The Potential of Lactiplantibacillus plantarum ATCC 14917 in the Development of Alginate-Based Gel Formulations with Anti–Staphylococcus aureus Properties
Abstract
:1. Introduction
2. Results
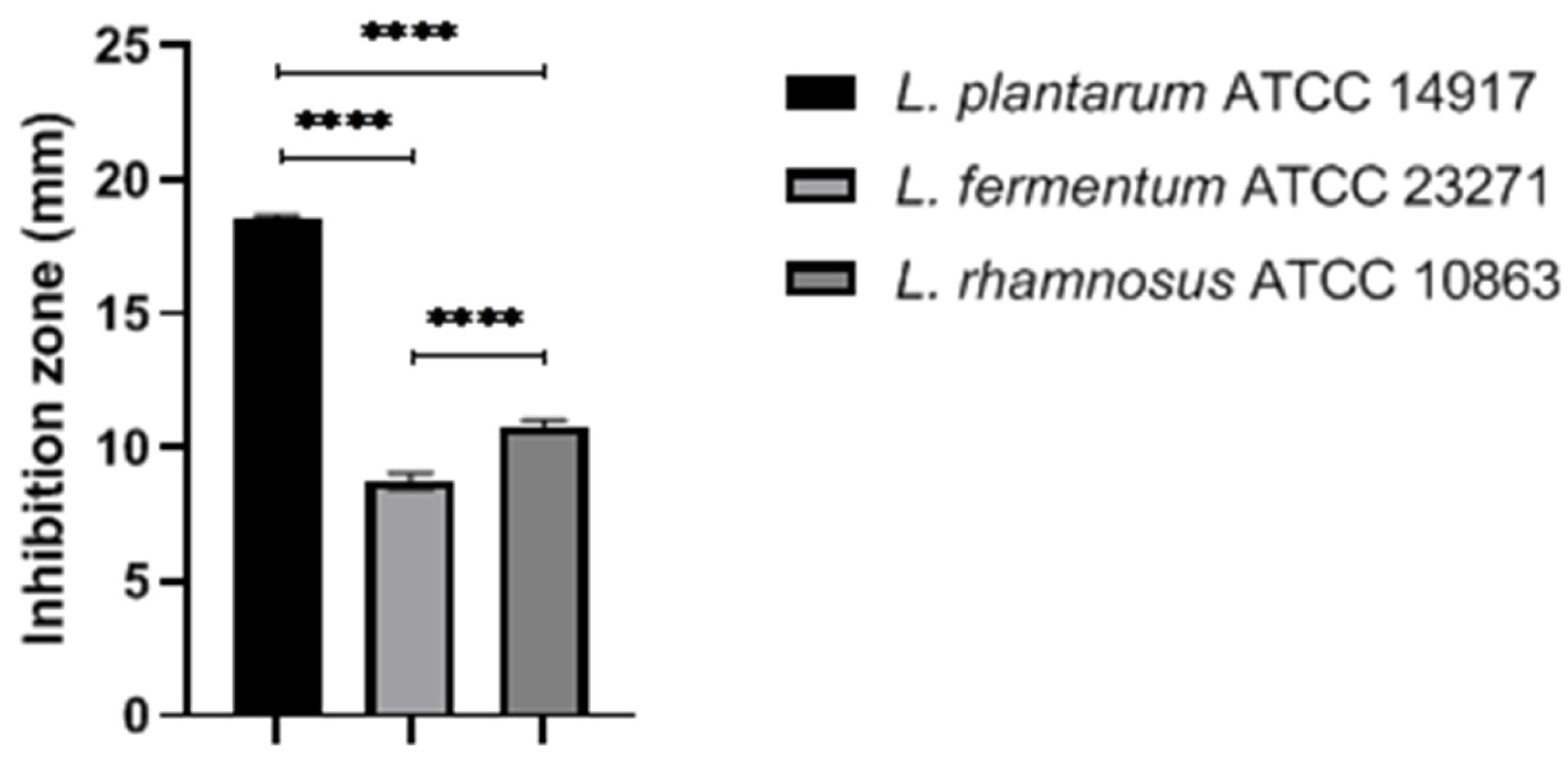
2.1. Antimicrobial Effects of Selected LAB against Staphylococcus aureus
2.2. Time-Death Assay
2.3. Antimicrobial Effects of Probiotic and Postbiotic Gels
2.4. Viablity of Lactiplantibacillus plantarum ATCC 14917 in the Formulation
2.5. Formulation Stability Tests
2.5.1. Preliminary Stability Test (PS)
2.5.2. Accelerated Stability Test (AS)
2.6. Microbiological Evaluation Test
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antagonism Test–Spot Overlay of LAB
- No inhibition capacity: R < 2 mm;
- Low inhibition capacity: R = 2–5 mm;
- High inhibition capacity: R > 6 mm.
