Results of Induction of Labor with Prostaglandins E1 and E2 (The RIPE Study): A Real-World Data Analysis of Obstetrical Effectiveness and Clinical Outcomes of Pharmacological Induction of Labor with Vaginal Inserts
Abstract
:1. Introduction
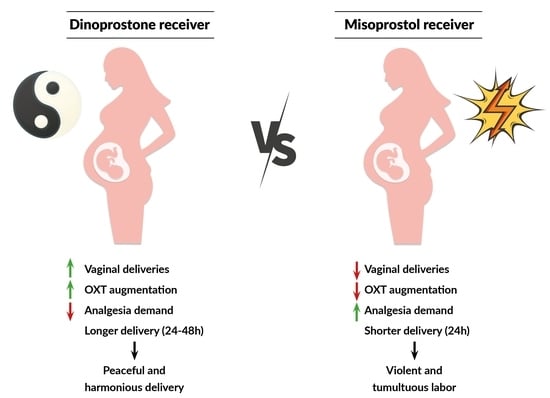
2. Results
2.1. Before 37 Weeks (Table 3; Table 4; Table 5)
2.2. Between 37 and 41 Weeks of Gestation
2.3. >41 Weeks of Gestation
3. Discussion
4. Materials and Methods
4.1. Ethical Review
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsakiridis, I.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Induction of Labor: An Overview of Guidelines. Obstet. Gynecol. Surv. 2020, 75, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Declercq, E.; Belanoff, C.; Iverson, R. Maternal perceptions of the experience of attempted labor induction and medically elective inductions: Analysis of survey results from listening to mothers in California. BMC Pregnancy Childbirth 2020, 20, 458. [Google Scholar] [CrossRef] [PubMed]
- Grobman, W.A.; Rice, M.M.; Reddy, U.M.; Tita, A.T.N.; Silver, R.M.; Mallett, G.; Hill, K.; Thom, E.A.; El-Sayed, Y.Y.; Perez-Delboy, A.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor Induction versus Expectant Management in Low-Risk Nulliparous Women. N. Engl. J. Med. 2018, 379, 513–523. [Google Scholar] [CrossRef]
- Chen, W.; Xue, J.; Peprah, M.K.; Wen, S.W.; Walker, M.; Gao, Y.; Tang, Y. A systematic review and network meta-analysis comparing the use of Foley catheters, Misoprostol, and Dinoprostone for cervical ripening in the induction of labor. BJOG 2016, 123, 346–354. [Google Scholar] [CrossRef]
- Liu, Y.R.; Pu, C.X.; Wang, X.Y.; Wang, X.-Y. Double-balloon catheter versus dinoprostone insert for labor induction: A meta-analysis. Arch. Gynecol. Obstet. 2019, 299, 7–12. [Google Scholar] [CrossRef]
- Hapangama, D.; Neilson, J.P. Mifepristone for induction of labor. Cochrane Database Syst. Rev. 2009, 2009, CD002865. [Google Scholar] [CrossRef]
- Jones, M.N.; Palmer, K.R.; Pathirana, M.M.; Cecatti, J.G.; Filho, O.B.M.; Marions, L.; Edlund, M.; Prager, M.; Pennell, C.; Dickinson, J.E.; et al. Balloon catheters versus vaginal prostaglandins for labor induction (CPI Collaborative): An individual participant data meta-analysis of randomised controlled trials. Lancet 2022, 400, 1681–1692. [Google Scholar] [CrossRef]
- Bakker, R.; Pierce, S.; Myers, D. The role of prostaglandins E1 and E2, Dinoprostone, and Misoprostol in cervical ripening and the induction of labor: A mechanistic approach. Arch. Gynecol. Obstet. 2017, 296, 167–179. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Austin, S.C.; Sanchez-Ramos, L.; Adair, C.D. Labor induction with intravaginal Misoprostol compared with the dinoprostone vaginal insert: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2010, 202, 624.e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Young, D.C.; Delaney, T.; Armson, B.A.; Fanning, C. Oral misoprostol, low dose vaginal misoprostol, and vaginal Dinoprostone for labor induction: Randomized controlled trial. PLoS ONE 2020, 15, e0227245. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.; Bakker, R.; Myers, D.A.; Edwards, R.K. Clinical Insights for Cervical Ripening and Labor Induction Using Prostaglandins. AJP Rep. 2018, 8, e307–e314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.; Fairclough, A.; Kavanagh, J.; Kelly, A.J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labor at term. Cochrane Database Syst. Rev. 2014, 2014, CD003101. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, E.G.; Plachouras, N.; Drougia, A.; Andronikou, S.; Vlachou, C.; Stefos, T.; Paraskevaidis, E.; Zikopoulos, K. Comparison of Misoprostol and Dinoprostone for elective induction of labor in nulliparous women at full term: A randomized prospective study. Reprod. Biol. Endocrinol. 2004, 2, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatsis, V.; Frey, N. Misoprostol for Cervical Ripening and Induction of Labour: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, USA, 2018.
