Cyclodextrins and Their Derivatives as Drug Stability Modifiers
Abstract
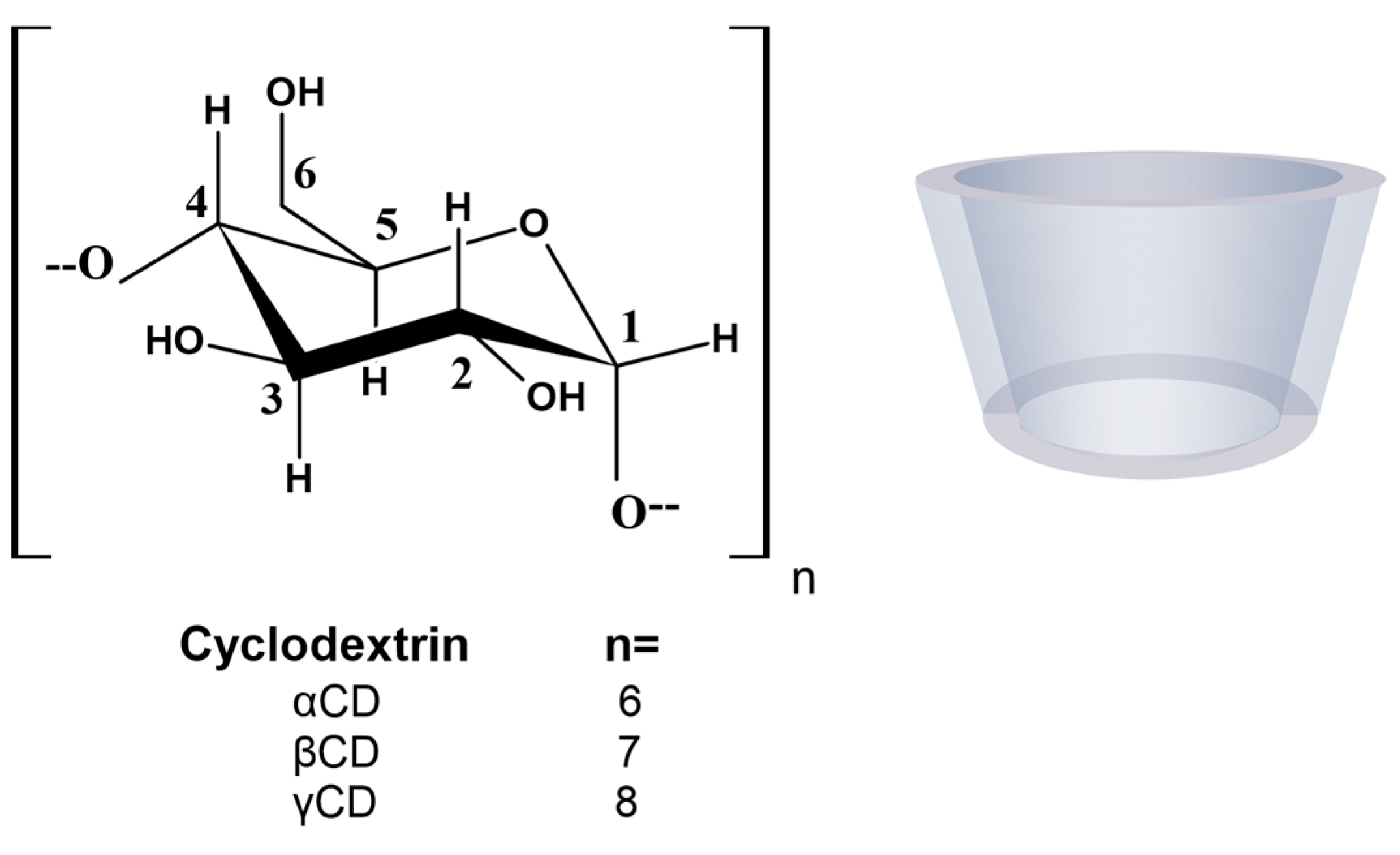
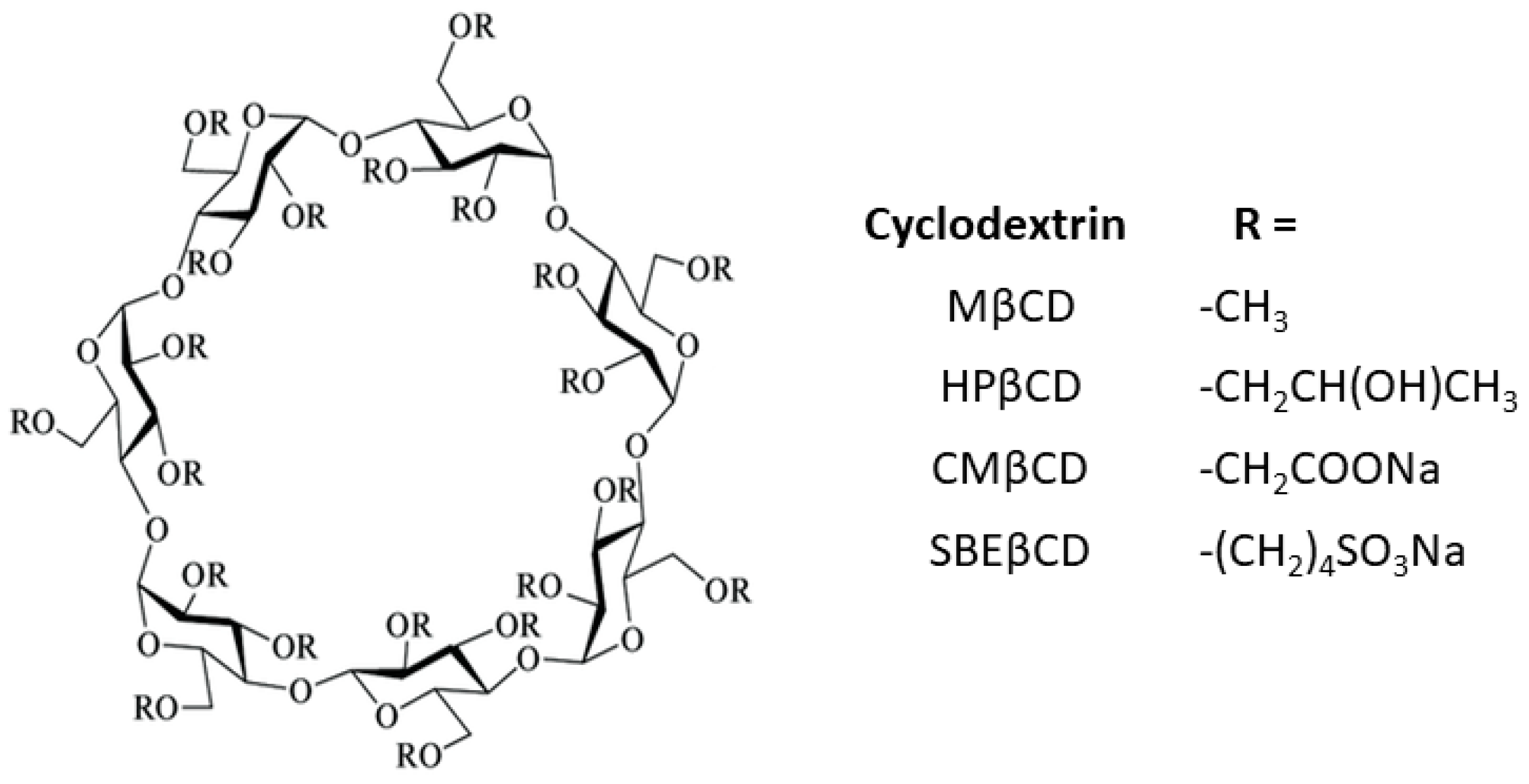
:1. Introduction
2. Enhancement of Drug Stability Induced by CDs
2.1. Biological Products
2.2. Herbal Compounds
2.3. Cosmetic Products
2.4. Photodynamic Therapy Drugs
2.5. Synthetic APIs
3. Degradation of Drugs Induced by CDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rehman, Q.; Akash, M.S.H.; Imran, I.; Rehman, K. Stability of Pharmaceutical Products. In Drug Stability and Chemical Kinetics; Akash, M.S.H., Rehman, K., Eds.; Springer: Singapore, 2020; pp. 147–154. ISBN 978-981-15-6426-0. [Google Scholar]
- Loftsson, T. Introduction. In Drug Stability for Pharmaceutical Scientists; Loftsson, T., Ed.; Academic Press: New York, NY, USA, 2014; pp. 1–3. ISBN 9780124115484. [Google Scholar]
- Yoshioka, S.; Stella, V.J. Chemical stability of drug substances. In Stability of Drugs and Dosage Forms; Yoshioka, S., Stella, V.J., Eds.; Springer Science & Business Media: New York, NY, USA, 2000; pp. 3–137. ISBN 0306464047. [Google Scholar]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez Pereira, A.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host-Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Samuelsen, L.; Holm, R.; Schönbeck, C. Simultaneous Determination of Cyclodextrin Stability Constants as a Function of pH and Temperature—A Tool for Drug Formulation and Process Design. J. Drug Deliv. Sci. Technol. 2021, 65, 102675. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins: Basic Science and Product Development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Popielec, A.; Loftsson, T. Effects of Cyclodextrins on the Chemical Stability of Drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Drug Solubilization and Stabilization by Cyclodextrin Drug Carriers. In Drug Delivery Strategies for Poorly Water-Soluble Drugs; Wiley: New York, NY, USA, 2013; pp. 67–101. [Google Scholar]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins. 1. Drug Solubilization and Stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef]
- Ioele, G.; de Luca, M.; Garofalo, A.; Ragno, G. Photosensitive Drugs: A Review on Their Photoprotection by Liposomes and Cyclodextrins. Drug Deliv. 2017, 24, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as functional excipients: Methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.C.; Martins, T.E.; Veiga, F.; Ferraz, H.G. Cyclodextrins and ternary complexes: Technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef] [Green Version]
- Suvarna, P.; Chaudhari, P.; Lewis, S.A. Cyclodextrin-Based Supramolecular Ternary Complexes: Emerging Role of Ternary Agents on Drug Solubility, Stability, and Bioavailability. Crit. Rev. Ther. Drug Carr. Syst. 2022, 39, 1–50. [Google Scholar] [CrossRef]
