The Additive Antinociceptive Effect of Resveratrol and Ketorolac in the Formalin Test in Mice
Abstract
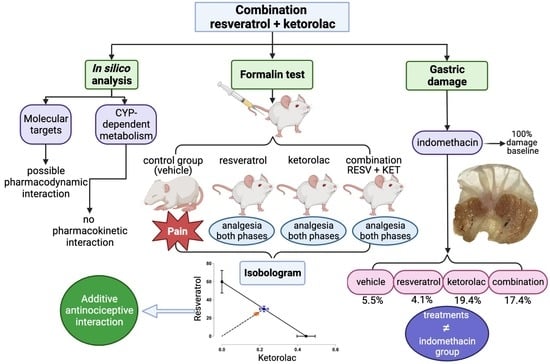
1. Introduction
2. Results
2.1. In Silico Analysis
2.1.1. Molecular Targets
2.1.2. In Silico Analysis of CYP-Dependent Metabolism
2.2. Evaluation of the Antinociceptive Activity of Individual Drugs in the Formalin Test
2.3. Evaluation of the Antinociceptive Activity of Drug Combinations in the Formalin Test
2.4. Isobolographic Analysis of the Combination of Resveratrol with Ketorolac
2.5. Evaluation of Gastric Damage Caused by the Drugs
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.2. Animals
4.3. Drugs
4.4. Formalin Test
4.5. Isobolographic Analysis
4.6. Gastric Damage Assessment
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- St. Sauver, J.L.; Warner, D.O.; Yawn, B.P.; Jacobson, D.J.; McGree, M.E.; Pankratz, J.J.; Melton III, L.J.; Roger, V.L.; Ebbert, J.O.; Rocca, W.A. Why Do Patients Visit Their Doctors? Assessing the Most Prevalent Conditions in a Defined US Population. Mayo Clin. Proc. 2014, 88, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Raffaeli, W.; Arnaudo, E. Pain as a Disease: An Overview. J. Pain Res. 2017, 10, 2003–2008. [Google Scholar] [CrossRef]
- IASP. IASP Announces Revised Definition of Pain. Available online: https://www.iasp-pain.org/publications/iasp-news/iasp-announces-revised-definition-of-pain/ (accessed on 22 December 2022).
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Adam, S.K.; Manan, N.A.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed]
- Salduker, S.; Allers, E.; Bechan, S.; Hodgson, R.E.; Meyer, F.; Meyer, H.; Smuts, J.; Vuong, E.; Webb, D. Practical Approach to a Patient with Chronic Pain of Uncertain Etiology in Primary Care. J. Pain Res. 2019, 12, 2651–2662. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, O.; Huffman, M.M.; Featherston, K. Pharmacologic Therapy for Acute Pain. Am. Fam. Physician 2021, 104, 63–72. [Google Scholar]
- Rafaniello, C.; Ferrajolo, C.; Sullo, M.G.; Sessa, M.; Sportiello, L.; Balzano, A.; Manguso, F.; Aiezza, M.L.; Rossi, F.; Scarpignato, C.; et al. Risk of Gastrointestinal Complications Associated to NSAIDs, Low-Dose Aspirin and Their Combinations: Results of a Pharmacovigilance Reporting System. Pharmacol. Res. 2016, 104, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.Y.F.; Yu, E.Y.T.; Chan, L.; Mok, A.H.Y.; Wang, Y.; Chan, E.W.Y.; Wong, I.C.K.; Lam, C.L.K. Comparative Risks of Nonsteroidal Anti-Inflammatory Drugs on CKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 898–907. [Google Scholar] [CrossRef]
- Martín Arias, L.H.; Martín González, A.; Sanz Fadrique, R.; Vazquez, E.S. Cardiovascular Risk of Nonsteroidal Anti-Inflammatory Drugs and Classical and Selective Cyclooxygenase-2 Inhibitors: A Meta-Analysis of Observational Studies. J. Clin. Pharmacol. 2019, 59, 55–73. [Google Scholar] [CrossRef]
- Raffa, R.B.; Pergolizzi, J.V.; Tallarida, R.J. The Determination and Application of Fixed-Dose Analgesic Combinations for Treating Multimodal Pain. J. Pain 2010, 11, 701–709. [Google Scholar] [CrossRef]
