Analysis of the Effect of the TRPC4/TRPC5 Blocker, ML204, in Sucrose-Induced Metabolic Imbalance
Abstract
:1. Introduction
2. Results
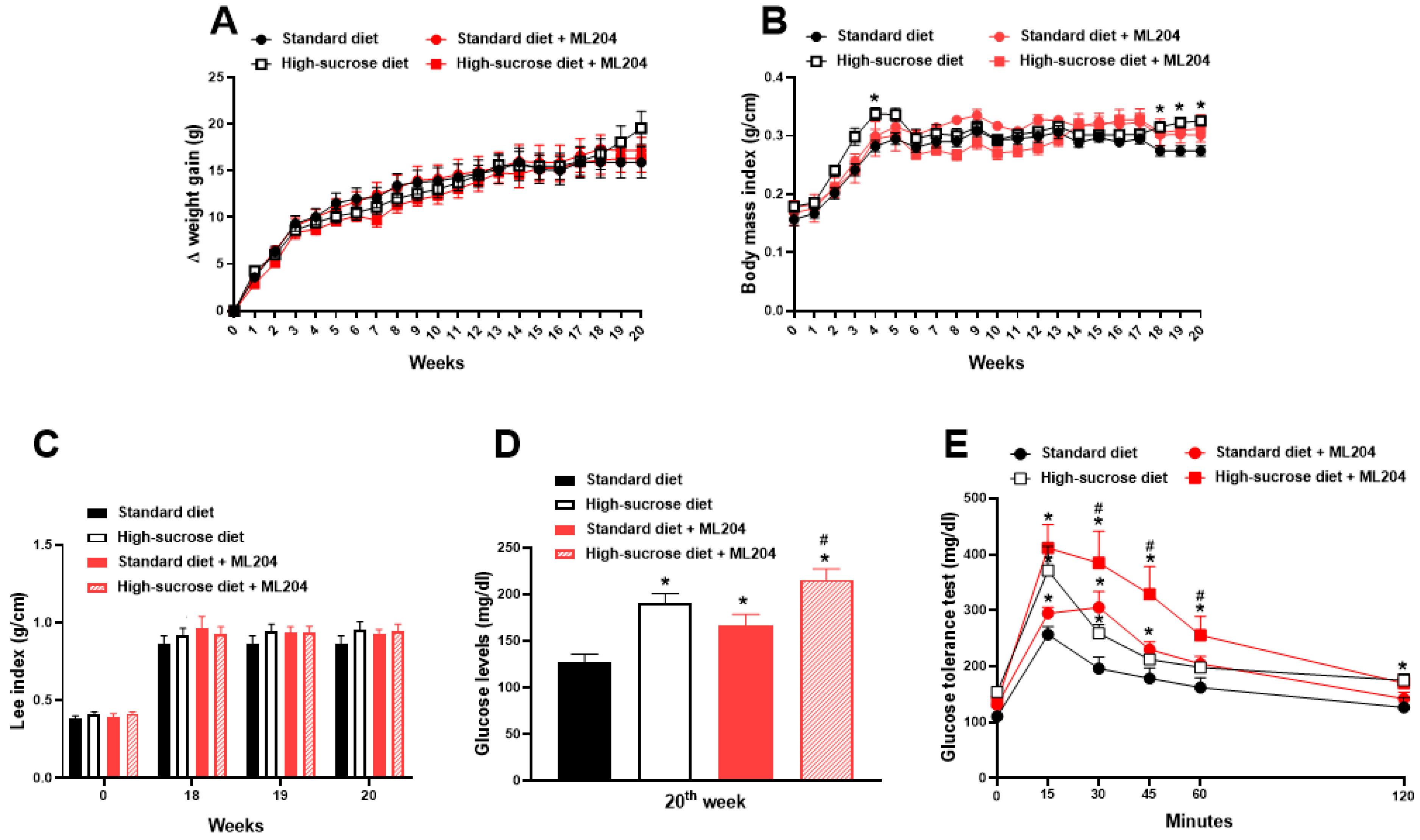
2.1. High Sucrose Induces Increased Body Mass Index, Fat Accumulation, and Glycaemia
2.2. ML204 Regulates Circulating Glucose and Lipid Levels
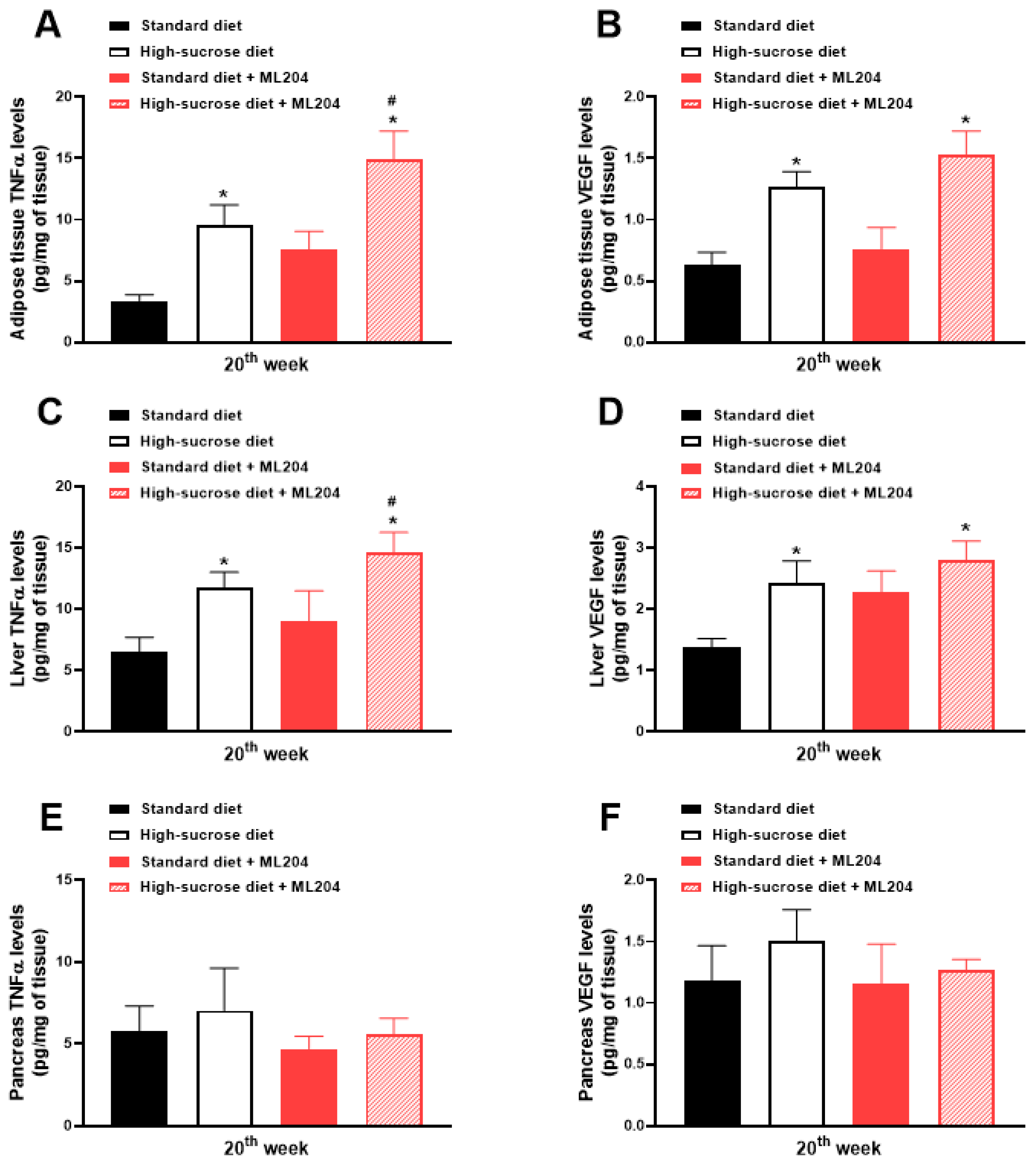
2.3. ML204 Increases High-Sucrose-Induced Adipose Tissue and Liver Inflammation by Modulating TNFα Production
2.4. ML204 Enhances Adipocyte Area and Mesenteric Fat Accumulation in High-Sucrose-Fed Mice and the Size of Adipocytes in Those Receiving Standard Diet
2.5. Non-Alcoholic Fatty Liver Disease Activity Score Is Enhanced by ML204 in High-Sucrose-Fed Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. High-Sucrose-Induced Metabolic Disturbances
4.3. Glucose Tolerance Test
4.4. Cholesterol and Triglyceride Quantifications
4.5. TNFα and VEGF Tissue Levels
4.6. Histology
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Li, R.; Park, S.; Galuska, D.A.; Sherry, B.; Freedman, D.S. A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics 2014, 134 (Suppl. 1), S29–S35. [Google Scholar] [PubMed] [Green Version]
- Geidl-Flueck, B.; Hochuli, M.; Németh, Á.; Eberl, A.; Derron, N.; Köfeler, H.C.; Tappy, L.; Berneis, K.; Spinas, G.A.; Gerber, P.A. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J. Hepatol. 2021, 75, 46–54. [Google Scholar]
- Shetty, A. Significance of sugar intake in young adults: A review. Int. J. Adolesc. Med. Health 2021, 33, 375–378. [Google Scholar]
- Witek, K.; Wydra, K.; Filip, M. A High-Sugar Diet Consumption, Metabolism and Health Impacts with a Focus on the Development of Substance Use Disorder: A Narrative Review. Nutrients 2022, 14, 2940. [Google Scholar]
- Huang, Y.; Chen, Z.; Chen, B.; Li, J.; Yuan, X.; Li, J.; Wang, W.; Dai, T.; Chen, H.; Wang, Y.; et al. Dietary sugar consumption and health: Umbrella review. BMJ 2023, 381, e071609. [Google Scholar]
- Raben, A.; Vasilaras, T.H.; Møller, A.C.; Astrup, A. Sucrose compared with artificial sweeteners: Different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am. J. Clin. Nutr. 2002, 76, 721–729. [Google Scholar]
- Maersk, M.; Belza, A.; Stødkilde-Jørgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar]
- Aeberli, I.; Gerber, P.A.; Hochuli, M.; Kohler, S.; Haile, S.R.; Gouni-Berthold, I.; Berthold, H.K.; Spinas, G.A.; Berneis, K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 479–485. [Google Scholar] [PubMed] [Green Version]