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin Multicomponent Complexes: Pharmaceutical Applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, M.A.; Zoppi, A. Current trends in molecular modeling methods applied to the study of cyclodextrin complexes. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 1–14. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Ye, Z.; Deng, J.; Ouyang, D. In silico formulation prediction of drug/cyclodextrin/polymer ternary complexes by machine learning and molecular modeling techniques. Carbohydr. Polym. 2022, 275, 118712. [Google Scholar] [CrossRef] [PubMed]
- Samra, H.S.; He, F.; Bhambhani, A.; Pipkin, J.D.; Zimmerer, R.; Joshi, S.B.; Russell Middaugh, C. The Effects of Substituted Cyclodextrins on the Colloidal and Conformational Stability of Selected Proteins. J. Pharm. Sci. 2010, 99, 2800–2818. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D.E.; Knudsen, B.R.; Aachmann, F.; Larsen, K.L.; Wimmer, R. Structural basis for cyclodextrins’ suppression of human growth hormone aggregation. Protein Sci. 2002, 11, 1779–1787. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, V.; Vatanara, A.; Seyedabadi, M.; Nabi Meibodi, M.; Fanaei, H. Application of Cyclodextrins in Antibody Microparticles: Potentials for Antibody Protection in Spray Drying. Drug Dev. Ind. Pharm. 2017, 43, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Matilainen, L.; Larsen, K.L.; Wimmer, R.; Keski-Rahkonen, P.; Auriola, S.; Järvinen, T.; Jarho, P. The Effect of Cyclodextrins on Chemical and Physical Stability of Glucagon and Characterization of Glucagon/γ-CD Inclusion Complexes. J. Pharm. Sci. 2008, 97, 2720–2729. [Google Scholar] [CrossRef]
- Uehata, K.; Anno, T.; Hayashida, K.; Motoyama, K.; Hirayama, F.; Ono, N.; Pipkin, J.D.; Uekama, K.; Arima, H. Effect of Sulfobutyl Ether-β-Cyclodextrin on Bioavailability of Insulin Glargine and Blood Glucose Level after Subcutaneous Injection to Rats. Int. J. Pharm. 2011, 419, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, S.; Zhao, Y.; Zhu, L.; Yu, S. Complexation of Z-ligustilide with hydroxypropyl-β-cyclodextrin to improve stability and oral bioavailability. Acta Pharm. 2014, 64, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kanabar, D.D.; Muth, A.; Gupta, V. Cyclodextrin Complexation for Enhanced Stability and Non-Invasive Pulmonary Delivery of Resveratrol—Applications in Non-Small Cell Lung Cancer Treatment. AAPS PharmSciTech 2020, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Krstić, L.; Jarho, P.; Ruponen, M.; Urtti, A.; González-García, M.J.; Diebold, Y. Improved Ocular Delivery of Quercetin and Resveratrol: A Comparative Study between Binary and Ternary Cyclodextrin Complexes. Int. J. Pharm. 2022, 624, 122028. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Guo, F.; Lin, L.; Chen, H.; Chen, J.; Cheng, Y.; Zheng, Z.