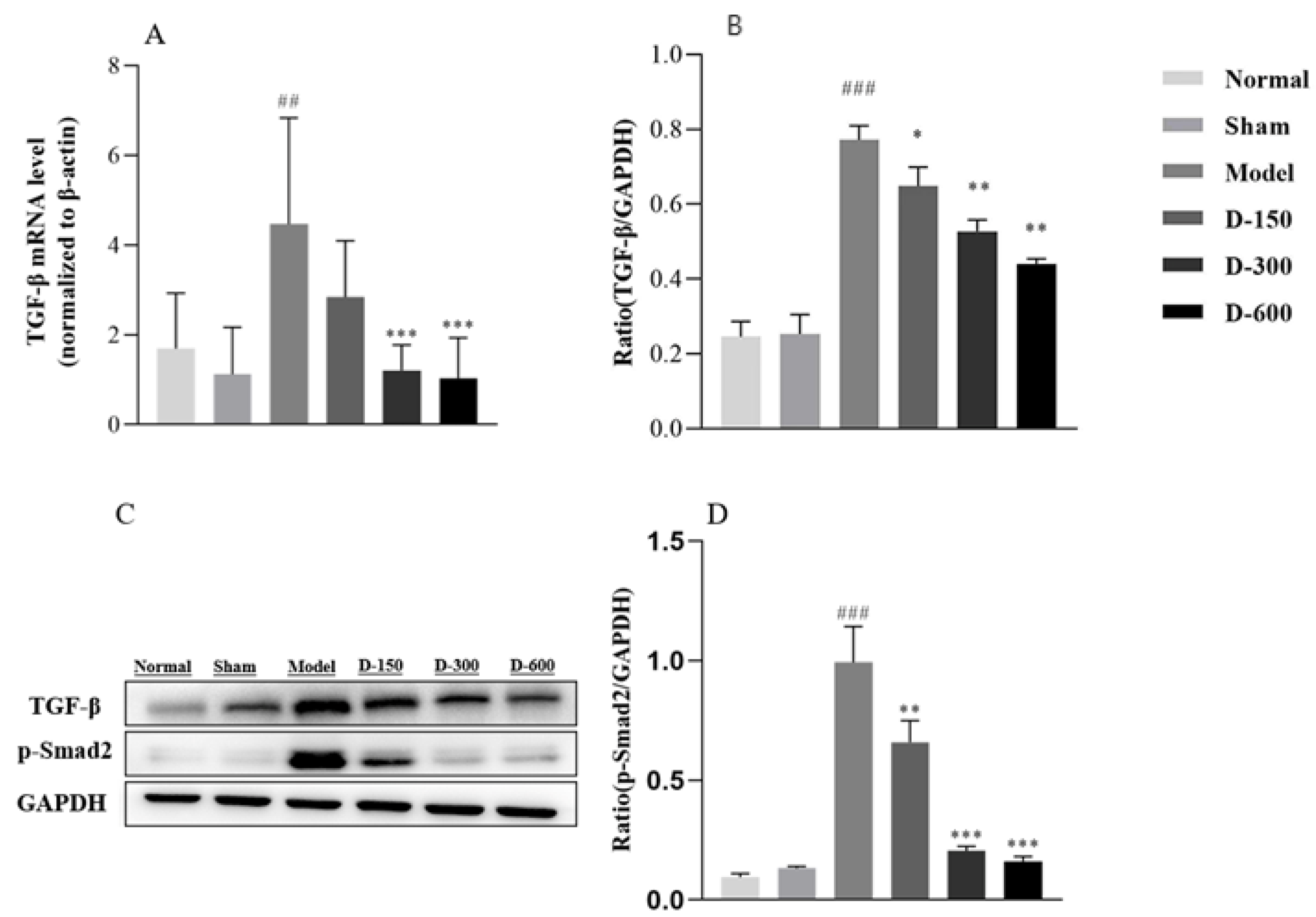
2.3. Dulaglutide May Ameliorate Fibrosis by Inhibiting Epithelial–Mesenchymal Transition via the TGF-β/Smad2 Signaling Pathway
3. Discussion
4. Methods
4.1. Animals and Chemicals
4.2. Animal Model and Experimental Groups
4.3. Histopathological Evaluation
4.4. RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.5. Western Blotting
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Lv, H.F.; Zhao, R.; Ying, M.F.; Samuriwo, A.T.; Zhao, Y.Z. Recent developments in bio-scaffold materials as delivery strategies for therapeutics for endometrium regeneration. Mater. Today Bio 2021, 11, 100101. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Liu, C.H.; Cheng, M.; Chang, W.H.; Liu, W.M.; Wang, P.H. Focus on the primary prevention of intrauterine adhesions: Current concept and vision. Int. J. Mol. Sci. 2021, 22, 5175. [Google Scholar] [CrossRef] [PubMed]
- Rout-Pitt, N.; Farrow, N.; Parsons, D.; Donnelley, M. Epithelial mesenchymal transition (EMT): A universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir. Res. 2018, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ding, M.; He, W. Emerging therapeutic strategies for attenuating tubular EMT and kidney fibrosis by targeting Wnt/β-catenin signaling. Front. Pharmacol. 2021, 12, 830340. [Google Scholar] [CrossRef] [PubMed]
- Lovisa, S.; Genovese, G.; Danese, S. Role of epithelial-to-mesenchymal transition in inflammatory bowel disease. J. Crohns. Colitis. 2019, 13, 659–668. [Google Scholar] [CrossRef]
- Shu, D.Y.; Lovicu, F.J. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-mesenchymal transition (EMT): The Type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Lih Yuan, T.; Sulaiman, N.; Nur Azurah, A.G.; Maarof, M.; Razali, R.A.; Yazid, M.D. Oestrogen-induced epithelial-mesenchymal transition (EMT) in endometriosis: Aetiology of vaginal agenesis in Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. Front. Physiol. 2022, 13, 937988. [Google Scholar] [CrossRef]
- Xu, Q.; Duan, H.; Gan, L.; Liu, X.; Chen, F.; Shen, X.; Tang, Y.Q.; Wang, S. MicroRNA-1291 promotes endometrial fibrosis by regulating the ArhGAP29-RhoA/ROCK1 signaling pathway in a murine model. Mol. Med. Rep. 2017, 16, 4501–4510. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.P.; Chen, L.M.; Chen, F.; Jiang, N.H.; Sui, L. Smad signaling coincides with epithelial-mesenchymal transition in a rat model of intrauterine adhesion. Am. J. Transl. Res. 2019, 11, 4726–4737. [Google Scholar]
