Dissecting the Role of SMYD2 and Its Inhibitor (LLY-507) in the Treatment of Chemically Induced Non-Small Cell Lung Cancer (NSCLC) by Using Fe3O4 Nanoparticles Drug Delivery System
Abstract
:1. Introduction
2. Results
2.1. Characterization of Uncoated IONPs
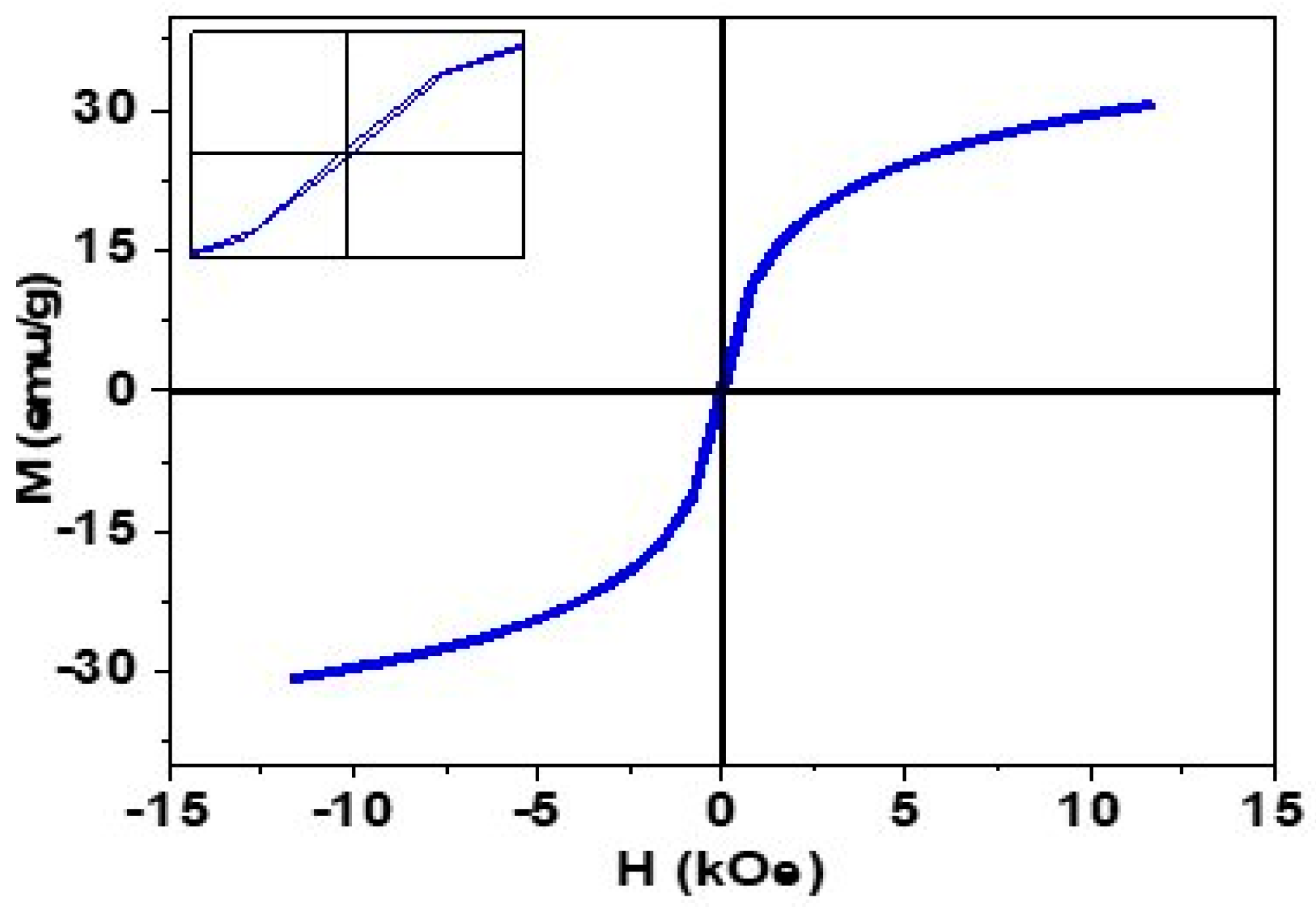
2.2. Magnetic Analysis
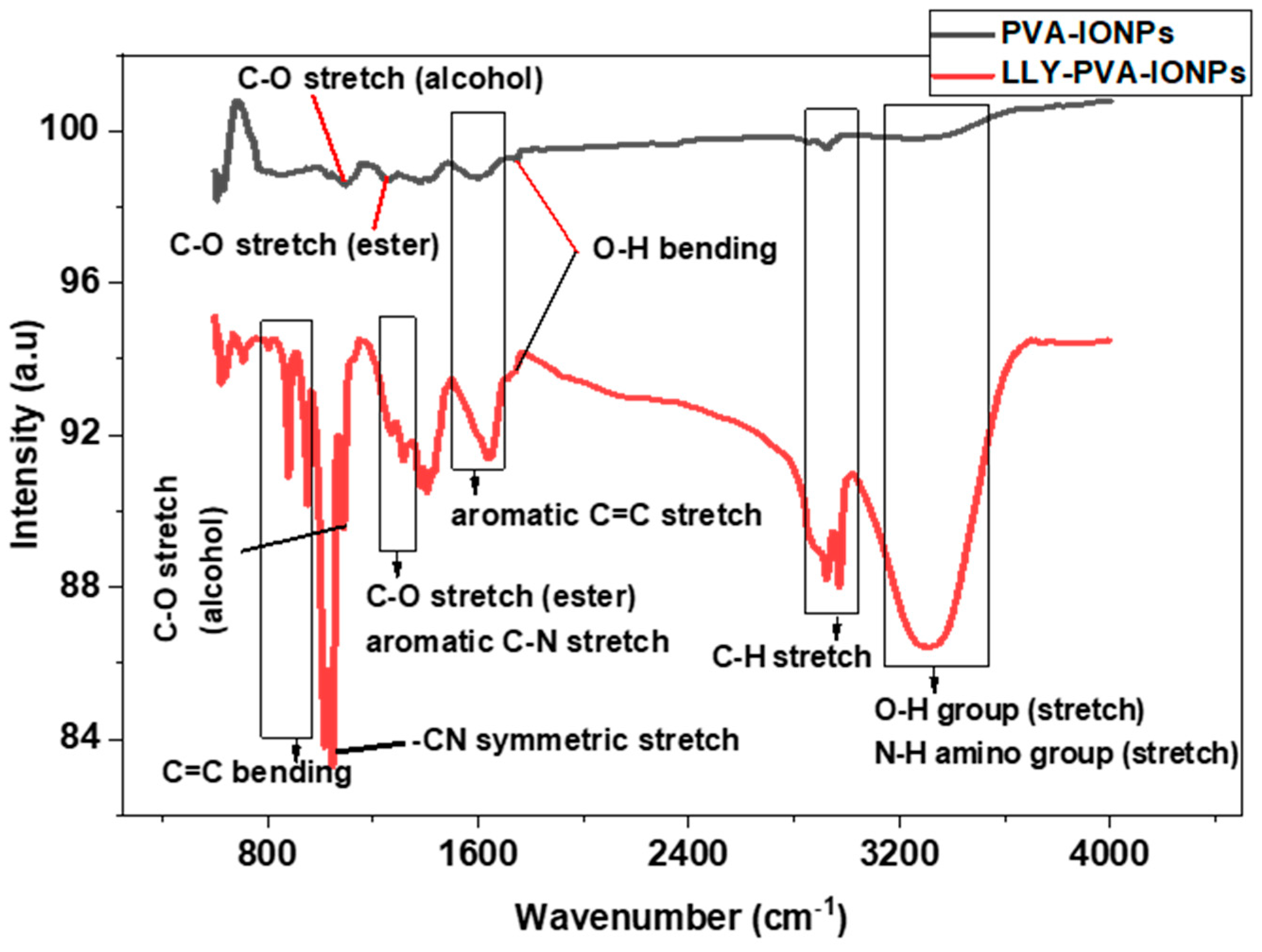
2.3. FTIR Analysis
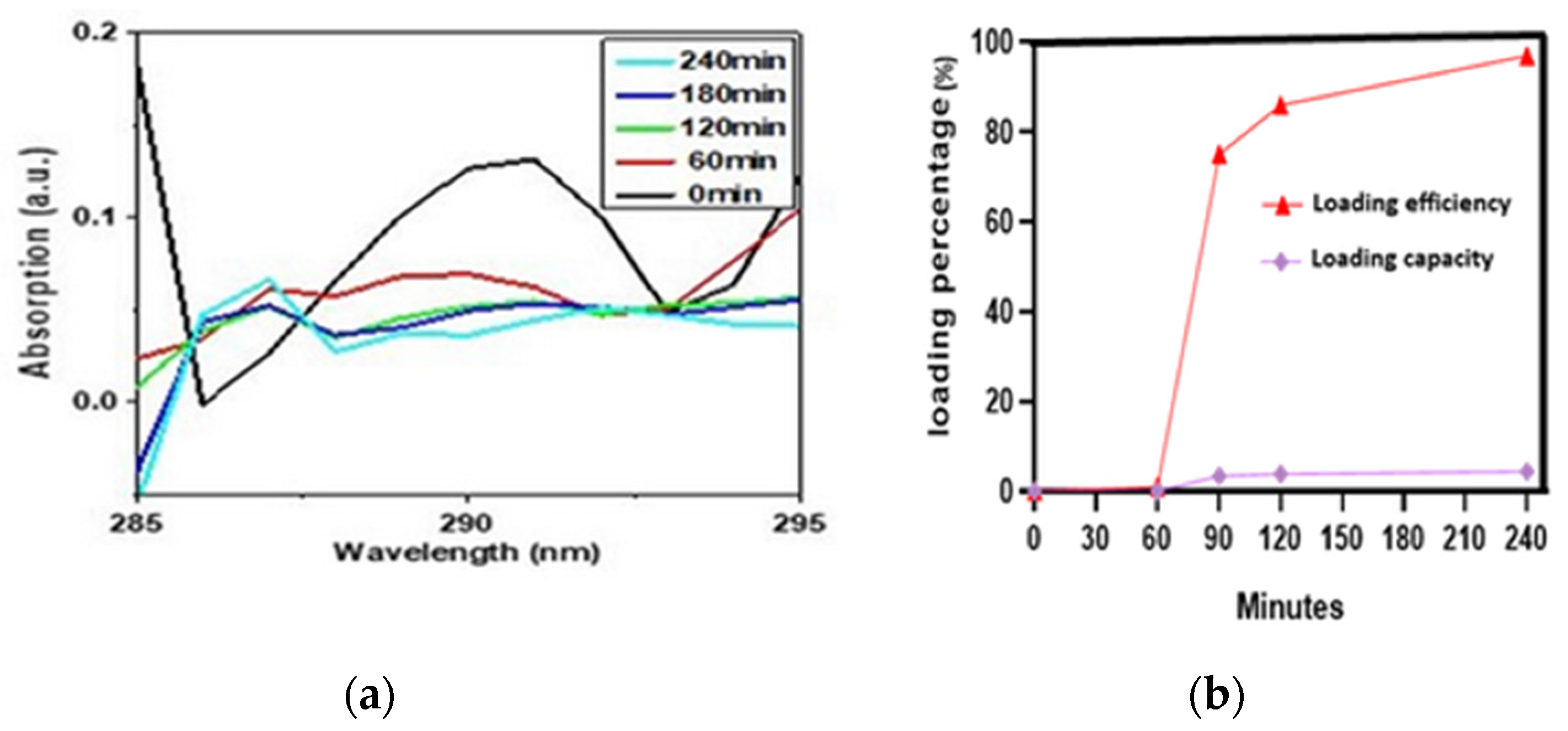
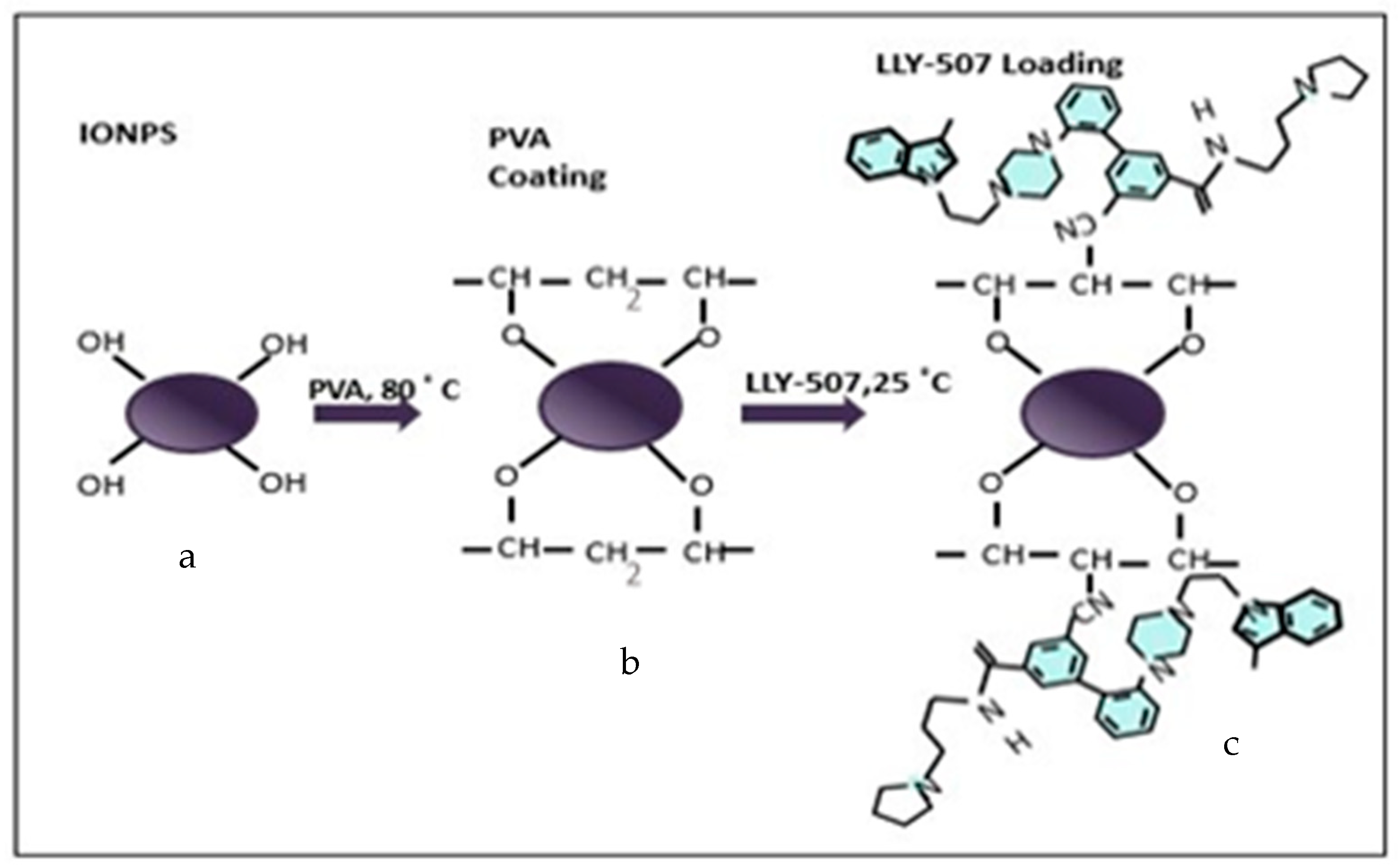
2.4. Drug Loading Analysis
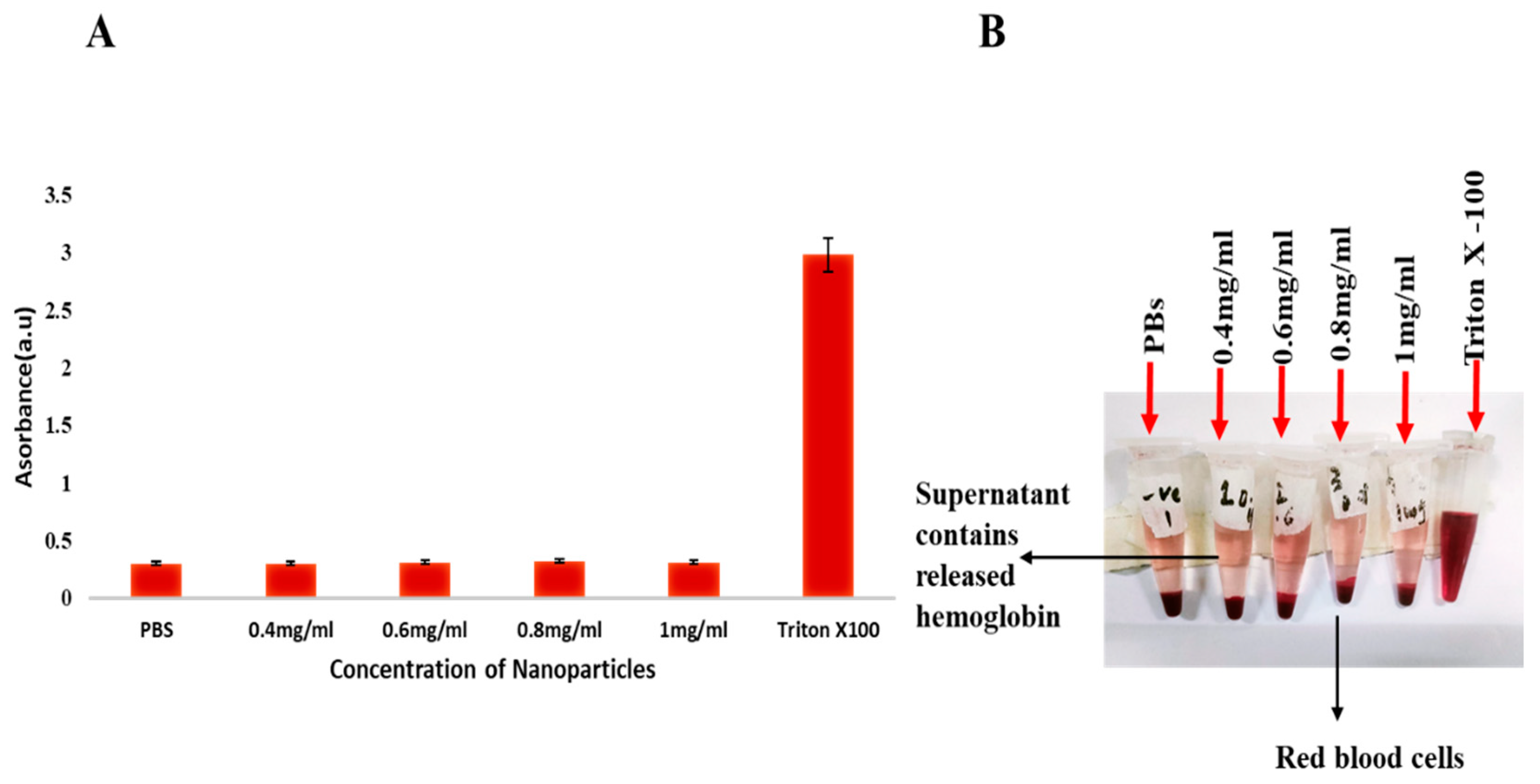
2.5. Hemolysis Activity
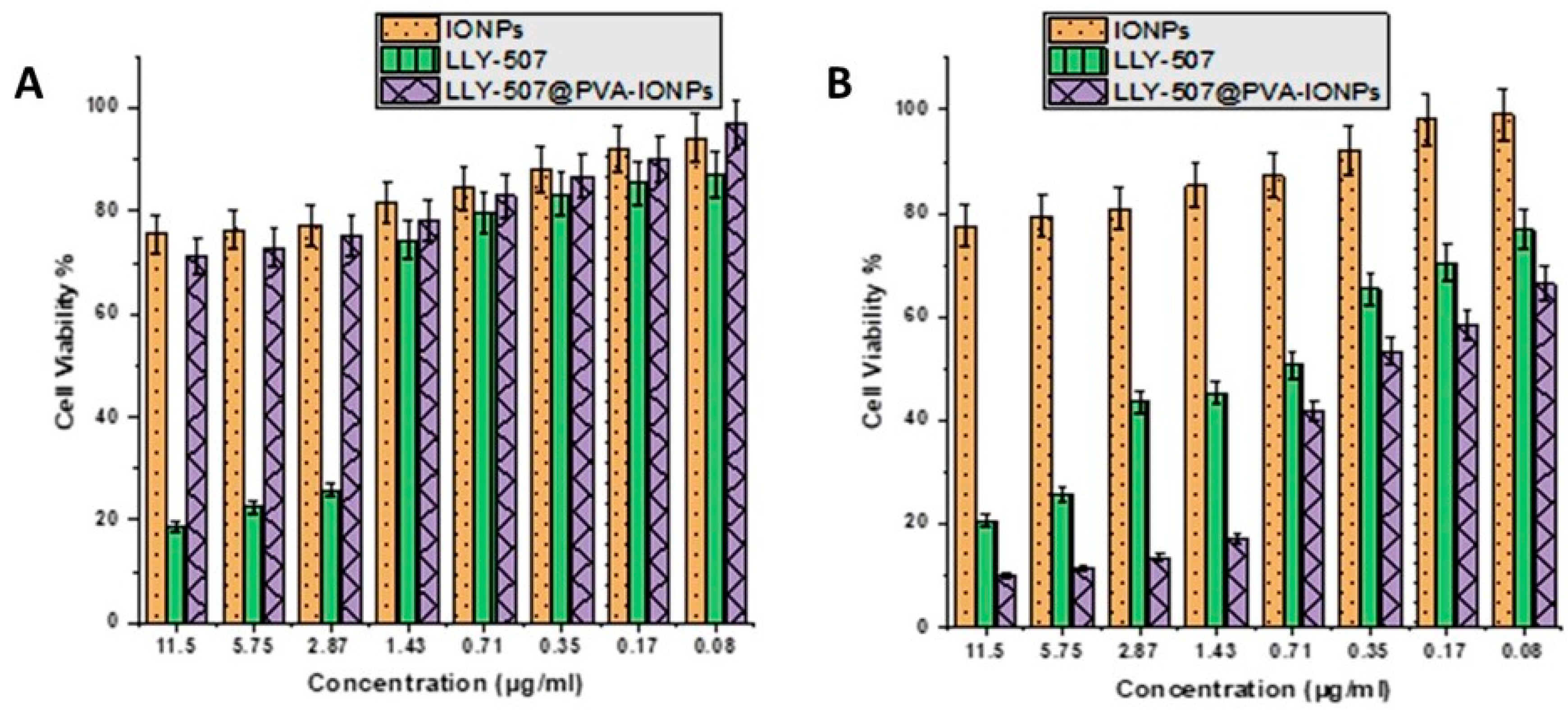
2.6. Cell Viability Assay
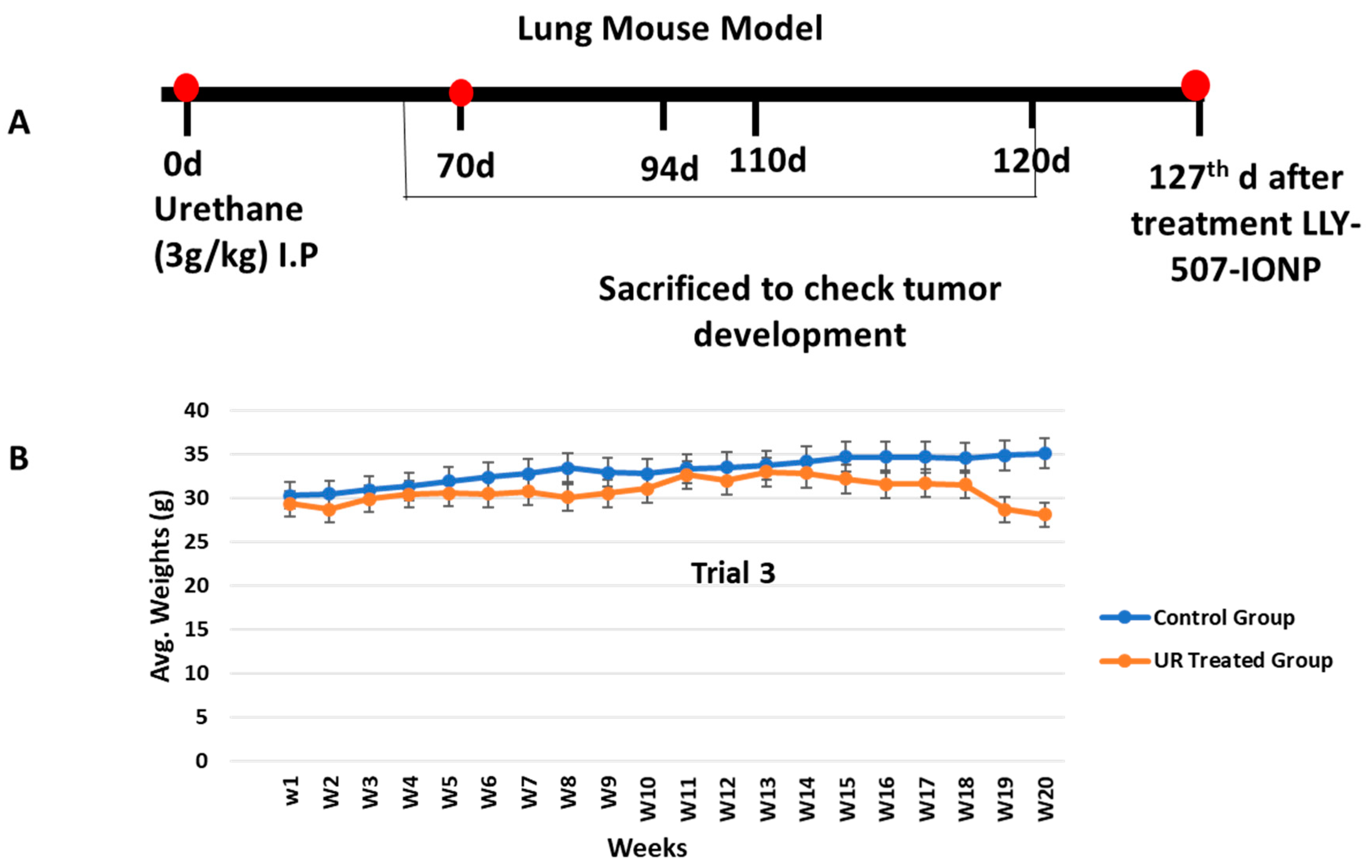
2.7. Lung Cancer Mouse Model Development and Its Treatment
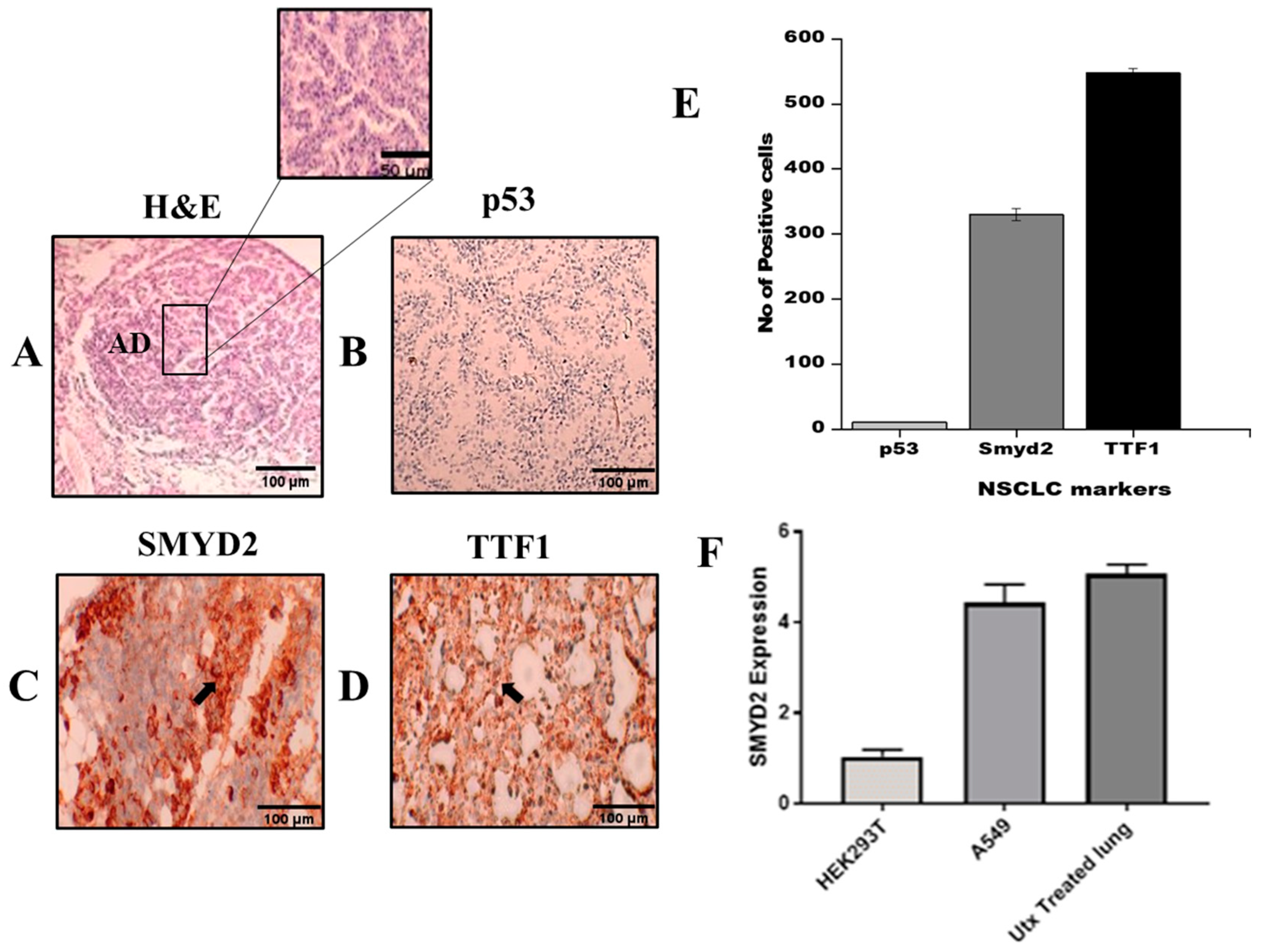
2.8. NSCLC Markers in Urethane Lung Cancer Mouse Model
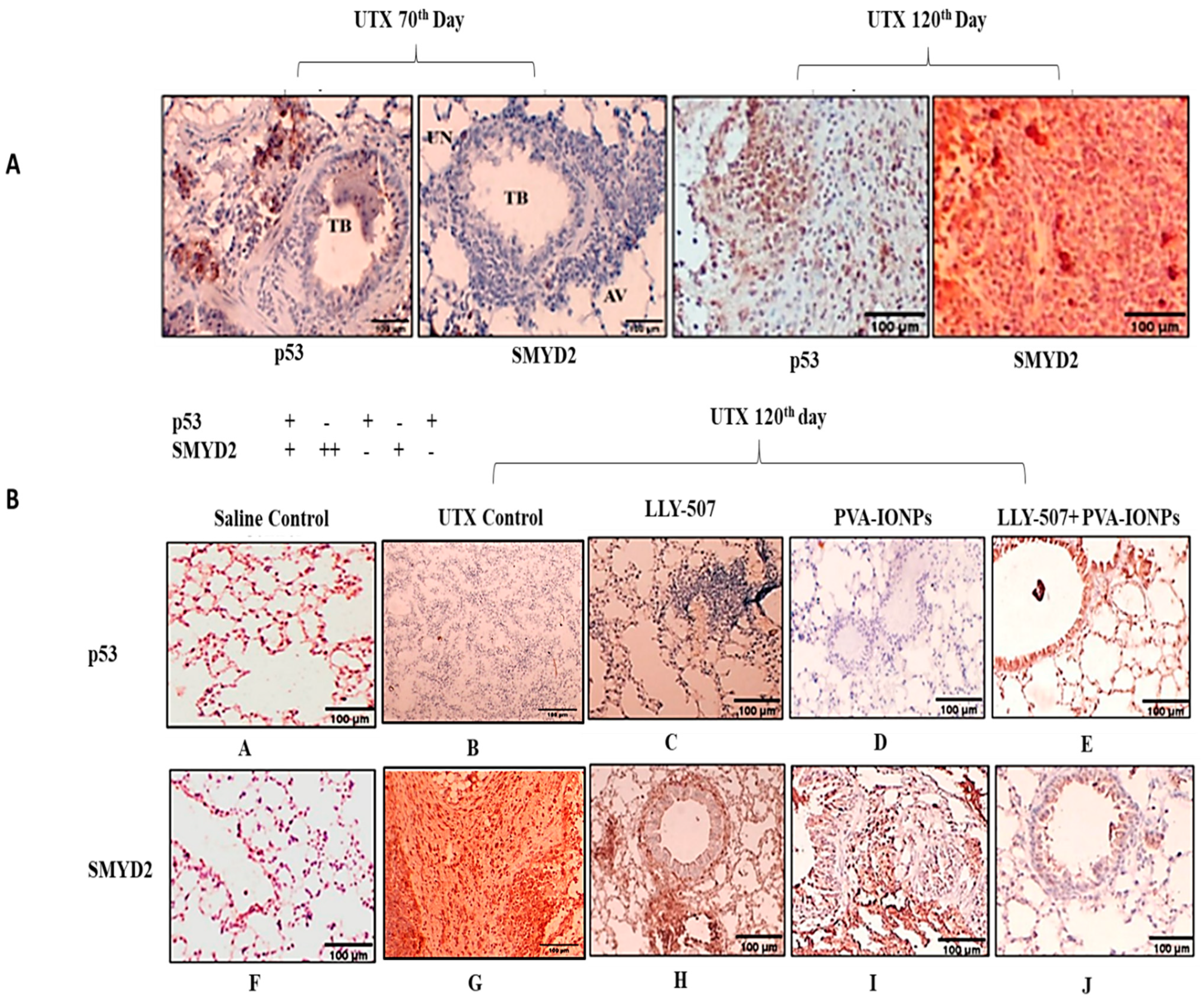
2.9. p53 Regulation in a Urethane-Induced Lung Cancer Mouse Model
3. Discussion
4. Material and Methods
4.1. Physiochemical Characterization of IONPs
4.2. Synthesis of Iron Oxide NPs
- (a)
- FeCl2 + 2NaOH Fe(OH)2 + 2NaClFe(OH)2 FeO + H2O
- (b)
- 2FeCl3 + 6NaOH 2Fe(OH)3 + 6NaCl2Fe(OH)3 Fe2O3 + 3H2O
4.3. PVA Functionalization of IONPs
4.4. LLY-507 Loading on PVA-Coated IONPs
4.5. In Vitro Hemocompatibility Test
4.6. Cell Viability Assay
4.7. Lung Cancer Mouse Model
4.8. Drug Administration for Treatment
4.9. Histology
4.10. Immunohistochemistry
4.11. SMYD2 Expression by RT-qPCR
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Xu, K.; Zhang, C.; Du, T.; Gabriel, A.N.A.; Wang, X.; Li, X.; Sun, L.; Wang, N.; Jiang, X.; Zhang, Y. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed. Pharmacother. 2021, 134, 111111. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Ahmad, M.; Akhtar, M.S.; Shaari, A.; Riaz, S.; Naseem, S.; Masood, M.; Saeed, M. Magnetic properties of polyvinyl alcohol and doxorubicine loaded iron oxide nanoparticles for anticancer drug delivery applications. PLoS ONE 2016, 11, e0158084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douziech-Eyrolles, L.; Marchais, H.; Hervé, K.; Munnier, E.; Souce, M.; Linassier, C.; Dubois, P.; Chourpa, I. Nanovectors for anticancer agents based on superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2007, 2, 541. [Google Scholar]