-P. Investigating the Oxyresveratrol β-Cyclodextrin and 2-Hydroxypropyl-β-Cyclodextrin Complexes: The Effects on Oxyresveratrol Solution, Stability, and Antibrowning Ability on Fresh Grape Juice. LWT 2019, 100, 263–270. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Sapino, S.; Ugazio, E.; Caron, G. On the Complexation of Quercetin with Methyl-β-Cyclodextrin: Photostability and Antioxidant Studies. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 81–90. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic-Gajic, I.M.; Nikolic, V.D.; Nikolic, L.B.; Radovanovic, B.C.; Milenkovic-Andjelkovic, A. Enhencemnet of Solubility and Photostability of Rutin by Complexation with β-Cyclodextrin and (2-Hydroxypropyl)-β-Cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 33–43. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Q.-S.; Chang, S.-L.; Chang, T.-R.; Tan, M.-H.; Zhao, B. Development of Cannabidiol Full-Spectrum Oil/2,6-Di-O-Methyl-β-Cyclodextrin Inclusion Complex with Enhanced Water Solubility, Bioactivity, and Thermal Stability. J. Mol. Liq. 2022, 347, 118318. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.F.; Al-Sadeh, K.S.; Al-Bitar, M.B. Inclusion Complexes of Sunscreen Agents with β-Cyclodextrin: Spectroscopic and Molecular Modeling Studies. J. Spectrosc. 2013, 2013, 841409. [Google Scholar] [CrossRef] [Green Version]
- Scalia, S.; Molinari, A.; Casolari, A.; Maldotti, A. Complexation of the Sunscreen Agent, Phenylbenzimidazole Sulphonic Acid with Cyclodextrins: Effect on Stability and Photo-Induced Free Radical Formation. Eur. J. Pharm. Sci. 2004, 22, 241–249. [Google Scholar] [CrossRef]
- Caddeo, C.; Manconi, M.; Valenti, D.; Pini, E.; Sinico, C. Photostability and Solubility Improvement of β-Cyclodextrin-Included Tretinoin. J. Incl. Phenom. Macrocycl. Chem. 2007, 59, 293–300. [Google Scholar] [CrossRef]
- Lu, S.; Wang, A.; Ma, Y.J.; Xuan, H.Y.; Zhao, B.; Li, X.D.; Zhou, J.H.; Zhou, L.; Wei, S.H. Cyclodextrin Type Dependent Host-Guest Interaction Mode with Phthalocyanine and Their Influence on Photodynamic Activity to Cancer. Carbohydr. Polym. 2016, 148, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, A.; Quevedo, M.A.; Longhi, M.R. Specific Binding Capacity of β-Cyclodextrin with Cis and Trans Enalapril: Physicochemical Characterization and Structural Studies by Molecular Modeling. Bioorg. Med. Chem. 2008, 16, 8403–8412. [Google Scholar] [CrossRef]
- Zoppi, A.; Garnero, C.; Linck, Y.G.; Chattah, A.K.; Monti, G.A.; Longhi, M.R. Enalapril:β-CD Complex: Stability Enhancement in Solid State. Carbohydr. Polym. 2011, 86, 716–721. [Google Scholar] [CrossRef]
- Davies, N.M.; Wang, G.; Tucker, I.G. Evaluation of a Hydrocortisone/Hydroxypropyl-β-Cyclodextrin Solution for Ocular Drug Delivery. Int. J. Pharm. 1997, 156, 201–209. [Google Scholar] [CrossRef]
- el Maghraby, G.M.; Alomrani, A.H. Effects of Hydroxypropyl-β-Cyclodextrin, Gel and in Situ Gelling Systems on the Stability of Hydrocortisone after Gamma Irradiation. J. Drug Deliv. Sci. Technol. 2010, 20, 385–389. [Google Scholar] [CrossRef]