- Aeberli, I.; Hochuli, M.; Gerber, P.A.; Sze, L.; Murer, S.B.; Tappy, L.; Spinas, G.A.; Berneis, K. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: A randomized controlled trial. Diabetes Care 2013, 36, 150–156. [Google Scholar]
- Kang, I.; Espín, J.C.; Carr, T.P.; Tomás-Barberán, F.A.; Chung, S. Raspberry seed flour attenuates high-sucrose diet-mediated hepatic stress and adipose tissue inflammation. J. Nutr. Biochem. 2016, 32, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, S.J.; Batdorf, H.M.; Martin, T.M.; Burk, D.H.; Noland, R.C.; Cooley, C.R.; Karlstad, M.D.; Johnson, W.D.; Collier, J.J. Liquid Sucrose Consumption Promotes Obesity and Impairs Glucose Tolerance without Altering Circulating Insulin Levels. Obesity 2018, 26, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Perazza, L.R.; Mitchell, P.L.; Jensen, B.A.H.; Daniel, N.; Boyer, M.; Varin, T.V.; Bouchareb, R.; Nachbar, R.T.; Bouchard, M.; Blais, M.; et al. Dietary sucrose induces metabolic inflammation and atherosclerotic cardiovascular diseases more than dietary fat in LDLr-/-ApoB100/100 mice. Atherosclerosis 2020, 304, 9–21. [Google Scholar]
- França, L.M.; Dos Santos, P.C.; Barroso, W.A.; Gondim, R.S.D.; Coêlho, C.F.F.; Flister, K.F.T.; Paes, A.M.A. Post-weaning exposure to high-sucrose diet induces early non-alcoholic fatty liver disease onset and progression in male mice: Role of dysfunctional white adipose tissue. J. Dev. Orig. Health Dis. 2020, 11, 509–520. [Google Scholar] [PubMed]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet Child Adolesc. Health 2022, 6, 158–170. [Google Scholar] [PubMed]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.J.; Sweet, T.B.; Clapham, D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef]
- Nilius, B.; Szallasi, A. Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient Receptor Potential (TRP) Channels. Subcell. Biochem. 2018, 87, 141–165. [Google Scholar]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [PubMed]
- Montell, C. Drosophila TRP channels. Pflugers Arch. 2005, 451, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.A.; Montell, C. Drosophila TRP channels and animal behavior. Life Sci. 2013, 92, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells. 2020, 9, 1983. [Google Scholar] [CrossRef]
