Synthesis, DFT Study, and In Vitro Evaluation of Antioxidant Properties and Cytotoxic and Cytoprotective Effects of New Hydrazones on SH-SY5Y Neuroblastoma Cell Lines
Abstract
1. Introduction
2. Results
2.1. Chemistry
2.1.1. Synthesis of the N-pyrrolyl hydrazides 7 (ethyl 5-(4-bromophenyl)-1-(2-hydrazinyl-2-oxoethyl)-2-methyl-1H-pyrrole-3-carboxylate) and 8 (ethyl 5-(4-bromophenyl)-1-(3-hydrazinyl-3-oxopropyl)-2-methyl-1H-pyrrole-3-carboxylate)
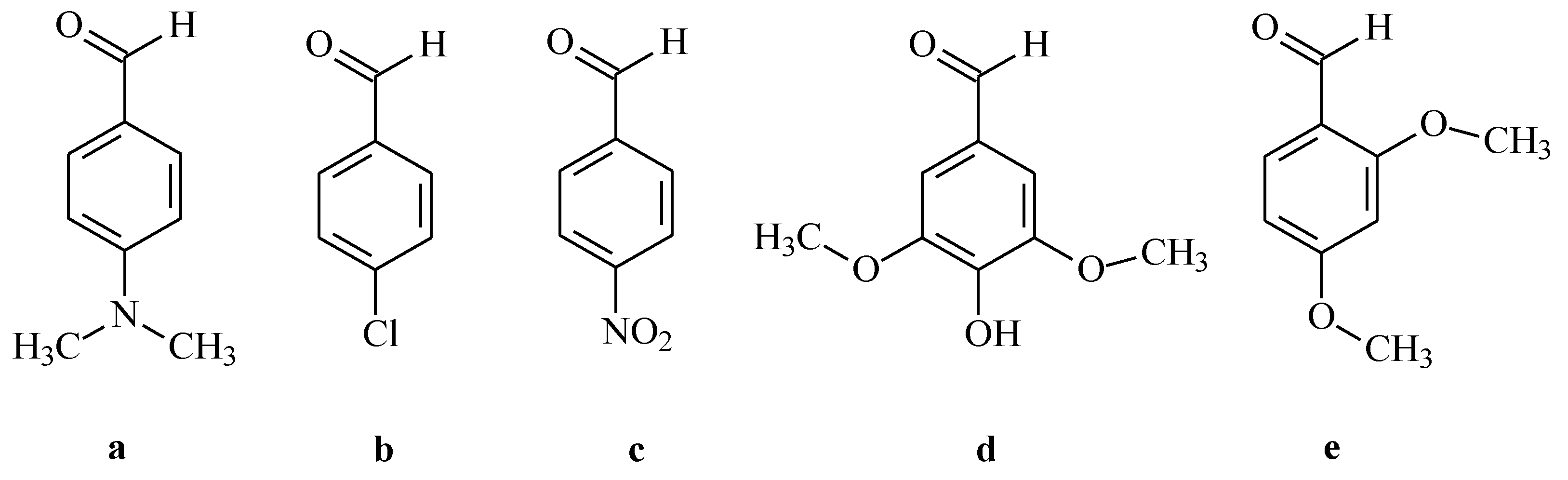
2.1.2. Synthesis of the New N–pyrrolylhydrazide–hydrazones 7a–e and 8a–e
2.2. Antioxidant Assays
2.2.1. DPPH Radical Scavenging Assay
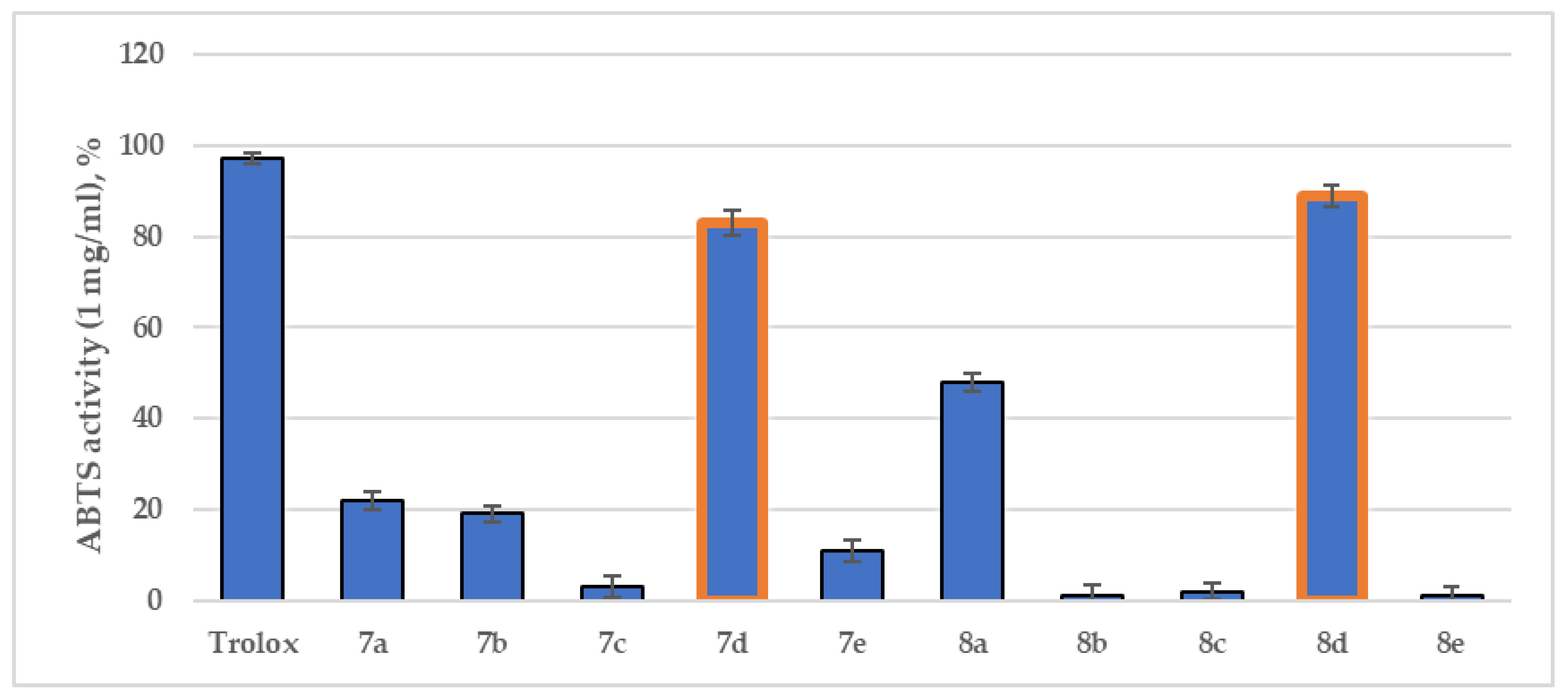
2.2.2. ABTS Radical Scavenging Assay
2.3. DFT Calculations
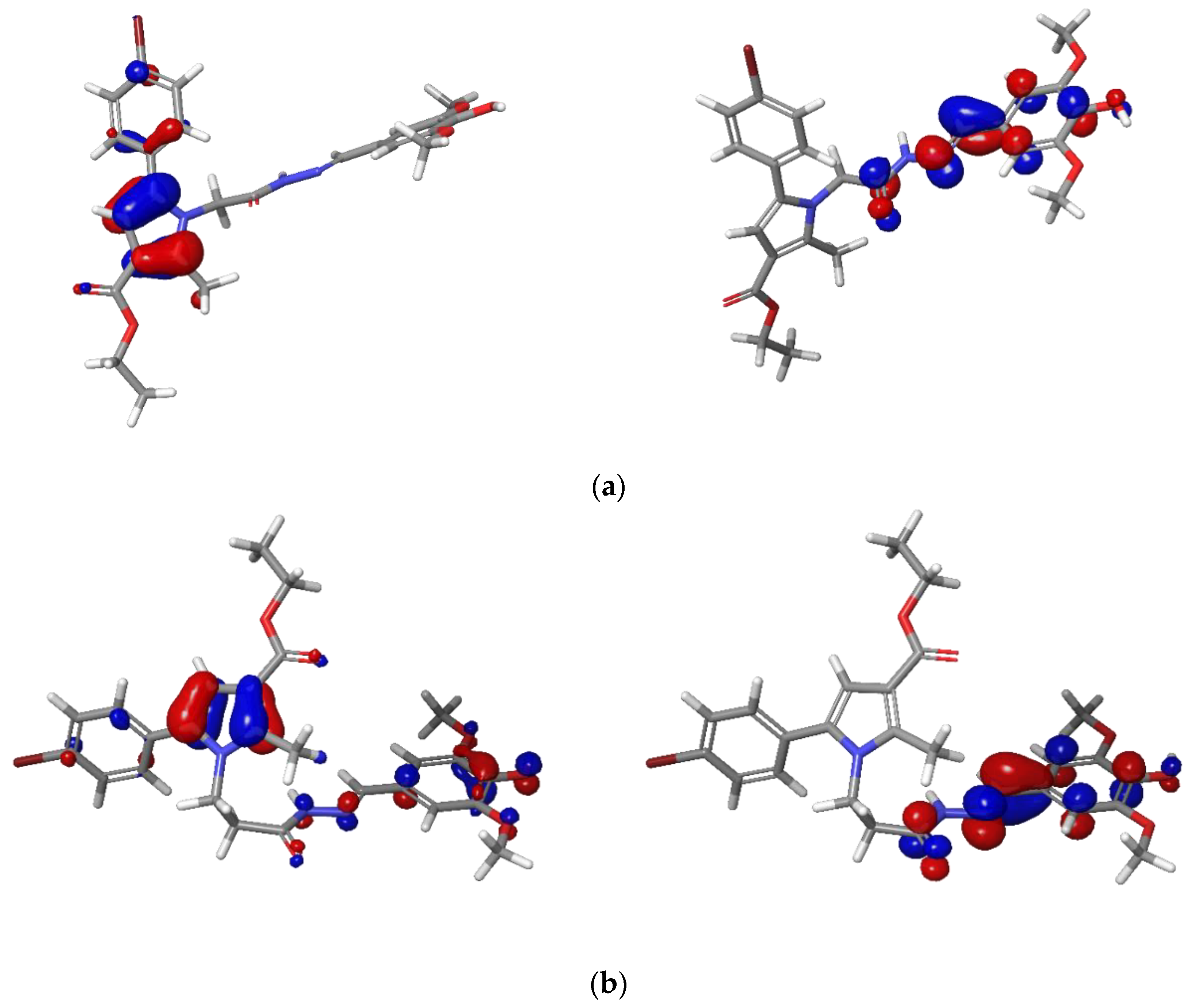
2.3.1. Optimized Geometries
2.3.2. Analysis of Frontier Molecular Orbitals FMOs and Global Reactivity Descriptors
2.3.3. Descriptors of the Antioxidant Properties
2.4. In Vitro Evaluations of the Cytotoxicity and Antioxidative Protective Activity on the SH-SY5Y Neuroblastoma Cell Line
2.4.1. Effects of the Newly Synthesized Derivatives 7a–e and 8a–e on the SH-SY5Y Cell Viability
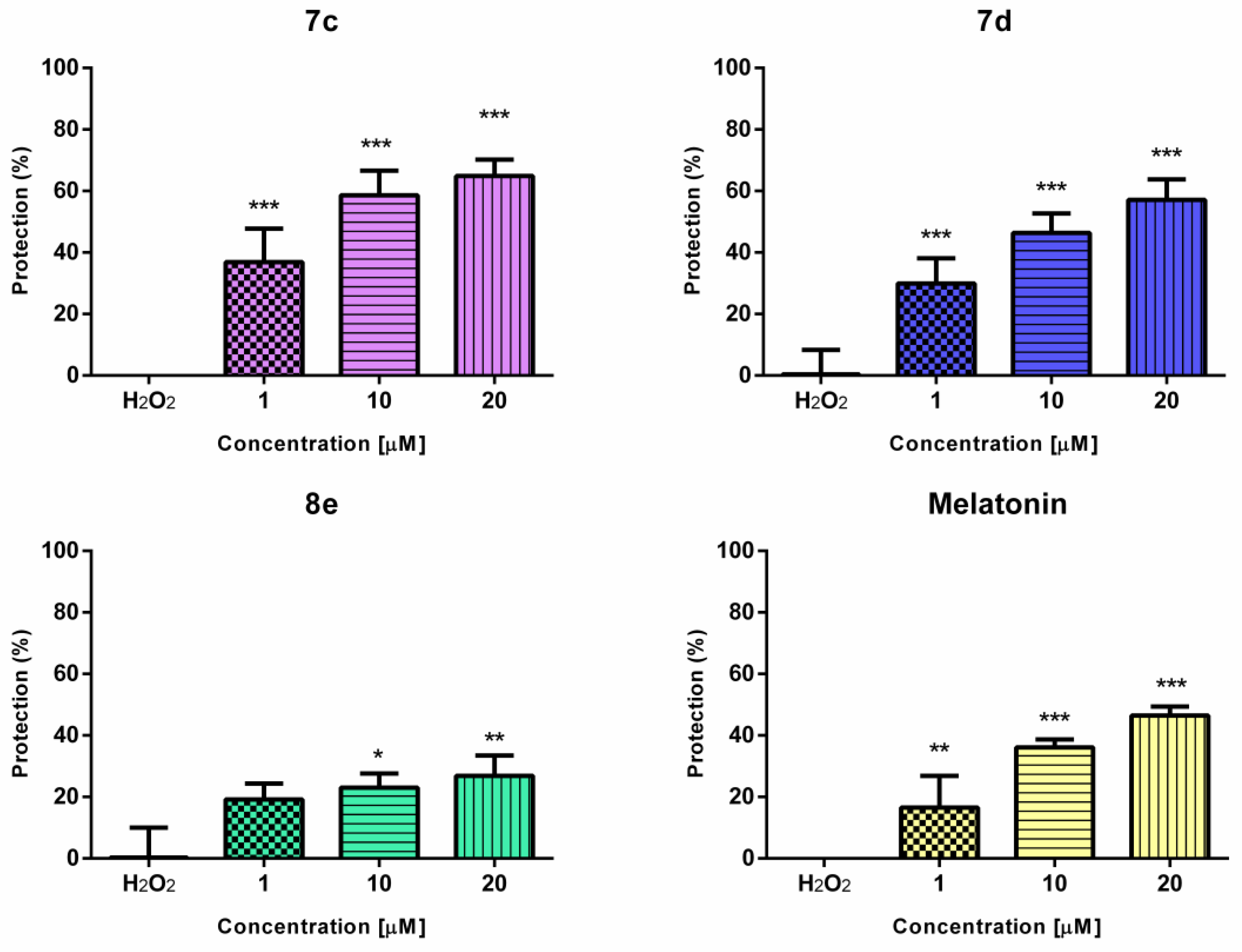
2.4.2. Effects of the Newly Synthesized Derivatives 7a–e and 8a–e in a Model of H2O2-Induced Oxidative Stress In Vitro
3. Discussion
3.1. Synthesis
3.2. Antioxidant Assays
3.2.1. DPPH Radical Scavenging Assay
3.2.2. ABTS Radical Scavenging Assay
3.3. DFT Calculations
3.4. In Vitro Cytotoxicity and Antioxidative Protective Activity on SH-SY5Y Cells
3.4.1. Effects of the Newly Synthesized Derivatives 7a–e and 8a–e on SH-SY5Y Cell Viability
3.4.2. Effects of the Newly Synthesized Derivatives 7a–e and 8a–e in a Model of H2O2-Induced Oxidative Stress In Vitro
3.5. Limitations and Implications Section
4. Materials and Methods
4.1. Materials
4.2. General Synthesis of the New Compounds
4.2.1. (E)-ethyl 5-(4-bromophenyl)-1-(2-(2-(4-(dimethylamino)benzylidene)hydrazinyl)-2-oxoethyl)-2-methyl-1H-pyrrole-3-carboxylate (7a)
4.2.2. (E)-ethyl 5-(4-bromophenyl)-1-(2-(2-(4-chlorobenzylidene)hydrazinyl)-2-oxoethyl)-2-methyl-1h-pyrrole-3-carboxylate (7b)
