Investigation of the Interaction between Mechanosynthesized ZnS Nanoparticles and Albumin Using Fluorescence Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solid-State Properties of Mechanochemically Synthesized ZnS—Minireview
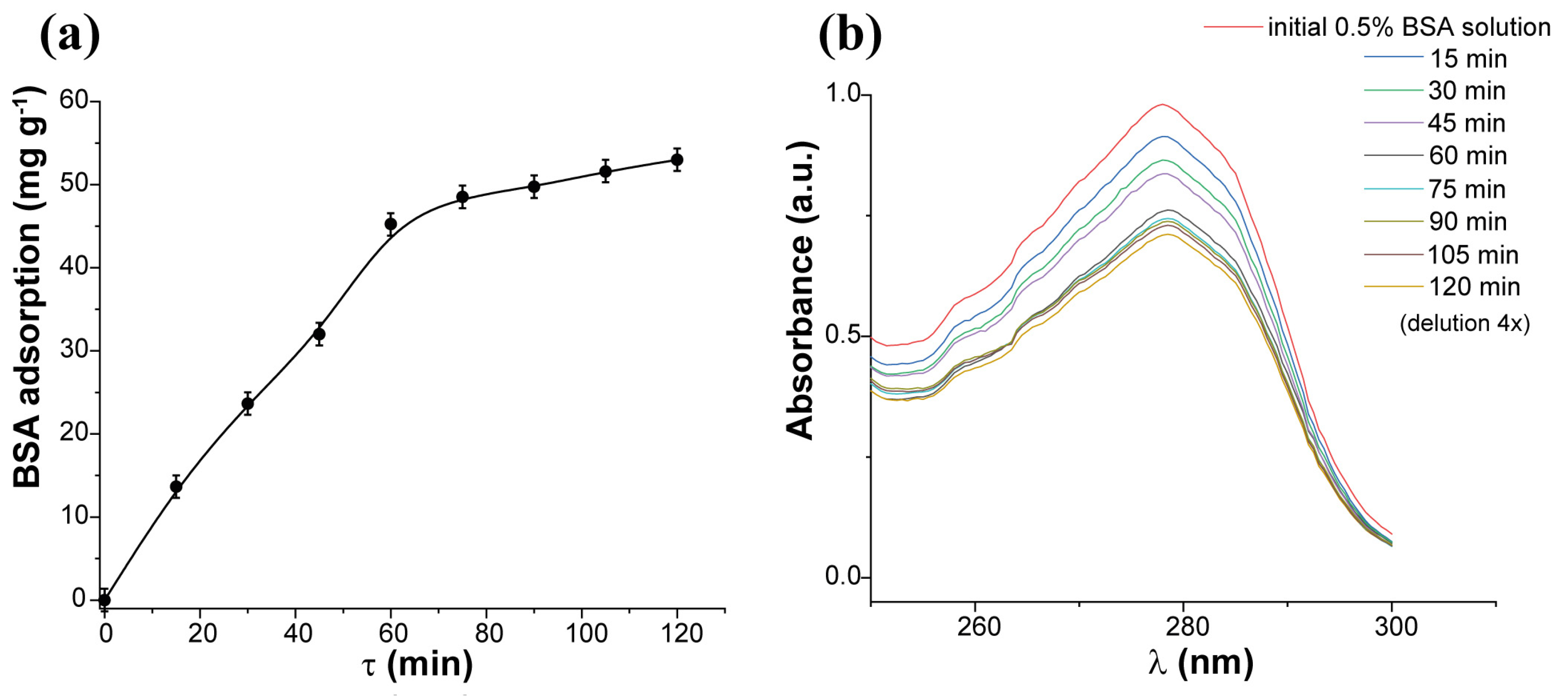
2.2. Sorption Kinetics and Isotherm
2.3. Preparation of ZnS–BSA Nanosuspension
2.4. Interaction Study between ZnS and BSA
2.4.1. UV–Vis Spectroscopy
2.4.2. Fluorescence Quenching Spectra
2.4.3. Stoichiometry of the Binding Process
2.4.4. Accessibility of BSA to ZnS Nanoparticles
2.4.5. Synchronous Fluorescence Spectra
2.4.6. CD Spectroscopy
2.4.7. Fourier Transform Infrared Spectroscopy—FTIR
2.5. Mechanisms of Binding
2.6. Stability Study
2.6.1. Thermal Stability
2.6.2. Long-Term Stability
2.7. Evaluation of the Cellular Effects of ZnS–BSA in Multiple Myeloma Cells
3. Materials and Methods
3.1. Synthesis of ZnS Nanoparticles
3.2. Preparation of ZnS–BSA Nanosuspension
3.3. Adsorption Studies
3.4. Characterization Methods
3.4.1. Particle Size Distribution
3.4.2. Zeta Potential
3.4.3. UV-Vis Specroscopy
3.4.4. Photoluminescence Spectroscopy
3.4.5. Circular Dichroisms Spectroscopy
3.4.6. Fourier-Transform Infrared Spectroscopy
3.4.7. Thermogravimetry
3.4.8. Determination of –SH Groups
3.5. Cell Cultures Studies
3.5.1. Spectrophotometry-Based Colorimetric MTT Cell Assay
3.5.2. Flow-Cytometry Based Fluorescence Cell Assays
3.5.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, B.C.; Guo, Y.; Su, Q.; Zhan, Y.F.; Chen, Z.; Li, Y.; You, B.G.; Dong, X.N.; Chen, S.M.; Wong, W.Y. Cadmium-Doped Zinc Sulfide Shell as a Hole Injection Springboard for Red, Green, and Blue Quantum Dot Light-Emitting Diodes. Adv. Sci. 2022, 9, 2104488. [Google Scholar] [CrossRef]
- Fang, X.S.; Bando, Y.; Liao, M.Y.; Gautam, U.K.; Zhi, C.Y.; Dierre, B.; Liu, B.D.; Zhai, T.Y.; Sekiguchi, T.; Koide, Y.; et al. Single-Crystalline ZnS Nanobelts as Ultraviolet-Light Sensors. Adv. Mater. 2009, 21, 2034–2039. [Google Scholar] [CrossRef]
- Boosagulla, D.; Mandati, S.; Misra, P.; Allikayala, R.; Sarada, B.V. Pulse electrodeposited zinc sulfide as an eco-friendly buffer layer for the cadmium-free thin-film solar cells. Superlattices Microstruct. 2021, 160, 107060. [Google Scholar] [CrossRef]
- Ang, E.H.X.; Zeng, J.L.; Subramanian, G.S.; Chellappan, V.; Sudhaharan, T.; Padmanabhan, P.; Gulyas, B.; Selvan, S.T. Silica-Coated Mn-Doped ZnS Nanocrystals for Cancer Theranostics. ACS Appl. Nano Mater. 2020, 3, 3088–3096. [Google Scholar] [CrossRef]
- Yang, H.M.; Huang, C.H.; Su, X.H.; Tang, A.D. Microwave-assisted synthesis and luminescent properties of pure and doped ZnS nanoparticles. J. Alloys Compd. 2005, 402, 274–277. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.F.; Liu, Y.; Song, Z.W.; Sun, Y.; Fang, J.R.; Liu, Z.S. Synthesis of ZnS nanoparticles via hydrothermal process assisted by microemulsion technique. J. Alloys Compd. 2009, 486, L40–L43. [Google Scholar] [CrossRef]
