Citrus By-Products as a Valuable Source of Biologically Active Compounds with Promising Pharmaceutical, Biological and Biomedical Potential
Abstract
:1. Introduction
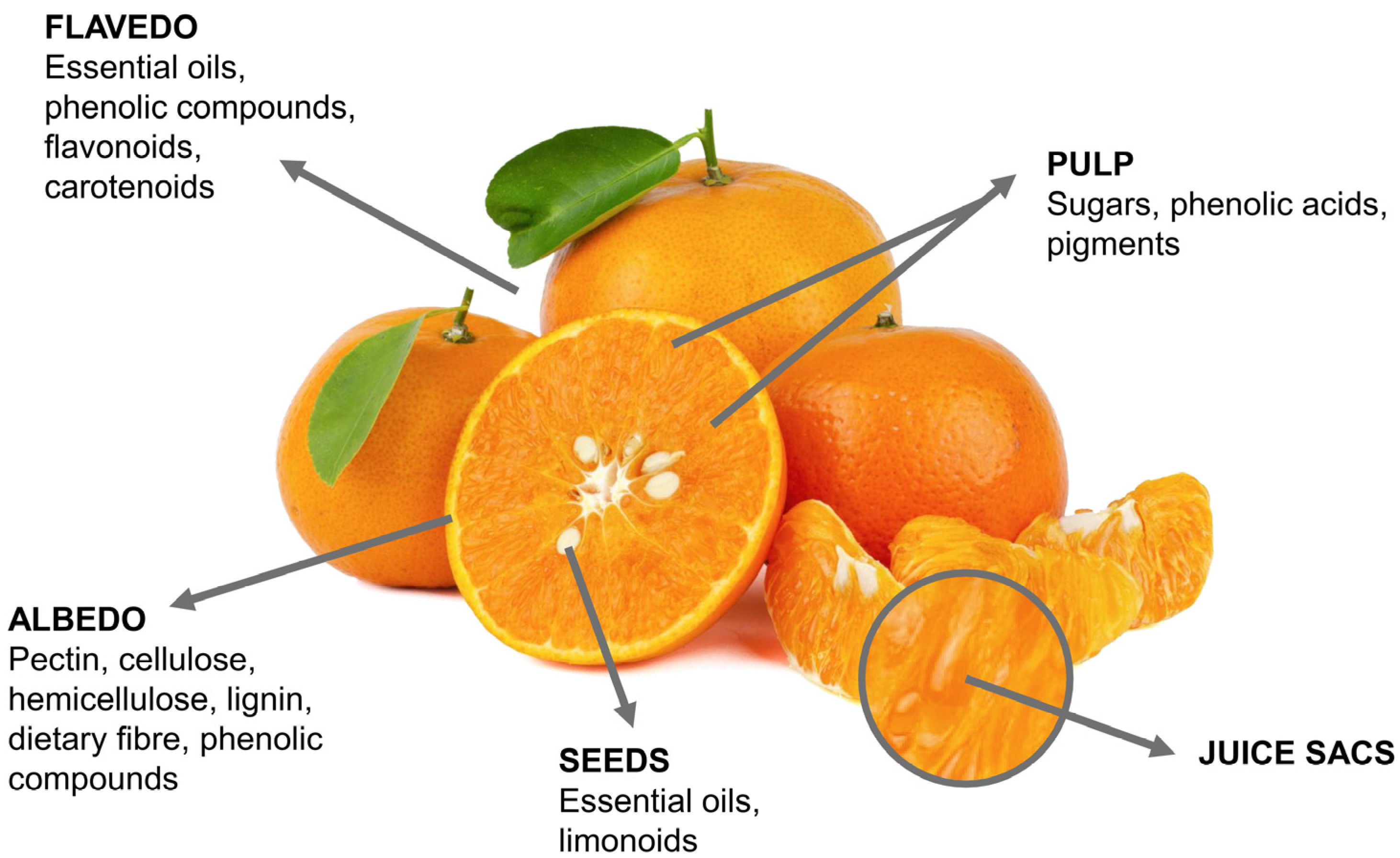
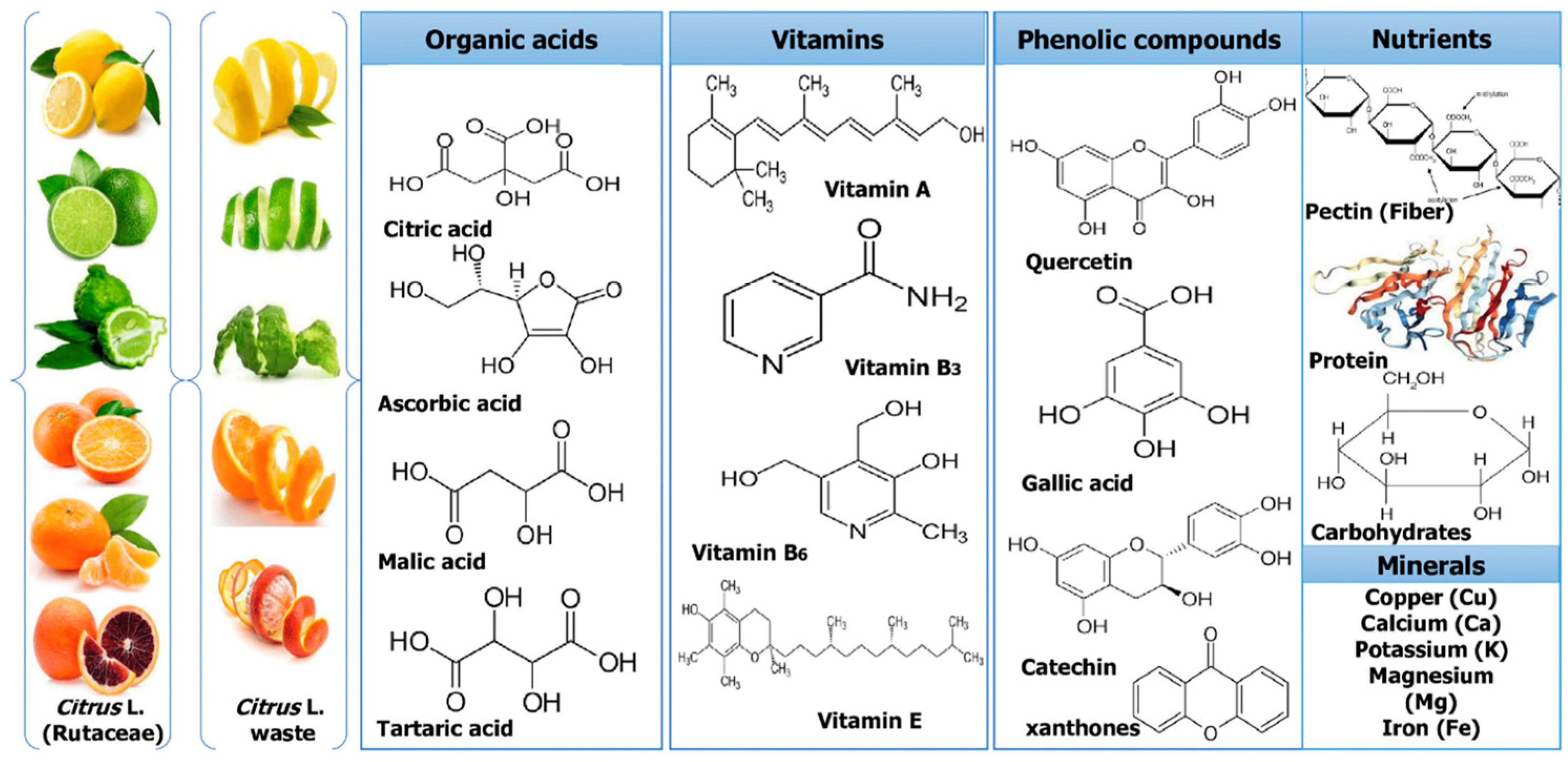
2. Structural and Chemical Characteristics of Citrus Fruits By-Products
3. Converting Waste into Treasure—Utilization of Citrus By-Products
4. Bioactivities of the Individual Groups of Compounds Present in Citrus By-Products
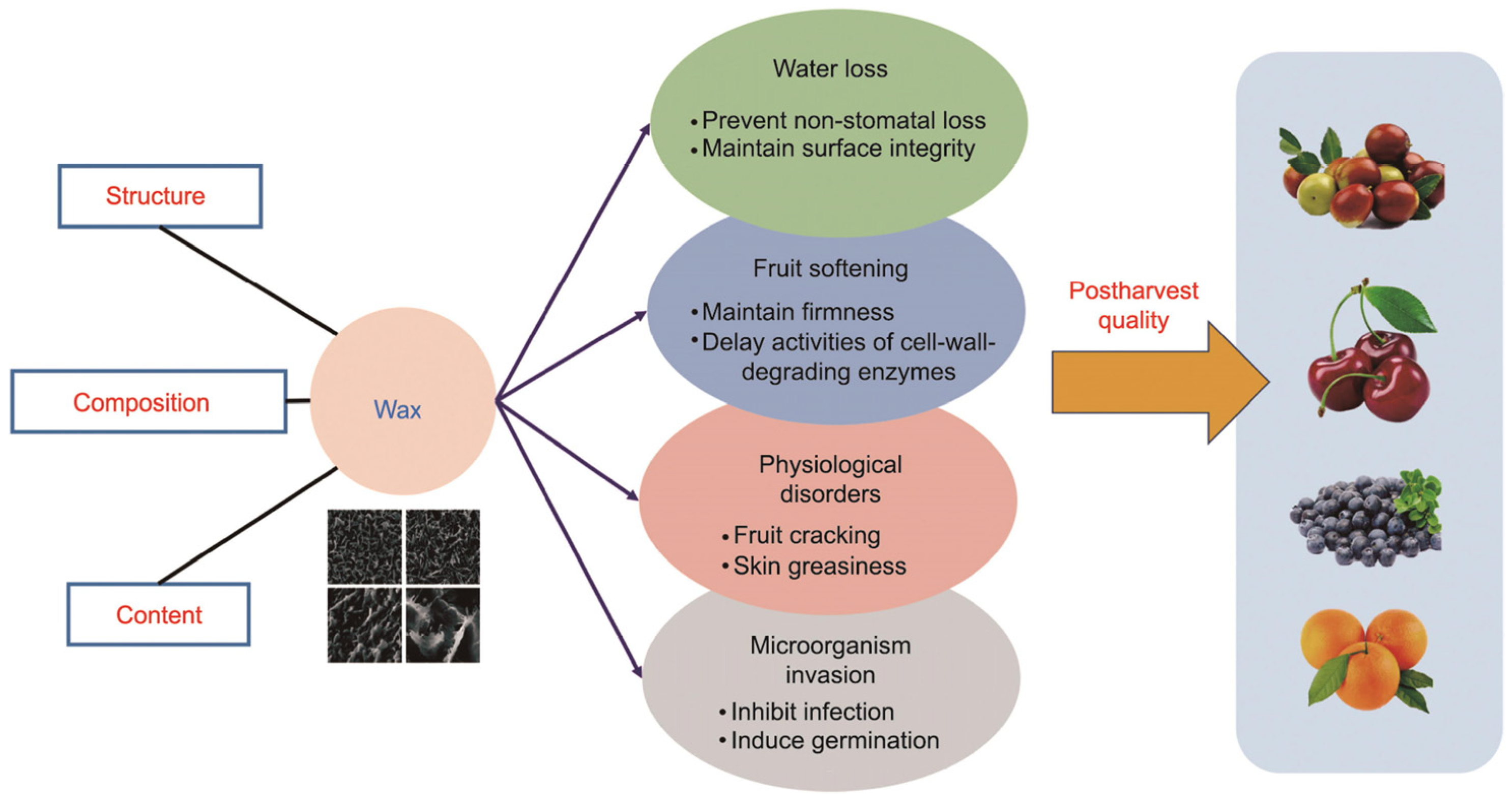
4.1. Waxes and Carotenoids
4.2. Aromatic Compounds—Essential Oils
4.3. Pectins
| Source | Formulation/Chemical Analyte | Bioactivity | Testing Subjects | References |
|---|---|---|---|---|
| C. unshiu peel | Extracted pectin (pH = 3; precipitation using 95% ethanol) | Antioxidant activity | Total phenolic content (TPC), DPPH•, ABTS•+, FRAP assay, ferrous ion chelating activity | [148] |
| Citrus peel 1 | Commercially purchased pectins; pectin-capped copper sulfide nanoparticles (pCuS NPs) | Antifungal activity | In vitro on Candida albicans | [149] |
| Citrus peel 1 | Pectin oligosaccharide fraction obtained by controlled chemical degradation of citrus peel pectin (commercial) | Prebiotic activity | In vitro on probiotic strains Bifidobacterium spp. and Lactobacillus spp./ | [145] |
| C. unshiu Marc. waste (remains from the canning processes) | Depolymerized RG-I-enriched pectin | Prebiotic activity | In vivo on male mice; Total serum cholesterol and triacylglycerol concentrations; Bacteroide thetaiotaomicron, Bifidobacterium Longum | [150] |
| Citrus (lime/lemon) peel 1 | High methoxylated citrus pectin nanoparticles (HMP-NPs), low methoxylated citrus pectin nanoparticles (LMP-NPs), and low methoxyamidated citrus pectin nanoparticles (AMP-NPs) | Oral drug delivery | In vitro cell viability tests on THP-1 (human leukemia monocytic cell line) cell line | [147] |
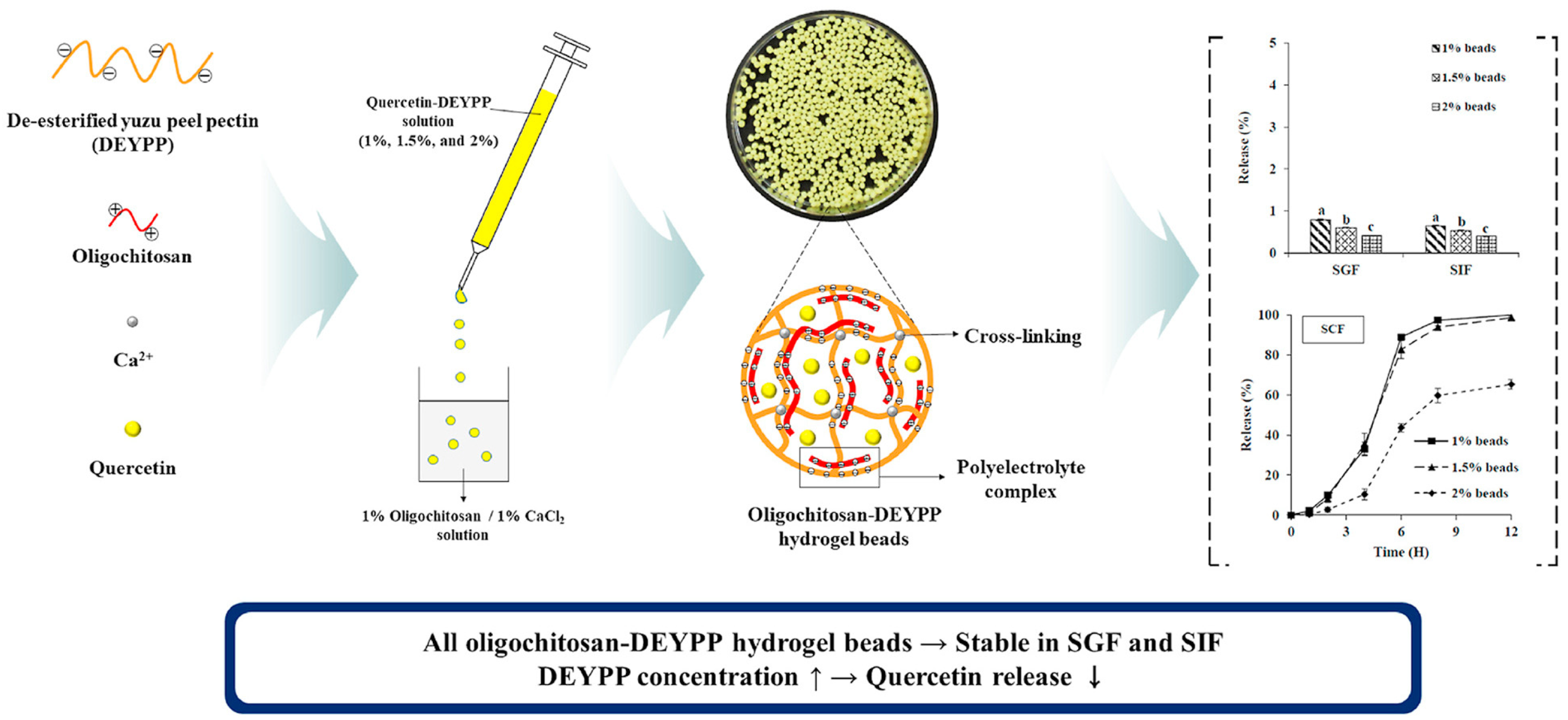
| Yuzu (C. junos) peel | Extracted pectin (pH = 3.5; precipitation using 95% ethanol)/de-esterification of pectin/oligochitosan/quercetin hydrogel beads preparation | Drug delivery/quercetin delivery system for the colon target | In vitro release study using simulated gastric, intestinal, and colonic fluids | [146] |
| C. reticulata peels | Extracted pectin (UAE 2; ammonium oxalate-oxalic acid—pH = 3.4; precipitation using 96% ethanol) | Potential antitumor activity | In vitro on the normal human embryonic kidney (HEK293) cells and colon cancer (HT29) cells | [151] |
| Lemon and lime peel 1 | Commercially purchased pectins | Anti-colitis activity/anti-inflammatory effect | In vivo on male C57BL/6 mice | [152] |
| C. sinensis peel (IntegroPectin) | Commercially purchased pectins/hesperidin-rich citrus pectin | Prevention and therapy of COVID-19 | Computational studies: molecular model of the 3-chymotrypsin-like protease (3CLpro) structure of the SARSCoV-2 | [153] |
| Citrus peel 1 | Citrus pectin oligosaccharides obtained by H2O2 degradation | Hypocholesterolemic effects | In vivo on male C57BL/6 mice | [154] |
| Grapefruit peel (IntegroPectin) 1 | IntegroPectin isolated by freeze-drying of water-based extract | Cardioprotective effects | In vivo on male Wistar rats | [155] |
4.4. Phenolic Compounds
| Source | Chemical Analytes | Bioactivity | Testing Subjects | References |
|---|---|---|---|---|
| Citrus by-products | ||||
| Finger lime peels 1 | Dominant phenolic acids: malic, citric, and quinic acid/phenolic compounds: neohesperidin, α-glucosyl hesperidin, (7S,8S)-4,7,9,9′-tetrahydroxy-3,3′-dimethoxy- 8-4′-oxyneolignan-9′-O-D-glucopyranoside, lyoniresinol 9′-O-glucoside and poncirin | Antioxidant, anti-inflammatory effect, neuronal cell protection | Antioxidant: DPPH•, ABTS•+, FRAP, ORAC/anti-inflammatory: in vitro on BV-2 (mouse microglial) cells and NO release analysis | [166] |
| Citrus (C. lumia Risso) albedo extract (peel and pulp) | Dominant phenolic acids: chlorogenic and ferulic acids/flavonoids: hesperidin and eriocitrin | Antioxidant and cytoprotective activity | Antioxidant: FRAP, TEAC, DPPH•, ORAC, β-Carotene bleaching/cell viability on lymphocytes (lactate dehydrogenase (LDH) activity) | [167] |
| C. unshiu (Chenpi) peel | Dominant flavonoid: hesperidin/Hesperidin (commercial product) | Analgesic activity and gastroprotective effect | In vitro on gastric tissue/in vivo on male ddY mice | [159] |
| C. amblycarpa peels and leaves | Phenolics: quercetin, rutin, and ɣ-aminobutyric acid (GABA) | Antihypertensive effects | ACE Inhibitory Activity Assay | [168] |
| Ougan peel extracts 1 | Flavonoids: nobiletin, tangeretin, and 5-demethylnobiletin | Antitumor activity | In vitro on gastric cancer cell line AGS, BGC-823 and SGC-7901/in vivo BALB/c nude mice | [163] |
| C. reticulata Cv. Suavissima peel extract | Flavonoids: nobiletin, tangeretin, and 5-demethylnobiletin | Anti-inflammatory effect | In vitro on BV-2 (mouse microglial) cells and NO release analysis, JAK2 inhibitor Ruxolitinib and the STAT3 inhibitor Stattic | [169] |
| C. reticulata Blanco, C. grandis, C. reticulata c.v. Kinnow, C. limetta, and C. sinensis peel extracts | Dominant flavonoids: hesperidin, naringin, quercetin, rutin, apigenin, nobiletin, tangeretin | Antioxidant activity, anti-inflammatory effect, neuroprotective effect | Antioxidant: DPPH• and ABTS•+ assay/Anti-inflammatory: protein denaturation assay (bovine serum albumin protein denaturation)/neuroprotective: Acetylcholinesterase inhibition assay | [96] |
| C. japonica var. Margarita peel | Detected phenolic acids: p-hydroxybenzoic acid, vanillic acid, protocatechuic acid, chlorogenic acid, sinapic acid, gallic acid, ferulic acid, caffeic acid | Antioxidant and antimicrobial activity | Antioxidant: DPPH•/Antimicrobial: E. coli, Salmonella (S.) typhimurium, S. aureus and Pseudomonas (P.) aeruginosa | [170] |
| C. sinensis (navel orange) | Hydroethanolic extract, naringin, naringenin | Hepatopreventive activity | In vivo on male Wistar rats; histopathological investigation and immunohistochemical detection of p53, Bax, Caspase-3, and Bcl-2 | [171] |
| C. reticulata peel | Extract (major components): hesperidin, nobiletin, narirutin, tangeretin, and sinensetin | Antiobesity- related effects. | In vitro on 3T3-L1 mouse preadipocytes | [172] |
| Ten citrus samples | Detected components: nobiletin, quercetin, diosmin, naringenin, hesperidin, hesperetin, rutin | Anti-estrogenic and anti-aromatase activity/antitumor activity | In vivo on immature female Swiss albino mice/in vitro on MCF-7 and T47D (breast cancer lines), as well as the normal human HFB4 cells | [33] |
| C. unshiu peel | Detected components: rutin, naringin, hesperidin, poncirin | Anti-inflammatory and antioxidant activity | In vitro on RAW 264.7 macrophages (originating from Abelson leukemia virus) | [173] |
| 14 Chinese cultivars (mandarins, oranges, pummelos, hybrids, citrons, kumquats) | Detected components: eriocitrin, naringin, hesperidin, didymin, poncirin, naringenin, hesperetin, sinensetin, nobiletin, tangeretin, and 5-O-demethylnobiletin | Antioxidant activity, and effects on intestinal microbiota | Antioxidant: DPPH•, ABTS•+, FRAP, CUPRAC/a-Glucosidase inhibition assay/bile salt binding capacity determination assay/investigation on fecal samples/in vitro on simulated intestinal fermentation | [13] |
| Sour orange, sweet orange, and lemon peels 1 | Dominant phenolic acids: o-coumaric acid, benzoic acid, ellagic acid, p-Hydroxybenzoic acid/flavonoids: myricetin, naringin, quercetin | Probiotic and symbiotic activity (Acidophilus-bifidus-thermophilus (ABT)-Type Synbiotic Yoghurt) | Antioxidant: DPPH•/antibacterial: S. aureus, Bacillus (B.) subtilis, and E. coli | [174] |
| C. limetta peel | Hesperidin-rich ethanol extract | Management of the rheumatoid arthritis | In vivo on Charles foster rats and Swiss albino mice | [175] |
| Individual components | ||||
| C. sinensis L. Osbeck peel and pulp | Hesperidin-rich extract | Antioxidant and antidiabetic activity | Antioxidant: DPPH•, ABTS•+, iron chelating activity/in vitro α-Amylase inhibition assay | [176] |
| Commercial product | Hesperetin and quercetin | Drug delivery | In vitro on MDCK II (Madin-Darby canine kidney cells) cell viability | [177] |
| C. uranium peel | Hesperidin | Anti-Helicobacter pylori activity | In vitro on human H. pylori strains/urease inhibition assay/molecular docking | [161] |
| C. reticulata peel | Hesperidin | Antihyperglycemic, antihyperlipidemic, and antioxidant activity | In vivo on male Wistar rats/biochemical assay and histological investigation | [17] |
| Commercial product | Hesperetin | Treatment and prevention of cardiovascular diseases | Ex vivo on porcine coronary arteries and human coronary artery smooth muscle cells | [178] |
| Commercial product | Hesperidin | Antitumor activity | In vivo on male-specified pathogen-free C57BL/6N mice/in vitro on Lewis lung carcinoma (LLC) cells | [179] |
| Commercial products | A mixture of hesperidin and naringenin | Treatment and prevention of cardiovascular diseases | In vivo and ex vivo on male Wistar rats and aortic rings | [180] |