- Andersen, F.M.; Bundgaard, H. The influence of b-cyclodextrin on the stability of hydrocortisone in aqueous solutions. Arch. Pharm. Chem. Sci. Ed. 1983, 11, 61–66. [Google Scholar]
- Islam, M.S.; Narurkar, M.M. The Effect of 2-Hydroxypropyl-SScyclodextrin on the Solubility, Stability and Dissolution Rate of Famotidine. Drug Dev. Ind. Pharm. 1991, 17, 1229–1239. [Google Scholar] [CrossRef]
- Jamrógiewicz, M.; Milewska, K. Studies on the Influence of β-Cyclodextrin Derivatives on the Physical Stability of Famotidine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 219, 346–357. [Google Scholar] [CrossRef]
- Mady, F.M.; Abou-Taleb, A.E.; Khaled, K.A.; Yamasaki, K.; Iohara, D.; Taguchi, K.; Anraku, M.; Hirayama, F.; Uekama, K.; Otagiri, M. Evaluation of Carboxymethyl-β-Cyclodextrin with Acid Function: Improvement of Chemical Stability, Oral Bioavailability and Bitter Taste of Famotidine. Int. J. Pharm. 2010, 397, 1–8. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, T.; Qi, J.; Zhang, J.; Wu, W. Enhanced Dissolution and Stability of Lansoprazole by Cyclodextrin Inclusion Complexation: Preparation, Characterization, and Molecular Modeling. AAPS PharmSciTech 2012, 13, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Kumar, V.; Yang, D.; Chowdhury, P.R.; Hohl, R.J. Cyclodextrin Complexation: Influence on the Solubility, Stability, and Cytotoxicity of Camptothecin, an Antineoplastic Agent. Eur. J. Pharm. Sci. 2002, 15, 163–170. [Google Scholar] [CrossRef]
- Vaidya, B.; Shukla, S.K.; Kolluru, S.; Huen, M.; Mulla, N.; Mehra, N.; Kanabar, D.; Palakurthi, S.; Ayehunie, S.; Muth, A.; et al. Nintedanib-Cyclodextrin Complex to Improve Bio-Activity and Intestinal Permeability. Carbohydr. Polym. 2019, 204, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.C.S.G.V.; Nadvorny, D.; da Rocha Passos, T.D.; de La Roca Soares, M.F.; Soares-Sobrinho, J.L. Influence of Cyclodextrin on Posaconazole Stability, Release and Activity: Improve the Utility of the Drug. J. Drug Deliv. Sci. Technol. 2019, 53, 101153. [Google Scholar] [CrossRef]
- Pomponio, R.; Gotti, R.; Fiori, J.; Cavrini, V.; Mura, P.; Cirri, M.; Maestrelli, F. Photostability Studies on Nicardipine–Cyclodextrin Complexes by Capillary Electrophoresis. J. Pharm. Biomed. Anal. 2004, 35, 267–275. [Google Scholar] [CrossRef]
- Kogawa, A.C.; Zoppi, A.; Quevedo, M.A.; Nunes Salgado, H.R.; Longhi, M.R. Increasing Doxycycline Hyclate Photostability by Complexation with β-Cyclodextrin. AAPS PharmSciTech 2014, 15, 1209–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, M.S.; Longhi, M.; Garnero, C. Pharmaceutical Systems as a Strategy to Enhance the Stability of Oxytetracycline Hydrochloride Polymorphs in Solution. Pharmaceutics 2023, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Liu, Y.; Zhang, H.; Cui, Y.; Zhai, G.; Chen, C. Photostability Study of Doxorubicin Aqueous Solution Enhanced by Inclusion Interaction between Doxorubicin and Hydroxypropyl-β-Cyclodextrin. Chin. J. Chem. 2010, 28, 1291–1295. [Google Scholar] [CrossRef]