- Di Francesco, M.; Celia, C.; Cristiano, M.C.; d’Avanzo, N.; Ruozi, B.; Mircioiu, C.; Cosco, D.; Di Marzio, L.; Fresta, M. Doxorubicin hydrochloride-loaded nonionic surfactant vesicles to treat metastatic and non-metastatic breast cancer. ACS Omega 2021, 6, 2973–2989. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Reddy, L.H.; Othman, M.; Gillet, B.; Desmaele, D.; Zouhiri, F.; Dosio, F.; Gref, R.; Couvreur, P. Squalene based nanocomposites: A new platform for the design of multifunctional pharmaceutical theragnostics. ACS Nano 2011, 5, 1513–1521. [Google Scholar] [CrossRef]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Phar. 2008, 5, 316–327. [Google Scholar] [CrossRef]
- Naqvi, S.; Samim, M.; Abdin, M.; Ahmed, F.J.; Maitra, A.; Prashant, C.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983. [Google Scholar] [CrossRef] [Green Version]
- Vinardell, M.P.; Mitjans, M. Antitumor Activities of Metal Oxide Nanoparticles. Nanomaterials 2015, 5, 1004–1021. [Google Scholar] [CrossRef] [Green Version]
- Alphandéry, E. Iron oxide nanoparticles for therapeutic applications. Drug Discov. Today 2020, 25, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Pai, J.A.; Shieh, M.J.; Chen, J.L.; Chen, K.C. Cisplatin-loaded gold nanoshells mediate chemo-photothermal therapy against primary and distal lung cancers growth. Biomed. Pharmacother. 2023, 158, 114146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278. [Google Scholar] [CrossRef]
- Javed, S.; Bouwmans, T.; Jung, S.K. Depth extended online RPCA with spatiotemporal constraints for robust background subtraction. In Proceedings of the 2015 21st Korea-Japan Joint Workshop on Frontiers of Computer Vision (FCV), Mokpo, Republic of Korea, 28–30 January 2015; pp. 1–6. [Google Scholar]