- De Logu, F.; Geppetti, P. Ion Channel Pharmacology for Pain Modulation. Handb. Exp. Pharmacol. 2019, 260, 161–186. [Google Scholar]
- Benemei, S.; Dussor, G. TRP Channels and Migraine: Recent Developments and New Therapeutic Opportunities. Pharmaceuticals 2019, 12, 54. [Google Scholar] [CrossRef] [Green Version]
- Inoue, R.; Kurahara, L.H.; Hiraishi, K. TRP channels in cardiac and intestinal fibrosis. Semin. Cell Dev. Biol. 2019, 94, 40–49. [Google Scholar] [CrossRef]
- Mukherjee, P.; Rahaman, S.G.; Goswami, R.; Dutta, B.; Mahanty, M.; Rahaman, S.O. Role of mechanosensitive channels/receptors in atherosclerosis. Am. J. Physiol. Cell. Physiol. 2022, 322, C927–C938. [Google Scholar] [CrossRef]
- Kytikova, O.Y.; Novgorodtseva, T.P.; Denisenko, Y.K.; Naumov, D.E.; Gvozdenko, T.A.; Perelman, J.M. Thermosensory Transient Receptor Potential Ion Channels and Asthma. Biomedicines 2021, 9, 816. [Google Scholar] [CrossRef]
- Müller, I.; Alt, P.; Rajan, S.; Schaller, L.; Geiger, F.; Dietrich, A. Transient Receptor Potential (TRP) Channels in Airway Toxicity and Disease: An Update. Cells 2022, 11, 2907. [Google Scholar] [CrossRef]
- Bishnoi, M.; Khare, P.; Brown, L.; Panchal, S.K. Transient receptor potential (TRP) channels: A metabolic TR(i)P to obesity prevention and therapy. Obes. Rev. 2018, 19, 1269–1292. [Google Scholar] [PubMed]
- Dhakal, S.; Lee, Y. Transient Receptor Potential Channels and Metabolism. Mol. Cells 2019, 42, 569–578. [Google Scholar] [PubMed]
- Araújo, M.C.; Soczek, S.H.S.; Pontes, J.P.; Marques, L.A.C.; Santos, G.S.; Simão, G.; Bueno, L.R.; Maria-Ferreira, D.; Muscará, M.N.; Fernandes, E.S. An Overview of the TRP-Oxidative Stress Axis in Metabolic Syndrome: Insights for Novel Therapeutic Approaches. Cells 2022, 11, 1292. [Google Scholar] [CrossRef]
- Qian, F.; Huang, P.; Ma, L.; Kuznetsov, A.; Tamarina, N.; Philipson, L.H. TRP genes: Candidates for nonselective cation channels and store-operated channels in insulin-secreting cells. Diabetes 2002, 51 (Suppl. 1), S183–S189. [Google Scholar] [CrossRef] [Green Version]