- Swift, E.M.; Gunnarsdottir, J.; Zoega, H.; Bjarnadottir, R.I.; Steingrimsdottir, T.; Einarsdottir, K. Trends in labor induction indications: A 20-year population-based study. Acta Obstet. Gynecol. Scand. 2022, 101, 1422–1430. [Google Scholar] [CrossRef]
- Hodnett, E.D.; Lowe, N.K.; Hannah, M.E.; Willan, A.R.; Stevens, B.; Weston, J.A.; Ohlsson, A.; Gafni, A.; Muir, H.A.; Myhr, T.L.; et al. Nursing Supportive Care in Labor Trial Group. Effectiveness of nurses as providers of birth labor support in North American hospitals: A randomized controlled trial. JAMA 2002, 288, 1373–1381. [Google Scholar] [CrossRef] [Green Version]
- Sydsjö, G.; Blomberg, M.; Palmquist, S.; Angerbjörn, L.; Bladh, M.; Josefsson, A. Effects of continuous midwifery labor support for women with severe fear of childbirth. BMC Pregnancy Childbirth 2015, 15, 115. [Google Scholar] [CrossRef] [Green Version]
- Maputle, M.S. Support provided by midwives to women during labor in a public hospital, Limpopo Province, South Africa: A participant observation study. BMC Pregnancy Childbirth 2018, 18, 210. [Google Scholar] [CrossRef] [Green Version]
- Anh, N.D.; Duc, T.A.; Ha, N.T.; Giang, D.T.; Dat, D.T.; Thuong, P.H.; Toan, N.K.; Duc, N.T.; Duc, N.M. Dinoprostone Vaginal Insert for Induction of Labor in Women with Low-Risk Pregnancies: A Prospective Study. Med. Arch. 2022, 76, 39–44. [Google Scholar] [CrossRef]
- Weeks, A.D.; Lightly, K.; Mol, B.W.; Frohlich, J.; Pontefract, S.; Williams, M.J. Royal College of Obstetricians and Gynaecologists. Evaluating Misoprostol and mechanical methods for induction of labor: Scientific Impact Paper No. 68 April 2022. BJOG 2022, 129, e61–e65. [Google Scholar] [CrossRef]
- Alfirevic, Z.; Keeney, E.; Dowswell, T.; Welton, N.J.; Dias, S.; Jones, L.V.; Navaratnam, K.; Caldwell, D.M. Labour induction with prostaglandins: A systematic review and network meta-analysis. BMJ 2015, 350, h217. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y.; Narumiya, S. Prostaglandin E receptors. J. Biol. Chem. 2007, 282, 11613–11617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicoll, A. The Physiology of Cervical Ripening and the Induction of Labour: A Potential Role for the Nitric Oxide Donor Isosorbide Mononitrate. Master’s Thesis, University of Glasgow, Glasgow, UK, 2001. [Google Scholar]
- Redling, K.; Schaedelin, S.; Huhn, E.A.; Hoesli, I. Efficacy and safety of Misoprostol vaginal insert vs. oral Misoprostol for induction of labor. J. Perinat. Med. 2019, 47, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyr, G.J.; Gülmezoglu, A.M.; Pileggi, C. Vaginal misoprostol for cervical ripening and induction of labor. Cochrane Database Syst. Rev. 2010, 2010, CD000941. [Google Scholar] [CrossRef] [PubMed]
- Gattás, D.S.M.B.; de Amorim, M.M.R.; Feitosa, F.E.L.; da Silva-Junior, J.R.; Ribeiro, L.C.G.; Souza, G.F.A.; Souza, A.S.R. Misoprostol administered sublingually at a dose of 12.5 μg versus vaginally at a dose of 25 μg for the induction of full-term labor: A randomized controlled trial. Reprod. Health 2020, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Czech, I.; Fuchs, P.; Fuchs, A.; Lorek, M.; Tobolska-Lorek, D.; Drosdzol-Cop, A.; Sikora, J. Pharmacological and Nonpharmacological Methods of Labour Pain Relief-Establishment of Effectiveness and Comparison. Int. J. Environ. Res. Public Health 2018, 15, 2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, L.S.; Gallo, R.B.; Ferreira, C.H.; Duarte, G.; Quintana, S.M.; Marcolin, A.C. Transcutaneous electrical nerve stimulation (TENS) reduces pain and postpones the need for pharmacological analgesia during labor: A randomised trial. J. Physiother. 