- Berry, C.C.; Wells, S.; Charles, S.; Aitchison, G.; Curtis, A.S. Cell response to dextran-derivatised iron oxide nanoparticles post internalisation. Biomaterials 2004, 25, 5405–5413. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; Zhang, L.M.; Jiang, W.; Li, W. Embedding Magnetic Nanoparticles into Polysaccharide-Based Hydrogels for Magnetically Assisted Bioseparation. ChemPhysChem 2007, 8, 2367–2372. [Google Scholar] [CrossRef]
- Lin, P.C.; Chou, P.H.; Chen, S.H.; Liao, H.K.; Wang, K.Y.; Chen, Y.J.; Lin, C.C. Ethylene glycol-protected magnetic nanoparticles for a multiplexed immunoassay in human plasma. Small 2006, 2, 485–489. [Google Scholar] [CrossRef]
- Aurich, K.; Schwalbe, M.; Clement, J.H.; Weitschies, W.; Buske, N. Polyaspartate coated magnetite nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2007, 311, 1–5. [Google Scholar] [CrossRef]
- Cavalieri, F.; Chiessi, E.; Villa, R.; Vigano, L.; Zaffaroni, N.; Telling, M.F.; Paradossi, G. Novel PVA-based hydrogel microparticles for doxorubicin delivery. Biomacromolecules 2008, 9, 1967–1973. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Milani, A.S.; Stroeve, P. Optimal design and characterization of superparamagnetic iron oxide nanoparticles coated with polyvinyl alcohol for targeted delivery and imaging. J. Phys. Chem. B 2008, 112, 14470–14481. [Google Scholar] [CrossRef] [PubMed]
- Kukita, A.; Sone, K.; Oda, K.; Hamamoto, R.; Kaneko, S.; Komatsu, M.; Wada, M.; Honjoh, H.; Kawata, Y.; Kojima, M. Histone methyltransferase SMYD2 selective inhibitor LLY-507 in combination with poly ADP ribose polymerase inhibitor has therapeutic potential against high-grade serous ovarian carcinomas. Biochem. Biophys. Res. Commun. 2019, 513, 340–346. [Google Scholar] [CrossRef]
- Deng, X.; Hamamoto, R.; Vougiouklakis, T.; Wang, R.; Yoshioka, Y.; Suzuki, T.; Dohmae, N.; Matsuo, Y.; Park, J.H.; Nakamura, Y. Critical roles of SMYD2-mediated beta-catenin methylation for nuclear translocation and activation of Wnt signaling. Oncotarget 2017, 8, 55837–55847. [Google Scholar] [CrossRef] [Green Version]