- Buckley, M.M.; Brogden, R.N. Ketorolac: A Review of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Potential. Drugs 1990, 39, 86–109. [Google Scholar] [CrossRef]
- Rooks, W.H.; Tomolonis, A.J.; Maloney, P.J.; Wallach, M.B.; Schuler, M.E. The Analgesic and Anti-Inflammatory Profile of (±)-5-Benzoyl-1,2-Dihydro-3H-Pyrrolo[1,2a]Pyrrole-1-Carboxylic Acid (RS-37619). Agents Actions 1982, 12, 684–690. [Google Scholar] [CrossRef]
- Catapano, M.S. The Analgesic Efficacy of Ketorolac for Acute Pain. J. Emerg. Med. 1996, 14, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, M. Resveratrol, a New Phenolic Compound, from Veratrum Grandiflorum. J. Chem. Soc. Jpn. 1939, 60, 1090–1100. [Google Scholar]
- Gambini, J.; López-Grueso, R.; Olaso-González, G.; Inglés, M.; Abdelazid, K.; El Alami, M.; Bonet-Costa, V.; Borrás, C.; Viña, J. Resveratrol: Distribución, Propiedades y Perspectivas. Rev. Esp. Geriatr. Gerontol. 2013, 48, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Konyalioglu, S.; Armagan, G.; Yalcin, A.; Atalayin, C.; Dagci, T. Effects of Resveratrol on Hydrogen Peroxide-Induced Oxidative Stress in Embryonic Neural Stem Cells. Neural Regen. Res. 2013, 8, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, X.; Cui, Y.; Zeng, G.; Chen, J.; Zhang, G. Resveratrol Alleviates Lysophosphatidylcholine-Induced Damage and Inflammation in Vascular Endothelial Cells. Mol. Med. Rep. 2018, 17, 4011–4018. [Google Scholar] [CrossRef]
- Lux, S.; Lobos, N.; Lespay-Rebolledo, C.; Salas-Huenuleo, E.; Kogan, M.J.; Flores, C.; Pinto, M.; Hernandez, A.; Pelissier, T.; Constandil, L. The Antinociceptive Effect of Resveratrol in Bone Cancer Pain Is Inhibited by the Silent Information Regulator 1 Inhibitor Selisistat. J. Pharm. Pharmacol. 2019, 71, 816–825. [Google Scholar] [CrossRef]
- Pham-Marcou, T.A.; Beloeil, H.; Sun, X.; Gentili, M.; Yaici, D.; Benoit, G.; Benhamou, D.; Mazoit, J.X. Antinociceptive Effect of Resveratrol in Carrageenan-Evoked Hyperalgesia in Rats: Prolonged Effect Related to COX-2 Expression Impairment. Pain 2008, 140, 274–283. [Google Scholar] [CrossRef]
- Montiel-Ruiz, R.M.; Granados-Soto, V.; García-Jiménez, S.; Reyes-García, G.; Flores-Murrieta, F.J.; Déciga-Campos, M. Synergistic Interaction of Diclofenac, Benfotiamine, and Resveratrol in Experimental Acute Pain. Drug Dev. Res. 2011, 72, 397–404. [Google Scholar] [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Long-Term Resveratrol Supplementation Improves Pain Perception, Menopausal Symptoms, and Overall Well-Being in Postmenopausal Women: Findings from a 24-Month Randomized, Controlled, Crossover Trial. Menopause 2020, 28, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Xiao, C.; Wei, Z.; Wang, J.; Yang, Z.; Fu, Y. Resveratrol Inhibits LPS-Induced Mice Mastitis through Attenuating the MAPK and NF-ΚB Signaling Pathway. Microb. Pathog. 2017, 107, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Calamini, B.; Ratia, K.; Malkowski, M.G.; Cuendet, M.; Pezzuto, J.M.; Santarsiero, B.D.; Mesecar, A.D. Pleiotropic Mechanisms Facilitated by Resveratrol and Its Metabolites. Biochem. J. 2010, 429, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, F.; Xu, Y.; Xiang, W.; Xie, C. TRPV1 Is Involved in the Antinociceptive Effects of Resveratrol in Paclitaxel-Induced Neuropathic Pain. All Life 2021, 14, 66–74. [Google Scholar] [CrossRef]