- Abraham-Miranda, J.; Garnero, C.; Zoppi, A.; Chattah, A.K.; Sterren, V.B.; Santiago de Oliveira, Y.; Ayala, A.P.; Longhi, M.R. Evaluating Ternary Systems with Oligosaccharides as a Strategy to Improve the Biopharmaceutical Properties of Furosemide. Mater. Sci. Eng. C 2020, 111, 110793. [Google Scholar] [CrossRef]
- Garnero, C.; Longhi, M. Study of Ascorbic Acid Interaction with Hydroxypropyl-β-Cyclodextrin and Triethanolamine, Separately and in Combination. J. Pharm. Biomed. Anal. 2007, 45, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Garnero, C.; Longhi, M. Development of HPLC and UV Spectrophotometric Methods for the Determination of Ascorbic Acid Using Hydroxypropyl-β-Cyclodextrin and Triethanolamine as Photostabilizing Agents. Anal. Chim. Acta. 2010, 659, 159–166. [Google Scholar] [CrossRef]
- Saokham, P.; Burapapadh, K.; Praphanwittaya, P.; Loftsson, T. Characterization and Evaluation of Ternary Complexes of Ascorbic Acid with γ-Cyclodextrin and Poly(Vinyl Alcohol). Int. J. Mol. Sci. 2020, 21, 4399. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Gu, J.; Guo, T.; Yang, S.; Guo, Z.; Zhang, X.; Zhu, W.; Zhang, J. Ternary System of Dihydroartemisinin with Hydroxypropyl-β-Cyclodextrin and Lecithin: Simultaneous Enhancement of Drug Solubility and Stability in Aqueous Solutions. J. Pharm. Biomed. Anal. 2013, 83, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Popielec, A.; Fenyvesi, É.; Yannakopoulou, K.; Loftsson, T. Effect of cyclodextrins on the degradation rate of benzylpenicillin. Pharmazie 2016, 71, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Popielec, A.; Agnes, M.; Yannakopoulou, K.; Fenyvesi, É.; Loftsson, T. Effect of β- and γ-cyclodextrins and their methylated derivatives on the degradation rate of benzylpenicillin. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 199–209. [Google Scholar] [CrossRef]
- Loftsson, T.; Johannesson, H.R. The influence of cyclodextrins on the stability of cephalothin and azteronam in aqueous solution. Die Pharm. 1994, 29, 292–293. [Google Scholar]
- Pamudji, J.S.; Mauludin, R. Nurhabibah Influence of β-Cyclodextrin on Cefixime Stability in Liquid Suspension Dosage Form. Procedia Chem. 2014, 13, 119–127. [Google Scholar] [CrossRef]
- Dan Córdoba, A.; Aiassa, V.; Dimmer, J.; Quevedo, M.; Longhi, M.; Zoppi, A. Development and Characterization of Pharmaceutical Systems Containing Rifampicin. Pharmaceutics 2023, 15, 198. [Google Scholar] [CrossRef] [PubMed]
- Garnero, C.; Chattah, A.K.; Aloisio, C.; Fabietti, L.; Longhi, M. Improving the Stability and the Pharmaceutical Properties of Norfloxacin Form C Through Binary Complexes with β-Cyclodextrin. AAPS PharmSciTech 2018, 19, 2255–2263. [Google Scholar] [CrossRef]