4.3. Preparation of Postbiotics of Lactiplantibacillus plantarum ATCC 14917
4.4. Inhibitory Action in Broth Media
4.5. Time-Death Curve
4.6. Preparation of Gel Formulation Containing Probiotics
4.7. Antimicrobial Activity of The Formulation
4.8. Viability of Probiotics in The Formulation
4.9. Stability Tests
4.9.1. Preliminary Stability Test
Centrifugation Test
Thermal Stress Test
4.9.2. Accelerated Stability Test (AS)
Evaluated Parameters
4.9.3. Physical–Chemical Evaluations
pH Value
Density
Spreadability
4.10. Microbiological Evaluation
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Xia, J.; Jiang, L.; Tan, Y.; An, Y.; Zhu, X.; Ruan, J.; Chen, Z.; Zhen, H.; Ma, Y.; et al. Characterization of the Human Skin Resistome and Identification of Two Microbiota Cutotypes. Microbiome 2021, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Dunaway, S.; Champer, J.; Kim, J.; Alikhan, A. Changing Our Microbiome: Probiotics in Dermatology. Br. J. Dermatol. 2020, 182, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beato, I.S. Impacto dos Cosméticos no Microbiota da Pele. Ph.D. Thesis, Universidade de Lisboa, Lisbon, Portugal, 2017. [Google Scholar]
- Prescott, S.L.; Larcombe, D.-L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Van Etten, E.; Horwitz, P.; Kozyrskyj, A.; et al. The Skin Microbiome: Impact of Modern Environments on Skin Ecology, Barrier Integrity, and Systemic Immune Programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Traisaeng, S.; Herr, D.R.; Kao, H.J.; Chuang, T.H.; Huang, C.M. A derivative of butyric acid, the fermentation metabolite of Staphylococcus epidermidis, inhibits the growth of a Staphylococcus aureus strain isolated from atopic dermatitis patients. Toxins 2019, 11, 311. [Google Scholar] [CrossRef] [Green Version]
- Sivamaruthi, B.; Kesika, P.; Chaiyasut, C. Probiotic based therapy for atopic dermatitis: Outcomes of clinical studies. Asian Pac. J. Trop. Biomed. 2018, 8, 328. [Google Scholar] [CrossRef]
- Imko-Walczuk, B.; Taraszkiewicz, A.; Mäyrä, A. Soothing Efficacy and Tolerability of a Skin Care Product Containing Live Lactobacillus rhamnosus Bacteria and Berry Seed Oils on Atopic Dermatitis Lesions. J. Cosmet. Dermatol. Sci. Appl. 2019, 9, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Rosignoli, C.; Thibaut de Ménonville, S.; Orfila, D.; Béal, M.; Bertino, B.; Aubert, J.; Mercenier, A.; Piwnica, D. A Topical Treatment Containing Heat-Treated Lactobacillus johnsonii NCC 533 Reduces Staphylococcus aureus Adhesion and Induces Antimicrobial Peptide Expression in an in vitro Reconstructed Human Epidermis Model. Exp. Dermatol. 2018, 27, 358–365. [Google Scholar] [CrossRef]
- Flesch, A.G.T.; Poziomyck, A.; Damin, D.D.C. The Therapeutic Use of Symbiotics. Arq. Bras. Cir. Dig. 2014, 27, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Tagliari, E. The impact of the use of symbiotics in the progression of nonalcoholic fatty liver disease in a rat model. Arq. Bras. Cir. Dig. 2017, 30, 211–215. [Google Scholar] [CrossRef]