- Mishra, D.; Prabhakar, P.; Lahiri, S.; Amritphale, S.S.; Chandra, N. Solvothermal synthesis and characterization of zinc sulphide nanoparticles. Indian J. Chem. Sect. A-Inorg. Bio-Inorg. Phys. Theor. Anal. Chem. 2013, 52, 1591–1594. [Google Scholar]
- Balaz, P.; Boldizarova, E.; Godocikova, E.; Briacin, J. Mechanochemical route for sulphide nanoparticles preparation. Mater. Lett. 2003, 57, 1585–1589. [Google Scholar] [CrossRef]
- Pathak, C.S.; Mandal, M.K.; Agarwala, V. Synthesis and characterization of zinc sulphide nanoparticles prepared by mechanochemical route. Superlattices Microstruct. 2013, 58, 135–143. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernandez, J.G. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. Chemsuschem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Biju, V.; Itoh, T.; Ishikawa, M. Delivering quantum dots to cells: Bioconjugated quantum dots for targeted and nonspecific extracellular and intracellular imaging. Chem. Soc. Rev. 2010, 39, 3031–3056. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, A.A.; Aguirre-alvarez, G.; Carino-Cortes, R.; Mendoza-Huizar, L.H.; Jimenez-Alvarado, R. Iron oxide nanoparticles: Synthesis, functionalization, and applications in diagnosis and treatment of cancer. Chem. Pap. 2020, 74, 3809–3824. [Google Scholar] [CrossRef]
- Naskar, M.K.; Patra, A.; Chatterjee, M. Understanding the role of surfactants on the preparation of ZnS nanocrystals. J. Colloid Interface Sci. 2006, 297, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Bujnakova, Z.; Dutkova, E.; Kello, M.; Mojzis, J.; Balaz, M.; Balaz, P.; Shpotyuk, O. Mechanochemistry of Chitosan-Coated Zinc Sulfide (ZnS) Nanocrystals for Bio-imaging Applications. Nanoscale Res. Lett. 2017, 12, 328. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef] [PubMed]
- Mullamuri, B.; Bhagavathula, S.D.; Kasturi, K.C.S.B.; Reddy, V. Facile synthesis of bovine serum albumin conjugated low-dimensional ZnS nanocrystals. Int. J. Biol. Macromol. 2017, 101, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Cen, D.; Ge, Q.W.; Xie, C.K.; Zheng, Q.; Guo, J.S.; Zhang, Y.Q.; Wang, Y.F.; Li, X.; Gu, Z.; Cai, X.J. ZnS@BSA Nanoclusters Potentiate Efficacy of Cancer Immunotherapy. Adv. Mater. 2021, 33, 2104037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, G.Y.; Yao, C.F.; Hu, S.; Hu, C.H.; Zhang, B.B. Paramagnetic albumin decorated CuInS2/ZnS QDs for CD133(+) glioma bimodal MR/fluorescence targeted imaging. J. Mater. Chem. B 2016, 4, 4110–4118. [Google Scholar] [CrossRef] [PubMed]
- Khani, O.; Rajabi, H.R.; Yousefi, M.H.; Khosravi, A.A.; Jannesari, M.; Shamsipur, M. Synthesis and characterizations of ultra-small ZnS and Zn(1-x)FexS quantum dots in aqueous media and spectroscopic study of their interactions with bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 361–369. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Yousefinejad, S. Interaction study of human serum albumin and ZnS nanoparticles using fluorescence spectrometry. J. Mol. Struct. 2013, 1037, 317–322. [Google Scholar] [CrossRef]