- Sukumar, P.; Sedo, A.; Li, J.; Wilson, L.A.; O'Regan, D.; Lippiat, J.D.; Porter, K.E.; Kearney, M.T.; Ainscough, J.F.; Beech, D.J. Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ. Res. 2012, 111, 191–200. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Gao, Y.; Lieu, L.; Afrin, S.; Cao, J.; Michael, N.J.; Dong, Y.; Sun, J.; Guo, H.; Williams, K.W. Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons—Implications for energy balance and glucose control. Mol. Metab. 2019, 28, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Carty, J.; Goldstein, N.; He, Z.; Hwang, E.; Chau, D.; Wallace, B.; Kabahizi, A.; Lieu, L.; Peng, Y.; et al. Time and metabolic state-dependent effects of GLP-1R agonists on NPY/AgRP and POMC neuronal activity in vivo. Mol. Metab. 2021, 54, 101352. [Google Scholar] [CrossRef]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food Groups and Risk of Overweight, Obesity, and Weight Gain: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2019, 10, 205–218. [Google Scholar]
- Tammi, R.; Männistö, S.; Harald, K.; Maukonen, M.; Eriksson, J.G.; Jousilahti, P.; Koskinen, S.; Kaartinen, N.E. Different carbohydrate exposures and weight gain-results from a pooled analysis of three population-based studies. Int. J. Obes. 2023, 47, 743–749. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Kengne, A.P.; Stathopoulou, M.G.; Azimi-Nezhad, M.; Siest, S. VEGF, the underlying factor for metabolic syndrome; fact or fiction? Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), S61–S64. [Google Scholar] [CrossRef]
- Mu, M.; Xu, L.F.; Hu, D.; Wu, J.; Bai, M.J. Dietary Patterns and Overweight/Obesity: A Review Article. Iran J. Public Health 2017, 46, 869–876. [Google Scholar]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- 2020 Dietary Guidelines Advisory Committee; Dietary Patterns Subcommittee. Dietary Patterns and Risk of Cardiovascular Disease: A Systematic Review; USDA Nutrition Evidence Systematic Review: Alexandria, VA, USA, 2020. [Google Scholar]
- Yeh, S.H.; Shie, F.S.; Liu, H.K.; Yao, H.H.; Kao, P.C.; Lee, Y.H.; Chen, L.M.; Hsu, S.M.; Chao, L.J.; Wu, K.W.; et al. A high-sucrose diet aggravates Alzheimer's disease pathology, attenuates hypothalamic leptin signaling, and impairs food-anticipatory activity in APPswe/PS1dE9 mice. Neurobiol. Aging 2020, 90, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, Y.; Liu, R.; Jia, X.L.; Hu, N.; An, X.W.; Zheng, C.G.; Chen, C.; Sun, H.T.; Chen, F.; et al. Rutaecarpine Ameliorated High Sucrose-Induced Alzheimer's Disease Like Pathological and Cognitive Impairments in Mice. Rejuvenation Res. 2021, 24, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Watts, R.; Quiroga, A.D.; Beggs, M.R.; Alexander, R.T.; Lehner, R. Ces1d