- Puebla-Barragan, S.; Reid, G. Probiotics in cosmetic and personal care products: Trends and challenges. Molecules 2021, 26, 1249. [Google Scholar] [CrossRef]
- Wagner, N.R. Postoperative changes in intestinal microbiota and use of probiotics in roux-en-y gastric bypass and sleeve vertical gastrectomy: An integrative review. Arq. Bras. Cir. Dig. 2018, 31, e1400. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. Publisher Correction: Publisher Correction: The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 671. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, L.; Spacova, I.; Van Malderen, J.; Oerlemans, E.; Claes, I.; Lebeer, S. The Role of Lactobacilli in Inhibiting Skin Pathogens. Biochem. Soc. Trans. 2021, 49, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Habeebuddin, M.; Karnati, R.K.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Khalid Anwer, M.; Fattepur, S. Topical Probiotics: More than a Skin Deep. Pharmaceutics 2022, 14, 557. [Google Scholar] [CrossRef]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The Microbiome in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, H.; Aguh, C.; Andriessen, A.; Benjamin, L.; Ferberg, A.S.; Hooper, D.; Jarizzo, J.L.; Lio, P.A.; Tlougan, B.; Woolery-Lloyd, H.C.; et al. Atopic Dermatitis and the Role of the Skin Microbiome in Choosing Prevention, Treatment, and Maintenance Options. J. Drugs Dermatol. 2020, 19, 935–940. [Google Scholar] [CrossRef]
- Sousa, M.A.D.S.D.; Ferreira, A.F.; da Silva, C.C.; Silva, M.A.; Bazan, T.A.X.N.; Monteiro, C.D.A.; Monteiro, A.D.S.; Sousa, J.C.D.S.; da Silva, L.C.N.; Zagmignan, A. Development and Characterization of Hydroxyethyl Cellulose-Based Gels Containing Lactobacilli Strains: Evaluation of Antimicrobial Effects in In Vitro and Ex Vivo Models. Pharmaceuticals 2023, 16, 468. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, S.; Yun, S.; Zhang, M.; Peng, L.; Li, Y.; Zhou, Y. Microencapsulating Alginate-Based Polymers for Probiotics Delivery Systems and Their Application. Pharmaceuticals 2022, 15, 644. [Google Scholar] [CrossRef]
- Kistaubayeva, A.; Abdulzhanova, M.; Zhantlessova, S.; Savitskaya, I.; Karpenyuk, T.; Goncharova, A.; Sinyavskiy, Y. The Effect of Encapsulating a Prebiotic-Based Biopolymer Delivery System for Enhanced Probiotic Survival. Polymers 2023, 15, 1752. [Google Scholar] [CrossRef]
- Vitória, H.M.; Suyenaga, E.S. An approach on the potential use of probiotics in the treatment of skin conditions: Acne and atopic dermatitis. Int. J. Dermatol. 2018, 12, 1425–1432. [Google Scholar]
- Werfel, T.; Allam, J.-P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.-U.; Wollenberg, A.; et al. Cellular and Molecular Immunologic Mechanisms in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of Atopic Dermatitis: Clinical Implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, M.B.; Furlaneto, M.C.; Maia, L.F. General aspects of bacteriocins. Braz. J. Food Technol. 2015, 18, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Franco-Robles, E.; Ramírez-Emiliano, J.; Blumenberg, M. Prebiotics and Probiotics: Potential Benefits in Nutrition and Health; BoD-Books on Demand: London, UK, 2020. [Google Scholar] [CrossRef]