- Li, L.X.; Zhou, J.X.; Calvet, J.P.; Godwin, A.K.; Jensen, R.A.; Li, X. Lysine methyltransferase SMYD2 promotes triple negative breast cancer progression. Cell Death Dis. 2018, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Zuo, S.R.; Zuo, X.C.; He, Y.; Fang, W.J.; Wang, C.J.; Zou, H.; Chen, P.; Huang, L.F.; Huang, L.H.; Xiang, H.; et al. Positive Expression of SMYD2 is Associated with Poor Prognosis in Patients with Primary Hepatocellular Carcinoma. J. Cancer 2018, 9, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.J.; Li, H.L.; Ma, H.; Shi, Y.; Yin, L.R.; Guo, S.J. SMYD2 promotes cervical cancer growth by stimulating cell proliferation. Cell Biosci. 2019, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Kang, D.; Suzuki, T.; Masuda, A.; Dohmae, N.; Nakamura, Y.; Hamamoto, R. The histone methyltransferase SMYD2 methylates PARP1 and promotes poly(ADP-ribosyl)ation activity in cancer cells. Neoplasia 2014, 16, 257–264.e252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saddic, L.A.; West, L.E.; Aslanian, A.; Yates, J.R., 3rd; Rubin, S.M.; Gozani, O.; Sage, J. Methylation of the retinoblastoma tumor suppressor by SMYD2. J. Biol. Chem. 2010, 285, 37733–37740. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Perez-Burgos, L.; Placek, B.J.; Sengupta, R.; Richter, M.; Dorsey, J.A.; Kubicek, S.; Opravil, S.; Jenuwein, T.; Berger, S.L. Repression of p53 activity by Smyd2-mediated methylation. Nature 2006, 444, 629–632. [Google Scholar] [CrossRef]
- Nakakido, M.; Deng, Z.; Suzuki, T.; Dohmae, N.; Nakamura, Y.; Hamamoto, R. Dysregulation of AKT pathway by SMYD2-mediated lysine methylation on PTEN. Neoplasia 2015, 17, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, A.D.; Larsen, N.A.; Howard, T.; Pollard, H.; Green, I.; Grande, C.; Cheung, T.; Garcia-Arenas, R.; Cowen, S.; Wu, J. Structural basis of substrate methylation and inhibition of SMYD2. Structure 2011, 19, 1262–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweis, R.F.; Wang, Z.; Algire, M.; Arrowsmith, C.H.; Brown, P.J.; Chiang, G.G.; Guo, J.; Jakob, C.G.; Kennedy, S.; Li, F. Discovery of A-893, a new cell-active benzoxazinone inhibitor of lysine methyltransferase SMYD2. ACS Med. Chem. Lett. 2015, 6, 695–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, G.G.; Koch, C.M.; Connors, L.H. Blood proteomic profiling in inherited (ATTRm) and acquired (ATTRwt) forms of transthyretin-associated cardiac amyloidosis. J. Proteome Res. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Nguyen, H.; Allali-Hassani, A.; Antonysamy, S.; Chang, S.; Chen, L.H.; Curtis, C.; Emtage, S.; Fan, L.; Gheyi, T.; Li, F. LLY-507, a cell-active, potent, and selective inhibitor of protein-lysine methyltransferase SMYD2. J. Biol. Chem. 2015, 290, 13641–13653. [Google Scholar] [CrossRef] [Green Version]
- Fabini, E.; Manoni, E.; Ferroni, C.; Rio, A.D.; Bartolini, M. Small-molecule inhibitors of lysine methyltransferases SMYD2 and SMYD3: Current trends. Future Med. Chem. 2019, 11, 901–921. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, Y.; Zhang, S.; Zhou, L.; Yan, G.; Wang, Y.; Zhang, M.; Wang, M.; Lin, H.; Tong, Q. Emodin regulates neutrophil phenotypes to prevent hypercoagulation and lung carcinogenesis. J. Transl. Med. 2019, 17, 90. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Kou, F.; Ji, Z.; Li, B.; Zhang, B.; Guo, Y.; Yang, L. SMYD2 promotes tumorigenesis and metastasis of lung adenocarcinoma through RPS7. Cell Death Dis. 2021, 12, 439. [Google Scholar] [CrossRef]
- Gurley, K.E.; Moser, R.D.; Kemp, C.J. Induction of Lung Tumors in Mice with Urethane. Cold Spring Harb. Protoc. 2015, 2015, pdb.prot077446. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Xu, Y.; Zhou, J.; Jiang, T.; Wang, J.; Li, C.; Sun, X.; Song, H.; Song, J. Targeting SMYD2 inhibits angiogenesis and increases the efficiency of apatinib by suppressing EGFL7 in colorectal cancer. Angiogenesis 2022, 26, 1–18. [Google Scholar] [CrossRef]
- Hu, J.; Qian, Y.; Wang, X.; Liu, T.; Liu, S. Drug-loaded and superparamagnetic iron oxide nanoparticle surface-embedded amphiphilic block copolymer micelles for integrated chemotherapeutic drug delivery and MR imaging. Langmuir 2012, 28, 2073–2082. [Google Scholar] [CrossRef]