| Commercial product | Hesperidin | Antitumor activity | In vitro on PC3 and DU145 (human prostate cancer) cell lines | [181] |
| Commercial product | Hesperetin and naringenin | Antitumor activity | In vitro on MIA PaCa-2, PANC-1, SNU-213 (pancreatic cancer cell lines), Detroit 551 (skin fibroblast), and human umbilical vein endothelial cells (HUVECs) | [182] |
| C. sinensis var. Valencia peel | Naringenin | Hepato- and renoprotective effects | In vivo on male Wistar rats/histological investigation of the liver and kidney tissues | [183] |
| Commercial product | Naringenin | Anti-proliferative effect., wound healing | In vitro on human A549 lung cancer cells | [184] |
| Commercial product | Naringenin, nobiletin, and hesperidin | Treatment of optic nerve injury, neuroprotective | In vivo on 6-weeks-old C57BL/6J mice/in vitro on HEK293T (human embryonic kidney cells) cells | [185] |
| Combination of commercial products (naringin and doxorubicin), orange peel 1 | Combination of naringin, doxorubicin, and orange peel extract | Antitumor activity | In vivo on mice models/in vitro on YM1 (human esophageal squamous cancer cell line)/ | [164] |
| C. junos Tanaka peel | Naringin | Preventive effect on pulmonary damage | In vivo on male 7-week-old BALB/c mice/in vitro on NCI-H460 (the human lung carcinoma cell lines) | [186] |
| C. maxima (Burm.) Merr peel | Naringin crystals + sericin | Treatment of psoriasis | In vitro on isolated human peripheral blood mononuclear cells, investigation on proinflammatory cytokines (TNF-α, IL-6, IL-12p40, and IL-23) | [165] |
| Commercial product | Narirutin | Antitumor activity | In vitro on PC-3 (prostate carcinoma and HEK-293 (embryonic kidney) cell lines | [187] |
| Grapefruit 1 peel | Narirutin-rich fractions | Neuroprotective effect (cerebral ischemia/reperfusion injury) | In vivo on male Wistar rats | [188] |
| Commercial product | Poncirin | Antidiabetic activity | PTP1B inhibitory assay, α-Glucosidase inhibitory assay, HRAR inhibition assay/in vitro on C2C12 cell (skeletal muscle cells) line | [189] |
| Commercial product | Poncirin and isosakuranetin | Beneficial effects on gut microbiota | In vivo on thirty C57Bl/6J male mice/fecal microbiota | [190] |
| C. sinensis peel | Rutin | Antimicrobial activity | In vitro on Aeromonas (A.) hydrophila strains | [191] |
| Citrus peel 1 | Tangeretin | Antitumor activity | In vitro on MCF-7 and MDA-MB-231 (breast cancer) cell lines | [34] |
| Commercial product | Diosmetin | Antihypertensive effects | In vivo on adult Sprague–Dawley rats/in vitro: vascular pathway inhibitors | [192] |
| Commercial product | Diosmetin and diosmin | Anti-inflammatory effect on atopic dermatitis | In vivo on six-week-old female SKH-1 hairless mice/in vitro: RBL-2H3 (basophilic leukemia) cell line | [193] |
5. Citrus By-Products Formulations with Enhanced Bioactivities
6. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, U.M.; Sameen, A.; Aadil, R.M.; Shahid, M.; Sezen, S.; Zarrabi, A.; Ozdemir, B.; Sevindik, M.; Kaplan, D.N.; Selamoglu, Z.; et al. Citrus Genus and Its Waste Utilization: A Review on Health-Promoting Activities and Industrial Application. Evid.-Based Complement. Altern. Med. 2021, 2021, 2488804. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus Fruits as a Treasure Trove of Active Natural Metabolites That Potentially Provide Benefits for Human Health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Lou, Y.; Li, Y.; Zhang, J.; Li, P.; Yang, B.; Gu, Q. Review of Phytochemical and Nutritional Characteristics and Food Applications of Citrus L. Fruits. Front. Nutr. 2022, 9, 968604. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.; Sadaka, C.; Generalić Mekinić, I.; Garzoli, S.; Švarc-Gajić, J.; Rodrigues, F.; Morais, S.; Moreira, M.M.; Ferreira, E.; Spigno, G.; et al. The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review. Antioxidants 2023, 12, 481. [Google Scholar] [CrossRef]
- Rao, M.J.; Zuo, H.; Xu, Q. Genomic Insights into Citrus Domestication and Its Important Agronomic Traits. Plant Commun. 2021, 2, 100138. [Google Scholar] [CrossRef]
- Goh, R.M.V.; Pua, A.; Luro, F.; Ee, K.H.; Huang, Y.; Marchi, E.; Liu, S.Q.; Lassabliere, B.; Yu, B. Distinguishing Citrus Varieties Based on Genetic and Compositional Analyses. PLoS ONE 2022, 17, e0267007. [Google Scholar] [CrossRef]
- Conti, G.; Xoconostle-Cázares, B.; Marcelino-Pérez, G.; Hopp, H.E.; Reyes, C.A. Citrus Genetic Transformation: An Overview of the Current Strategies and Insights on the New Emerging Technologies. Front. Plant Sci. 2021, 12, 768197. [Google Scholar] [CrossRef]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the Origin and Evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Suri, S.; Singh, A.; Nema, P.K. Current Applications of Citrus Fruit Processing Waste: A Scientific Outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef] [PubMed]
- Hussien Abou Baker, D.; Ahmed Ibrahim, E.; Abd El-Rhaman Salama, Z. Citrus Peels as a Source of Bioactive Compounds with Industrial and Therapeutic Applications. In Biochemistry; Badria, F.A., Ed.; IntechOpen: London, UK, 2022; Volume 26, ISBN 978-1-83969-346-5. [Google Scholar]
- Li, P.; Yao, X.; Zhou, Q.; Meng, X.; Zhou, T.; Gu, Q. Citrus Peel Flavonoid Extracts: Health-Beneficial Bioactivities and Regulation of Intestinal Microecology in Vitro. Front. Nutr. 2022, 9, 888745. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Nakajima, A.; Guo, Y.; Ohizumi, Y. A Narrative Review of the Effects of Citrus Peels and Extracts on Human Brain Health and Metabolism. Nutrients 2022, 14, 1847. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Guo, C.; Shan, Y.; Yang, Z.; Zhang, L.; Ling, W.; Liang, Y.; Ouyang, Z.; Zhong, B.; Zhang, J. Chemical Composition, Antioxidant, Antibacterial, and Tyrosinase Inhibition Activity of Extracts from Newhall Navel Orange (Citrus sinensis Osbeck Cv. Newhall) Peel. J. Sci. Food Agric. 2020, 100, 2664–2674. [Google Scholar] [CrossRef]
- Ali, A.M.; Gabbar, M.A.; Abdel-Twab, S.M.; Fahmy, E.M.; Ebaid, H.; Alhazza, I.M.; Ahmed, O.M. Antidiabetic Potency, Antioxidant Effects, and Mode of Actions of Citrus reticulata Fruit Peel Hydroethanolic Extract, Hesperidin, and Quercetin in Nicotinamide/Streptozotocin-Induced Wistar Diabetic Rats. Oxidative Med. Cell. Longev. 2020, 2020, 1730492. [Google Scholar] [CrossRef]
- Lin, X.; Cao, S.; Sun, J.; Lu, D.; Zhong, B.; Chun, J. The Chemical Compositions, and Antibacterial and Antioxidant Activities of Four Types of Citrus Essential Oils. Molecules 2021, 26, 3412. [Google Scholar] [CrossRef]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El- Aziz, N.M.; Youssef, M.M. Antioxidant and Antimicrobial Activities and UPLC-ESI-MS/MS Polyphenolic Profile of Sweet Orange Peel Extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef]
- Hou, H.-S.; Bonku, E.M.; Zhai, R.; Zeng, R.; Hou, Y.-L.; Yang, Z.-H.; Quan, C. Extraction of Essential Oil from Citrus Reticulate Blanco Peel and Its Antibacterial Activity against Cutibacterium acnes (Formerly Propionibacterium acnes). Heliyon 2019, 5, e02947. [Google Scholar] [CrossRef] [Green Version]
- Degirmenci, H.; Erkurt, H. Chemical Profile and Antioxidant Potency of Citrus aurantium L. Flower Extracts with Antibacterial Effect against Foodborne Pathogens in Rice Pudding. LWT 2020, 126, 109273. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.; Oalđe, M.; Alimpić-Aradski, A. In vitro Evaluation of Antioxidant, Antineurodegenerative and Antidiabetic Activities of Ocimum basilicum L., Laurus nobilis L. Leaves and Citrus reticulata Blanco Peel Extracts. Lek. Sirovine 2019, 39, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Ani, P.N.; Ochu, K.E. Anti-Diabetic, Anti-Hyperlipidemic and Hepatoprotective Potential of Shaddock (Citrus maxima) Peel Extract. Acta Sci. Pol. Technol. Aliment. 2020, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bendaha, H.; Bnouham, M.; et al. Phytochemical Profile, α-Glucosidase, and α-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules 2021, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Choi, G.; Kim, Y.; Lee, G.; Hyun, K. Neuroprotective properties of ethanolic extract of Citrus unshiu Markovich peel through NADPH oxidase 2 inhibition in chemotherapy-induced neuropathic pain animal model. Phytother. Res. 2021, 35, 6918–6931. [Google Scholar] [CrossRef]
- Furukawa, Y.; Okuyama, S.; Amakura, Y.; Sawamoto, A.; Nakajima, M.; Yoshimura, M.; Igase, M.; Fukuda, N.; Tamai, T.; Yoshida, T. Isolation and Characterization of Neuroprotective Components from Citrus Peel and Their Application as Functional Food. Chem. Pharm. Bull. 2021, 69, 2–10. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Malik, A.; Najda, A.; Bains, A.; Nurzyńska-Wierdak, R.; Chawla, P. Characterization of Citrus nobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Molecules 2021, 26, 4310. [Google Scholar] [CrossRef]
- Lala, M.; Modak, D.; Paul, S.; Sarkar, I.; Dutta, A.; Kumar, A.; Bhattacharjee, S.; Sen, A. Potent Bioactive Methanolic Extract of Wild Orange (Citrus macroptera Mont.) Shows Antioxidative, Anti-Inflammatory, and Antimicrobial Properties in in Vitro, in Vivo, and in Silico Studies. Bull. Natl. Res. Cent. 2020, 44, 81. [Google Scholar] [CrossRef]