2016, 62, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Levett, K.M.; Smith, C.A.; Dahlen, H.G.; Bensoussan, A. Acupuncture and acupressure for pain management in labor and birth: A critical narrative review of current systematic review evidence. Complement Ther. Med. 2014, 22, 523–540. [Google Scholar] [CrossRef]
- Beyable, A.A.; Bayable, S.D.; Ashebir, Y.G. Pharmacologic and non-pharmacologic labor pain management techniques in a resource-limited setting: A systematic review. Ann. Med. Surg. (Lond) 2022, 74, 103312. [Google Scholar] [CrossRef]
- Zuarez-Easton, S.; Erez, O.; Zafran, N.; Carmeli, J.; Garmi, G.; Salim, R. Pharmacologic and nonpharmacologic options for pain relief during labor: An expert review. Am. J. Obstet. Gynecol. 2023, 228 (Suppl. S5), S1246–S1259. [Google Scholar] [CrossRef]
- Eberle, R.L.; Norris, M.C. Labour analgesia: A risk-benefit analysis. Drug. Saf. 1996, 14, 239–251. [Google Scholar] [CrossRef]
- Aziato, L.; Kyei, A.A.; Deku, G. Experiences of midwives on pharmacological and nonpharmacological labor pain management in Ghana. Reprod. Health 2017, 14, 128. [Google Scholar] [CrossRef] [Green Version]
- Borders, N.; Wendland, C.; Haozous, E.; Leeman, L.; Rogers, R. Midwives’ verbal support of nulliparous women in second-stage labor. J. Obstet. Gynecol. Neonatal. Nurs. 2013, 42, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, L.; Thorsell, T.; Hertfelt Wahn, E.; Ekström, A. Factors influencing positive birth experiences of first-time mothers. Nurs. Res. Pract. 2013, 2013, 349124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildingsson, I.; Westlund, K.; Wiklund, I. Burnout in Swedish midwives. Sex. Reprod. Healthc. 2013, 4, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Tournaire, M.; Theau-Yonneau, A. Complementary and alternative approaches to pain relief during labor. Evid. Based Complement. Altern. Med. 2007, 4, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Yu, Y.; Li, M.; Fang, P.; Gan, S.; Yao, Y.; Zhou, Y.; Kang, X. The efficacy and safety of Remifentanil patient-controlled versus epidural analgesia in labor: A meta-analysis and systematic review. PLoS ONE 2022, 17, e0275716. [Google Scholar] [CrossRef]
- Blajic, I.; Zagar, T.; Semrl, N.; Umek, N.; Lucovnik, M.; Stopar Pintaric, T. Analgesic efficacy of Remifentanil patient-controlled analgesia versus combined spinal-epidural technique in multiparous women during labor. Ginekol. Pol. 2021, 92, 797–803. [Google Scholar] [CrossRef]
- Egarter, C.H.; Husslein, P.W.; Rayburn, W.F. Uterine hyperstimulation after low-dose prostaglandin E2 therapy: Tocolytic treatment in 181 cases. Am. J. Obstet. Gynecol. 1990, 163, 794–796. [Google Scholar] [CrossRef]
- Bebbington, M.; Pevzner, L.; Schmuel, E.; Bernstein, P.; Dayal, A.; Barnhard, J.; Chazotte, C.; Merkatz, I. Uterine tachysystole and hyperstimulation during induction of labor. Am. J. Obstet. Gynecol. 2003, 189, S211. [Google Scholar] [CrossRef]