deficiency protects against high-sucrose diet-induced hepatic triacylglycerol accumulation. J. Lipid Res. 2019, 60, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Flister, K.F.T.; Pinto, B.A.S.; França, L.M.; Coêlho, C.F.F.; Dos Santos, P.C.; Vale, C.C.; Kajihara, D.; Debbas, V.; Laurindo, F.R.M.; Paes, A.M.A. Long-term exposure to high-sucrose diet down-regulates hepatic endoplasmic reticulum-stress adaptive pathways and potentiates de novo lipogenesis in weaned male mice. J. Nutr. Biochem. 2018, 62, 155–166. [Google Scholar] [CrossRef]
- Norman, J.E.; Nuthikattu, S.; Milenkovic, D.; Rutledge, J.C.; Villablanca, A.C. A high sucrose diet modifies brain oxylipins in a sex-dependent manner. Prostaglandins Leukot. Essent. Fat. Acids 2022, 186, 102506. [Google Scholar] [CrossRef]
- Elias, I.; Franckhauser, S.; Ferré, T.; Vilà, L.; Tafuro, S.; Muñoz, S.; Roca, C.; Ramos, D.; Pujol, A.; Riu, E.; et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 2012, 61, 1801–1813. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.K.; Doh, K.O.; Son, J.E.; Park, J.G.; Bae, Y.; Choi, S.; Nelson, S.M.; Cowling, R.; Nagy, K.; Michael, I.P.; et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013, 17, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Shi, J.; Wu, M.; Engers, J.; Hopkins, C.R.; Lindsley, C.W.; Salovich, J.M.; Zhu, Y.; Tian, J.B.; Zhu, M.X.; et al. Novel Chemical Inhibitor of TRPC4 Channels. In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. [Google Scholar]
- Miller, M.; Shi, J.; Zhu, Y.; Kustov, M.; Tian, J.B.; Stevens, A.; Wu, M.; Xu, J.; Long, S.; Yang, P.; et al. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J. Biol. Chem. 2011, 286, 33436–33446. [Google Scholar] [CrossRef] [Green Version]
- Alawi, K.M.; Russell, F.A.; Aubdool, A.A.; Srivastava, S.; Riffo-Vasquez, Y.; Baldissera Jr, L.; Thakore, P.; Saleque, N.; Fernandes, E.S.; Walsh, D.A.; et al. Transient receptor potential canonical 5 (TRPC5) protects against pain and vascular inflammation in arthritis and joint inflammation. Ann. Rheum. Dis. 2017, 76, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.S.; Mendes, S.J.F.; Alawi, K.; Thakore, P.; Aubdool, A.; Sousa, N.C.F.; da Silva, J.F.R.; Castro, J.A., Jr.; Pereira, I.C.P.; Silva, L.C.N.; et al. Transient Receptor Potential Canonical Channels 4 and 5 Mediate Escherichia coli-Derived Thioredoxin Effects in Lipopolysaccharide-Injected Mice. Oxid. Med. Cell. Longev. 