- Trung, L.Q.; Espinoza, J.L.; An, D.T.; Viet, N.H.; Shimoda, K.; Nakao, S. Resveratrol Selectively Induces Apoptosis in Malignant Cells with the JAK2V617F Mutation by Inhibiting the JAK2 Pathway. Mol. Nutr. Food Res. 2015, 59, 2143–2154. [Google Scholar] [CrossRef]
- Jiang, M.; He, J.; Gu, H.; Yang, Y.; Huang, Y.; Xu, X.; Liu, L. Protective Effect of Resveratrol on Obesity-Related Osteoarthritis via Alleviating JAK2/STAT3 Signaling Pathway Is Independent of SOCS3. Toxicol. Appl. Pharmacol. 2020, 388, 114871. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, Y.; Chen, H.; Meng, L.; Chen, B.; Gong, H.; Zhao, Y.; Qi, R. Resveratrol Inhibits MMP3 and MMP9 Expression and Secretion by Suppressing TLR4/NF- κ B/STAT3 Activation in Ox-LDL-Treated HUVECs. Oxidative Med. Cell. Longev. 2019, 2019, 9013169. [Google Scholar] [CrossRef]
- Yin, Y.; Guo, R.; Shao, Y.; Ge, M.; Miao, C.; Cao, L.; Hu, L.; Yang, Y. Pretreatment with Resveratrol Ameliorate Trigeminal Neuralgia by Suppressing Matrix Metalloproteinase-9/2 in Trigeminal Ganglion. Int. Immunopharmacol. 2019, 72, 339–347. [Google Scholar] [CrossRef]
- Youn, J.; Lee, J.S.; Na, H.K.; Kundu, J.K.; Surh, Y.J. Resveratrol and Piceatannol Inhibit INOS Expression and NF-KappaB Activation in Dextran Sulfate Sodium-Induced Mouse Colitis. Nutr. Cancer 2009, 61, 847–854. [Google Scholar] [CrossRef]
- Bishayee, A.; Waghray, A.; Barnes, K.F.; Mbimba, T.; Bhatia, D.; Chatterjee, M.; Darvesh, A.S. Suppression of the Inflammatory Cascade Is Implicated in Resveratrol Chemoprevention of Experimental Hepatocarcinogenesis. Pharm. Res. 2010, 27, 1080–1091. [Google Scholar] [CrossRef]
- Devaux, P.F. Perspectives in Biochemistry Static and Dynamic Lipid Asymmetry in Cell Membranes? Biochemistry 1991, 30, 1163–1173. [Google Scholar] [CrossRef]
- Vance, D.E.; Vance, J.E. Phospholipid Biosynthesis in Eukaryotes. In Biochemistry of Lipids, Lipoproteins and Membranes; Ridgway, N., McLeod, R., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2008; Volume 36, pp. 213–244. [Google Scholar]
- Pérez-Chacón, G.; Astudillo, A.M.; Balgoma, D.; Balboa, M.A.; Balsinde, J. Control of Free Arachidonic Acid Levels by Phospholipases A2 and Lysophospholipid Acyltransferases. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2009, 1791, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Cardounel, A.J.; Zweier, J.L. Endogenous Methylarginines Regulate Neuronal Nitric-Oxide Synthase and Prevent Excitotoxic Injury. J. Biol. Chem. 2002, 277, 33995–34002. [Google Scholar] [CrossRef]
- D’Mello, R.; Sand, C.A.; Pezet, S.; Leiper, J.M.; Gaurilcikaite, E.; McMahon, S.B.; Dickenson, A.H.; Nandi, M. Dimethylarginine Dimethylaminohydrolase 1 Is Involved in Spinal Nociceptive Plasticity. Pain 2015, 156, 2052–2060. [Google Scholar] [CrossRef]