- Roko, O.G.; Dougnon, V.; Hounkpatin, A.; Klotoé, J.R.; Baba-Moussa, L. Anti-Inflammatory, Analgesic and Antipyretic Properties of Ethanolic Extracts of Three Plants of Beninese’s Pharmacopoeia: Euphorbia hirta, Citrus aurantifolia and Heterotis rotundifolia. AJOB 2020, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Matuka, T.; Oyedeji, O.; Gondwe, M.; Oyedeji, A. Chemical Composition and In Vivo Anti-Inflammatory Activity of Essential Oils from Citrus sinensis (L.) Osbeck Growing in South Africa. J. Essent. Oil Bear. Plants 2020, 23, 638–647. [Google Scholar] [CrossRef]
- Ho, Y.; Suphrom, N.; Daowtak, K.; Potup, P.; Thongsri, Y.; Usuwanthim, K. Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 476. [Google Scholar] [CrossRef]
- El-Kersh, D.M.; Ezzat, S.M.; Salama, M.M.; Mahrous, E.A.; Attia, Y.M.; Ahmed, M.S.; Elmazar, M.M. Anti-Estrogenic and Anti-Aromatase Activities of Citrus Peels Major Compounds in Breast Cancer. Sci. Rep. 2021, 11, 7121. [Google Scholar] [CrossRef]
- Ko, Y.-C.; Choi, H.S.; Liu, R.; Kim, J.-H.; Kim, S.-L.; Yun, B.-S.; Lee, D.-S. Inhibitory Effects of Tangeretin, a Citrus Peel-Derived Flavonoid, on Breast Cancer Stem Cell Formation through Suppression of Stat3 Signaling. Molecules 2020, 25, 2599. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Sun, L.; An, R.; Zhang, W.; Xiang, L.; Li, Q.; Lai, X.; Huo, M.; Li, D.; Sun, S. A Combination of Citrus reticulata Peel and Black Tea Inhibits Migration and Invasion of Liver Cancer via PI3K/AKT and MMPs Signaling Pathway. Mol. Biol. Rep. 2020, 47, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Caggia, C.; Palmeri, R.; Russo, N.; Timpone, R.; Randazzo, C.L.; Todaro, A.; Barbagallo, S. Employ of Citrus By-Product as Fat Replacer Ingredient for Bakery Confectionery Products. Front. Nutr. 2020, 7, 46. [Google Scholar] [CrossRef]
- Magalhães, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional Ingredients and Additives from Lemon By-Products and Their Applications in Food Preservation: A Review. Foods 2023, 12, 1095. [Google Scholar] [CrossRef]
- Taghavi Kevij, H.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Mechanical, Physical, and Bio-Functional Properties of Biopolymer Films Based on Gelatin as Affected by Enriching with Orange Peel Powder. Polym. Bull. 2021, 78, 4387–4402. [Google Scholar] [CrossRef]
- Bhandari, D.P.; Poudel, D.K.; Satyal, P.; Khadayat, K.; Dhami, S.; Aryal, D.; Chaudhary, P.; Ghimire, A.; Parajuli, N. Volatile Compounds and Antioxidant and Antimicrobial Activities of Selected Citrus Essential Oils Originated from Nepal. Molecules 2021, 26, 6683. [Google Scholar] [CrossRef]
- Manzur, M.; Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Citrus sinensis Essential Oils an Innovative Antioxidant and Antipathogenic Dual Strategy in Food Preservation against Spoliage Bacteria. Antioxidants 2023, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Jiménez-Belenguer, A.; Settanni, L.; Perdones, A.; Moschetti, M.; Palazzolo, E.; Guarrasi, V.; Vargas, M.; Germanà, M.A.; Moschetti, G. Antilisterial Effect of Citrus Essential Oils and Their Performance in Edible Film Formulations. Food Control 2016, 59, 750–758. [Google Scholar] [CrossRef] [Green Version]
- Sarma, R.; Adhikari, K.; Mahanta, S.; Khanikor, B. Insecticidal Activities of Citrus aurantifolia Essential Oil against Aedes aegypti (Diptera: Culicidae). Toxicol. Rep. 2019, 6, 1091–1096. [Google Scholar] [CrossRef]
- Asadollahi, A.; Khoobdel, M.; Zahraei-Ramazani, A.; Azarmi, S.; Mosawi, S.H. Effectiveness of Plant-Based Repellents against Different Anopheles Species: A Systematic Review. Malar. J. 2019, 18, 436. [Google Scholar] [CrossRef] [PubMed]
- Misni, N.; Mohamed Nor, Z.; Ahmad, R.; Ithnin, N.R.; Zasmy Unyah, N. Microencapsulation Preservation of the Stability and Efficacy of Citrus Grandis Oil-Based Repellent Formulation against Aedes aegypti during Storage. Molecules 2021, 26, 3599. [Google Scholar] [CrossRef] [PubMed]
- Changbunjong, T.; Boonmasawai, S.; Sungpradit, S.; Weluwanarak, T.; Leesombun, A. Contact and Fumigant Activities of Citrus aurantium Essential Oil against the Stable Fly Stomoxys calcitrans (Diptera: Muscidae). Plants 2022, 11, 1122. [Google Scholar] [CrossRef] [PubMed]
- Ostovar, E.; Khodayari, S.; Aramideh, S. Fumigant toxicity of essential oils of Citrus limon L., Citrus sinensis L. and Citrus aurantium L. peels on three important stored products pests. IJMAPR 2021, 37, 859–871. [Google Scholar] [CrossRef]
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and Delivery of Bioactive Citrus Pomace Polyphenols: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8028–8044. [Google Scholar] [CrossRef]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. IJMS 2023, 24, 3813. [Google Scholar] [CrossRef]
- Dadwal, V.; Gupta, M. Recent Developments in Citrus Bioflavonoid Encapsulation to Reinforce Controlled Antioxidant Delivery and Generate Therapeutic Uses: Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1187–1207. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Zheng, T.; Liu, Q.; Zhu, J.; Huang, Q. Evaluation of Oral Bioaccessibility of Aged Citrus Peel Extracts Encapsulated in Different Lipid-Based Systems: A Comparison Study Using Different in Vitro Digestion Models. J. Agric. Food Chem. 2020, 68, 97–105. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Al-Maqtari, Q.A.; Mohammed, J.K.; Al-Ansi, W.; Cui, H.; Lin, L. Enhancement of Antioxidant Activity, Antifungal Activity, and Oxidation Stability of Citrus reticulata Essential Oil Nanocapsules by Clove and Cinnamon Essential Oils. Food Biosci. 2021, 43, 101226. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic Gajic, I.M.; Milovanovic, M.G.; Zerajic, S.; Gajic, D.G. Optimization of Ultrasound-Assisted Extraction and Encapsulation of Antioxidants from Orange Peels in Alginate-Chitosan Microparticles. Antioxidants 2022, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Ghosh, N.N.; Das, M.; Adhikary, R.; Mandal, V.; Chattopadhyay, A.P. Green Synthesis of Antibacterial and Antifungal Silver Nanoparticles Using Citrus limetta Peel Extract: Experimental and Theoretical Studies. J. Environ. Chem. Eng. 2020, 8, 104019. [Google Scholar] [CrossRef]
- Khane, Y.; Benouis, K.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; Al Ali, A.; Aouf, D.; Fenniche, F.; Khane, S.; Chaibi, W.; et al. Green Synthesis of Silver Nanoparticles Using Aqueous Citrus limon Zest Extract: Characterization and Evaluation of Their Antioxidant and Antimicrobial Properties. Nanomaterials 2022, 12, 2013. [Google Scholar] [CrossRef]
- Jahan, I.; Erci, F.; Isildak, I. Facile Microwave-Mediated Green Synthesis of Non-Toxic Copper Nanoparticles Using Citrus sinensis Aqueous Fruit Extract and Their Antibacterial Potentials. J. Drug Deliv. Sci. Technol. 2021, 61, 102172. [Google Scholar] [CrossRef]
- Purushothaman, B.K.; Uma Maheswari, P.; Meera Sheriffa Begum, K.M. Magnetic Casein-CaFe2O4 Nanohybrid Carrier Conjugated with Progesterone for Enhanced Cytotoxicity of Citrus Peel Derived Hesperidin Drug towards Breast and Ovarian Cancer. Int. J. Biol. Macromol. 2020, 151, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, M.; Maleki, H.; Barani, M.; Fahmidehkar, M.; Mahmoodi, M.; Torkzadeh-Mahani, M. In Vitro Cytotoxicity Assay of D-Limonene Niosomes: An Efficient Nano-Carrier for Enhancing Solubility of Plant-Extracted Agents. Res. Pharma Sci. 2019, 14, 448. [Google Scholar] [CrossRef]
- Medina-Rodríguez, A.C.; Ávila-Sierra, A.; Ariza, J.J.; Guillamón, E.; Baños-Arjona, A.; Vicaria, J.M.; Jurado, E. Clean-in-Place Disinfection of Dual-Species Biofilm (Listeria and Pseudomonas) by a Green Antibacterial Product Made from Citrus Extract. Food Control 2020, 118, 107422. [Google Scholar] [CrossRef]
- Majumdar, M.; Khan, S.A.; Biswas, S.C.; Roy, D.N.; Panja, A.S.; Misra, T.K. In vitro and in silico Investigation of Anti-Biofilm Activity of Citrus macroptera Fruit Extract Mediated Silver Nanoparticles. J. Mol. Liq. 2020, 302, 112586. [Google Scholar] [CrossRef]
- Pradeep, M.; Kruszka, D.; Kachlicki, P.; Mondal, D.; Franklin, G. Uncovering the Phytochemical Basis and the Mechanism of Plant Extract-Mediated Eco-Friendly Synthesis of Silver Nanoparticles Using Ultra-Performance Liquid Chromatography Coupled with a Photodiode Array and High-Resolution Mass Spectrometry. ACS Sustain. Chem. Eng. 2022, 10, 562–571. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, J.; Medhi, T.; Kumar, A. Phytochemical Screening, Quantification, FT-IR Analysis, and In Silico Characterization of Potential Bio-Active Compounds Identified in HR-LC/MS Analysis of the Polyherbal Formulation from Northeast India. ACS Omega 2022, 7, 33067–33078. [Google Scholar] [CrossRef]
- Berk, Z. Citrus Fruit Processing; Academic Press: London, UK, 2016; ISBN 978-0-12-803133-9. [Google Scholar]
- Martínez-Nicolas, J.J.; Núñez-Gómez, D.; Lidón, V.; Martínez-Font, R.; Melgarejo, P.; Hernández, F.; Legua, P. Physico-Chemical Attributes of Lemon Fruits as Affected by Growing Substrate and Rootstock. Foods 2022, 11, 2487. [Google Scholar] [CrossRef]