4.2.3. (E)-ethyl 5-(4-bromophenyl)-2-methyl-1-(2-(2-(4-nitrobenzylidene)hydrazinyl)-2-oxo-ethyl)-1H-pyrrole-3-carboxylate (7c)
4.2.4. (E)-ethyl 5-(4-bromophenyl)-1-(2-(2-(4-hydroxy-3,5-dimethoxybenzylidene)hydrazine-yl)-2-oxoethyl)-2-methyl-1H-pyrrole-3-carboxylate (7d)
4.2.5. (E)-ethyl 5-(4-bromophenyl)-1-(2-(2-(2,4-dimethoxybenzylidene)hydrazinyl)-2-oxo-ethyl)-2-methyl-1H-pyrrole-3-carboxylate (7e)
4.2.6. (E)-ethyl 5-(4-bromophenyl)-1-(3-(2-(4-(dimethylamino)benzylidene)hydrazinyl)-3-oxopropyl)-2-methyl-1H-pyrrole-3-carboxylate (8a)
4.2.7. (E)-ethyl 5-(4-bromophenyl)-1-(3-(2-(4-chlorobenzylidene)hydrazinyl)-3-oxopropyl)-2-methyl-1H-pyrrole-3-carboxylate (8b)
4.2.8. (E)-ethyl 5-(4-bromophenyl)-2-methyl-1-(3-(2-(4-nitrobenzylidene)hydrazinyl)-3-oxopropyl)-1H-pyrrole-3-carboxylate (8c)
4.2.9. (E)-ethyl 5-(4-bromophenyl)-1-(3-(2-(4-hydroxy-3,5-dimethoxybenzylidene) hydra zine-yl)-3-oxopropyl)-2-methyl-1H-pyrrole-3-carboxylate (8d)
4.2.10. (E)-ethyl 5-(4-bromophenyl)-1-(3-(2-(2,4-dimethoxybenzylidene)hydrazinyl)-3-oxopropyl)-2-methyl-1H-pyrrole-3-carboxylate (8e)
4.3. Antioxidant Activity Evaluation
4.3.1. DPPH Radical Scavenging Assay
4.3.2. ABTS Radical Scavenging Assay
4.4. DFT Theoretical Calculations
4.5. In Vitro Pharmacological Evaluations
4.5.1. Cell Line
4.5.2. Cell Viability Assay
4.5.3. H2O2-Induced Oxidative Stress Model in SH-SY5Y Cells
4.5.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Singh, R.P. In vitro methods of assay of antioxidants: An overview. Food Rev. Int. 2008, 24, 392–415. [Google Scholar] [CrossRef]
- Linseman, D.A. Targeting oxidative stress for neuroprotection. Antioxid. Redox Signal. 2009, 11, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef] [PubMed]
- Thurkauf, A.; Yuan, J.; Chen, N.; Wasley, J.W.; Meade, R.; Woodruff, K.H.; Ross, P.C. 1-Phenyl-3-(aminomethyl) pyrroles as potential antipsychotic agents. Synthesis and dopamine receptor binding. J. Med. Chem. 1995, 38, 4950–4952. [Google Scholar] [CrossRef]
- de Oliveira, K.N.; Costa, P.; Santin, J.R.; Mazzambani, L.; Bürger, C.; Mora, C.; Nunes, R.J.; de Souza, M.M. Synthesis and antidepressant-like activity evaluation of sulphonamides and sulphonyl-hydrazones. Bioorg. Med. Chem. 2011, 19, 4295–4306. [Google Scholar] [CrossRef]