- Schmidt, M.; Neophytou, M.; Hars, O.; Freudenberg, J.; Kühnert, M. Clinical experience with Misoprostol vaginal insert for induction of labor: A prospective clinical observational study. Arch. Gynecol. Obstet. 2019, 299, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Rouzi, A.A.; Alsibiani, S.; Mansouri, N.; Alsinani, N.; Darhouse, K. Randomized clinical trial between hourly titrated oral Misoprostol and vaginal Dinoprostone for induction of labor. Am. J. Obstet. Gynecol. 2014, 210, e1–e56. [Google Scholar] [CrossRef]
- Rugarn, O.; Tipping, D.; Powers, B.; Wing, D.A. Induction of labor with retrievable prostaglandin vaginal inserts: Outcomes following retrieval due to an intrapartum adverse event. BJOG 2017, 124, 796–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlodawski, J.; Mlodawska, M.; Armanska, J.; Swiercz, G.; Gluszek, S. Misoprostol vs. dinoprostone vaginal insert in labor induction: Comparison of obstetrical outcome. Sci. Rep. 2021, 11, 9077. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.; Kellar, L.; Henning, G.; Waheed, A.; Colon-Gonzalez, M.; Ural, S. The association between the regular use of preventive labor induction and improved term birth outcomes: Findings of a systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 773–784. [Google Scholar] [CrossRef] [PubMed]
- ACOG. ACOG Practice Bulletin No. 107: Induction of labor. Obstet. Gynecol. 2009, 114, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Zahran, K.M.; Shahin, A.Y.; Abdellah, M.S.; Elsayh, K.I. Sublingual versus vaginal Misoprostol for induction of labor at term: A randomized prospective placebo-controlled study. J. Obstet. Gynaecol. Res. 2009, 35, 1054–1060. [Google Scholar] [CrossRef]
- Sharami, S.H.; Milani, F.; Faraji, R.; Bloukimoghadam, K.; Salamat, F.; Momenzadeh, S.; Ebrahimi, H. Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: A randomized controlled equivalence trial. Arch. Iran. Med. 2014, 17, 652–656. [Google Scholar]
- Nassar, A.H.; Awwad, J.; Khalil, A.M.; Abu-Musa, A.; Mehio, G.; Usta, I.M. A randomised comparison of patient satisfaction with vaginal and sublingual Misoprostol for induction of labour at term. BJOG 2007, 114, 1215–1221. [Google Scholar] [CrossRef]
- Singer, A.; Jordan, J.A. The Functional Anatomy of the Cervix, the Cervical Epithelium and the Stroma. In The Cervix, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 13–37. [Google Scholar]
- Garavito, R.M.; Dewitt, D.L. The Cyclooxygenase Isoforms: Structural Insights into the Conversion of Arachidonic Acid to Prostaglandins. Biochim. Biophys. Acta 1999, 1441, 278–287. [Google Scholar] [CrossRef]
- Roos, N.; Blesson, C.S.; Stephansson, O.; Masironi, B.; Vladic Stjernholm, Y.; Ekman-Ordeberg, G.; Sahlin, L. The expression of prostaglandin receptors EP3 and EP4 in human cervix in post-term pregnancy differs between failed and successful labor induction. Acta Obstet. Gynecol. Scand. 2014, 93, 159–167. [Google Scholar] [CrossRef] [PubMed]
- El Maradny, E.; Kanayama, N.; Halim, A.; Maehara, K.; Sumimoto, K.; Terao, T. Biochemical changes in the cervical tissue of rabbit induced by interleukin-8, interleukin-1beta, dehydroepiandrosterone sulphate and prostaglandin E2: A comparative study. Hum. Reprod. 1996, 11, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkinheimo, T.-L.; Saukkonen, K.; Narko, K.; Jalkanen, J.; Ylikorkala, O.; Ristimäki, A. Expression of Cyclooxygenase-2 and Prostanoid Receptors by Human Myometrium. J. Clin. Endocrinol. Metab. 2000, 85, 3468–3475. [Google Scholar] [PubMed]