- Moraes, M.C. Assessment of probiotic bacterial strains effect on S. aureus biofilm on titanium discs with treated surfaces. Rev. Odontol. UNESP 2019, 48, e20190096. [Google Scholar] [CrossRef]
- Costa, G. Antimicrobial activity of Lactobacillus and Bifidobacterium strains against pathogenic microorganisms “in vitro”. Semin. Ciências Agrárias 2012, 33, 1839–1846. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-Y.; Lim, S.-D. Probiotic characteristics of Lactobacillus plantarum FH185 isolated from human feces. Korean J. Food Sci. Anim. Resour. 2015, 35, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-E.; Lee, N.-K.; Paik, H.-D. Antimicrobial and Anti-Biofilm Effects of Probiotic Lactobacillus plantarum KU200656 Isolated from Kimchi. Food Sci. Biotechnol. 2021, 30, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Dicks, L.M.T. Lactobacillus plantarum Isolated from Molasses Produces Bacteriocins Active against Gram-Negative Bacteria. Enzyme Microb. Technol. 2005, 36, 318–326. [Google Scholar] [CrossRef]
- Todorov, S.D.; Nyati, H.; Meincken, M.; Dicks, L.M.T. Partial Characterization of Bacteriocin AMA-K, Produced by Lactobacillus plantarum AMA-K Isolated from Naturally Fermented Milk from Zimbabwe. Food Control 2007, 18, 656–664. [Google Scholar] [CrossRef]
- Todorov, S.D.; Franco, B.D.G.D.M. Lactobacillus plantarum: Characterization of the Species and Application in Food Production. Food Rev. Int. 2010, 26, 205–229. [Google Scholar] [CrossRef]
- Hata, T.; Tanaka, R.; Ohmomo, S. Isolation and characterization of plantaricin ASM1: A new bacteriocin produced by Lactobacillus plantarum A-1. Int. J. Food Microbiol. 2010, 137, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.S.; Meng, X.C.; Wang, H. Plantaricin MG active against Gram-negative bacteria produced by Lactobacillus plantarum KLDS1. 0391 isolated from “Jiaoke”, a traditional fermented cream from China. Food Control 2010, 21, 89–96. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Wachsman, M.; Torres, N.I.; LeBlanc, J.G.; Todorov, S.D.; de Melo Franco, B.D.G. Biochemical, Antimicrobial and Molecular Characterization of a Noncytotoxic Bacteriocin Produced by Lactobacillus plantarum ST71KS. Food Microbiol. 2013, 34, 376–381. [Google Scholar] [CrossRef]
- Buntin, N.; Hongpattarakere, T. Antimicrobial activity and plantaricin (pln) encoding genes of Lactobacillus plantarum isolated from various sources. J. Biotechnol. 2014, 185, S74. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Y.; Sun, Y.; Gu, Q. Purification and characterization of Plantaricin ZJ008, a novel bacteriocin against Staphylococcus spp. from Lactobacillus plantarum ZJ008. Food Chem. 2014, 165, 216–223. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, F.; Wan, C.; Xiong, Y.; Shah, N.P.; Wei, H.; Tao, X. Evaluation of Probiotic Properties of Lactobacillus plantarum WLPL04 Isolated from Human Breast Milk. J. Dairy Sci. 2016, 99, 1736–1746. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-W.; Liu, W.