- Ragavendran, J.V.; Sriram, D.; Patel, S.K.; Reddy, I.V.; Bharathwajan, N.; Stables, J.; Yogeeswari, P. Design and synthesis of anticonvulsants from a combined phthalimide–GABA–anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007, 42, 146–151. [Google Scholar] [CrossRef]
- Zlatanova, H.; Vladimirova, S.; Kandilarov, I.; Kostadinov, I.; Delev, D.; Kostadinova, I.; Bijev, A. Analgesic effect of a newly synthesized N-pyrrolylcarboxylic acid in experimental conditions. Europ. Neuropsychopharmacol. 2019, 29, S468–S469. [Google Scholar] [CrossRef]
- MacLean, P.D.; Chapman, E.E.; Dobrowolski, S.L.; Thompson, A.; Barclay, L.R.C. Pyrroles as antioxidants: Solvent effects and the nature of the attacking radical on antioxidant activities and mechanisms of pyrroles, dipyrrinones, and bile pigments. J. Org. Chem. 2008, 73, 6623–6635. [Google Scholar] [CrossRef]
- Bhosale, J.D.; Dabur, R.; Jadhav, G.P.; Bendre, R.S. Facile syntheses and molecular-docking of novel substituted 3,4-dimethyl-1H-pyrrole-2-carboxamide/ carbohydrazide analogues with antimicrobial and antifungal properties. Molecules 2018, 23, 875. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, G.; Zeng, Q.H.; Li, Y.; Wu, Y.; Liu, H.; Wang, J.J.; Zhao, Y. Synthesis, antioxidant and anti-tyrosinase activity of 1,2,4-triazole hydrazones as antibrowning agents. Food Chem. 2021, 341, 128265. [Google Scholar] [CrossRef]
- Boulebd, H.; Zine, Y.; Khodja, I.A.; Mermer, A.; Demir, A.; Debache, A. Synthesis and radical scavenging activity of new phenolic hydrazone/hydrazide derivatives: Experimental and theoretical studies. J. Mol. Struct. 2022, 1249, 131546. [Google Scholar] [CrossRef]
- Khodja, A.; Bensouici, C.; Bouleb, H. Combined experimental and theoretical studies of the structure-antiradical activity relationship of heterocyclic hydrazone compounds. J. Mol. Struct. 2020, 1221, 128858. [Google Scholar] [CrossRef]
- Tzankova, D.G.; Vladimirova, S.P.; Peikova, L.P.; Georgieva, M.B. Synthesis and preliminary antioxidant activity evaluation of new pyrrole based aryl hydrazones. Bulg. Chem. Commun. 2019, 51, 179–185. [Google Scholar]