- Diering, N.L.; Ulrich, A.; Scapini, T.; Müller, C.; Gasparetto, I.G.; Júnior, F.W.R.; Treichel, H.; Mossi, A.J. Microbial Natural Bioactive Formulations in Citrus Development. Biotechnol. Rep. 2022, 34, e00718. [Google Scholar] [CrossRef]
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros-Berruezo, G.; Viuda-Martos, M. Valorization of Citrus Co-Products: Recovery of Bioactive Compounds and Application in Meat and Meat Products. Plants 2021, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus By-Products: Valuable Source of Bioactive Compounds for Food Applications. Antioxidants 2022, 12, 38. [Google Scholar] [CrossRef]
- Casas Cardoso, L.; Cejudo Bastante, C.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Application of Citrus By-Products in the Production of Active Food Packaging. Antioxidants 2022, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Petracek, P.D. Peel Morphology and Fruit Blemishes. In Citrus Flowering & Fruiting Short Course; Citrus Research and Education Center: Lake Alfred, FL, USA, 1997. [Google Scholar]
- Inthachat, W.; Temviriyanukul, P.; On-Nom, N.; Kanoongon, P.; Thangsiri, S.; Chupeerach, C.; Suttisansanee, U. Optimization of Phytochemical-Rich Citrus Maxima Albedo Extract Using Response Surface Methodology. Molecules 2023, 28, 4121. [Google Scholar] [CrossRef]
- Nomura, R.; Ohata, J.; Otsugu, M.; Okawa, R.; Naka, S.; Matsumoto-Nakano, M.; Nakano, K. Inhibitory Effects of Flavedo, Albedo, Fruits, and Leaves of Citrus unshiu Extracts on Streptococcus mutans. Arch. Oral. Biol. 2021, 124, 105056. [Google Scholar] [CrossRef]
- Ahmed, M.; Saeid, A. Citrus Fruits: Nutritive Value and Value-Added Products. In Citrus—Research, Development and Biotechnology; Sarwar Khan, M., Ahmad Khan, I., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83968-723-5. [Google Scholar]
- Wang, J.; Hao, H.; Liu, R.; Ma, Q.; Xu, J.; Chen, F.; Cheng, Y.; Deng, X. Comparative Analysis of Surface Wax in Mature Fruits between Satsuma Mandarin (Citrus unshiu) and ‘Newhall’ Navel Orange (Citrus sinensis) from the Perspective of Crystal Morphology, Chemical Composition and Key Gene Expression. Food Chem. 2014, 153, 177–185. [Google Scholar] [CrossRef]
- Romero, P.; Lafuente, M.T. Abscisic Acid Deficiency Alters Epicuticular Wax Metabolism and Morphology That Leads to Increased Cuticle Permeability during Sweet Orange (Citrus sinensis) Fruit Ripening. Front. Plant Sci. 2020, 11, 594184. [Google Scholar] [CrossRef] [PubMed]
- Pashova, S. Application of Plant Waxes in Edible Coatings. Coatings 2023, 13, 911. [Google Scholar] [CrossRef]
- García-Coronado, H.; Tafolla-Arellano, J.C.; Hernández-Oñate, M.Á.; Burgara-Estrella, A.J.; Robles-Parra, J.M.; Tiznado-Hernández, M.E. Molecular Biology, Composition and Physiological Functions of Cuticle Lipids in Fleshy Fruits. Plants 2022, 11, 1133. [Google Scholar] [CrossRef] [PubMed]
- Lado, J.; Alós, E.; Manzi, M.; Cronje, P.J.R.; Gómez-Cadenas, A.; Rodrigo, M.J.; Zacarías, L. Light Regulation of Carotenoid Biosynthesis in the Peel of Mandarin and Sweet Orange Fruits. Front. Plant Sci. 2019, 10, 1288. [Google Scholar] [CrossRef] [Green Version]
- Kato, M. Mechanism of Carotenoid Accumulation in Citrus Fruit. J. Jpn. Soc. Hort. Sci. 2012, 81, 219–233. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Lado, J.; Zacarías, L. Biochemical Bases and Molecular Regulation of Pigmentation in the Peel of Citrus Fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Agarwal, P.; Sebghatollahi, Z.; Kamal, M.; Dhyani, A.; Shrivastava, A.; Singh, K.K.; Sinha, M.; Mahato, N.; Mishra, A.K.; Baek, K.-H. Citrus Essential Oils in Aromatherapy: Therapeutic Effects and Mechanisms. Antioxidants 2022, 11, 2374. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Ambrosio, C.M.S.; Diaz-Arenas, G.L.; Agudelo, L.P.A.; Stashenko, E.; Contreras-Castillo, C.J.; da Gloria, E.M. Chemical Composition and Antibacterial and Antioxidant Activity of a Citrus Essential Oil and Its Fractions. Molecules 2021, 26, 2888. [Google Scholar] [CrossRef]
- Mehl, F.; Marti, G.; Boccard, J.; Debrus, B.; Merle, P.; Delort, E.; Baroux, L.; Raymo, V.; Velazco, M.I.; Sommer, H.; et al. Differentiation of Lemon Essential Oil Based on Volatile and Non-Volatile Fractions with Various Analytical Techniques: A Metabolomic Approach. Food Chem. 2014, 143, 325–335. [Google Scholar] [CrossRef]
- Van den Bruinhorst, A.; Kouris, P.; Timmer, J.; De Croon, M.; Kroon, M. Exploring Orange Peel Treatment with Deep Eutectic Solvents and Diluted Organic Acids. Nat. Prod. Chem. Res. 2016, 4, 1–5. [Google Scholar] [CrossRef]
- Singhal, S.; Swami Hulle, N.R. Citrus Pectins: Structural Properties, Extraction Methods, Modifications and Applications in Food Systems—A Review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Sharma, P.; Dadwal, V.; Rahmatkar, S.N.; Gupta, M.; Singh, D. Flavonoid Composition and Antioxidant Efficacy of Citrus Peels: An Integrated in Vitro and in Silico Approach toward Potential Neuroprotective Agents. J. Sci. Ind. Res. 2022, 81, 445–454. [Google Scholar]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Drincovich, M.F.; Voll, L.M.; Maurino, V.G. Editorial: On the Diversity of Roles of Organic Acids. Front. Plant Sci. 2016, 7, 1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bampidis, V.A.; Robinson, P.H. Citrus By-Products as Ruminant Feeds: A Review. Anim. Feed. Sci. Technol. 2006, 128, 175–217. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef] [PubMed]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2021, 12, 29. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Costanzo, G.; Iesce, M.R.; Naviglio, D.; Ciaravolo, M.; Vitale, E.; Arena, C. Comparative Studies on Different Citrus Cultivars: A Revaluation of Waste Mandarin Components. Antioxidants 2020, 9, 517. [Google Scholar] [CrossRef]
- Bocco, A.; Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidant Activity and Phenolic Composition of Citrus Peel and Seed Extracts. J. Agric. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and Extraction Optimization of Citrus Seeds for Food and Functional Food Applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef]
- Ahmed, R.H.A.; Mariod, A.A. Citrus Seeds Fixed Oil, Composition and Its Biological Activities. In Multiple Biological Activities of Unconventional Seed Oils; Elsevier: Amsterdam, The Netherlands, 2022; pp. 229–235. ISBN 978-0-12-824135-6. [Google Scholar]
- Sharma, P.; Vishvakarma, R.; Gautam, K.; Vimal, A.; Kumar Gaur, V.; Farooqui, A.; Varjani, S.; Younis, K. Valorization of Citrus Peel Waste for the Sustainable Production of Value-Added Products. Bioresour. Technol. 2022, 351, 127064. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent Progress in the Utilization of Industrial Waste and By-products of Citrus Fruits: A Review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Ramos, M.; Hamzaoui, M.; Kohnen, S.; Jiménez, A.; Garrigós, M.C. Optimisation of Sequential Microwave-Assisted Extraction of Essential Oil and Pigment from Lemon Peels Waste. Foods 2020, 9, 1493. [Google Scholar] [CrossRef]
- Tunç, M.T.; Odabaş, H.İ. Single-Step Recovery of Pectin and Essential Oil from Lemon Waste by Ohmic Heating Assisted Extraction/Hydrodistillation: A Multi-Response Optimization Study. Innov. Food Sci. Emerg. Technol. 2021, 74, 102850. [Google Scholar] [CrossRef]
- Sandhu, H.K.; Sinha, P.; Emanuel, N.; Kumar, N.; Sami, R.; Khojah, E.; Al-Mushhin, A.A.M. Effect of Ultrasound-Assisted Pretreatment on Extraction Efficiency of Essential Oil and Bioactive Compounds from Citrus Waste By-Products. Separations 2021, 8, 244. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Tiruta-Barna, L.; Ahmadi, A.; Hamelin, L.; Thomsen, M. A Step Closer to Circular Bioeconomy for Citrus Peel Waste: A Review of Yields and Technologies for Sustainable Management of Essential Oils. J. Environ. Manag. 2021, 280, 111832. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Recent Trends on the Valorization Strategies for the Management of Citrus By-Products. Food Rev. Int. 2021, 37, 91–120. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Kim, H.-J.; Ko, M.-J.; Chung, M.-S. Recovery of Hesperidin and Narirutin from Waste Citrus unshiu Peel Using Subcritical Water Extraction Aided by Pulsed Electric Field Treatment. Food Sci. Biotechnol. 2021, 30, 217–226. [Google Scholar] [CrossRef]
- Idamokoro, E.M.; Hosu, Y.S. Out-Look on Worldwide Trends of Related Studies on Citrus Waste as Feed for Livestock Production: A Scientometric Analysis. Front. Res. Metr. Anal. 2022, 7, 869974. [Google Scholar] [CrossRef]
- Mamma, D.; Christakopoulos, P. Biotransformation of Citrus By-Products into Value Added Products. Waste Biomass Valor. 2014, 5, 529–549. [Google Scholar] [CrossRef]
- Yun, D.; Liu, J. Recent Advances on the Development of Food Packaging Films Based on Citrus Processing Wastes: A Review. J. Agric. Food Res. 2022, 9, 100316. [Google Scholar] [CrossRef]
- Meydanju, N.; Pirsa, S.; Farzi, J. Biodegradable Film Based on Lemon Peel Powder Containing Xanthan Gum and TiO2–Ag Nanoparticles: Investigation of Physicochemical and Antibacterial Properties. Polym. Test. 2022, 106, 107445. [Google Scholar] [CrossRef]