- Rossiter, S.; Smith, C.L.; Malaki, M.; Nandi, M.; Gill, H.; Leiper, J.M.; Vallance, P.; Selwood, D.L. Selective Substrate-Based Inhibitors of Mammalian Dimethylarginine Dimethylaminohydrolase. J. Med. Chem. 2005, 48, 4670–4678. [Google Scholar] [CrossRef] [PubMed]
- Leiper, J.; Nandi, M.; Torondel, B.; Murray-Rust, J.; Malaki, M.; O’Hara, B.; Rossiter, S.; Anthony, S.; Madhani, M.; Selwood, D.; et al. Disruption of Methylarginine Metabolism Impairs Vascular Homeostasis. Nat. Med. 2007, 13, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Toriyabe, M.; Omote, K.; Kawamata, T.; Namiki, A. Contribution of Interaction between Nitric Oxide and Cyclooxygenases to the Production of Prostaglandins in Carrageenan-Induced Inflammation. Anesthesiology 2004, 101, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Frombaum, M.; Therond, P.; Djelidi, R.; Beaudeux, J.L.; Bonnefont-Rousselot, D.; Borderie, D. Piceatannol Is More Effective than Resveratrol in Restoring Endothelial Cell Dimethylarginine Dimethylaminohydrolase Expression and Activity after High-Glucose Oxidative Stress. Free Radic. Res. 2011, 45, 293–302. [Google Scholar] [CrossRef]
- Tsikas, D.; Böger, R.H.; Sandmann, J.; Bode-Böger, S.M.; Frölich, J.C. Endogenous Nitric Oxide Synthase Inhibitors Are Responsible for the L-Arginine Paradox. FEBS Lett. 2000, 478, 1–3. [Google Scholar] [CrossRef]
- Kielstein, A.; Tsikas, D.; Galloway, G.P.; Mendelson, J.E. Asymmetric Dimethylarginine (ADMA)-A Modulator of Nociception in Opiate Tolerance and Addiction? Nitric Oxide Biol. Chem. 2007, 17, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.; Böger, R.; Lüneburg, N. ADMA and the Role of the Genes: Lessons from Genetically Modified Animals and Human Gene Polymorphisms. Pharmacol. Res. 2009, 60, 475–480. [Google Scholar] [CrossRef]
- Waterbury, L.D.; Silliman, D.; Jolas, T. Comparison of Cyclooxygenase Inhibitory Activity and Ocular Anti-Inflammatory Effects of Ketorolac Tromethamine and Bromfenac Sodium. Curr. Med. Res. Opin. 2006, 22, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Aumont, V.; Krisa, S.; Richard, T.; Mérillon, J.M.; Battaglia, E.; Netter, P.; Magdalou, J.; Sabolovic, N. Regioselective and Stereospecific Glucuronidation of Trans- and Cis-Resveratrol in Human. Arch. Biochem. Biophys. 2001, 393, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Maier-Salamon, A.; Böhmdrfer, M.; Thalhammer, T.; Szekeres, T.; Jaeger, W. Hepatic Glucuronidation of Resveratrol: Interspecies Comparison of Enzyme Kinetic Profiles in Human, Mouse, Rat, and Dog. Drug Metab. Pharmacokinet. 2011, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Miksits, M.; Maier-Salamon, A.; Aust, S.; Thalhammer, T.; Reznicek, G.; Kunert, O.; Haslinger, E.; Szekeres, T.; Jaeger, W. Sulfation of Resveratrol in Human Liver: Evidence of a Major Role for the Sulfotransferases SULT1A1 and SULT1E1. Xenobiotica 2005, 35, 1101–1119. [Google Scholar] [CrossRef] [PubMed]