- Bijev, A.; Georgieva, M. Pyrrole-based hydrazones synthesized and evaluated in vitro as potential tuberculostatics. Lett. Drug Des. Discov. 2010, 7, 430–437. [Google Scholar] [CrossRef]
- Georgieva, M.; Bijev, A.; Prodanova, P. Synthesis and comparative study of tuberculostatic activity of pyrrole-based hydrazones related to structural variations. Pharmacia 2010, 52, 3–14. [Google Scholar]
- Tsuneda, T.; Song, J.W.; Suzuki, S.; Hirao, K. On Koopmans’ theorem in density functional theory. J. Chem. Phys. 2010, 133, 174101. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Wang, Y.; Wang, X.; Zhang, Z.; Xin, Y. Neuroprotective effects of melatonin-mediated mitophagy through nucleotide-binding oligomerization domain and leucine-rich repeat-containing protein X1 in neonatal hypoxic–ischemic brain damage. FASEB J. 2023, 37, e22784. [Google Scholar] [CrossRef]
- Joshi, S.D.; Kumar, D.; Dixit, S.R.; Tigadi, N.; More, U.A.; Lherbet, C.; Aminabhavi, T.M.; Yang, K.S. Synthesis, characterization and antitubercular activities of novel pyrrolyl hydrazones and their Cu-complexes. Eur. J. Med. Chem. 2016, 121, 21–39. [Google Scholar] [CrossRef]
- Manvar, A.; Bavishi, A.; Radadiya, A.; Patel, J.; Vora, V.; Dodia, N.; Rawal, K.; Shah, A. Diversity oriented design of various hydrazides and their in vitro evaluation against Mycobacterium tuberculosis H37Rv strains. Bioorg. Med. Chem. Lett. 2011, 21, 4728–4731. [Google Scholar] [CrossRef]
- Nassiri, K.M.; Assarzadeh, M.J.; Almasirad, A.; Ghasemi-Niri, S.F.; Amini, M.; Kebriaeezadeh, A.; Nassiri, K.N.; Ghadimi, M.; Tabei, A. Synthesis and analgesic activity of novel hydrazide and hydrazine derivatives. Iran. J. Pharm. Res. 2013, 12, 721–727. [Google Scholar]
- Rawat, P.; Singh, R.N. Synthesis, spectral and chemical reactivity analysis of 2,4-dinitrophenyl hydrazone having pyrrole moiety. J. Mol. Struct. 2015, 1097, 214–225. [Google Scholar] [CrossRef]