- Spigno, G.; Donsì, F.; Amendola, D.; Sessa, M.; Ferrari, G.; De Faveri, D.M. Nanoencapsulation Systems to Improve Solubility and Antioxidant Efficiency of a Grape Marc Extract into Hazelnut Paste. J. Food Eng. 2013, 114, 207–214. [Google Scholar] [CrossRef]
- Jones, D.; Caballero, S.; Davidov-Pardo, G. Bioavailability of Nanotechnology-Based Bioactives and Nutraceuticals. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 88, pp. 235–273. ISBN 978-0-12-816073-2. [Google Scholar]
- Todorović, A.; Šturm, L.; Salević-Jelić, A.; Lević, S.; Osojnik Črnivec, I.G.; Prislan, I.; Skrt, M.; Bjeković, A.; Poklar Ulrih, N.; Nedović, V. Encapsulation of Bilberry Extract with Maltodextrin and Gum Arabic by Freeze-Drying: Formulation, Characterisation, and Storage Stability. Processes 2022, 10, 1991. [Google Scholar] [CrossRef]
- Papoutsis, K.; Golding, J.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.; Scarlett, C.; Bowyer, M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Mahato, N.; Sharma, K.; Sinha, M.; Dhyani, A.; Pathak, B.; Jang, H.; Park, S.; Pashikanti, S.; Cho, S. Biotransformation of Citrus Waste-I: Production of Biofuel and Valuable Compounds by Fermentation. Processes 2021, 9, 220. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Nanjundaswamy, A.; Bansal, S.; Singh, S.; Kaur, S.; Babbar, N. Enhanced Ethanol Production from Kinnow Mandarin (Citrus reticulata) Waste via a Statistically Optimized Simultaneous Saccharification and Fermentation Process. Bioresour. Technol. 2011, 102, 1593–1601. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, B.; Liu, R.; Han, Y.; Fang, X.; Mu, H.; Farag, M.A.; Simal-Gandara, J.; Prieto, M.A.; Chen, H.; et al. Structures and Functions of Cuticular Wax in Postharvest Fruit and Its Regulation: A Comprehensive Review with Future Perspectives. Engineering 2023, 23, 118–129. [Google Scholar] [CrossRef]
- Zhu, M.; Ji, J.; Wang, M.; Zhao, M.; Yin, Y.; Kong, J.; Liu, M.; Li, Y.-F. Cuticular Wax of Mandarin Fruit Promotes Conidial Germination and Germ Tube Elongation, and Impairs Colony Expansion of the Green Mold Pathogen, Penicillium Digitatum. Postharvest Biol. Technol. 2020, 169, 111296. [Google Scholar] [CrossRef]
- Multari, S.; Licciardello, C.; Caruso, M.; Martens, S. Monitoring the Changes in Phenolic Compounds and Carotenoids Occurring during Fruit Development in the Tissues of Four Citrus Fruits. Food Res. Int. 2020, 134, 109228. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Zografaki, M.-E.; Marka, S.; Myrtsi, E.D.; Giamouri, E.; Christodoulou, C.; Evergetis, E.; Iliopoulos, V.; Koulocheri, S.D.; Moschopoulou, G.; et al. Effect of a Carotenoid Extract from Citrus reticulata By-Products on the Immune-Oxidative Status of Broilers. Antioxidants 2022, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Barman, K.; Chowdhury, D.; Baruah, P.K. Development of Β-carotene Loaded Nanoemulsion Using the Industrial Waste of Orange (Citrus reticulate) Peel to Improve in Vitro Bioaccessibility of Carotenoids and Use as Natural Food Colorant. J. Food Process Preserv. 2020, 44, e14429. [Google Scholar] [CrossRef]
- Sedeek, M.S.; Al-Mahallawi, A.M.; Hussien, R.A.A.; Ali, A.M.A.; Naguib, I.A.; Mansour, M.K. Hexosomal Dispersion: A Nano-Based Approach to Boost the Antifungal Potential of Citrus Essential Oils against Plant Fungal Pathogens. Molecules 2021, 26, 6284. [Google Scholar] [CrossRef]
- Abdel-Bar, H.M.; Khater, S.E.; Ghorab, D.M.; Al-mahallawi, A.M. Hexosomes as Efficient Platforms for Possible Fluoxetine Hydrochloride Repurposing with Improved Cytotoxicity against HepG2 Cells. ACS Omega 2020, 5, 26697–26709. [Google Scholar] [CrossRef]
- Feng, K.; Zhu, X.; Liu, G.; Kan, Q.; Chen, T.; Chen, Y.; Cao, Y. Dietary Citrus Peel Essential Oil Ameliorates Hypercholesterolemia and Hepatic Steatosis by Modulating Lipid and Cholesterol Homeostasis. Food Funct. 2020, 11, 7217–7230. [Google Scholar] [CrossRef]
- Asikin, Y.; Shimizu, K.; Iwasaki, H.; Oku, H.; Wada, K. Stress Amelioration and Anti-Inflammatory Potential of Shiikuwasha (Citrus depressa Hayata) Essential Oil, Limonene, and γ-Terpinene. J. Food Drug Anal. 2022, 30, 454–465. [Google Scholar] [CrossRef]
- Torimiro, N.; Adegun, B.R.; Abioye, O.E.; Omole, R.K. Antibacterial Activity of Essential Oil from Citrus aurantifolia (Christm.) Swingle Peels against Multidrug-Resistant Bacterial Isolates. AiM 2020, 10, 214–223. [Google Scholar] [CrossRef]
- Sreepian, A.; Popruk, S.; Nutalai, D.; Phutthanu, C.; Sreepian, P.M. Antibacterial Activities and Synergistic Interaction of Citrus Essential Oils and Limonene with Gentamicin against Clinically Isolated Methicillin-Resistant Staphylococcus aureus. Sci. World J. 2022, 2022, 8418287. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Z.; Li, G.; Fu, F.; Liang, Z.; Zhu, H.; Shan, Y. Antimicrobial and Antibiofilm Efficacy and Mechanism of Essential Oil from Citrus Changshan-Huyou Y. B. Chang against Listeria monocytogenes. Food Control 2019, 105, 256–264. [Google Scholar] [CrossRef]
- Restuccia, C.; Oliveri Conti, G.; Zuccarello, P.; Parafati, L.; Cristaldi, A.; Ferrante, M. Efficacy of Different Citrus Essential Oils to Inhibit the Growth and B1 Aflatoxin Biosynthesis of Aspergillus flavus. Environ. Sci. Pollut. Res. 2019, 26, 31263–31272. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Castagna, F.; Piras, C.; Palma, E.; Cringoli, G.; Musolino, V.; Lupia, C.; Perri, M.R.; Statti, G.; Britti, D.; et al. In Vitro Evaluation of Acute Toxicity of Five Citrus Spp. Essential Oils towards the Parasitic Mite Varroa destructor. Pathogens 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.O.; Okunowo, W.O.; Osuntoki, A.A.; Olabode, T.B.; Ayo-folorunso, F. Insecticidal and Biochemical Activity of Essential Oil from Citrus sinensis Peel and Constituents on Callosobrunchus maculatus and Sitophilus zeamais. Pestic. Biochem. Physiol. 2020, 168, 104643. [Google Scholar] [CrossRef]
- Visakh, N.U.; Pathrose, B.; Narayanankutty, A.; Alfarhan, A.; Ramesh, V. Utilization of Pomelo (Citrus maxima) Peel Waste into Bioactive Essential Oils: Chemical Composition and Insecticidal Properties. Insects 2022, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Fadilah, N.Q.; Jittmittraphap, A.; Leaungwutiwong, P.; Pripdeevech, P.; Dhanushka, D.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Virucidal Activity of Essential Oils From Citrus x aurantium L. Against Influenza A Virus H1N1: Limonene as a Potential Household Disinfectant Against Virus. Nat. Product. Commun. 2022, 17, 1934578X2110727. [Google Scholar] [CrossRef]
- Asaad, G.; Abdelhameed, M.; Elraey, M.; Roshdy, W.; Elgamal, A.; Moamen, Y. Citrus clementine Peels Essential Oil Exhibited Anti-SARS-CoV-2 and Its Modulatory Effect against Cytokine Storm: Evidence from in Vitro and in Silico Studies. Egypt. J. Chem. 2022, 10, 419–427. [Google Scholar] [CrossRef]
- Uçar, Y. Antioxidant Effect of Nanoemulsions Based on Citrus Peel Essential Oils: Prevention of Lipid Oxidation in Trout. Eur. J. Lipid Sci. Technol. 2020, 122, 1900405. [Google Scholar] [CrossRef]
- Kosker, A.R. The Effects of Nanoemulsions Based on Citrus Essential Oils on the Formation of Biogenic Amines in Trout Fillets Stored at 4 ± 2 °C. J. Food Saf. 2020, 40, e12762. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Chuang, C.-H.; Chen, H.-C.; Yang, K.-M. Lime (Citrus aurantifolia (Christm.) Swingle) Essential Oils: Volatile Compounds, Antioxidant Capacity, and Hypolipidemic Effect. Foods 2019, 8, 398. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, M.L.; Ionta, M.; Ferreira, G.Á.; Campidelli, M.L.L.; Nelson, D.L.; Ferreira, V.R.F.; Rezende, D.A.D.C.S.; Cardoso, M.D.G. Biological Activities of the Essential Oil from the Moro Orange Peel (Citrus sinensis (L.) Osbeck). Flavour. Fragr. J. 2020, 35, 294–301. [Google Scholar] [CrossRef]
- Viana, M.D.M.; Silva Neto, G.J.D.; Lima, A.A.D.; Leite, A.B.; Souza, I.T.; Santana, A.E.G.; Campesatto, E.A.; Moreira, M.S.A. Citrus limon (L.) Burm f. Essential Oil Has Anxiolytic and Sedative Properties by Modulating GABAA-Receptors. Braz. Arch. Biol. Technol. 2020, 63, e20200206. [Google Scholar] [CrossRef]
- Kwangjai, J.; Cheaha, D.; Manor, R.; Sa-ih, N.; Samerphob, N.; Issuriya, A.; Wattanapiromsakul, C.; Kumarnsit, E. Modification of Brain Waves and Sleep Parameters by Citrus reticulata Blanco. Cv. Sai-Nam-Phueng Essential Oil. Biomed. J. 2021, 44, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Zhou, J.; Zhao, L.; Wang, C.; Wu, W.; Zhang, L.; Ji, B.; Zhang, N.; Zhou, F. Preventive Effect of Different Citrus Essential Oils on Primary Dysmenorrhea: In Vivo and in Vitro Study. Food Biosci. 2021, 42, 101135. [Google Scholar] [CrossRef]