- Poór, M.; Kaci, H.; Bodnárová, S.; Mohos, V.; Fliszár-Nyúl, E.; Kunsági-Máté, S.; Özvegy-Laczka, C.; Lemli, B. Interactions of Resveratrol and Its Metabolites (Resveratrol-3-Sulfate, Resveratrol-3-Glucuronide, and Dihydroresveratrol) with Serum Albumin, Cytochrome P450 Enzymes, and OATP Transporters. Biomed. Pharmacother. 2022, 151, 113136. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.S.; Garland, L.; Hsu, C.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D. Resveratrol Modulates Drug and Carcinogen Metabolizing Enzymes in a Healthy Volunteer Study. Nutr. Cancer 2010, 3, 1168–1175. [Google Scholar] [CrossRef]
- Välitalo, P.A.; Kemppainen, H.; Kulo, A.; Smits, A.; van Calsteren, K.; Olkkola, K.T.; de Hoon, J.; Knibbe, C.A.J.; Allegaert, K. Body Weight, Gender and Pregnancy Affect Enantiomer-Specific Ketorolac Pharmacokinetics. Br. J. Clin. Pharmacol. 2017, 83, 1966–1975. [Google Scholar] [CrossRef]
- Mroszczak, E.J.; Lee, F.W.; Combs, D.; Sarnquist, F.H.; Huang, B.L.; Wu, A.T.; Tokes, L.G.; Maddox, M.L.; Cho, D.K. Ketorolac Tromethamine Absorption, Distribution, Metabolism, Excretion, and Pharmacokinetics in Animals and Humans. Drug Metab. Dispos. 1987, 15, 618–626. [Google Scholar]
- Jung, D.; Mroszczak, E.J.; Wu, A.; Ling, T.L.; Sevelius, H.; Bynum, L. Pharmacokinetics of Ketorolac and P-Hydroxyketorolac Following Oral and Intramuscular Administration of Ketorolac Tromethamine. Pharm Res 1989, 6, 62–65. [Google Scholar] [CrossRef]
- Granados-Soto, V.; Argüelles, C.F.; Ortiz, M.I. The Peripheral Antinociceptive Effect of Resveratrol Is Associated with Activation of Potassium Channels. Neuropharmacology 2002, 43, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, Y.; Li, J.; Wang, L.; Li, Z.; Zhang, C.; Zhen, L.; Ding, L.; Wang, G.; Sun, X.; et al. The Analgesic Effect of Trans-Resveratrol Is Regulated by Calcium Channels in the Hippocampus of Mice. Metab. Brain Dis. 2017, 32, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Torres-López, J.E.; Ortiz, M.I.; Castañeda-Hernández, G.; Alonso-López, R.; Asomoza-Espinosa, R.; Granados-Soto, V. Comparison of the Antinociceptive Effect of Celecoxib, Diclofenac and Resveratrol in the Formalin Test. Life Sci. 2002, 70, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, S.; Kogure, Y.; Yamamoto, S.; Noguchi, K.; Dai, Y. Modulation of TRP Channels by Resveratrol and Other Stilbenoids. Mol. Pain 2013, 9, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, Y.; Chen, Z.; Blumberg, P.M.; Kozikowski, A.P.; Wang, Z.J. Antinociceptive Pharmacology of N-(4-Chlorobenzyl)-N′-(4-Hydroxy-3- Iodo-5-Methoxybenzyl) Thiourea, a High-Affinity Competitive Antagonist of the Transient Receptor Potential Vanilloid 1 Receptor. J. Pharmacol. Exp. Ther. 2007, 321, 791–798. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 Mediates Formalin-Induced Pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef]
- Fischer, M.; Carli, G.; Raboisson, P.; Reeh, P. The Interphase of the Formalin Test. Pain 2014, 155, 511–521. [Google Scholar] [CrossRef]