- Sujarwo, W.; Keim, A.P. Spondias pinnata (L. f.) Kurz. (Anacardiaceae): Profiles and Applications to Diabetes. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-813822-9. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy, M.; Jóźwik-Dolęba, M. Importance of solvent association in the estimation of antioxidant properties of phenolic compounds by DPPH method. J. Food Sci. Technol. 2015, 52, 4523–4529. [Google Scholar] [CrossRef]
- Brizzolari, A.; Foti, M.C.; Saso, L.; Ciuffreda, P.; Lazarević, J.; Santaniello, E. Evaluation of the radical scavenging activity of some representative isoprenoid and aromatic cytokinin ribosides (N6-substituted adenosines) by in vitro chemical assays. Nat. Prod. Res. 2022, 36, 6443–6447. [Google Scholar] [CrossRef]
- Schaich, K.M. Lipid oxidation in specialty oils. In Nutraceutical and Specialty Oils; Shahidi., F., Ed.; CRC Press/Taylor & Francis: London, UK, 2006; pp. 401–448. [Google Scholar]
- Amarowicz, R.; Pegg, R.B. Functional Food Ingredients from Plants. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Dasgupta, A.; Klein, K. Methods for Measuring Oxidative Stress in the Laboratory. In Antioxidants in Food, Vitamins and Supplements; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Mateev, E.; Georgieva, M.; Zlatkov, A. Design, microwave-assisted synthesis, biological evaluation, molecular docking, and adme studies of pyrrole-based hydrazide-hydrazones as potential antioxidant agents. Maced. J. Chem. Chem. Eng. 2022, 41, 175–186. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Farrokhnia, M. Density Functional Theory Studies on the Antioxidant Mechanism and Electronic Properties of Some Bioactive Marine Meroterpenoids: Sargahydroquionic Acid and Sargachromanol. ACS Omega 2020, 5, 20382–20390. [Google Scholar] [CrossRef]
- Safna Hussan, K.P.; Shahin Thayyil, M.; Rajan, V.K.; Muraleedharan, K. DFT studies on global parameters, antioxidant mechanism and molecular docking of amlodipine besylate. Comput. Biol. Chem. 2019, 80, 46–53. [Google Scholar] [CrossRef]