- Ayuningtyas, N.F.; Hendarti, H.T.; Soebadi, B.; Condro Surboyo, M.D.; Hadi, P.; Ganesha, R.; Ernawati, D.S.; Marsetyo, R.I. Expression of VEGF and CD-31 in Traumatic Ulcer of Diabetic Wistar Rats after Application of Citrus limon Peel Essential Oil. J. Oral. Biol. Craniofacial Res. 2023, 13, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Disson, O.; Lecuit, M. Targeting of the Central Nervous System by Listeria monocytogenes. Virulence 2012, 3, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Minzanova, S.; Mironov, V.; Arkhipova, D.; Khabibullina, A.; Mironova, L.; Zakirova, Y.; Milyukov, V. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Míguez, B.; Gómez, B.; Gullón, P.; Gullón, B.; Alonso, J.L. Pectic Oligosaccharides and Other Emerging Prebiotics. In Probiotics and Prebiotics in Human Nutrition and Health; Rao, V., Rao, L.G., Eds.; InTech: Houston, TX, USA, 2016; ISBN 978-953-51-2475-7. [Google Scholar]
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and Prebiotic Potential of Pectin Oligosaccharides Obtained from Citrus Peel Pectin. Food Chem. 2018, 244, 232–237. [Google Scholar] [CrossRef]
- Lee, T.; Chang, Y.H. Structural, Physicochemical, and in-Vitro Release Properties of Hydrogel Beads Produced by Oligochitosan and de-Esterified Pectin from Yuzu (Citrus junos) Peel as a Quercetin Delivery System for Colon Target. Food Hydrocoll. 2020, 108, 106086. [Google Scholar] [CrossRef]
- Jacob, E.M.; Borah, A.; Jindal, A.; Pillai, S.C.; Yamamoto, Y.; Maekawa, T.; Kumar, D.N.S. Synthesis and Characterization of Citrus-Derived Pectin Nanoparticles Based on Their Degree of Esterification. J. Mater. Res. 2020, 35, 1514–1522. [Google Scholar] [CrossRef]
- Han, H.; Song, K.B. Antioxidant Activities of Mandarin (Citrus unshiu) Peel Pectin Films Containing Sage (Salvia officinalis) Leaf Extract. Int. J. Food Sci. Technol. 2020, 55, 3173–3181. [Google Scholar] [CrossRef]
- Al-Enazi, N.M.; Alsamhary, K.; Ameen, F. Evaluation of Citrus Pectin Capped Copper Sulfide Nanoparticles against Candidiasis Causing Candida Biofilms. Environ. Res. 2023, 225, 115599. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Li, S.; Orfila, C.; Shen, X.; Zhou, S.; Linhardt, R.J.; Ye, X.; Chen, S. Depolymerized RG-I-Enriched Pectin from Citrus Segment Membranes Modulates Gut Microbiota, Increases SCFA Production, and Promotes the Growth of Bifidobacterium spp., Lactobacillus spp. and Faecalibaculum spp. Food Funct. 2019, 10, 7828–7843. [Google Scholar] [CrossRef] [PubMed]
- Rajulapati, V.; Dhillon, A.; Goyal, A. Enzymatically Produced Pectic-Oligosaccharides from Fruit Waste of Citrus reticulata (Mandarin) Peels Display Cytotoxicity against Colon Cancer Cells. Bioresour. Technol. Rep. 2021, 15, 100740. [Google Scholar] [CrossRef]
- Ishisono, K.; Mano, T.; Yabe, T.; Kitaguchi, K. Dietary Fiber Pectin Ameliorates Experimental Colitis in a Neutral Sugar Side Chain-Dependent Manner. Front. Immunol. 2019, 10, 2979. [Google Scholar] [CrossRef] [Green Version]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, S.; Liu, F.; Zhang, P.; Muhammad, Z.; Pan, S. Role of the Gut Microbiota and Their Metabolites in Modulating the Cholesterol-Lowering Effects of Citrus Pectin Oligosaccharides in C57BL/6 Mice. J. Agric. Food Chem. 2019, 67, 11922–11930. [Google Scholar] [CrossRef]
- Flori, L.; Albanese, L.; Calderone, V.; Meneguzzo, F.; Pagliaro, M.; Ciriminna, R.; Zabini, F.; Testai, L. Cardioprotective Effects of Grapefruit IntegroPectin Extracted via Hydrodynamic Cavitation from By-Products of Citrus Fruits Industry: Role of Mitochondrial Potassium Channels. Foods 2022, 11, 2799. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Torres-Giner, S.; Quiles-Carrillo, L.; Gomez-Caturla, J.; Garcia-Garcia, D.; Balart, R. On the Use of Phenolic Compounds Present in Citrus Fruits and Grapes as Natural Antioxidants for Thermo-Compressed Bio-Based High-Density Polyethylene Films. Antioxidants 2020, 10, 14. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-919-5. [Google Scholar]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus Flavonoids: Molecular Structure, Biological Activity and Nutritional Properties: A Review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
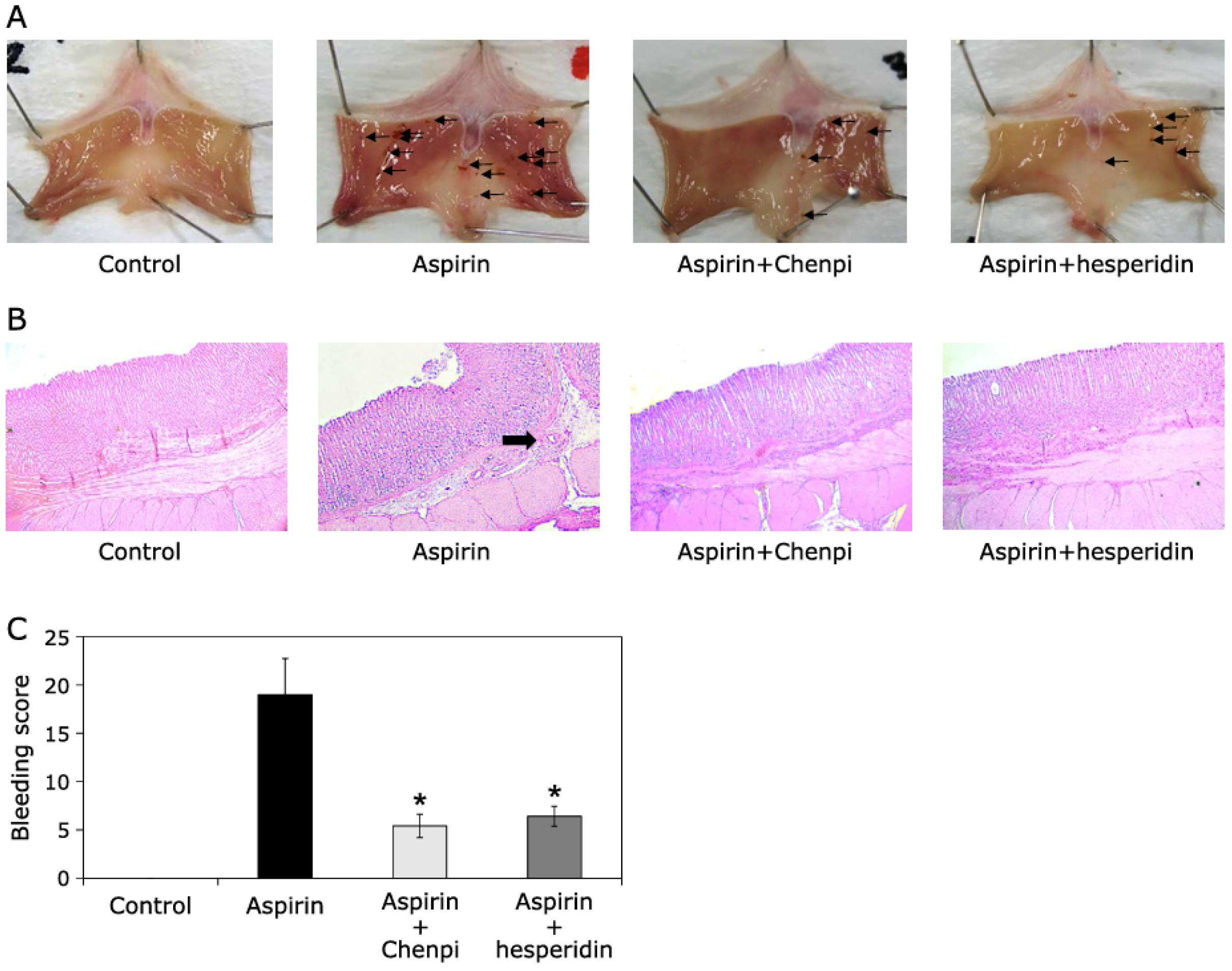
- Shimamura, Y.; Sei, S.; Nomura, S.; Masuda, S. Protective Effects of Dried Mature Citrus unshiu Peel (Chenpi) and Hesperidin on Aspirin-Induced Oxidative Damage. J. Clin. Biochem. Nutr. 2021, 68, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Cryer, B.; Mahaffey, K. Gastrointestinal Ulcers, Role of Aspirin, and Clinical Outcomes: Pathobiology, Diagnosis, and Treatment. JMDH 2014, 7, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Sharaf, M.; Arif, M.; Hamouda, H.I.; Khan, S.; Abdalla, M.; Shabana, S.; Rozan, H.E.; Khan, T.U.; Chi, Z.; Liu, C. Preparation, Urease Inhibition Mechanisms, and Anti-Helicobacter pylori Activities of Hesperetin-7-Rhamnoglucoside. Curr. Res. Microb. Sci. 2022, 3, 100103. [Google Scholar] [CrossRef] [PubMed]
- Chandra Babu, T.M.; Rajesh, S.S.; Bhaskar, B.V.; Devi, S.; Rammohan, A.; Sivaraman, T.; Rajendra, W. Molecular Docking, Molecular Dynamics Simulation, Biological Evaluation and 2D QSAR Analysis of Flavonoids from Syzygium alternifolium as Potent Anti-Helicobacter pylori Agents. RSC Adv. 2017, 7, 18277–18292. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, Y.; Zhang, H.; Chen, J.; Cao, J.; Chen, Q.; Li, X.; Sun, C. Polymethoxyflavones from Citrus Inhibited Gastric Cancer Cell Proliferation through Inducing Apoptosis by Upregulating RARβ, Both in Vitro and in Vivo. Food Chem. Toxicol. 2020, 146, 111811. [Google Scholar] [CrossRef]
- Tajaldini, M.; Samadi, F.; Khosravi, A.; Ghasemnejad, A.; Asadi, J. Protective and Anticancer Effects of Orange Peel Extract and Naringin in Doxorubicin Treated Esophageal Cancer Stem Cell Xenograft Tumor Mouse Model. Biomed. Pharmacother. 2020, 121, 109594. [Google Scholar] [CrossRef]
- Deenonpoe, R.; Prayong, P.; Thippamom, N.; Meephansan, J.; Na-Bangchang, K. Anti-Inflammatory Effect of Naringin and Sericin Combination on Human Peripheral Blood Mononuclear Cells (HPBMCs) from Patient with Psoriasis. BMC Complement. Altern. Med. 2019, 19, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ji, S.; Zang, W.; Wang, N.; Cao, J.; Li, X.; Sun, C. Identification of Phenolic Compounds from a Unique Citrus Species, Finger Lime (Citrus australasica) and Their Inhibition of LPS-Induced NO-Releasing in BV-2 Cell Line. Food Chem. Toxicol. 2019, 129, 54–63. [Google Scholar] [CrossRef]
- Smeriglio, A.; Cornara, L.; Denaro, M.; Barreca, D.; Burlando, B.; Xiao, J.; Trombetta, D. Antioxidant and Cytoprotective Activities of an Ancient Mediterranean Citrus (Citrus lumia Risso) Albedo Extract: Microscopic Observations and Polyphenol Characterization. Food Chem. 2019, 279, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Kusumawati, I.G.A.W.; Putra, I.M.W.A.; Yogeswara, I.B.A. In Vitro ACE Inhibitory Activity and Bioactive Compounds of Aqueous Extract of Citrus amblycarpa. Trad. Med. J. 2021, 26, 117. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, W.; Ji, S.; Cao, J.; Sun, C. Three Polymethoxyflavones Purified from Ougan (Citrus reticulata Cv. Suavissima) Inhibited LPS-Induced NO Elevation in the Neuroglia BV-2 Cell Line via the JAK2/STAT3 Pathway. Nutrients 2019, 11, 791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Saman, M.A.; Abdella, A.; Mazrou, K.E.; Tayel, A.A.; Irmak, S. Antimicrobial and Antioxidant Activities of Different Extracts of the Peel of Kumquat (Citrus japonica Thunb). Food Meas. 2019, 13, 3221–3229. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Fahim, H.I.; Ahmed, H.Y.; Al-Muzafar, H.M.; Ahmed, R.R.; Amin, K.A.; El-Nahass, E.-S.; Abdelazeem, W.H. The Preventive Effects and the Mechanisms of Action of Navel Orange Peel Hydroethanolic Extract, Naringin, and Naringenin in N-Acetyl-p-Aminophenol-Induced Liver Injury in Wistar Rats. Oxidative Med. Cell. Longev. 2019, 2019, 2745352. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.; Suh, J.H.; Wang, Y. Effects of Heat Treatment of Mandarin Peel on Flavonoid Profiles and Lipid Accumulation in 3T3-L1 Adipocytes. J. Food Drug Anal. 2019, 27, 729–735. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Kim, H.H.; Preethi, V.; Moniruzzaman, M.; Lee, K.H.; Kalaiselvi, S.; Kim, G.S.; Min, T. Assessment of Anti-Inflammatory and Antioxidant Effects of Citrus unshiu Peel (CUP) Flavonoids on LPS-Stimulated RAW 264.7 Cells. Plants 2021, 10, 2209. [Google Scholar] [CrossRef] [PubMed]
- Fathy, H.M.; Abd El-Maksoud, A.A.; Cheng, W.; Elshaghabee, F.M.F. Value-Added Utilization of Citrus Peels in Improving Functional Properties and Probiotic Viability of Acidophilus-bifidus-thermophilus (ABT)-Type Synbiotic Yoghurt during Cold Storage. Foods 2022, 11, 2677. [Google Scholar] [CrossRef]
- Babu, V.; Binwal, M.; Ranjana; Kumari, R.; Sen, S.; Kumar, A.; Mugale, M.N.; Shanker, K.; Kumar, N.; Bawankule, D.U. Hesperidin-rich Ethanol Extract from Waste Peels of Citrus limetta Mitigates Rheumatoid Arthritis and Related Complications. Phytother. Res. 2021, 35, 3325–3336. [Google Scholar] [CrossRef]
- Ben Abdallah, M.; Chadni, M.; M’hiri, N.; Brunissen, F.; Rokbeni, N.; Allaf, K.; Besombes, C.; Ioannou, I.; Boudhrioua, N. Intensifying Effect of Instant Controlled Pressure Drop (DIC) Pre-Treatment on Hesperidin Recovery from Orange Byproducts: In Vitro Antioxidant and Antidiabetic Activities of the Extracts. Molecules 2023, 28, 1858. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Goda, N.; Tenno, T.; Kotake, A.; Inotsume, Y.; Amaya, M.; Hiroaki, H. Pharmacologic Comparison of High-Dose Hesperetin and Quercetin on MDCK II Cell Viability, Tight Junction Integrity, and Cell Shape. Antioxidants 2023, 12, 952. [Google Scholar] [CrossRef]