- Yu, M.K.; Jeong, Y.Y.; Park, J.; Park, S.; Kim, J.W.; Min, J.J.; Kim, K.; Jon, S. Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew. Chem. Int. Ed. 2008, 47, 5362–5365. [Google Scholar] [CrossRef]
- Sajjad, A.; Novoyatleva, T.; Vergarajauregui, S.; Troidl, C.; Schermuly, R.T.; Tucker, H.O.; Engel, F.B. Lysine methyltransferase Smyd2 suppresses p53-dependent cardiomyocyte apoptosis. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2556–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Milani, A.S.; Stroeve, P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2009, 336, 510–518. [Google Scholar] [CrossRef]
- Hornsby, T.K.; Kashkooli, F.M.; Jakhmola, A.; Kolios, M.C.; Tavakkoli, J.J. Multiphysics Modeling of Low-Intensity Pulsed Ultrasound Induced Chemotherapeutic Drug Release from the Surface of Gold Nanoparticles. Cancers 2023, 15, 523. [Google Scholar] [CrossRef]
- Filippi, M.; Nguyen, D.V.; Garello, F.; Perton, F.; Begin-Colin, S.; Felder-Flesch, D.; Power, L.; Scherberich, A. Metronidazole-functionalized iron oxide nanoparticles for molecular detection of hypoxic tissues. Nanoscale 2019, 11, 22559–22574. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cha, R.; Zhang, Y.; Guo, H.; Long, K.; Gao, P.; Wang, X.; Zhou, F.; Jiang, X. Iron oxide nanoparticles for targeted imaging of liver tumors with ultralow hepatotoxicity. J. Mater. Chem. B 2018, 6, 6413–6423. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, L.; Lu, L.; Chen, T.; Wei, S.; Lin, X.; Lian, X. Inflammation has a role in urethane-induced lung cancer in C57BL/6J mice. Mol. Med. Rep. 2016, 14, 3323–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaynagetdinov, R.; Sherrill, T.P.; Polosukhin, V.V.; Han, W.; Ausborn, J.A.; McLoed, A.G.; McMahon, F.B.; Gleaves, L.A.; Degryse, A.L.; Stathopoulos, G.T. A critical role for macrophages in promotion of urethane-induced lung carcinogenesis. J. Immunol. 2011, 187, 5703–5711. [Google Scholar] [CrossRef] [Green Version]
- Kojima, M.; Sone, K.; Oda, K.; Hamamoto, R.; Kaneko, S.; Oki, S.; Kukita, A.; Kawata, A.; Honjoh, H.; Kawata, Y. The histone methyltransferase SMYD2 is a novel therapeutic target for the induction of apoptosis in ovarian clear cell carcinoma cells. Oncol. Lett. 2020, 20, 153. [Google Scholar] [CrossRef]