- Wu, D.D.; Chen, Z.; Liu, X.G. Study of the interaction between bovine serum albumin and ZnS quantum dots with spectroscopic techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 84, 178–183. [Google Scholar] [CrossRef]
- Liang, C.S.; Liu, X.Q.; Chen, C.Y.; Chen, X.M.; Cai, C.Q. Vitro toxicity assessments of nano-ZnS on bovine serum albumin by multispectroscopic methods. Can. J. Chem. 2016, 94, 877–881. [Google Scholar] [CrossRef]
- Abniki, M.; Azizi, Z.; Panahi, H.A. Design of 3-aminophenol-grafted polymer-modified zinc sulphide nanoparticles as drug delivery system. Iet Nanobiotechnol. 2021, 15, 664–673. [Google Scholar] [CrossRef]
- Li, L.S.; Zhang, Z.Q.; Zhang, Y.B.; Liu, Y.F.; Zhao, M.X. Efficient drug delivery by polyethylenimine capped CdSe/ZnS quantum dots and their biological activity. Mater. Des. 2022, 220, 110890. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Han, Y.H.; Yoo, J.; Seok, H.Y.; Park, J.H.; Kim, Y.C. Evaluation of cell penetrating peptide coated Mn:ZnS nanoparticles for paclitaxel delivery to cancer cells (vol 8, 1899, 2018). Sci. Rep. 2018, 8, 13488. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principle of Fluorescence Spectroscopy; Springer: New York, NY, USA, 1999. [Google Scholar]
- Mariam, J.; Dongre, P.M.; Kothari, D.C. Study of Interaction of Silver Nanoparticles with Bovine Serum Albumin Using Fluorescence Spectroscopy. J. Fluoresc. 2011, 21, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Manjubaashini, N.; Kesavan, M.P.; Rajesh, J.; Thangadurai, T.D. Multispectroscopic and bioimaging approach for the interaction of rhodamine 6G capped gold nanoparticles with bovine serum albumin. J. Photochem. Photobiol. B Biol. 2018, 183, 374–384. [Google Scholar] [CrossRef]
- Shi, X.J.; Li, D.; Xie, J.; Wang, S.; Wu, Z.Q.; Chen, H. Spectroscopic investigation of the interactions between gold nanoparticles and bovine serum albumin. Chin. Sci. Bull. 2012, 57, 1109–1115. [Google Scholar] [CrossRef]
- Hao, C.C.; Xu, G.K.; Feng, Y.; Lu, L.H.; Sun, W.Y.; Sun, R.G. Fluorescence quenching study on the interaction of ferroferric oxide nanoparticles with bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 184, 191–197. [Google Scholar] [CrossRef]
- Prasad, A.R.; Basheer, S.M.; Gupta, I.R.; Elyas, K.K.; Joseph, A. Investigation on Bovine Serum Albumin (BSA) binding efficiency and antibacterial activity of ZnO nanoparticles. Mater. Chem. Phys. 2020, 240, 122115. [Google Scholar] [CrossRef]
- Eixenberger, J.E.; Anders, C.B.; Hermann, R.J.; Brown, R.J.; Reddy, K.M.; Punnoose, A.; Wingett, D.G. Rapid Dissolution of ZnO Nanoparticles Induced by Biological Buffers Significantly Impacts Cytotoxicity. Chem. Res. Toxicol. 2017, 30, 1641–1651. [Google Scholar] [CrossRef]