-H.; Wu, C.-C.; Juan, Y.-C.; Wu, Y.-C.; Tsai, H.-P.; Wang, S.; Tsai, Y.-C. Psychotropic Effects of Lactobacillus plantarum PS128 in Early Life-Stressed and Naïve Adult Mice. Brain Res. 2016, 1631, R713–R715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.S.; Philip, K.; Ajam, N. Purification, characterization and mode of action of plantaricin K25 produced by Lactobacillus plantarum. Food Control 2016, 60, 430–439. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Jiang, Y.H.; Li, H.W.; Li, X.Z.; Zhang, Q.L. Purification and characterization of Lactobacillus plantarum—derived bacteriocin with activity against Staphylococcus argenteus planktonic cells and biofilm. J. Food Sci. 2022, 87, 2718–2731. [Google Scholar] [CrossRef]
- Bonin, M.C.B.; Penteado, A.L.; de Queiroz, S.C.D.N. Avaliação da atividade antagonista de bactérias ácido lácticas e seus metabólitos frente a patógenos de origem animal/Evaluation of the antagonistic activity of lactic acid bacteria and their metabolites against animal pathogens. Braz. J. Dev. 2019, 5, 18511–18525. [Google Scholar] [CrossRef]
- Onbas, T.; Osmanagaoglu, O.; Kiran, F. Potential Properties of Lactobacillus plantarum F-10 as a Bio-Control Strategy for Wound Infections. Probiotics Antimicrob. Proteins 2019, 11, 1110–1123. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gutiérrez, L.; San Vicente, L.; Sáenz, J.; Barron, L.J.R.; Chávarri, M. Characterisation of the probiotic potential of Lactiplantibacillus plantarum K16 and its ability to produce the postbiotic metabolite γ-aminobutyric acid. J. Funct. Foods. 2022, 97, 105230. [Google Scholar] [CrossRef]
- Hernández-Granados, M.J.; Franco-Robles, E. Postbiotics in Human Health: Possible New Functional Ingredients? Food Res. Int. 2020, 137, 109660. [Google Scholar] [CrossRef] [PubMed]
- Blanchet-Réthoré, S.; Bourdès, V.; Mercenier, A.; Haddar, C.H.; Verhoeven, P.O.; Andres, P. Effect of a Lotion Containing the Heat-Treated Probiotic Strain Lactobacillus Johnsonii NCC 533 on Staphylococcus aureus Colonization in Atopic Dermatitis. Clin. Cosmet. Investig. Dermatol. 2017, 10, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Khmaladze, I.; Butler, É.; Fabre, S.; Gillbro, J.M. Lactobacillus reuteri DSM 17938—A Comparative Study on the Effect of Probiotics and Lysates on Human Skin. Exp. Dermatol. 2019, 28, 822–828. [Google Scholar] [CrossRef]
- Levi, S.; Rac, V.; Manojlovi, V.; Raki, V.; Bugarski, B.; Flock, T.; Krzyczmonik, K.E.; Nedovi, V. Limonene Encapsulation in Alginate/Poly (Vinyl Alcohol). Procedia Food Sci. 2011, 1, 1816–1820. [Google Scholar] [CrossRef] [Green Version]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and characteristics of alginate microparticles for food, pharmaceutical and cosmetic applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Pál, P.; Németh, Á. Investigation and Characterisation of New Eco-Friendly Cosmetic Ingredients Based on Probiotic Bacteria Ferment Filtrates in Combination with Alginite Mineral. Processes 2022, 10, 2672. [Google Scholar]