- Yao, Y.; Chen, R.; Wang, G.; Zhang, Y.; Liu, F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res. Ther. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, J.; Pan, Y.; Wang, J.; Yang, J.; Shen, S.; Hou, Y. Melatonin-primed MSCs alleviate intrauterine adhesions by affecting MSC-expressed galectin-3 on macrophage polarization. Stem. Cells 2022, 40, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Wu, Y.H.; Ali, M.; Wu, X.Q.; Nie, M.F. Endometrial stromal cells treated by tumor necrosis factor-α stimulate macrophages polarized toward M2 via interleukin-6 and monocyte chemoattractant protein-1. J. Obstet. Gynaecol. Res. 2020, 46, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Xie, Y.; Lu, W.; Chen, Y.; Lu, Z.; Zhen, J.; Wang, W.; Shang, A. Engineering an enhanced thrombin-based GLP-1 analog with long-lasting glucose-lowering and efficient weight reduction. RSC Adv. 2019, 9, 30707–30714. [Google Scholar] [CrossRef] [PubMed]
- Basalay, M.V.; Davidson, S.M.; Yellon, D.M. Neuroprotection in rats following ischaemia-reperfusion injury by GLP-1 analogues-liraglutide and semaglutide. Cardiovasc. Drugs Ther. 2019, 33, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Seufert, J.; Gallwitz, B. The extra-pancreatic effects of GLP-1 receptor agonists: A focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes. Metab. 2014, 16, 673–688. [Google Scholar] [CrossRef]
- Wang, L.; Ding, J.; Zhu, C.; Guo, B.; Yang, W.; He, W.; Li, X.; Wang, Y.; Li, W.; Wang, F.; et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole-kindled mice. Int. J. Mol. Med. 2021, 48, 219. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Toba, L.; Fandiño, J.; González-Matías, L.C.; Garcia-Segura, L.M.; Mallo, F. The GLP-1 analog, liraglutide prevents the increase of proinflammatory mediators in the hippocampus of male rat pups submitted to maternal perinatal food restriction. J. Neuroinflammation 2018, 15, 337. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.K.; Ma, D.X.; Wang, Z.M.; Hu, X.F.; Li, S.L.; Tian, H.Z.; Wang, M.J.; Shu, Y.W.; Yang, J. The glucagon-like peptide-1 (GLP-1) analog liraglutide attenuates renal fibrosis. Pharmacol. Res. 2018, 131, 102–111. [Google Scholar] [CrossRef]
- Withaar, C.; Meems, L.M.G.; Markousis-Mavrogenis, G.; Boogerd, C.J.; Silljé, H.H.W.; Schouten, E.M.; Dokter, M.M.; Voors, A.A.; Westenbrink, B.D.; Lam, C.S.P.; et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc. Res. 2021, 117, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.Q.; Luo, L.Z.; Xue, P.P.; Han, Y.H.; Wang, L.F.; Zhuge, D.L.; Yao, Q.; Chen, B.; Zhao, Y.Z.; Xu, H.L. Glucose-responsive hydrogel enhances the preventive effect of insulin and liraglutide on diabetic nephropathy of rats. Acta Biomater. 2021, 122, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.F.; Cao, Y.J.; Chen, X.; Zhang, Y.; Fan, Y.P.; Liu, J.; Chen, X.L. Lixisenatide protects doxorubicin-induced renal fibrosis by activating wNF-κB/TNF-α and TGF-β/Smad pathways. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4017–4026. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Ding, Y.; Wu, L.M.; Wang, Y.X.; Yao, Y.; Wang, Y.X.; Zhang, Y.G.; Niu, J.Q.; He, X.X.; Wang, Y.Q. The glucagon-like peptide-1 (GLP-1) analog exenatide ameliorates intrauterine adhesions in mice. Peptides 2021, 137, 170481. [Google Scholar] [CrossRef]