- Lu, Q.; Kishi, H.; Zhang, Y.; Morita, T.; Kobayashi, S. Hesperetin Inhibits Sphingosylphosphorylcholine-Induced Vascular Smooth Muscle Contraction by Regulating the Fyn/Rho-Kinase Pathway. J. Cardiovasc. Pharmacol. 2022, 79, 456–466. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, M.; Liu, Z.; Liu, M.; Zhang, S.; Zhang, Y. Hesperidin Inhibits Lung Cancer In Vitro and In Vivo Through PinX1. Front. Pharmacol. 2022, 13, 918665. [Google Scholar] [CrossRef]
- Sánchez-Recillas, A.; González-Rivero, N.A.; Barrera-Canto, V.; Ibarra-Barajas, M.; Estrada-Soto, S.; Ortiz-Andrade, R. Vasorelaxant and Antihypertensive Activities of Citroflavonoids (Hesperidin/Naringenin Mixture): Potential Prophylactic of Cardiovascular Endothelial Dysfunction. Pharmacogn. Mag. 2019, 15, 84. [Google Scholar]
- Jeong, S.A.; Yang, C.; Song, J.; Song, G.; Jeong, W.; Lim, W. Hesperidin Suppresses the Proliferation of Prostate Cancer Cells by Inducing Oxidative Stress and Disrupting Ca2+ Homeostasis. Antioxidants 2022, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, D.-H.; Kim, J.H. Combined Administration of Naringenin and Hesperetin with Optimal Ratio Maximizes the Anti-Cancer Effect in Human Pancreatic Cancer via down Regulation of FAK and P38 Signaling Pathway. Phytomedicine 2019, 58, 152762. [Google Scholar] [CrossRef] [PubMed]
- Ammar, N.M.; Hassan, H.A.; Abdallah, H.M.I.; Afifi, S.M.; Elgamal, A.M.; Farrag, A.R.H.; El-Gendy, A.E.-N.G.; Farag, M.A.; Elshamy, A.I. Protective Effects of Naringenin from Citrus sinensis (Var. Valencia) Peels against CCl4-Induced Hepatic and Renal Injuries in Rats Assessed by Metabolomics, Histological and Biochemical Analyses. Nutrients 2022, 14, 841. [Google Scholar] [CrossRef]
- Shi, X.; Luo, X.; Chen, T.; Guo, W.; Liang, C.; Tang, S.; Mo, J. Naringenin Inhibits Migration, Invasion, Induces Apoptosis in Human Lung Cancer Cells and Arrests Tumour Progression in Vitro. J. Cell. Mol. Med. 2021, 25, 2563–2571. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Yang, C.; He, Y.; Arai, T.; Huang, Q.; Liu, X.; Miao, L. Citrus Naringenin Increases Neuron Survival in Optic Nerve Crush Injury Model by Inhibiting JNK-JUN Pathway. IJMS 2021, 23, 385. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Woo, J.-K.; Heo, W.; Huang, W.-Y.; Kim, Y.; Chung, S.; Lee, G.-H.; Park, J.-W.; Han, B.-K.; Shin, E.-C.; et al. Citrus junos Tanaka Peel Extract and Its Bioactive Naringin Reduce Fine Dust-Induced Respiratory Injury Markers in BALB/c Male Mice. Nutrients 2022, 14, 1101. [Google Scholar] [CrossRef]
- Singh, S.; Maurya, A.K.; Meena, A.; Mishra, N.; Luqman, S. Narirutin. A Flavonoid Found in Citrus Fruits Modulates Cell Cycle Phases and Inhibits the Proliferation of Hormone-Refractory Prostate Cancer Cells by Targeting Hyaluronidase. Food Chem. Toxicol. 2023, 174, 113638. [Google Scholar] [CrossRef]
- Patel, P.; Barve, K.; Bhatt, L.K. Narirutin-Rich Fraction from Grape Fruit Peel Protects against Transient Cerebral Ischemia Reperfusion Injury in Rats. Nutr. Neurosci. 2022, 25, 920–930. [Google Scholar] [CrossRef]
- Yousof Ali, M.; Zaib, S.; Mizanur Rahman, M.; Jannat, S.; Iqbal, J.; Kyu Park, S.; Seog Chang, M. Poncirin, an Orally Active Flavonoid Exerts Antidiabetic Complications and Improves Glucose Uptake Activating PI3K/Akt Signaling Pathway in Insulin Resistant C2C12 Cells with Anti-Glycation Capacities. Bioorganic Chem. 2020, 102, 104061. [Google Scholar] [CrossRef]
- Cao, X.; Guo, X.; Fang, X.; Ru, S.; Li, E. Effects of Poncirin, a Citrus Flavonoid and Its Aglycone, Isosakuranetin, on the Gut Microbial Diversity and Metabolomics in Mice. Molecules 2022, 27, 3641. [Google Scholar] [CrossRef] [PubMed]
- Deepika, M.S.; Thangam, R.; Vijayakumar, T.S.; Sasirekha, R.; Vimala, R.T.V.; Sivasubramanian, S.; Arun, S.; Babu, M.D.; Thirumurugan, R. Antibacterial Synergy between Rutin and Florfenicol Enhances Therapeutic Spectrum against Drug Resistant Aeromonas Hydrophila. Microb. Pathog. 2019, 135, 103612. [Google Scholar] [CrossRef]
- Ahmad, T.; Javed, A.; Khan, T.; Althobaiti, Y.S.; Ullah, A.; Almutairi, F.M.; Shah, A.J. Investigation into the Antihypertensive Effects of Diosmetin and Its Underlying Vascular Mechanisms Using Rat Model. Pharmaceuticals 2022, 15, 951. [Google Scholar] [CrossRef]
- Park, S.; Bong, S.-K.; Lee, J.W.; Park, N.-J.; Choi, Y.; Kim, S.M.; Yang, M.H.; Kim, Y.K.; Kim, S.-N. Diosmetin and Its Glycoside, Diosmin, Improve Atopic Dermatitis- Like Lesions in 2,4-Dinitrochlorobenzene-Induced Murine Models. Biomol. Ther. 2020, 28, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. Au 2023, 3, 58–75. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Niluxsshun, M.C.D.; Masilamani, K.; Mathiventhan, U. Green Synthesis of Silver Nanoparticles from the Extracts of Fruit Peel of Citrus tangerina, Citrus sinensis, and Citrus limon for Antibacterial Activities. Bioinorg. Chem. Appl. 2021, 2021, 6695734. [Google Scholar] [CrossRef] [PubMed]
- Osonga, F.J.; Akgul, A.; Yazgan, I.; Akgul, A.; Eshun, G.B.; Sakhaee, L.; Sadik, O.A. Size and Shape-Dependent Antimicrobial Activities of Silver and Gold Nanoparticles: A Model Study as Potential Fungicides. Molecules 2020, 25, 2682. [Google Scholar] [CrossRef]
- Alkhulaifi, M.M.; Alshehri, J.H.; Alwehaibi, M.A.; Awad, M.A.; Al-Enazi, N.M.; Aldosari, N.S.; Hatamleh, A.A.; Abdel- Raouf, N. Green Synthesis of Silver Nanoparticles Using Citrus limon Peels and Evaluation of Their Antibacterial and Cytotoxic Properties. Saudi J. Biol. Sci. 2020, 27, 3434–3441. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver Nanoparticles: Mechanism of Antimicrobial Action, Synthesis, Medical Applications, and Toxicity Effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Rizzello, L.; Pompa, P.P. Nanosilver-Based Antibacterial Drugs and Devices: Mechanisms, Methodological Drawbacks, and Guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Song, W.; Zhao, B.; Yang, B. Spectroscopic Studies of the Optical Properties of Carbon Dots: Recent Advances and Future Prospects. Mater. Chem. Front. 2020, 4, 472–488. [Google Scholar] [CrossRef]
- Šafranko, S.; Goman, D.; Stanković, A.; Medvidović-Kosanović, M.; Moslavac, T.; Jerković, I.; Jokić, S. An Overview of the Recent Developments in Carbon Quantum Dots—Promising Nanomaterials for Metal Ion Detection and (Bio)Molecule Sensing. Chemosensors 2021, 9, 138. [Google Scholar] [CrossRef]
- Gudimella, K.K.; Appidi, T.; Wu, H.-F.; Battula, V.; Jogdand, A.; Rengan, A.K.; Gedda, G. Sand Bath Assisted Green Synthesis of Carbon Dots from Citrus Fruit Peels for Free Radical Scavenging and Cell Imaging. Colloids Surf. B Biointerfaces 2021, 197, 111362. [Google Scholar] [CrossRef]
- Šafranko, S.; Stanković, A.; Hajra, S.; Kim, H.-J.; Strelec, I.; Dutour-Sikirić, M.; Weber, I.; Herak Bosnar, M.; Grbčić, P.; Kraljević Pavelić, S.; et al. Preparation of Multifunctional N-Doped Carbon Quantum Dots from Citrus clementina Peel: Investigating Targeted Pharmacological Activities and the Potential Application for Fe3+ Sensing. Pharmaceuticals 2021, 14, 857. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Zhao, X.; Wang, S.; Yang, Q.; Zhang, X. Crystal Structure, Solubility, and Pharmacokinetic Study on a Hesperetin Cocrystal with Piperine as Coformer. Pharmaceutics 2022, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.; Liu, L.H.B.; Dos Santos, R.V.; Costa, A.F.; Durán, N.; Tasic, L. New Sustainable Process for Hesperidin Isolation and Anti-Ageing Effects of Hesperidin Nanocrystals. Molecules 2020, 25, 4534. [Google Scholar] [CrossRef]
- Elghani, E.M.A.; Omar, F.A.; Emam, M.M.A.-A.; Al-Mahallawi, A.M.; Tadros, S.H.; Soliman, F.M.; ElSayed, A.M. Hesperidin Hexosomal Loaded Nanodispersion: Insights of Its Antimycobacterial, Cytotoxic and Anti-HCoV Effects. Nat. Product. Res. 2023, 37, 1719–1724. [Google Scholar] [CrossRef]
- Gao, L.; Mei, S.; Ma, H.; Chen, X. Ultrasound-Assisted Green Synthesis of Gold Nanoparticles Using Citrus Peel Extract and Their Enhanced Anti-Inflammatory Activity. Ultrason. Sonochemistry 2022, 83, 105940. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.P.; Sahoo, S.; Behera, A.; Sahoo, R.; Sahu, P.K. Memory Amelioration by Hesperidin Conjugated Gold Nanoparticles in Diabetes Induced Cognitive Impaired Rats. J. Drug Deliv. Sci. Technol. 2022, 69, 103145. [Google Scholar] [CrossRef]
- Tshireletso, P.; Ateba, C.N.; Fayemi, O.E. Spectroscopic and Antibacterial Properties of CuONPs from Orange, Lemon and Tangerine Peel Extracts: Potential for Combating Bacterial Resistance. Molecules 2021, 26, 586. [Google Scholar] [CrossRef] [PubMed]
- Julianti Wijayadi, L.; Rusliati Rusli, T. Encapsulated Lime Peel Essential Oil (Citrus hystrix) Into Chitosan Nanoparticle: New Entity to Enhanced Effectivity Against Propionilbacterium acne in Vitro. IOP Conf. Ser. Mater. Sci. Eng. 2020, 852, 012016. [Google Scholar] [CrossRef]