- Choudhary, V.K.; Bhatt, A.K.; Dash, D.; Sharma, N. DFT calculations on molecular structures, HOMO–LUMO study, reactivity descriptors and spectral analyses of newly synthesized diorganotin (IV) 2-chloridophenylacetohydroxamate complexes. J. Comput. Chem. 2019, 40, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, J.Z.; Valderrama-Negrón, A.C.; Barrios, J.R.; Freitas, V.L.S.; Ribeiro da Silva, M.D.M.C. Energetic and structural properties of two phenolic antioxidants: Tyrosol and hydroxytyrosol. J. Phys. Chem. A 2018, 122, 4130–4137. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Lu, L.; Qiang, M.; Li, F.; Zhang, H.; Zhang, S. Theoretical investigation on the antioxidative activity of anthocyanidins: A DFT/B3LYP study. Dyes Pigment. 2014, 103, 175–182. [Google Scholar] [CrossRef]
- Lopez-Suarez, L.; Al Awabdh, S.; Coumoul, X.; Chauvet, C. The SH-SY5Y human neuroblastoma cell line, a relevant in vitro cell model for investigating neurotoxicology in human: Focus on organic pollutants. Neurotoxicology 2022, 92, 131–155. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Gao, Q.; Ping, D.; Wang, Y.; Wu, W.; Lin, X.; Fang, Y.; Zhang, J.; Shao, A. The Role of exosomal microRNAs and oxidative stress in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2020, 2020, 3232869. [Google Scholar] [CrossRef]
- Ratan, R.R.; Murphy, T.H.; Baraban, J.M. Oxidative stress induces apoptosis in embryonic cortical neurons. J. Neurochem. 1994, 62, 376–379. [Google Scholar] [CrossRef]
- Floyd, R.A.; Carney, J.M. Free radical damage to protein and DNA: Mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann. Neurol. 1992, 32 (Suppl. S1), S22–S27. [Google Scholar] [CrossRef]
- Kane, D.J.; Sarafian, T.A.; Anton, R.; Hahn, H.; Gralla, E.B.; Valentine, J.S.; Ord, T.; Bredesen, D.E. Bcl-2 inhibition of neural death: Decreased generation of reactive oxygen species. Science 1993, 262, 1274–1281. [Google Scholar] [CrossRef]
- Amoroso, S.; Gioielli, A.; Cataldi, M.; Di Renzo, G.; Annunziato, L. In the neuronal cell line SH-SY5Y, oxidative stress-induced free radical overproduction causes cell death without any participation of intracellular Ca2+ increase. Biochim. Biophys. Acta Mol. Cell Res. 1999, 1452, 151–160. [Google Scholar] [CrossRef][Green Version]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J.; García-Cánovas, F.; Acosta, M. Inhibition by L-ascorbic acid and other antioxidants of the 2.2’-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) oxidation catalyzed by peroxidase: A new approach for determining total antioxidant status of foods. Anal. Biochem. 