- Malmberg, A.B.; Yaksh, T. Pharmacology of the Spinal Action of Ketorolac, Morphine, ST-91, U50488H, and L-PIA on the Formalin Test and an Isobolographic Analysis of the NSAID Interaction. Anesthesiology 1993, 79, 270–281. [Google Scholar] [CrossRef]
- Lázaro-Ibáñez, G.G.; Torres-López, J.E.; Granados-Soto, V. Participation of the Nitric Oxide-Cyclic GMP-ATP-Sensitive K+ Channel Pathway in the Antinociceptive Action of Ketorolac. Eur. J. Pharmacol. 2001, 426, 39–44. [Google Scholar] [CrossRef]
- Korkmaz, H.A.; Maltepe, F.; Erbayraktar, S.; Yilmaz, O.; Güray, M.; Canda, M.Ş.; Günerli, A.; Gökmen, N. Antinociceptive and Neurotoxicologic Screening of Chronic Intrathecal Administration of Ketorolac Tromethamine in the Rat. Anesth. Analg. 2004, 98, 148–152. [Google Scholar] [CrossRef]
- Rocha-González, H.I.; Sánchez-Mendoza, M.E.; Cruz-Antonio, L.; Flores-Murrieta, F.J.; Cornelio-Huerta, X.I.; Arrieta, J. Antinociceptive Interaction and Pharmacokinetics of the Combination Treatments of Methyleugenol Plus Diclofenac or Ketorolac. Molecules 2020, 25, 5106. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The Formalin Test in Mice: Dissociation between Inflammatory and Non-Inflamatory Pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Munro, G. Pharmacological Assessment of the Rat Formalin Test Utilizing the Clinically Used Analgesic Drugs Gabapentin, Lamotrigine, Morphine, Duloxetine, Tramadol and Ibuprofen: Influence of Low and High Formalin Concentrations. Eur. J. Pharmacol. 2009, 605, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Chavarria-Bolaños, D.; Perez-Urizar, J.; Grandfils, C.; Pozos-Guillén, A. Peripheral Synergism between Tramadol and Ibuprofen in the Formalin Test. Drug Dev. Res. 2014, 75, 224–230. [Google Scholar] [CrossRef]
- Uphouse, L.A.; Welch, S.P.; Ward, C.R.; Ellis, E.F.; Embrey, J.P. Antinociceptive Activity of Intrathecal Ketorolac Is Blocked by the K-Opioid Receptor Antagonist, nor-Binaltorphimine. Eur. J. Pharmacol. 1993, 242, 53–58. [Google Scholar] [CrossRef]
- Tripathi, A.; Welch, S.P. Blockade of the Antinociceptive Activity of Centrally Administered Ketorolac by Nor-Binaltorphimine. Eur. J. Pharmacol. 1995, 278, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Tsiklauri, N.; Nozadze, I.; Gurtskaia, G.; Tsagareli, M.G. Antinociceptive Tolerance to NSAIDs in the Rat Formalin Test Is Mediated by the Opioid Mechanism. Pharmacol. Rep. 2017, 69, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.K.; Sharma, M.; Briyal, S. Antinociceptive Effect of Trans-Resveratrol in Rats: Involvement of an Opioidergic Mechanism. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Villalobos, K.L.; Déciga-Campos, M.; Aguilar-Mariscal, H.; González-Trujano, M.E.; Martínez-Salazar, M.F.; de los Ramírez-Cisneros, M.Á.; Rios, M.Y.; López-Muñoz, F.J. Synergistic Antinociceptive Interaction of Syzygium Aromaticum or Rosmarinus Officinalis Coadministered with Ketorolac in Rats. Biomed. Pharmacother. 2017, 94, 858–864. [Google Scholar] [CrossRef]
- López-Muñoz, F.J.; Díaz-Reval, M.I.; Terrón, J.A.; Déciga Campos, M. Analysis of the Analgesic Interactions between Ketorolac and Tramadol during Arthritic Nociception in Rat. Eur. J. Pharmacol. 2004, 484, 157–165. [Google Scholar] [CrossRef]