- Stanly, C.; Moubarak, M.; Fiume, I.; Turiák, L.; Pocsfalvi, G. Membrane Transporters in Citrus clementina Fruit Juice-Derived Nanovesicles. IJMS 2019, 20, 6205. [Google Scholar] [CrossRef] [Green Version]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef]

| Source | Formulation/Chemical Analyte | Bioactivity | Testing Subjects | References |
|---|---|---|---|---|
| C. aurantifolia peel | The essential oil isolated by hydrodistillation using a Clevenger apparatus | Antimicrobial activity | In vitro on multi-drug resistant bacterial isolates | [124] |
| C. reticulata Blanco, C. aurantifolia (Christm.) Swingle peel | Essential oils prepared by hydrodistillation | Antimicrobial activity | In vitro against S. aureus, including MSSA 1, MRSA 2, and MDR 3 phenotypes, and clinically isolated MRSA and MSSA | [125] |
| C. aurantium “Changshan-huyou” peel | Essential oils isolated by steam distillation | Antimicrobial activity | In vitro against L. monocytogenes | [126] |
| C. lemon, C. aurantifolia, C. maxima, and C. sinensis peels | Nano-hexosomal dispersions of citrus essential oils | Antifungal activity | In vitro against phytopathogenic fungi (R. solani, S. rolfsii, F. solani, F. oxysporum, F. semtectium, B. cinerea, and A. alternata) | [120] |
| C. bergamia Risso, C. aurantium L., C. sinensis (L.) Osbeck., C. deliciosa Ten., and C. limon (L.) Burm. f. peels | Cold-pressed essential oils | Antifungal activity | In vitro against aflatoxin B1 (AFB1) | [127] |
| C. bergamia, C. sinensis, C. limon, C. reticulata, and C. paradisi peel | Essential oils obtained by distillation | Antiparasitic activity | In vitro against Varroa destructor | [128] |
| C. sinensis peel | The essential oil isolated by hydrodistillation using Clevenger apparatus | Insecticidal activity | In vitro against Callosobrunchus maculatus and Sitophilus zeamais; studies on the inhibitory effects on acetylcholinesterase (AChE), Na+/K+-ATPase and glutathione-S- transferase (GST) activity | [129] |
| C. maxima peel | Essential oils prepared by hydrodistillation | Insecticidal (larvicidal) activity | In vitro against Culex tritaeniorhynchus and Aedes aegypti species of mosquitoes | [130] |
| C. aurantium peel | Essential oils prepared by solvent extraction | Antiviral activity | In vitro against influenza A virus H1N1 | [131] |
| C. clementine peel | Essential oil prepared by solvent extraction | Antiviral activity | In vitro on Vero-E6 cell lines; SARS-CoV-2 propagated in tested cell line | [132] |
| Orange, lemon, mandarin, and grapefruit peels 4 | Commercially purchased essential oils; prepared nanoemulsions | Antioxidant activity | Lipid and fatty acid methyl ester analysis; trout | [133] |
| C. reticulata peel | Essential oil prepared by continuous phase transition extraction | Prevention of hypercholesterolemia and hepatic steatosis | In vivo on male Sprague-Dawley rats on a high-fat diet | [122] |
| Orange, lemon, mandarin, and grapefruit peels 4 | Commercially purchased essential oils; prepared nanoemulsions | Suppressive effect on the biogenic amine formation | Trout fillets | [134] |
| C. aurantifolia (Christm.) Swingle peel | Essential oils prepared by steam distillation | Antioxidant capacity and hypolipidemic effect | DPPH•, ABTS•+ assay; lipid-induced hyperlipidemia in a rat model | [135] |
| C. sinensis (L.) Osbeck | The essential oil isolated by hydrodistillation using a Clevenger apparatus | Antifungal and antitumor activity | Antifungal: Aspergillus carbonarius and Aspergillus flavus/antitumor: Tumor cells (A549, lung adenocarcinoma; MCF-7, breast adenocarcinoma; and HT-144, melanoma) and normal cells (fibroblasts derived from normal human skin, CCD-1059Sk) | [136] |
| C. depressa Hayata pulp | The essential oil isolated by hydrodistillation using a Clevenger apparatus | Stress reduction activity and anti-inflammatory potential | In vivo on nine healthy female panelists (ECG and EEG monitoring); nitric oxide (NO) and interleukin-1β markers | [123] |
| C. limon (L.) Burm f. peel | Commercially purchased essential oil | Anxiolytic and sedative properties | In vivo on Swiss mice model | [137] |
| C. reticulata Blanco peels | The essential oil obtained by supercritical CO2 extraction | Mood disorder/relaxing agent | In vivo on adult male Wistar rats; inhalation | [138] |
| C. sinensis, C. bergamia, C. paradisi, C. grandis, C. reticulata Blanco, C. japonica, C. limon, C. aurantifolia, and immature C. aurantium L. peels | Essential oils prepared by hydrodistillation | Treatment of dysmenorrhea | In vivo on female Sprague Dawley rats/in vitro on the RL95-2 (human endometrial carcinoma) cells | [139] |
| C. limon peel | Essential oil prepared by steam distillation | The healing effect of traumatic ulcers induced by diabetes | In vivo on diabetic Wistar rats | [140] |
| Source | Formulation | Application/Bioactivity | Testing Subjects | References |
|---|---|---|---|---|
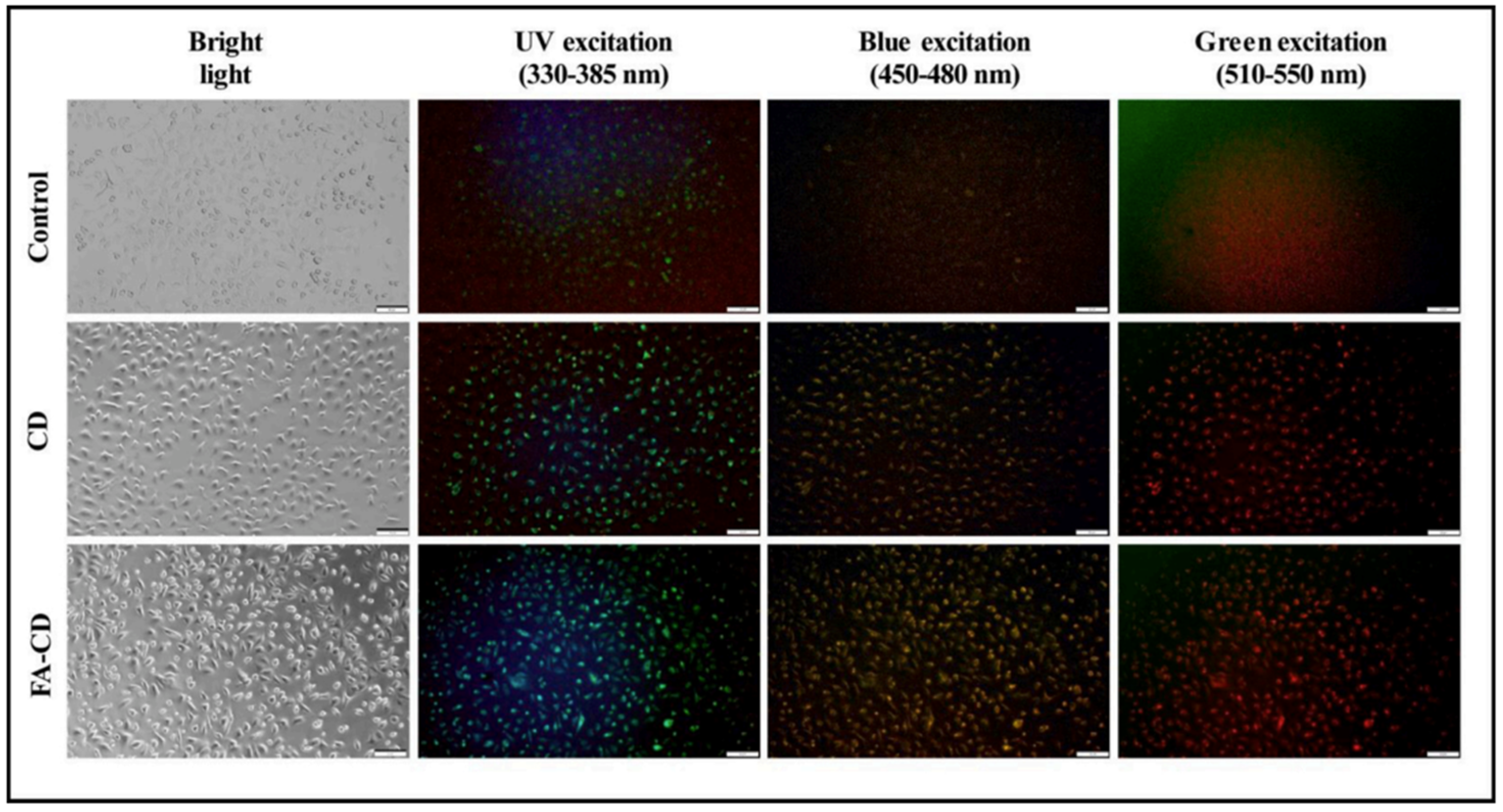
| Citrus peel 1 | Carbon quantum dots conjugated with folic acid | Bioimaging in MCF-7 cell lines, antiradical activity | In vitro on MCF-7 (human breast carcinoma), L929 (mice fibroblasts) | [203] |
| C. clementina peel | Amino acid-functionalized carbon quantum dots | Antiradical activity, bioimaging in MCF-7 cell lines, antitumor activity in pancreatic cancer cell lines | Antiradical activity: DPPH•/in vitro on HepG2 (hepatocellular carcinoma), MCF-7 (breast adenocarcinoma, metastatic), HCT-116 (colorectal carcinoma), CFPAC-1 (cystic fibrosis pancreatic adenocarcinoma, metastatic), and HFF-1 (human foreskin fibroblasts) | [204] |
| Commercial product | Hesperetin cocrystals with piperine | Drug delivery | In vivo bioavailability on Sprague–Dawley rats | [205] |
| C. sinensis peel | Hesperidin nanocrystals | Cosmetics | In vitro on artificial skin | [206] |
| C. sinensis L. Osbeck var. Valencia peel | Hesperidin hexosomal loaded nanodispersion | Antimycobacterial, cytotoxic, and anti-HCov activity | Antimycobacterial: Mycobacterium (M.) tuberculosis (MTB)/cytotoxic: against A-549 (human pulmonary adenocarcinoma) cell lines/antiviral: human coronavirus 229E | [207] |
| C. reticulata peel | Hesperidin encapsulated in magnetic casein-CaFe2O4 nanohybrid carrier | Drug delivery, antitumor activity | In vitro drug release/in vitro on SKOV-3 (human ovarian cancer cell line) and MDA-MB-231 TNBC (human breast cancer cell line) | [56] |
| C. sinensis var. Valencia peel | Gold nanoparticles (AuNPs) | Anti-inflammatory activity | Nitric oxide inhibitory activity, qRT-PCR 2, Western blot | [208] |
| Orange peel 1 | Hesperidin gold nanoparticles (Hes-AuNPs) | Neuroprotective and antioxidant effects | In vivo on Wistar rats/antioxidant: DPPH• and in vivo studies | [209] |
| C. tangerina, C. sinensis, and C. limon peel | Silver nanoparticles (AgNPs) | Antimicrobial activity | Antimicrobial: E. coli and S. aureus | [196] |
| C. limon peel | Silver nanoparticles (AgNPs) | Antimicrobial activity | Antimicrobial: A. baumannii, S. typhimurium, E. coli, P. aeruginosa, S. aureus, and P. vulgaris | [198] |
| Lemon, tangerine, and orange peel 1 | Copper oxide nanoparticles (CuONPs) | Antimicrobial activity | Antimicrobial: five strains of Gram-positive (Enterococcus (E.) faecalis, S. aureus, L. monocytogenes, S. pneumonia and Clostridium (C.) perfringens) and five strains of Gram-negative (E. coli, Moraxella (M.) catarrhalis, Salmonella (S.) enterica subsp. diarizonae, Campylobacter (C.) coli, and P. aeruginosa) bacteria | [210] |
| C. hystrix peel | Encapsulated essential oil into chitosan nanoparticle | Antimicrobial activity | Antimicrobial: Propionilbacterium (P.) Acnes | [211] |
| C. clementine vesicles | Exosome-like nano-sized vesicles | Molecular delivery | Proteomic and bioinformatic studies | [212] |
| C. sinensis, C. limon, C. paradise, C. aurantium isolated vesicles | Micro- and nano-sized vesicles | Antitumor activity | In vitro on breast adenocarcinoma (MCF7), human melanoma (A375), lung adenocarcinoma (A549), and human normal skin keratinocyte (HaCat) cells | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šafranko, S.; Šubarić, D.; Jerković, I.; Jokić, S. Citrus By-Products as a Valuable Source of Biologically Active Compounds with Promising Pharmaceutical, Biological and Biomedical Potential. Pharmaceuticals 2023, 16, 1081. https://doi.org/10.3390/ph16081081
Šafranko S, Šubarić D, Jerković I, Jokić S. Citrus By-Products as a Valuable Source of Biologically Active Compounds with Promising Pharmaceutical, Biological and Biomedical Potential. Pharmaceuticals. 2023; 16(8):1081. https://doi.org/10.3390/ph16081081
Chicago/Turabian StyleŠafranko, Silvija, Drago Šubarić, Igor Jerković, and Stela Jokić. 2023. "Citrus By-Products as a Valuable Source of Biologically Active Compounds with Promising Pharmaceutical, Biological and Biomedical Potential" Pharmaceuticals 16, no. 8: 1081. https://doi.org/10.3390/ph16081081
APA StyleŠafranko, S., Šubarić, D., Jerković, I., & Jokić, S. (2023). Citrus By-Products as a Valuable Source of Biologically Active Compounds with Promising Pharmaceutical, Biological and Biomedical Potential. Pharmaceuticals, 16(8), 1081. https://doi.org/10.3390/ph16081081