- Mohamad, H.E.; Abdelhady, M.A.; Abdel Aal, S.M.; Elrashidy, R.A. Dulaglutide mitigates high dietary fructose-induced renal fibrosis in rats through suppressing epithelial-mesenchymal transition mediated by GSK-3β/TGF-β1/Smad3 signaling pathways. Life Sci. 2022, 309, 120999. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, Q.; Guo, F.; Zhao, Y.; Ji, L.; An, T.; Song, Y.; Liu, Y.; He, Y.; Qin, G. FoxO1-mediated inhibition of STAT1 alleviates tubulointerstitial fibrosis and tubule apoptosis in diabetic kidney disease. EBiomedicine 2019, 48, 491–504. [Google Scholar] [CrossRef] [Green Version]
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial-mesenchymal transition and its transcription factors. Biosci. Rep. 2022, 42, BSR20211754. [Google Scholar] [CrossRef]
- Shrestha, N.; Chand, L.; Han, M.K.; Lee, S.O.; Kim, C.Y.; Jeong, Y.J. Glutamine inhibits CCl4 induced liver fibrosis in mice and TGF-β1 mediated epithelial-mesenchymal transition in mouse hepatocytes. Food Chem. Toxicol. 2016, 93, 129–137. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The pathogenesis of endometriosis: Molecular and cell biology insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [Green Version]
- Gan, L.; Duan, H.; Xu, Q.; Tang, Y.Q.; Li, J.J.; Sun, F.Q.; Wang, S. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy 2017, 19, 603–616. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Tsai, N.C.; Chen, Y.J.; Weng, P.L.; Chang, Y.C.; Cheng, J.H.; Ko, J.Y.; Kang, H.Y.; Lan, K.C. Extracorporeal shock wave therapy combined with platelet-rich plasma during preventive and therapeutic stages of intrauterine adhesion in a rat model. Biomedicines 2022, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Habener, J.F.; Holst, J.J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Investig. 2017, 127, 4217–4227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, T.D.; Finan, B.; Bloom, S.R. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Chen, J.; Xie, J.J.; Shi, K.S. Glucagon-like peptide-1 receptor regulates endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and the progression of osteoarthritis in rat. Cell Death Dis. 2018, 9, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Jia, F.; Zhu, Z.; Huang, L. Lixisenatide attenuates advanced glycation end products (AGEs)-induced degradation of extracellular matrix in human primary chondrocytes. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1256–1264. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Sun, H. Glucagon-like peptide-1 modulates RAW264.7 macrophage polarization by interfering with the JNK/STAT3 signaling pathway. Exp. Ther. Med. 2019, 17, 3573–3579. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Parlevliet, E.T.; Geerling, J.J.; van der Tuin, S.J.; Zhang, H.; Bieghs, V.; Jawad, A.H.; Shiri-Sverdlov, R.; Bot, I.; de Jager, S.C.; et al. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br. J. Pharmacol. 2014, 171, 723–734. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wu, L.; Liu, L.; Ruan, Q.; Zhang, X.; Hong, W.; Wu, S.; Jin, G.; Bai, Y. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem. Pharmacol. 2018, 154, 203–212. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, D.; Chen, S.; Yang, Q. The role of miR-34c-5p/Notch in epithelial-mesenchymal transition (EMT) in endometriosis. Cell Signal 2020, 72, 109666. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, H.; Zhang, X.; Song, M.; Yao, S.; Jiang, P.; Liu, D.; Wang, Z.; Lv, H.; Li, R.; et al. Defective autophagy contributes to endometrial epithelial-mesenchymal transition in intrauterine adhesions. Autophagy. 2022, 18, 2427–2442. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, X.Q.; Ma, A.; He, J.Z.; Wang, L.; Jia, Y.L.; Shao, G.Y.; Li, M.; Zhou, H.; Lin, S.Q.; Ran, J.H.; et al. Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways. Acta Pharmacol. Sin. 2020, 41, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lu, H.; Wu, C.; Liang, Y.; Wang, S.; Lin, C.; Chen, B.; Xia, P. Resveratrol inhibits epithelial-mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochem. Pharmacol. 2014, 92, 484–493. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Lian, X.; Zhu, Z.; Chen, X.; Pei, W.; Li, S.; Abbas, A.; Wang, Y.; Tian, L. MicroRNA-29b mediates lung mesenchymal-epithelial transition and prevents lung fibrosis in the silicosis model. Mol. Ther. Nucleic Acids. 2019, 14, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Andugulapati, S.B.; Gourishetti, K.; Tirunavalli, S.K.; Shaikh, T.B.; Sistla, R. Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-β mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems. Phytomedicine 2020, 78, 153298. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Liu, X.; Xu, D.; Wang, F. MicroRNA-29b regulates TGF-β1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells by targeting AKT2. Exp. Cell Res. 2016, 345, 115–124. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, H.; Yang, H. MicroRNA-326 inhibits endometrial fibrosis by regulating TGF-β1/Smad3 pathway in intrauterine adhesions. Mol. Med. Rep. 2018, 18, 2286–2292. [Google Scholar] [CrossRef] [Green Version]
- Abudukeyoumu, A.; Li, M.Q.; Xie, F. Transforming growth factor-β1 in intrauterine adhesion. Am. J. Reprod. Immunol. 2020, 84, e13262. [Google Scholar] [CrossRef]
- Cao, J.; Liu, D.; Zhao, S.; Yuan, L.; Huang, Y.; Ma, J.; Yang, Z.; Shi, B.; Wang, L.; Wei, J. Estrogen attenuates TGF-β1-induced EMT in intrauterine adhesion by activating Wnt/β-catenin signaling pathway. Braz. J. Med. Biol. Res. 2020, 53, e9794. [Google Scholar] [CrossRef]