- Bueno, M.S.; Chierentin, L.; Bongioanni, A.; Nunes Salgado, H.R.; Longhi, M.R.; Garnero, C. β-cyclodextrin complexation as an approach to enhance thebiopharmaceutical properties of Norfloxacin B Hydrate. Carbohydr. Res. 2019, 485, 107818. [Google Scholar] [CrossRef]
- El-Badry, M.; Taha, E.I.; Alanazi, F.K.; Alsarra, I.A. Study of Omeprazole Stability in Aqueous Solution: Influence of Cyclodextrins. J. Drug Deliv. Sci. Technol. 2009, 19, 347–351. [Google Scholar] [CrossRef]
- Hirayama, F.; Kurihara, M.; Uekama, K. Improving the aqueous stability of prostaglandin E2 and prostaglandin A2 by inclusion complexation with methylated-beta-cyclodextrin. Chem. Pharm. Bull. 1984, 32, 4237–4240. [Google Scholar] [CrossRef] [Green Version]
- Jansook, P.; Hnin, H.M.; Praphanwittaya, P.; Loftsson, T.; Stefansson, E. Effect of Salt Formation on γ-Cyclodextrin Solubilization of Irbesartan and Candesartan and the Chemical Stability of Their Ternary Complexes. J. Drug Deliv. Sci. Technol. 2022, 67, 102980. [Google Scholar] [CrossRef]
- Wang, W. Protein Aggregation and Its Inhibition in Biopharmaceutics. Int. J. Pharm. 2005, 289, 1–30. [Google Scholar] [CrossRef]
- Dahabra, L.; Broadberry, G.; le Gresley, A.; Najlah, M.; Khoder, M. Sunscreens Containing Cyclodextrin Inclusion Complexes for Enhanced Efficiency: A Strategy for Skin Cancer Prevention. Molecules 2021, 26, 1698. [Google Scholar] [CrossRef]
- Inbaraj, J.J.; Bilski, P.; Chignell, C.F. Photophysical and Photochemical Studies of 2-Phenylbenzimidazole and UVB Sunscreen 2-Phenylbenzimidazole-5-Sulfonic Acid. Photochem. Photobiol. 2002, 75, 107–116. [Google Scholar] [CrossRef]
- Macdonald, I.J.; Dougherty, T.J. Basic Principles of Photodynamic Therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Kubiak, M.; Łysenko, L.; Gerber, H.; Nowak, R. Cell Reactions and Immune Responses to Photodynamic Therapy in Oncology. Adv. Hyg. Exp. Med. Postep. Hig. I Med. Dosw. 2016, 70, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, Z.; Du, J.; Li, W.; Pei, Z. Host–Guest Interaction Based Supramolecular Photodynamic Therapy Systems: A Promising Candidate in the Battle against Cancer. Chem. Commun. 2020, 56, 5865–5876. [Google Scholar] [CrossRef]
- de Araújo, J.I.R.; de Moura França, L.; Soares, M.F.L.R.; Nadvorny, D.; Bedor, D.C.G.; Rolim, L.A.; Soares-Sobrinho, J.L. Stability Study and Oxidative Degradation Kinetics of Posaconazole. Microchem. J. 2019, 151, 104181. [Google Scholar] [CrossRef]
- Mumtaz, S.; Mumtaz, S.; Ali, S.; Tahir, H.M.; Kazmi, S.A.R.; Mughal, T.A.; Younas, M. Evaluation of Antibacterial Activity of Vitamin C against Human Bacterial Pathogens. Braz. J. Biol. 2021, 83, e247165. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Stability Testing of New Drug Substances and Products (Q1AR2). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 2 November 2003.