2018, 2018, 4904696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, L.; Guo, G.; Qi, Y.; Xiong, Y.; Ma, X.; Wu, N.; Dong, C.; Yang, C. Inhibition of Canonical Transient Receptor Potential 5 Channels Polarizes Macrophages to an M1 Phenotype. Pharmacology 2020, 105, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Krauthamer, M.; Bjalme-Evans, M. Wegovy (semaglutide): A new weight loss drug for chronic weight management. J. Investig. Med. 2022, 70, 5–13. [Google Scholar] [CrossRef]
- Idrees, Z.; Cancarevic, I.; Huang, L. FDA-Approved Pharmacotherapy for Weight Loss Over the Last Decade. Cureus 2022, 14, e29262. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar]
- Sousa, R.M.L.; Ribeiro, N.L.X.; Pinto, B.A.S.; Sanches, J.R.; da Silva, M.U.; Coêlho, C.F.F.; França, L.M.; de Figueiredo Neto, J.A.; Paes, A.M.A. Long-term high-protein diet intake reverts weight gain and attenuates metabolic dysfunction on high-sucrose-fed adult rats. Nutr. Metab. 2018, 15, 53. [Google Scholar] [CrossRef] [Green Version]
- Marshall, N.J.; Liang, L.; Bodkin, J.; Dessapt-Baradez, C.; Nandi, M.; Collot-Teixeira, S.; Smillie, S.J.; Lalgi, K.; Fernandes, E.S.; Gnudi, L.; et al. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension 2013, 61, 246–252. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, M.C.; Soczek, S.H.S.; Pontes, J.P.; Pinto, B.A.S.; França, L.M.; Soley, B.d.S.; Santos, G.S.; Saminez, W.F.d.S.; Fernandes, F.K.M.; Lima, J.L.d.C.; et al. Analysis of the Effect of the TRPC4/TRPC5 Blocker, ML204, in Sucrose-Induced Metabolic Imbalance. Pharmaceuticals 2023, 16, 1100. https://doi.org/10.3390/ph16081100
Araújo MC, Soczek SHS, Pontes JP, Pinto BAS, França LM, Soley BdS, Santos GS, Saminez WFdS, Fernandes FKM, Lima JLdC, et al. Analysis of the Effect of the TRPC4/TRPC5 Blocker, ML204, in Sucrose-Induced Metabolic Imbalance. Pharmaceuticals. 2023; 16(8):1100. https://doi.org/10.3390/ph16081100
Chicago/Turabian StyleAraújo, Mizael C., Suzany H. S. Soczek, Jaqueline P. Pontes, Bruno A. S. Pinto, Lucas M. França, Bruna da Silva Soley, Gabriela S. Santos, Warlison F. de Silva Saminez, Fernanda K. M. Fernandes, João L. do Carmo Lima, and et al. 2023. "Analysis of the Effect of the TRPC4/TRPC5 Blocker, ML204, in Sucrose-Induced Metabolic Imbalance" Pharmaceuticals 16, no. 8: 1100. https://doi.org/10.3390/ph16081100