- Yedomon, B.; Fessi, H.; Charcosset, C. Preparation of Bovine Serum Albumin (BSA) nanoparticles by desolvation using a membrane contactor: A new tool for large scale production. Eur. J. Pharm. Biopharm. 2013, 85, 398–405. [Google Scholar] [CrossRef]
- He, C.B.; Hu, Y.P.; Yin, L.C.; Tang, C.; Yin, C.H. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Godin, B.; Tanaka, T.; Lee, S.Y.; Chiappini, C.; Liu, X.; Ferrari, M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 2010, 141, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Xiang, C.Y.; Xu, Y.; Chen, S.Z.; Zeng, W.W.; Liu, K.; Jin, X.; Zhou, X.; Zhang, B.B. Albumin-constrained large-scale synthesis of renal clearable ferrous sulfide quantum dots for T-1-Weighted MR imaging and phototheranostics of tumors. Biomaterials 2020, 255, 120186. [Google Scholar] [CrossRef] [PubMed]
- Lukacova Bujnakova, Z.; Melnyk, I.; Dutkova, E.; Varhac, R.; Jakubikova, J.; Cholujova, D.; Tothova, E.; Storozhuk, L.; Briancin, J. Nano-bio interface between As4S4 nanoparticles and albumin influenced by wet stirred media milling. Int. J. Pharm. 2023, 640, 123046. [Google Scholar] [CrossRef]
- Dutkova, E.; Balaz, P.; Pourghahramani, P.; Velumani, S.; Ascencio, J.A.; Kostova, N.G. Properties of Mechanochemically Synthesized ZnS Nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 6600–6605. [Google Scholar] [CrossRef]
- Bujnakova, Z.; Balaz, M.; Zduriencikova, M.; Sedlak, J.; Caplovicova, M.; Caplovic, L.; Dutkova, E.; Zorkovska, A.; Turianicova, E.; Balaz, P.; et al. Preparation, properties and anticancer effects of mixed As4S4/ZnS nanoparticles capped by Poloxamer 407. Mater. Sci. Eng. C 2017, 71, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Dutkova, E.; Daneu, N.; Bujnakova, Z.L.; Balaz, M.; Kovac, J.; Kovac, J.; Balaz, P. Mechanochemical Synthesis and Characterization of CuInS2/ZnS Nanocrystals. Molecules 2019, 24, 1031. [Google Scholar] [CrossRef]
- Liu, J.C.; Huang, C.P. Electrokinetic Characteristics of Some Metal Sulfide Water Interfaces. Langmuir 1992, 8, 1851–1856. [Google Scholar] [CrossRef]
- Pugh, R.J.; Tjus, K. Electrokinetic Studies on Cu(Ii) Hydroxy Coated Zinc-Sulfide Particles. J. Colloid Interface Sci. 1987, 117, 231–241. [Google Scholar] [CrossRef]
- Williams, R.; Labib, M.E. Zinc-Sulfide Surface-Chemistry—An Electrokinetic Study. J. Colloid Interface Sci. 1985, 106, 251–254. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef]
- Wieczorek-Ciurowa, K.; Gamrat, K. Some aspects of mechanochemical reactions. Mater. Sci. Pol. 2007, 25, 219–232. [Google Scholar]
- An, F.F.; Zhang, X.H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Zhao, X.C.; Liu, R.T.; Chi, Z.X.; Teng, Y.; Qin, P.F. New Insights into the Behavior of Bovine Serum Albumin Adsorbed onto Carbon Nanotubes: Comprehensive Spectroscopic Studies. J. Phys. Chem. B 2010, 114, 5625–5631. [Google Scholar] [CrossRef]