| Drug | Trade Name | Administration Route | Company |
|---|---|---|---|
| αCD | |||
| Alprostadil (PGE1) | Caverject Prostavasin | injection injection | Pfizer Sidus |
| Cefotiam-hexetil | Pansporin T | oral | Takeda |
| βCD | |||
| Aceclofenac | Aceclofenac-B-Cyclodextrin | oral | Taj Pharmaceuticals India |
| Cholecalciferol Glucagon | Vitamin D3 Baqsimi | oral nasal | Natures Aid, U.K. Eli Lilly |
| γCD | |||
| Benzoyl peroxide | Nujevi Acne | dermal | Nujevi |
| HPβCD | |||
| Indometacin | Indocid | ocular | Chauvin |
| Hydrocortisone | Dexocort | buccal | Actavis |
| SBEβCD | |||
| Posaconazole | Noxafil | injection | Merck Sharp & Dohme |
| Voriconazole | Vfend | injection | Pfizer |
| API | CD Used | Effect Observed | Ref. |
|---|---|---|---|
| Clostridium difficile Toxoid A V antigen Fibroblast growth factor 10 | αCD βCD HPβCD SBEβCD γCD | inhibit protein aggregation | [19] |
| Human growth hormone | αCD, HPβCD, SBEβCD, Sulfated βCD, Monoglycosyl-βCD, Monomaltosyl-βCD, Monoacetyl-βCD, γCD | inhibit protein aggregation | [20] |
| IgG | βCD, HPβCD | inhibit protein aggregation | [21] |
| Glucagon | γCD | chemical and physical stability improved | [22] |
| Insulin glargine | SBEβCD | enzymatic degradation at the injection site reduced | [23] |
| Z-ligustilide | HPβCD | photostability improved | [24] |
| Resveratrol | SBEβCD | degradation kinetics in biological matrices inhibited | [25] |
| HPβCD | stability improved | [26] | |
| multicomponent: HPβCD and hyaluronic acid | improved stability dependent on the polysaccharide concentration | [26] | |
| Oxyresveratrol | HPβCD | thermal stability increased | [27] |
| Quercetin | αCD, βCD | photostability improved | [28] |
| HPβCD | stability improved | [26] | |
| multicomponent: HPβCD and hyaluronic acid | improved stability dependent of polysaccharide concentration | [26] | |
| Rutin | βCD, HPβCD | photostability improved | [29] |
| Ethanol extract of Cannabis sativa | DMβCD | thermal stability increased | [30] |
| UV filters (oxybenzone, octocrylene, and ethylhexyl-methoxycinnamate) | βCD | photostability increased | [31] |
| Phenylbenzimidazole sulfonic acid | HPβCD | photostability increased | [32] |
| Tretinoin | βCD | photostability increased | [33] |
| Tetra-1,2-diethylamino substituted zinc (II) phthalocyanine | αCD, βCD, γCD | aggregation decreased | [34] |
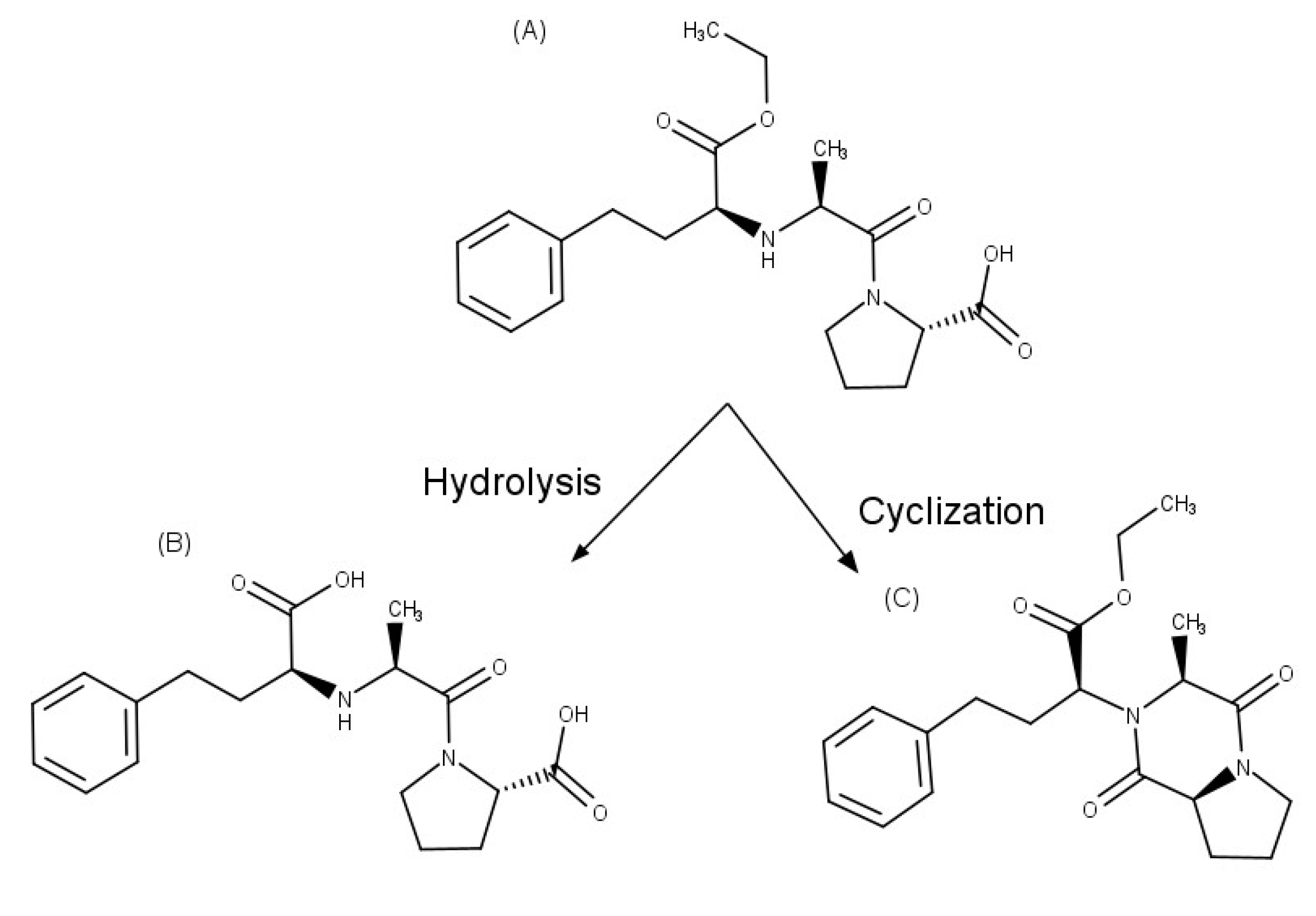
| Enalapril | βCD | hydrolysis and cyclization decreased | [35,36] |
| Hydrocortisone | HPβCD | hydrolysis decreased | [37] |
| significantly increased stability after gamma irradiation | [38] | ||
| βCD | accelerated decomposition under alkaline conditions | [39] | |
| Famotidine | HPβCD, CMβCD | degradation reduced under acidic conditions | [40] |
| physical stability improved | [41] | ||
| SBEβCD | destabilizing effect induced | [42] | |
| physical stability improved | [41] | ||
| Lansoprazole | HPβCD, βCD | stabilization effects under light, heat, and humidity exposition | [43] |