1996, 236, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Bochevarov, A.D.; Harder, E.; Hughes, T.F.; Greenwood, J.R.; Braden, D.A.; Philipp, D.M.; Rinaldo, D.; Halls, M.D.; Zhang, J.; Friesner, R.A. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. [Google Scholar] [CrossRef]
- Spiegel, M.; Gamian, A.; Sroka, Z. A statistically supported antioxidant activity dft benchmark-the effects of hartree-fock exchange and basis set selection on accuracy and resources uptake. Molecules 2021, 26, 5058. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]




| Reaction Media | Reaction Temperature °C | Reaction Time (min) | Yields % |
|---|---|---|---|
| Ethanol | Heating | 50–60 | 46–80 |
| Ethanol + HCl | Room temperature | 20–30 | 56–94 |
| Methanol + conc. HCl | Room temperature | 1440 | 15–56 |
| Ethanol + glacial acetic acid | 100 °C | 30–50 | 26–64 |
| Glacial acetic acid | 100 °C | 20–30 | 68–84 |
| IDs | m.p. °C | Rf | MS Data [M+H]+ (m/z) | Yields % |
|---|---|---|---|---|
| 7a | 211.4–213.6 | 0.38 | 511.13 | 84 |
| 7b | 229.4–231.2 | 0.33 | 502.05 | 78 |
| 7c | 245.9–247.2 | 0.33 | 512.07 | 76 |
| 7d | 191.9–194.4 | 0.29 | 544.10 | 68 |
| 7e | 206.0–207.6 | 0.40 | 528.11 | 72 |
| 8a | 212.0–213.3 | 0.35 | 525.15 | 86 |
| 8b | 181.4–184.6 | 0.31 | 517.06 | 74 |
| 8c | 214.4–217.1 | 0.33 | 527.09 | 82 |
| 8d | 196.6–197.6 | 0.28 | 558.12 | 64 |
| 8e | 170.0–171.9 | 0.34 | 542.13 | 74 |
| Electronic Parameter | 7d | 8d |
|---|---|---|
| EHOMO | −0.2013 | −0.1976 |
| ELUMO | −0.0564 | −0.0470 |
| ΔEHOMO−LUMO | 0.1449 | 0.1506 |
| Ionization Energy (IP) | 0.2013 | 0.1976 |
| Electron Affinity | 0.0564 | 0.0470 |
| Chemical Hardness | 0.0724 | 0.0753 |
| Softness | 6.9060 | 6.6401 |
| Electronegativity | 0.1288 | 0.1223 |
| Chemical Potential | −0.1288 | −0.1223 |
| Electrophilicity Index | 0.1145 | 0.0992 |
| Compound | Bond | BDE (Kcal/mol) | IP (Kcal/mol) |
|---|---|---|---|
| 7d | O31-H | 83.55 | |
| C7-H | 90.4 | 126.31 | |