- Warner, T.D.; Giuliano, F.; Vojnovic, I.; Bukasa, A.; Mitchell, J.A.; Vane, J.R. Nonsteroid Drug Selectivities for Cyclo-Oxygenase-1 Rather than Cyclo-Oxygenase-2 Are Associated with Human Gastrointestinal Toxicity: A Full In Vitro Analysis. Proc. Natl. Acad. Sci. USA 1999, 96, 7563–7568. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Dey, A.; Chatterjee, A.; Chattopadhyay, S.; Bandyopadhyay, S.K. Pro-Ulcer Effects of Resveratrol in Mice with Indomethacin-Induced Gastric Ulcers Are Reversed by L-Arginine. Br. J. Pharmacol. 2010, 159, 726–734. [Google Scholar] [CrossRef]
- Kirimlioglu, V.; Ara, C.; Yilmaz, M.; Ozgor, D.; Isik, B.; Sogutlu, G.; Kirimlioglu, H.; Karabulut, A.B.; Yilmaz, S.; Kayaalp, C.; et al. Resveratrol, a Red Wine Constituent Polyphenol, Protects Gastric Tissue against the Oxidative Stress in Cholestatic Rats. Dig. Dis. Sci. 2006, 51, 298–302. [Google Scholar] [CrossRef]
- Weininger, D. SMILES, a Chemical Language and Information System: 1: Introduction to Methodology and Encoding Rules. J. Chem. Inf. Comput. Sci. 1988, 28, 31–36. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, 357–364. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. AdmetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The Formalin Test: A Quantitative Study of the Analgesic Effects of Morphine, Meperidine, and Brain Stem Stimulation in Rats and Cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J.; Murray, R.B. Manual of Pharmacologic Calculations with Computer Programs, 2nd ed.; Springer: New York, NY, USA, 1986. [Google Scholar]
- Tallarida, R.J. Drug Synergism and Dose-Effect Data Analysis, 1st ed.; Chapman & Hall/CRC: New York, NY, USA, 2000; ISBN 1584880457. [Google Scholar]
- Tallarida, R.J. The Interaction Index: A Measure of Drug Synergism. Pain 2002, 98, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Campos, M.; Guevara López, U.; Díaz Reval, M.I.; López-Muñoz, F.J. Enhancement of Antinociception by Co-Administration of an Opioid Drug (Morphine) and a Preferential Cyclooxygenase-2 Inhibitor (Rofecoxib) in Rats. Eur. J. Pharmacol. 2003, 460, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Tamaddonfard, E.; Erfanparast, A.; Farshid, A.A.; Imani, M.; Mirzakhani, N.; Salighedar, R.; Tamaddonfard, S. Safranal, a Constituent of Saffron, Exerts Gastro-Protective Effects against Indomethacin-Induced Gastric Ulcer. Life Sci. 2019, 224, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Chen, H.M.; Liu, Y.H.; Zhang, Z.B.; Zheng, Y.F.; Su, Z.Q.; Zhang, X.; Xie, J.H.; Liang, Y.Z.; Fu, L.D.; et al. The Gastroprotective Effect of Pogostone from Pogostemonis Herba against Indomethacin-Induced Gastric Ulcer in Rats. Exp. Biol. Med. 2016, 241, 193–204. [Google Scholar] [CrossRef] [PubMed]






| Probability | Molecular Target | Common Nomenclature | Software |
|---|---|---|---|
| 1.0 | Cyclooxygenase 1 | COX-1/PTGS1 | SwissTargetPrediction |
| 1.0 | Cyclooxygenase 2 | COX-2/PTGS2 | SwissTargetPrediction |