| Camptothecin | RDMβCD | hydrolysis decreased | [44] |
| Nintedanib | SBEβCD | stability in simulated intestinal fluid enhanced | [45] |
| Posaconazole | βCD | oxidative degradation decreased | [46] |
| Nicardipine | βCD, HPαCD, 2-hydroxyethyl-βCD | photoprotective effect | [47] |
| γCD, MβCD, HPβCD, HPγCD | no effect on photostability | [47] | |
| αCD | photodegradation effect | [47] | |
| Doxycycline hyclate | βCD | photoprotective effect | [48] |
| Oxytetracycline hydrochloride | βCD | degradation rate reduced only for Form III | [49] |
| Doxorubicin | HPβCD | photostability increased | [50] |
| Furosemide | multicomponent:βCD and triethanolamine | chemical degradation reduced | [51] |
| Ascorbic acid | HPβCD | stabilizing effect pH-dependent in solution | [52] |
| multicomponent: HPβCD and triethanolamine | stability in aqueous solutions improved, photodegradation reduced | [53] | |
| multicomponent: γCD and polyvinyl alcohol | oxidation reduced in aqueous solutions | [54] | |
| Dihydroartemisinin | multicomponent: HPβCD and soybean lecithin | stability in aqueous solutions improved | [55] |
| Benzylpenicillin | HPβCD, RMβCD | hydrolysis reduced under acidic conditions | [56] |
| HPβCD | hydrolysis accelerated under neutral and basic conditions | [56] | |
| RMβCD | catalytic effect on hydrolysis reduced under basic solution | [56] | |
| γCD | catalytic effect of hydrolysis | [56] | |
| randomly methylated γ-CD, octakis(2,3,6-triO-methyl)-γCD | catalytic effect of hydrolysis reduced | [57] | |
| heptakis(2,3,6-tri-O-methyl) βCD | degradation reduced by null catalytic effect | [57] | |
| RMβCD, heptakis(2,6-di-O-methyl)-βCD | catalytic effect of hydrolysis reduced | [57] | |
| β-lactam antibiotics | βCD | destabilizing effect | [58] |
| Cefixime | βCD | destabilizing effect | [59] |
| Rifampicin | γCD | destabilizing effect | [60] |
| multicomponent: γCD and arginine | stabilizing effect | [60] | |
| Norfloxacin | βCD | photostability of Form C increased | [61] |
| chemical stability of Form B hydrate decreased | [62] | ||
| Omeprazole | βCD, DMβCD, HPβCD, MaβCD | hydrolysis accelerated | [63] |
| Prostaglandins | βCD | destabilizing effect | [64] |
| DMβCD | stabilizing effect | [64] | |
| Irbesartan Candesartan cilexetil | multicomponent: γCD and organic salts | hydrolysis increased | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. https://doi.org/10.3390/ph16081074
Aiassa V, Garnero C, Zoppi A, Longhi MR. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals. 2023; 16(8):1074. https://doi.org/10.3390/ph16081074
Chicago/Turabian StyleAiassa, Virginia, Claudia Garnero, Ariana Zoppi, and Marcela R. Longhi. 2023. "Cyclodextrins and Their Derivatives as Drug Stability Modifiers" Pharmaceuticals 16, no. 8: 1074. https://doi.org/10.3390/ph16081074
APA StyleAiassa, V., Garnero, C., Zoppi, A., & Longhi, M. R. (2023). Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals, 16(8), 1074. https://doi.org/10.3390/ph16081074