| N22-H | 95.10 | ||
| 8d | O30-H | 83.09 | |
| C7-H | 88.9 | 123.99 | |
| N21-H | 96.2 |
| Compound IDs | IC50 [µM] | 95% Confidence Intervals (CI) |
|---|---|---|
| 7a | 55.75 | 55.65–61.37 |
| 7b | 67.60 | 54.31–79.64 |
| 7c | >500 | NA |
| 7d | 99.56 | 88.87–103.23 |
| 7e | 63.08 | 55.56–73.23 |
| 8a | 56.33 | 45.25–67.63 |
| 8b | 57.36 | 46.26–68.36 |
| 8c | 58.23 | 47.36–69.23 |
| 8d | 57.26 | 46.29–68.39 |
| 8e | 91.07 | 83.65–105-36 |
| Melatonin | >500 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzankova, D.; Kuteva, H.; Mateev, E.; Stefanova, D.; Dzhemadan, A.; Yordanov, Y.; Mateeva, A.; Tzankova, V.; Kondeva-Burdina, M.; Zlatkov, A.; et al. Synthesis, DFT Study, and In Vitro Evaluation of Antioxidant Properties and Cytotoxic and Cytoprotective Effects of New Hydrazones on SH-SY5Y Neuroblastoma Cell Lines. Pharmaceuticals 2023, 16, 1198. https://doi.org/10.3390/ph16091198
Tzankova D, Kuteva H, Mateev E, Stefanova D, Dzhemadan A, Yordanov Y, Mateeva A, Tzankova V, Kondeva-Burdina M, Zlatkov A, et al. Synthesis, DFT Study, and In Vitro Evaluation of Antioxidant Properties and Cytotoxic and Cytoprotective Effects of New Hydrazones on SH-SY5Y Neuroblastoma Cell Lines. Pharmaceuticals. 2023; 16(9):1198. https://doi.org/10.3390/ph16091198
Chicago/Turabian StyleTzankova, Diana, Hristina Kuteva, Emilio Mateev, Denitsa Stefanova, Alime Dzhemadan, Yordan Yordanov, Alexandrina Mateeva, Virginia Tzankova, Magdalena Kondeva-Burdina, Alexander Zlatkov, and et al. 2023. "Synthesis, DFT Study, and In Vitro Evaluation of Antioxidant Properties and Cytotoxic and Cytoprotective Effects of New Hydrazones on SH-SY5Y Neuroblastoma Cell Lines" Pharmaceuticals 16, no. 9: 1198. https://doi.org/10.3390/ph16091198
APA StyleTzankova, D., Kuteva, H., Mateev, E., Stefanova, D., Dzhemadan, A., Yordanov, Y., Mateeva, A., Tzankova, V., Kondeva-Burdina, M., Zlatkov, A., & Georgieva, M. (2023). Synthesis, DFT Study, and In Vitro Evaluation of Antioxidant Properties and Cytotoxic and Cytoprotective Effects of New Hydrazones on SH-SY5Y Neuroblastoma Cell Lines. Pharmaceuticals, 16(9), 1198. https://doi.org/10.3390/ph16091198