- Oliveira, T.A.; Melo, E.M.; Aquino, M.M.; Mariani Neto, C. Eficácia de dinoprostone e misoprostol para indução do trabalho de parto em nulíparas [Efficacy of dinoprostone and misoprostol for labor induction in nulliparous women]. Rev. Bras. Ginecol. Obstet. 2011, 33, 118–122. [Google Scholar]
- Aghideh, F.K.; Mullin, P.M.; Ingles, S.; Ouzounian, J.G.; Opper, N.; Wilson, M.L.; Miller, D.A.; Lee, R.H. A comparison of obstetrical outcomes with labor induction agents used at term. J. Matern. Fetal. Neonatal. Med. 2014, 27, 592–596. [Google Scholar] [CrossRef]
- Wing, D.A.; Brown, R.; Plante, L.A.; Miller, H.; Rugarn, O.; Powers, B.L. Misoprostol vaginal insert and time to vaginal delivery: A randomized controlled trial. Obstet. Gynecol. 2013, 122 Pt 1, 201–209. [Google Scholar] [CrossRef]
- Maggi, C.; Mazzoni, G.; Gerosa, V.; Fratelli, N.; Prefumo, F.; Sartori, E.; Lojacono, A. Labor induction with Misoprostol vaginal insert compared with dinoprostone vaginal insert. Acta Obstet. Gynecol. Scand. 2019, 98, 1268–1273. [Google Scholar] [CrossRef]
- Bomba-Opoń, D.; Drews, K.; Huras, H.; Laudański, P.; Paszkowski, T.; Wielgoś, M. Polish Gynecological Society Recommendations for Labor Induction. Ginekol. Pol. 2017, 88, 224–234. [Google Scholar] [CrossRef] [Green Version]


| Dinoprostone (n = 212) | Misoprostol (n = 190) | All (n = 402) | p-Value 1 | |
|---|---|---|---|---|
| <37 weeks of gestation (n = 53/42) | 0.3536 | |||
| Vaginal delivery (VD) | 34 (64.2%) | 23 (54.8%) | 57 (60.0%) | |
| Cesarean section (CS) | 19 (35.8%) | 19 (45.2%) | 38 (40.0%) | |
| 37 to 41 weeks of gestation (n = 86/76) | 0.0394 | |||
| Vaginal delivery (VD) | 63 (73.3%) | 44 (57.9%) | 107 (66.0%) | |
| Cesarean section (CS) | 23 (26.7%) | 32 (42.1%) | 55 (34.0%) | |
| >41 weeks of gestation (n = 73/72) | 0.8134 | |||
| Vaginal delivery (VD) | 46 (63.0%) | 44 (61.1%) | 90 (62.1%) | |
| Cesarean section (CS) | 27 (37.0%) | 28 (38.9%) | 55 (37.9%) |
| Dinoprostone (n = 212) | Misoprostol (n = 190) | All (n = 402) | p-Value 1 | |
|---|---|---|---|---|
| OXT augmentation | ||||
| <37 weeks (n = 53/42) | 43 (81.1%) | 15 (35.7%) | 58 (61.1%) | <0.0001 |
| 37 to 41 weeks (n = 86/76) | 57 (66.3%) | 15 (19.7%) | 72 (44.4%) | <0.0001 |
| >41 weeks (n = 73/72) | 43 (58.9%) | 8 (11.1%) | 51 (35.2%) | <0.0001 |
| Intrapartum analgesia | ||||
| <37 weeks (n = 53/42) | 40 (75.5%) | 39 (92.9%) | 79 (83.2%) | 0.0245 |
| 37 to 41 weeks(n = 86/76) | 61 (70.9%) | 70 (92.1%) | 131 (80.9%) | 0.0006 |
| >41 weeks (n = 73/72) | 50 (68.5%) | 64 (88.9%) | 114 (78.6%) | 0.0027 |
| Hyperstimulation | ||||
| <37 weeks (n = 53/42) | 0 (0.0%) | 2 (4.8%) | 2 (2.1%) | 0.1084 |
| 37 to 41 weeks (n = 86/76) | 2 (2.3%) | 6 (7.9%) | 8 (4.9%) | 0.1025 |
| >41 weeks (n = 73/72) | 1 (1.4%) | 4 (5.6%) | 5 (3.4%) | 0.1672 |
| Dinoprostone (n = 53) | Misoprostol (n = 42) | p-Value 1 | |
|---|---|---|---|
| Time to delivery (VD and CS) | <0.0001 | ||
| 24 h | 9 (17.0%) | 27 (64.3%) | |
| 24–48 h | 32 (60.4%) | 6 (14.3%) | |
| >48 h | 12 (22.6%) | 9 (21.4%) | |
| Parturition type | 0.3536 | ||
| Vaginal delivery (VD) | 34 (64.2%) | 23 (54.8%) | |
| Cesarean section (CS) | 19 (35.8%) | 19 (45.2%) |
| Dinoprostone (n = 34) | Misoprostol (n = 23) | p-Value 1 | |
|---|---|---|---|
| Time to VD | 0.0004 | ||