- Shang, L.; Wei, M. Inhibition of SMYD2 sensitized cisplatin to resistant cells in NSCLC through activating p53 pathway. Front. Oncol. 2019, 9, 306. [Google Scholar] [CrossRef]
- Walia, R.; Jain, D.; Madan, K.; Sharma, M.C.; Mathur, S.R.; Mohan, A.; Iyer, V.K.; Kumar, L. p40 & thyroid transcription factor-1 immunohistochemistry: A useful panel to characterize non-small cell lung carcinoma-not otherwise specified (NSCLC-NOS) category. Indian J. Med. Res. 2017, 146, 42. [Google Scholar]
- Chou, C.-W.; Lin, C.-H.; Hsiao, T.-H.; Lo, C.-C.; Hsieh, C.-Y.; Huang, C.-C.; Sher, Y.-P. Therapeutic effects of statins against lung adenocarcinoma via p53 mutant-mediated apoptosis. Sci. Rep. 2019, 9, 20403. [Google Scholar] [CrossRef] [Green Version]
- Joerger, A.C.; Fersht, A.R. The p53 pathway: Origins, inactivation in cancer, and emerging therapeutic approaches. Annu. Rev. Biochem. 2016, 85, 375–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, C.Y.; Duan, F.G.; Fan, X.-X.; Yao, X.-J.; Parks, R.J.; Tang, Y.-J.; Wang, M.-F.; Liu, L.; Tsang, B.K. p53 sensitizes chemoresistant non-small cell lung cancer via elevation of reactive oxygen species and suppression of EGFR/PI3K/AKT signaling. Cancer Cell Int. 2019, 19, 188. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D. A study on iron oxide nanoparticles coated with dextrin obtained by coprecipitation. Dig. J. Nanomater. Biostructures 2007, 2, 169–173. [Google Scholar]
- Nadeem, M.; Ahmad, M.; Saeed, M.; Shaari, A.; Riaz, S.; Naseem, S.; Rashid, K. Uptake and clearance analysis of Technetium99m labelled iron oxide nanoparticles in a rabbit brain. IET Nanobiotechnol. 2015, 9, 136–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, K.; Gogoi, B.; Buragohain, A.K.; Deb, P. Fe2O3/C nanocomposites having distinctive antioxidant activity and hemolysis prevention efficiency. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Riaz, A.; Wei, W.; Sarfraz, I.; Hassan, M.; Li, J.; Asif, F.; Adem, Ş.; Bukhari, S.A.; Asrar, M. Mangifera indica extracts as novel PKM2 inhibitors for treatment of triple negative breast cancer. BioMed Res. Int. 2021, 2021, 5514669. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Bielecki, L.C.; Ribeiro, J.M.; Andrade Balsalobre, F.; Teodoro, W.R.; Capelozzi, V.L. Association between decreases in type V collagen and apoptosis in mouse lung chemical carcinogenesis: A preliminary model to study cancer cell behavior. Clinics 2010, 65, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reymao, M.S.; Cury, P.M.; Lichtenfels, A.J.; Lemos, M.; Battlehner, C.N.; Conceicao, G.M.; Capelozzi, V.L.; Montes, G.S.; Junior, M.F.; Martins, M.A.; et al. Urban air pollution enhances the formation of urethane-induced lung tumors in mice. Environ. Res. 1997, 74, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ruan, J.; Weng, W.; Tang, Y. Overexpression of SET and MYND domain-containing protein 2 (SMYD2) is associated with poor prognosis in pediatric B lineage acute lymphoblastic leukemia. Leuk. Lymphoma 2020, 61, 437–444. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munawwar, A.; Sajjad, A.; Rasul, A.; Sattar, M.; Jabeen, F. Dissecting the Role of SMYD2 and Its Inhibitor (LLY-507) in the Treatment of Chemically Induced Non-Small Cell Lung Cancer (NSCLC) by Using Fe3O4 Nanoparticles Drug Delivery System. Pharmaceuticals 2023, 16, 986. https://doi.org/10.3390/ph16070986
Munawwar A, Sajjad A, Rasul A, Sattar M, Jabeen F. Dissecting the Role of SMYD2 and Its Inhibitor (LLY-507) in the Treatment of Chemically Induced Non-Small Cell Lung Cancer (NSCLC) by Using Fe3O4 Nanoparticles Drug Delivery System. Pharmaceuticals. 2023; 16(7):986. https://doi.org/10.3390/ph16070986
Chicago/Turabian StyleMunawwar, Aasma, Amna Sajjad, Azhar Rasul, Mehran Sattar, and Farhat Jabeen. 2023. "Dissecting the Role of SMYD2 and Its Inhibitor (LLY-507) in the Treatment of Chemically Induced Non-Small Cell Lung Cancer (NSCLC) by Using Fe3O4 Nanoparticles Drug Delivery System" Pharmaceuticals 16, no. 7: 986. https://doi.org/10.3390/ph16070986