- Bao, M.; Feng, Q.; Zou, L.; Huang, J.; Zhu, C.; Xia, W. Endoplasmic reticulum stress promotes endometrial fibrosis through the TGF-β/SMAD pathway. Reproduction 2023, 165, 171–182. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| TGF-β | CTCCCGTGGCTTCTAGTGC | GCCTTAGTTTGGACAGGATCTG |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| IL-6 | CCAAGAGGTGAGTGCTTCCC | CTGTTGTTCAGACTCTCTCCCT |
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| ADGRE1 | TTGTACGTGCAACTCAGGACT | CCACGTCTCACCATTGGGG |
| COL1A1 | GCTCCTCTTAGGGGCCACT | GATCCCAGAGTGTTGATGCAA |
| FN1 | TTCAAGTGTGATCCCCATGAAG | CAGGTCTACGGCAGTTGTCA |
| VIM | CGGCTGCGAGAGAAATTGC | CCACTTTCCGTTCAAGGTCAAG |
| β-actin | CTGAGAGGGAAATCGTGCGT | TGTTGGCATAGAGGTCTTTACGG |
| Antibody | Host | Dilution | Company | Catalog No. |
|---|---|---|---|---|
| Anti-TGF-β | Rabbit | 1:1000 | Cell Signaling Technology (MA, USA) | #3709 |
| Anti-p-Smad2 | Rabbit | 1:2000 | Cell Signaling Technology (MA, USA) | #8828 |
| Anti-COL1A1 | Rabbit | 1:1000 | Cell Signaling Technology (MA, USA) | #72026 |
| Anti-E-cadherin | Mouse | 1:1000 | Cell Signaling Technology (MA, USA) | #14472 |
| Anti-vimentin | Mouse | 1:1000 | Cell Signaling Technology (MA, USA) | #5741 |
| Anti-F4/80 | Mouse | 1:1000 | Bioss (Beijing, China) | bs-11182R |
| Anti-TNF-α | Mouse | 1:1000 | Cell Signaling Technology (MA, USA) | #11948 |
| Anti-IL-1β | Mouse | 1:200 | Santa Cruz (Dallas, USA) | sc-12742 |
| Anti-IL-6 | Mouse | 1:1000 | Cell Signaling Technology (MA, USA) | #12912 |
| Anti-GAPDH mouse monoclonal antibody | Mouse | 1:5000 | TransGene Biotech (Beijing, China) | HC301-01 |
| Goat-anti-mouse IgG | Goat | 1:10,000 | OriGene (Wuxi, China) | ZB-2305 |
| Goat-anti-rabbit IgG | Goat | 1:10,000 | OriGene (Wuxi, China) | ZB-2301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Y.; Wu, Y.; Wang, Y. Dulaglutide Ameliorates Intrauterine Adhesion by Suppressing Inflammation and Epithelial–Mesenchymal Transition via Inhibiting the TGF-β/Smad2 Signaling Pathway. Pharmaceuticals 2023, 16, 964. https://doi.org/10.3390/ph16070964
Wang Y, Wang Y, Wu Y, Wang Y. Dulaglutide Ameliorates Intrauterine Adhesion by Suppressing Inflammation and Epithelial–Mesenchymal Transition via Inhibiting the TGF-β/Smad2 Signaling Pathway. Pharmaceuticals. 2023; 16(7):964. https://doi.org/10.3390/ph16070964
Chicago/Turabian StyleWang, Yifan, Yixiang Wang, Yang Wu, and Yiqing Wang. 2023. "Dulaglutide Ameliorates Intrauterine Adhesion by Suppressing Inflammation and Epithelial–Mesenchymal Transition via Inhibiting the TGF-β/Smad2 Signaling Pathway" Pharmaceuticals 16, no. 7: 964. https://doi.org/10.3390/ph16070964
APA StyleWang, Y., Wang, Y., Wu, Y., & Wang, Y. (2023). Dulaglutide Ameliorates Intrauterine Adhesion by Suppressing Inflammation and Epithelial–Mesenchymal Transition via Inhibiting the TGF-β/Smad2 Signaling Pathway. Pharmaceuticals, 16(7), 964. https://doi.org/10.3390/ph16070964