- Pál, P.; Németh, Á. Investigations Into the usage of the mineral alginite fermented with Lactobacillus paracasei for cosmetic purposes. Hung. J. Ind. Chem. 2022, 50, 17–21. [Google Scholar]
- Oerlemans, E.F.M.; Bellen, G.; Claes, I.; Henkens, T.; Allonsius, C.N.; Wittouck, S.; van den Broek, M.F.L.; Wuyts, S.; Kiekens, F.; Donders, G.G.G.; et al. Impact of a Lactobacilli-Containing Gel on Vulvovaginal Candidosis and the Vaginal Microbiome. Sci. Rep. 2020, 10, 7976. [Google Scholar] [CrossRef]
- Kyser, A.J.; Masigol, M.; Mahmoud, M.Y.; Ryan, M.; Lewis, W.G.; Lewis, A.L.; Frieboes, H.B.; Steinbach-Rankins, J.M. Fabrication and characterization of bioprints with Lactobacillus crispatus for vaginal application. J. Control. Release 2023, 357, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Calixto, L.S.; Maia Campos, P.M.B.G. Physical-Mechanical Characterization of Cosmetic Formulations and Correlation Between Instrumental Measurements and Sensorial Properties. Int. J. Cosmet. Sci. 2017, 39, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Ainurofiq, A.; Maharani, A.; Fatonah, F.; Halida, H.N.; Nurrodlotiningtyas, T. Pre-formulation study on the preparation of skin cosmetics. Sci. Technol. Indones. 2021, 6, 273–284. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, T.; Chen, Z.; Ren, H.; Song, P.; Zhu, Y.; Liang, S.; Tzeng, C. Development and Evaluation of a Thermosensitive In Situ Gel Formulation for Intravaginal Delivery of Lactobacillus gasseri. Pharmaceutics 2022, 14, 1934. [Google Scholar] [CrossRef]
- Chew, S.Y. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J. Appl. Microbiol. 2015, 118, 1180–1190. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-S. Antimicrobial activity of Lactobacillus salivarius and Lactobacillus fermentum against Staphylococcus aureus. Pathog. Dis. 2017, 75, ftx009. [Google Scholar] [CrossRef] [Green Version]
- Brasil Agência Nacional de Vigilância Sanitária. Formulário Nacional da Farmacopeia Brasileira, 2nd ed.; Brasil Agência Nacional de Vigilância Sanitária: Guará, Brasil, 2012. [Google Scholar]


| Percentages Analyzed | Cells | Sonicated | Supernatant pH 5.0 | Supernatant pH 7.0 |
|---|---|---|---|---|
| 5% | +* | + | + | + |
| 10% | +* | + | + | + |
| 20% | +* | + | − | + |
| 30% | − | + | − | + |
| 40% | − | + | − | + |
| 50% | − | + | − | + |
| Percentages Added to the Gel | Inhibition Zones (mm ± SD) | ||
|---|---|---|---|
| Gel with Cells | Gel with Sonicated Cells | Gel with Supernatant (pH 5.0) | |
| 5% | 0 ± 0 a,1 | 0 ± 0 a,1 | 0 ± 0 a,1 |
| 10% | 4 ± 0 a,2 | 0 ± 0 b,1 | 0 ± 0 b,1 |
| 20% | 4.9 ± 0.02 a,3 | 0 ± 0 b,1 | 6 ± 0 c,2 |
| 30% | 5.9 ± 0.4 a,4 | 0 ± 0 b,1 | 6.9 ± 0.007 c,3 |
| Time | Aspect | Color | Odor | pH | Density |
|---|---|---|---|---|---|
| T0 | N | N | N | 5.3 | 0.890 g/mL |
| T2 | N | N | N | 4.9 | 1.154 g/mL |
| T4 | N | N | N | 5.0 | 0.720 g/mL |
| T6 | N | N | N | 4.7 | 0.788 g/mL |
| T8 | N | N | N | 5.1 | 0.885 g/mL |
| T10 | N | N | N | 5.0 | 0.624 g/mL |
| T12 | N | N | N | 4.9 | 0.606 g/mL |
| T14 | N | N | N | 4.9 | 0.741 g/mL |