- Prasanth, S.; RitheshRaj, D.; Vineeshkumar, T.V.; Sudarsanakumar, C. Spectroscopic exploration of interaction between PEG-functionalized Ag2S nanoparticles with bovine serum albumin. Chem. Phys. Lett. 2018, 700, 15–21. [Google Scholar] [CrossRef]
- Li, H.L.; Wu, F.; Tan, J.; Wang, K.; Zhang, C.P.; Zheng, H.Q.; Hu, F.L. Caffeic acid phenethyl ester exhibiting distinctive binding interaction with human serum albumin implies the pharmacokinetic basis of propolis bioactive components. J. Pharm. Biomed. Anal. 2016, 122, 21–28. [Google Scholar] [CrossRef]
- Bhogale, A.; Patel, N.; Sarpotdar, P.; Mariam, J.; Dongre, P.M.; Miotello, A.; Kothari, D.C. Systematic investigation on the interaction of bovine serum albumin with ZnO nanoparticles using fluorescence spectroscopy. Colloids Surf. B Biointerfaces 2013, 102, 257–264. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, D.D. Spectroscopic studies on the interaction between ZnSe nanoparticles with bovine serum albumin. J. Lumin. 2012, 132, 2968–2974. [Google Scholar] [CrossRef]
- Saikia, J.; Saha, B.; Das, G. Interpreting the adsorption of serum albumin and lactoglobulin onto ZnS nanopaticles: Effect of conformational rigidity of the proteins. J. Colloid Interface Sci. 2014, 416, 235–242. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, V.; Carlucci, C.; Cascione, M.; Lorusso, C.; Conciauro, F.; Scremin, B.F.; Congedo, P.M.; Cannazza, G.; Citti, C.; Ciccarella, G. Interaction between Human Serum Albumin and Different Anatase TiO2 Nanoparticles: A Nano-bio Interface Study. Nanomater. Nanotechnol. 2015, 5, 30. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, S.; Piludu, M.; Casula, M.F.; Vallet-Regi, M.; Monduzzi, M.; Salis, A. Interactions between bovine serum albumin and mesoporous silica nanoparticles functionalized with biopolymers. Chem. Eng. J. 2018, 340, 42–50. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Y.Y.; Zhang, X.J.; Yu, C.S.; Zhao, Y.; Sun, S.K. BSA-directed synthesis of CuS nanoparticles as a biocompatible photothermal agent for tumor ablation in vivo. Dalton Trans. 2015, 44, 13112–13118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, X.P. Fabrication of vascular endothelial growth factor antibody bioconjugated ultrasmall near-infrared fluorescent Ag2S quantum dots for targeted cancer imaging in vivo. Chem. Commun. 2013, 49, 3324–3326. [Google Scholar] [CrossRef]
- Maciazek-Jurczyk, M.; Rownicka-Zubik, J.; Dyja, R.; Sulkowska, A. Comparative Analysis of KP-HSA Complex by Spectroscopic Methods. Acta Phys. Pol. A 2013, 123, 673–680. [Google Scholar] [CrossRef]
- Varlan, A.; Hillebrand, M. Bovine and Human Serum Albumin Interactions with 3-Carboxyphenoxathiin Studied by Fluorescence and Circular Dichroism Spectroscopy. Molecules 2010, 15, 3905–3919. [Google Scholar] [CrossRef] [PubMed]