| 24 h | 5 (14.7%) | 15 (65.2%) | |
| 24–48 h | 22 (64.7%) | 5 (21.7%) | |
| >48 h | 7 (20.6%) | 3 (13.0%) |
| Dinoprostone (n = 19) | Misoprostol (n = 19) | p-Value 1 | |
|---|---|---|---|
| Time to CS | 0.0033 | ||
| 24 h | 4 (21.1%) | 12 (63.2%) | |
| 24–48 h | 10 (52.6%) | 1 (5.3%) | |
| >48 h | 5 (26.3%) | 6 (31.6%) |
| Dinoprostone (n = 86) | Misoprostol (n = 76) | p-Value 1 | |
|---|---|---|---|
| Time to delivery (VD and CS) | <0.0001 | ||
| 24 h | 29 (33.7%) | 61 (80.3%) | |
| 24–48 h | 46 (53.5%) | 7 (9.2%) | |
| >48 h | 11 (12.8%) | 8 (10.5%) | |
| Parturition type | 0.0394 | ||
| Vaginal delivery (VD) | 63 (73.3%) | 44 (57.9%) | |
| Cesarean section (CS) | 23 (26.7%) | 32 (42.1%) |
| Dinoprostone (n = 63) | Misoprostol (n = 44) | p-Value 1 | |
|---|---|---|---|
| Time to VD | <0.0001 | ||
| 24 h | 20 (31.7%) | 41 (93.2%) | |
| 24–48 h | 37 (58.7%) | 1 (2.3%) | |
| >48 h | 6 (9.5%) | 2 (4.5%) |
| Dinoprostone (n = 23) | Misoprostol (n = 32) | p-Value 1 | |
|---|---|---|---|
| Time to CS | 0.1752 | ||
| 24 h | 9 (39.1%) | 20 (62.5%) | |
| 24–48 h | 9 (39.1%) | 6 (18.8%) | |
| >48 h | 5 (21.7%) | 6 (18.8%) |
| Dinoprostone (n = 73) | Misoprostol (n = 72) | p-Value 1 | |
|---|---|---|---|
| Time to delivery (VD and CS) | <0.0001 | ||
| 24 h | 30 (41.1%) | 64 (88.9%) | |
| 24–48 h | 33 (45.2%) | 5 (6.9%) | |
| >48 h | 10 (13.7%) | 3 (4.2%) | |
| Parturition type | 0.8134 | ||
| Vaginal delivery (VD) | 46 (63.0%) | 44 (61.1%) | |
| Cesarean delivery (CS) | 27 (37.0%) | 28 (38.9%) |
| Dinoprostone (n = 46) | Misoprostol (n = 44) | p-Value 1 | |
|---|---|---|---|
| Time to VD | <0.0001 | ||
| 24 h | 19 (41.3%) | 43 (97.7%) | |
| 24–48 h | 26 (56.5%) | 1 (2.3%) | |
| >48 h | 1 (2.2%) | 0 (0.0%) |
| Dinoprostone (n = 27) | Misoprostol (n = 28) | p-Value 1 | |
|---|---|---|---|
| Time to CS | 0.0313 | ||
| 24 h | 11 (40.7%) | 21 (75.0%) | |
| 24–48 h | 7 (25.9%) | 4 (14.3%) | |
| >48 h | 9 (33.3%) | 3 (10.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socha, M.W.; Flis, W.; Pietrus, M.; Wartęga, M. Results of Induction of Labor with Prostaglandins E1 and E2 (The RIPE Study): A Real-World Data Analysis of Obstetrical Effectiveness and Clinical Outcomes of Pharmacological Induction of Labor with Vaginal Inserts. Pharmaceuticals 2023, 16, 982. https://doi.org/10.3390/ph16070982
Socha MW, Flis W, Pietrus M, Wartęga M. Results of Induction of Labor with Prostaglandins E1 and E2 (The RIPE Study): A Real-World Data Analysis of Obstetrical Effectiveness and Clinical Outcomes of Pharmacological Induction of Labor with Vaginal Inserts. Pharmaceuticals. 2023; 16(7):982. https://doi.org/10.3390/ph16070982
Chicago/Turabian StyleSocha, Maciej W., Wojciech Flis, Miłosz Pietrus, and Mateusz Wartęga. 2023. "Results of Induction of Labor with Prostaglandins E1 and E2 (The RIPE Study): A Real-World Data Analysis of Obstetrical Effectiveness and Clinical Outcomes of Pharmacological Induction of Labor with Vaginal Inserts" Pharmaceuticals 16, no. 7: 982. https://doi.org/10.3390/ph16070982
APA StyleSocha, M. W., Flis, W., Pietrus, M., & Wartęga, M. (2023). Results of Induction of Labor with Prostaglandins E1 and E2 (The RIPE Study): A Real-World Data Analysis of Obstetrical Effectiveness and Clinical Outcomes of Pharmacological Induction of Labor with Vaginal Inserts. Pharmaceuticals, 16(7), 982. https://doi.org/10.3390/ph16070982