| 0.912 | Janus kinase 2 expression inhibitor | JAK2 | PASS Online |
| 0.773 | Phosphatidylserine decarboxylase inhibitor | PISD | PASS Online |
| 0.772 | Matrix metallopeptidase 9 expression inhibitor | MMP9 | PASS Online |
| 0.681 | Dimethylargininase inhibitor | DDAH | PASS Online |
| 0.654 | Tumor necrosis factor expression inhibitor | TNF | PASS Online |
| 0.603 | Nitric oxide synthase 2 expression inhibitor | NOS2 | PASS Online |
| 0.587 | Transcription factor RelA expression inhibitor | RelA/p65 | PASS Online |
| Probability | Molecular Target | Common Name |
|---|---|---|
| 1.0 | Cyclooxygenase 1 | COX 1/PTGS1 |
| 1.0 | Cyclooxygenase 2 | COX 2/PTGS2 |
| Inhibitor | |||
|---|---|---|---|
| CYP Isoform | admetSAR | % Probability | Drugs |
| CYP2D6 | No | 0.9226 | Resveratrol |
| No | 0.9333 | Ketorolac | |
| CYP3A4 | Yes | 0.7539 | Resveratrol |
| No | 0.9621 | Ketorolac | |
| CYP1A2 | Yes | 0.9106 | Resveratrol |
| Yes | 0.5483 | Ketorolac | |
| CYP2C19 | Yes | 0.8052 | Resveratrol |
| No | 0.9225 | Ketorolac | |
| CYP2C9 | Yes | 0.7068 | Resveratrol |
| No | 0.9081 | Ketorolac | |
| Substrate | |||
|---|---|---|---|
| CYP Isoform | admetSAR | % Probability | Drugs |
| CYP2D6 | No | 0.6927 | Resveratrol |
| No | 0.8801 | Ketorolac | |
| CYP3A4 | No | 0.7342 | Resveratrol |
| No | 0.6185 | Ketorolac | |
| CYP1A2 | -- | -- | Resveratrol |
| -- | -- | Ketorolac | |
| CYP2C19 | -- | -- | Resveratrol |
| -- | -- | Ketorolac | |
| CYP2C9 | No | 0.5955 | Resveratrol |
| No | 0.6206 | Ketorolac | |
| Combination | Dose | ||
|---|---|---|---|
| Resveratrol mg/kg | Ketorolac mg/kg | Total mg/kg | |
| ED50 + ED50 | 59.9 | 0.44 | 60.34 |
| (ED50 + ED50)/2 | 29.95 | 0.22 | 30.17 |
| (ED50 + ED50)/4 | 14.97 | 0.11 | 15.08 |
| (ED50 + ED50)/8 | 7.48 | 0.05 | 7.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Aguilar, F.A.; Briones-Aranda, A.; Jaramillo-Morales, O.A.; Romero-Nava, R.; Esquinca-Avilés, H.A.; Espinosa-Juárez, J.V. The Additive Antinociceptive Effect of Resveratrol and Ketorolac in the Formalin Test in Mice. Pharmaceuticals 2023, 16, 1078. https://doi.org/10.3390/ph16081078
Rojas-Aguilar FA, Briones-Aranda A, Jaramillo-Morales OA, Romero-Nava R, Esquinca-Avilés HA, Espinosa-Juárez JV. The Additive Antinociceptive Effect of Resveratrol and Ketorolac in the Formalin Test in Mice. Pharmaceuticals. 2023; 16(8):1078. https://doi.org/10.3390/ph16081078
Chicago/Turabian StyleRojas-Aguilar, Fidencio Abner, Alfredo Briones-Aranda, Osmar Antonio Jaramillo-Morales, Rodrigo Romero-Nava, Héctor Armando Esquinca-Avilés, and Josué Vidal Espinosa-Juárez. 2023. "The Additive Antinociceptive Effect of Resveratrol and Ketorolac in the Formalin Test in Mice" Pharmaceuticals 16, no. 8: 1078. https://doi.org/10.3390/ph16081078
APA StyleRojas-Aguilar, F. A., Briones-Aranda, A., Jaramillo-Morales, O. A., Romero-Nava, R., Esquinca-Avilés, H. A., & Espinosa-Juárez, J. V. (2023). The Additive Antinociceptive Effect of Resveratrol and Ketorolac in the Formalin Test in Mice. Pharmaceuticals, 16(8), 1078. https://doi.org/10.3390/ph16081078