- Marsch, G.A.; Carlson, B.T.; Guengerich, F.P. 7,8-benzoflavone binding to human cytochrome P450 3A4 reveals complex fluorescence quenching, suggesting binding at multiple protein sites. J. Biomol. Struct. Dyn. 2018, 36, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Q.; Jaffe, R. Interaction between Hg(II) and natural dissolved organic matter: A fluorescence spectroscopy based study. Water Res. 2001, 35, 1793–1803. [Google Scholar] [CrossRef]
- Wyatt, W.A.; Bright, F.V.; Hieftje, G.M. Characterization and Comparison of 3 Fiberoptic Sensors for Iodide Determination Based on Dynamic Fluorescence Quenching of Rhodamine-6g. Anal. Chem. 1987, 59, 2272–2276. [Google Scholar] [CrossRef]
- Ballin, J.D.; Prevas, J.P.; Bharill, S.; Gryczynski, I.; Gryczynski, Z.; Wilson, G.M. Local RNA conformational dynamics revealed by 2-aminopurine solvent accessibility. Biochemistry 2008, 47, 7043–7052. [Google Scholar] [CrossRef]
- Miller, J.N. Recent advances in molecular luminescence analysis. Proc. Anal. Div. Chem. Soc. 1979, 16, 203–208. [Google Scholar]
- Huang, P.; Li, Z.M.; Hu, H.Y.; Cui, D.X. Synthesis and Characterization of Bovine Serum Albumin-Conjugated Copper Sulfide Nanocomposites. J. Nanomater. 2010, 2010, 641545. [Google Scholar] [CrossRef]
- Sripriyalakshmi, S.; Anjali, C.H.; Doss, C.G.P.; Rajith, B.; Ravindran, A. BSA Nanoparticle Loaded Atorvastatin Calcium—A New Facet for an Old Drug. PLoS ONE 2014, 9, e86317. [Google Scholar] [CrossRef]
- Retnakumari, A.; Setua, S.; Menon, D.; Ravindran, P.; Muhammed, H.; Pradeep, T.; Nair, S.; Koyakutty, M. Molecular-receptor-specific, non-toxic, near-infrared-emitting Au cluster-protein nanoconjugates for targeted cancer imaging. Nanotechnology 2010, 21, 055103. [Google Scholar] [CrossRef]
- Kollath, V.O.; De Geest, B.G.; Mullens, S.; De Koker, S.; Luyten, J.; Persoons, R.; Traina, K.; Remon, J.P.; Cloots, R. Systematic Processing of beta-Tricalcium Phosphate for Efficient Protein Loading and In Vitro Analysis of Antigen Uptake. Adv. Eng. Mater. 2013, 15, 295–301. [Google Scholar] [CrossRef]
- Kaintura, R.; Sharma, P.; Singh, S.P.; Rawat, K.; Solanski, P.R. Gelatin nanoparticles as a delivery system for proteins. J. Nanomed. Res. 2015, 2, 00018. [Google Scholar]
- Militello, V.; Casarino, C.; Emanuele, A.; Giostra, A.; Pullara, F.; Leone, M. Aggregation kinetics of bovine serum albumin studied by FTIR spectroscopy and light scattering. Biophys. Chem. 2004, 107, 175–187. [Google Scholar] [CrossRef]
- Raussens, V.; Ruysschaert, J.M.; Goormaghtigh, E. Analysis of H-1/H-2 exchange kinetics using model infrared spectra. Appl. Spectrosc. 2004, 58, 68–82. [Google Scholar] [CrossRef]
- Hamedimehr, S.; Aghbash, K.O.; Pesyan, N.N. Zn Complex on Tryptophan-Functionalized MCM-41 as an Efficient and Promising Reusable Nanocatalyst in One-Pot Three-Component Synthesis of Amino Benzyl Quinolinols and Naphthols via a Betti Reaction. ACS Omega 2023, 8, 8227–8236. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, A.E.; Chemes, D.M.; Frias, M.D.; Torres, M.D.G. Spectroscopic characterization and docking studies of ZnO nanoparticle modified with BSA. Appl. Surf. Sci. 2017, 412, 177–188. [Google Scholar] [CrossRef]