| Temperature | Time | Aspect | Color | Odor | pH | Density | Spreadability |
|---|---|---|---|---|---|---|---|
| 37 ± 2 °C | T0 | N | N | N | 5.3 | 0.890 g/mL | 3957 mm |
| T7 | LM | LM | LM | 5.0 | 0.714 g/mL | X | |
| T15 | M | LM | LM | 5.1 | 0.994 g/mL | X | |
| T30 | M | M | LM | 5.4 | 0.705 g/mL | X | |
| T60 | M | M | LM | 4.4 | 0.652 g/mL | 3956 mm | |
| 25 ± 2 °C | T0 | N | N | N | 5.3 | 0.890 g/mL | 3957 mm |
| T7 | N | N | N | 4.9 | 0.905 g/mL | X | |
| T15 | N | N | N | 5.4 | 0.990 g/mL | X | |
| T30 | N | LM | LM | 5.0 | 0.610 g/mL | X | |
| T60 | N | LM | LM | 4.7 | 0.766 g/mL | 3367 mm | |
| 5 ± 2 °C | T0 | N | N | N | 5.3 | 0.890 g/mL | 3957 mm |
| T7 | N | N | N | 5.0 | 0.931 g/mL | X | |
| T15 | N | N | N | 5.4 | 0.938 g/mL | X | |
| T30 | N | N | N | 5.1 | 0.733 g/mL | X | |
| T60 | N | N | N | 4.6 | 0.759 g/mL | 3471 mm |
| Microorganism | 37 °C (0 Day) | 37 °C (60 Days) | 5 °C (0 Day) | 5 °C (60 Days) |
|---|---|---|---|---|
| Bacteria Mesophiles Total aerobics | <103 CFU | <103 CFU | <103 CFU | <103 CFU |
| Fungi/yeasts | <103 CFU | 52 × 102 CFU | <103 CFU | <103 CFU |
| Parameters | Results |
|---|---|
| Aspect | Medium viscosity gel |
| Color | Brown |
| Odor (fragrance) | Characteristic |
| pH | 5.3 |
| Density | 0.890 g/mL |
| Spreadability | 3957 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodré, M.T.C.; Ferraz, F.A.; Alencar, A.K.V.; Silva, K.F.; Silva, D.H.d.S.; Silva, L.d.S.; Araújo Carneiro, J.S.d.S.; Monteiro, C.A.; Silva, L.C.N.; Monteiro, A.d.S. The Potential of Lactiplantibacillus plantarum ATCC 14917 in the Development of Alginate-Based Gel Formulations with Anti–Staphylococcus aureus Properties. Pharmaceuticals 2023, 16, 1112. https://doi.org/10.3390/ph16081112
Sodré MTC, Ferraz FA, Alencar AKV, Silva KF, Silva DHdS, Silva LdS, Araújo Carneiro JSdS, Monteiro CA, Silva LCN, Monteiro AdS. The Potential of Lactiplantibacillus plantarum ATCC 14917 in the Development of Alginate-Based Gel Formulations with Anti–Staphylococcus aureus Properties. Pharmaceuticals. 2023; 16(8):1112. https://doi.org/10.3390/ph16081112
Chicago/Turabian StyleSodré, Monaliza Teresa Campos, Fernanda Avelino Ferraz, Amanda Karoline Vieira Alencar, Karinny Farias Silva, Douglas Henrique dos Santos Silva, Lucas dos Santos Silva, Jéssica Silva dos Santos Araújo Carneiro, Cristina Andrade Monteiro, Luis Cláudio Nascimento Silva, and Andrea de Souza Monteiro. 2023. "The Potential of Lactiplantibacillus plantarum ATCC 14917 in the Development of Alginate-Based Gel Formulations with Anti–Staphylococcus aureus Properties" Pharmaceuticals 16, no. 8: 1112. https://doi.org/10.3390/ph16081112
APA StyleSodré, M. T. C., Ferraz, F. A., Alencar, A. K. V., Silva, K. F., Silva, D. H. d. S., Silva, L. d. S., Araújo Carneiro, J. S. d. S., Monteiro, C. A., Silva, L. C. N., & Monteiro, A. d. S. (2023). The Potential of Lactiplantibacillus plantarum ATCC 14917 in the Development of Alginate-Based Gel Formulations with Anti–Staphylococcus aureus Properties. Pharmaceuticals, 16(8), 1112. https://doi.org/10.3390/ph16081112