- Dengo, N.; Vittadini, A.; Natile, M.M.; Gross, S. In-Depth Study of ZnS Nanoparticle Surface Properties with a Combined Experimental and Theoretical Approach. J. Phys. Chem. C 2020, 124, 7777–7789. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Lehrer, S.S. Solute Perturbation of Protein Fluorescence-Quenching of Tryptophyl Fluorescence of Model Compounds and of Lysozyme by Iodide Ion. Biochemistry 1971, 10, 3254–3263. [Google Scholar] [CrossRef]
- Badyal, J.P.; Cameron, A.M.; Cameron, N.R.; Coe, D.M.; Cox, R.; Davis, B.G.; Oates, L.J.; Oye, G.; Steel, P.G. A simple method for the quantitative analysis of resin bound thiol groups. Tetrahedron Lett. 2001, 42, 8531–8533. [Google Scholar] [CrossRef]











| The BSA Kinetic Sorption Parameters | ||||||
| Pseudo-First Order | Pseudo-Second Order | |||||
| Aeq mg g−1 | k1 min−1 | R2 | Aeq mg g−1 | k2 mg−1 min−1 | h mg min−1 g−1 | R2 |
| 69.7 | 0.0353 | 0.968 | 93.8 | 0.0001 | 1.143 | 0.920 |
| The BSA adsorption parameters estimated by Langmuir and Freundlich isotherm models | ||||||
| Sorption capacity mg g−1 | Langmuir equation | Freundlich equation | ||||
| Amax mg g−1 | KL L mg−1 | R2 | KF mg g−1 | n | R2 | |
| 120.0 | 408.2 | 0.041 | 0.244 | 18.69 | 1.24 | 0.956 |
| Kapp (×103 M−1) | KSV (×104 M−1) | kq (×1011 M−1s−1) | m | Km (×106 M−1) | fa | Ka (×103 M−1) |
|---|---|---|---|---|---|---|
| 2 | 2 | 4.3 | 1.51 | 3.1 | 1.73 | 8.3 |
| BSA | ZnS–BSA after Milling | ZnS–BSA after Adsorption | ||||
|---|---|---|---|---|---|---|
| Vibration (cm−1) | Content (%) | Vibration (cm−1) | Content (%) | Vibration (cm−1) | Content (%) | |
| antiparallel β-sheet | 1686 | 22 | 1689 | 10 | 1680 | 2 |
| α-helix | 1655 | 72 | 1656 | 66 | 1660 | 3 |
| random coil | 1633 | 3 | 1634 | 10 | 1637 | 91 |
| β-sheet | 1620 | 3 | 1620 | 14 | 1618 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukáčová Bujňáková, Z.; Dutková, E.; Jakubíková, J.; Cholujová, D.; Varhač, R.; Borysenko, L.; Melnyk, I. Investigation of the Interaction between Mechanosynthesized ZnS Nanoparticles and Albumin Using Fluorescence Spectroscopy. Pharmaceuticals 2023, 16, 1219. https://doi.org/10.3390/ph16091219
Lukáčová Bujňáková Z, Dutková E, Jakubíková J, Cholujová D, Varhač R, Borysenko L, Melnyk I. Investigation of the Interaction between Mechanosynthesized ZnS Nanoparticles and Albumin Using Fluorescence Spectroscopy. Pharmaceuticals. 2023; 16(9):1219. https://doi.org/10.3390/ph16091219
Chicago/Turabian StyleLukáčová Bujňáková, Zdenka, Erika Dutková, Jana Jakubíková, Danka Cholujová, Rastislav Varhač, Larysa Borysenko, and Inna Melnyk. 2023. "Investigation of the Interaction between Mechanosynthesized ZnS Nanoparticles and Albumin Using Fluorescence Spectroscopy" Pharmaceuticals 16, no. 9: 1219. https://doi.org/10.3390/ph16091219






