Enhanced Antidepressant Activity of Nanostructured Lipid Carriers Containing Levosulpiride in Behavioral Despair Tests in Mice
Abstract
:1. Introduction
2. Results and Discussion
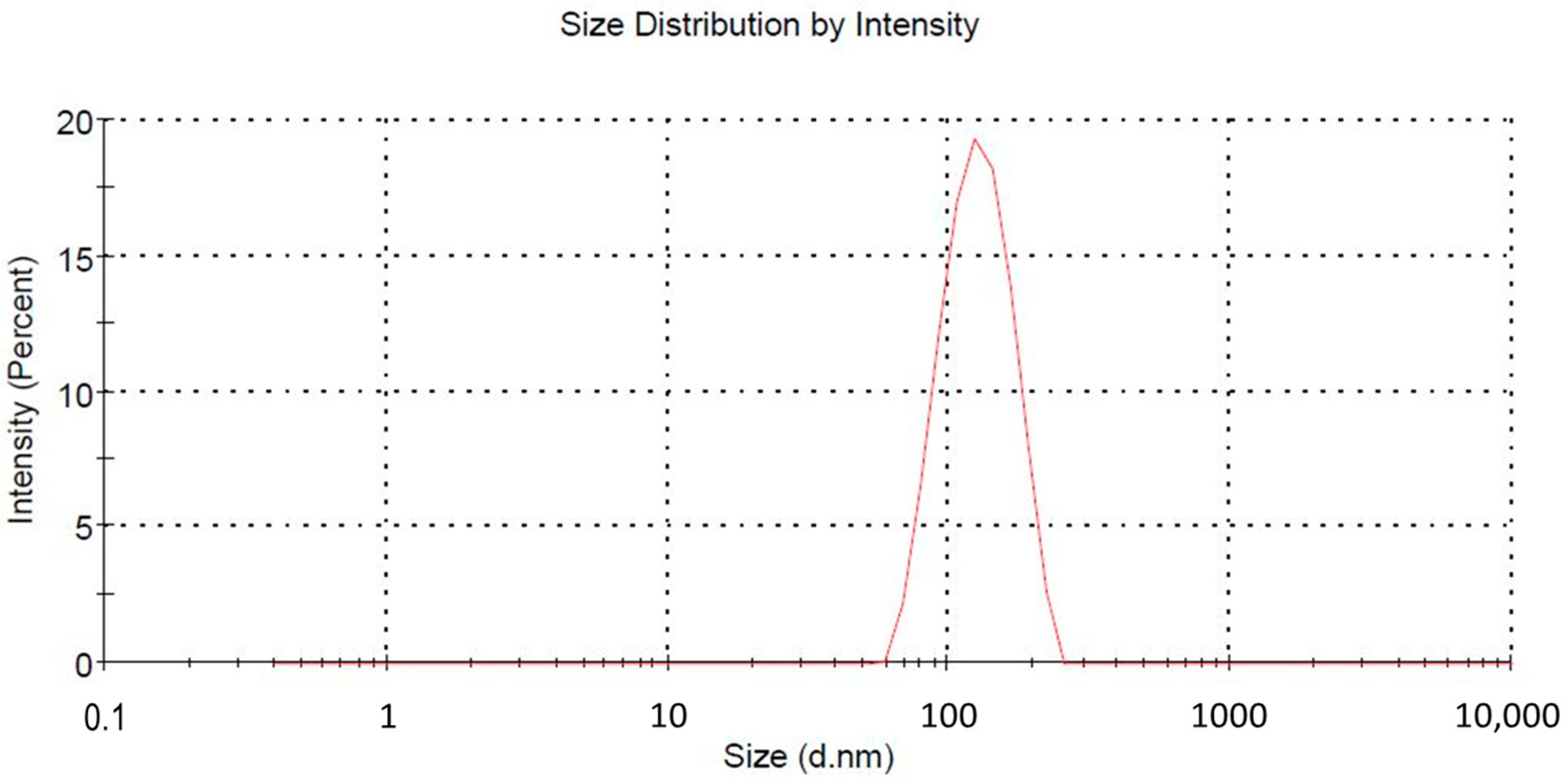
2.1. Particle Characterization and Entrapment Efficiency
2.2. Biodistribution Study
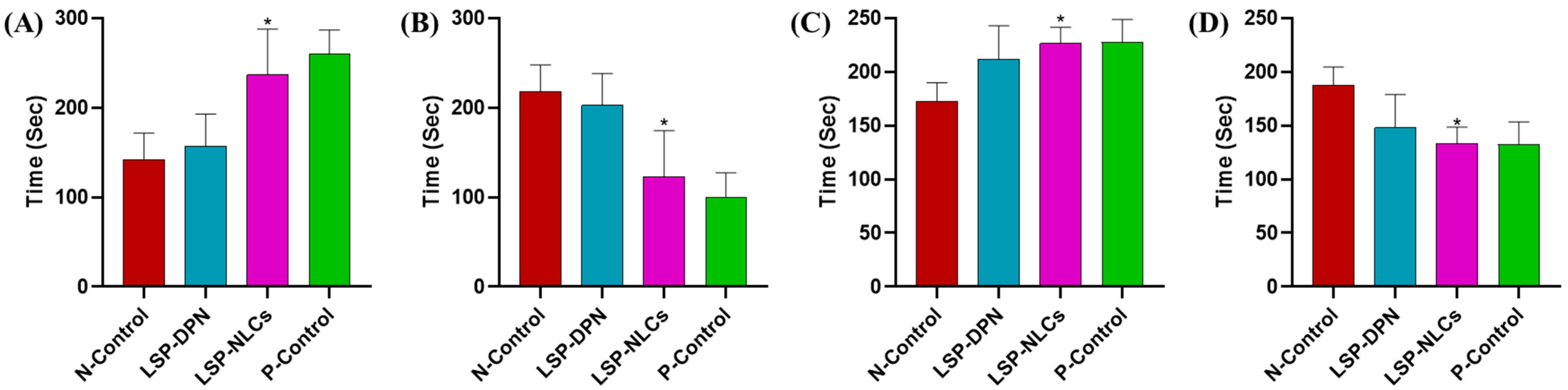
2.3. Antidepressant and Anxiolytic Activity
2.4. Acute Oral Toxicity Study
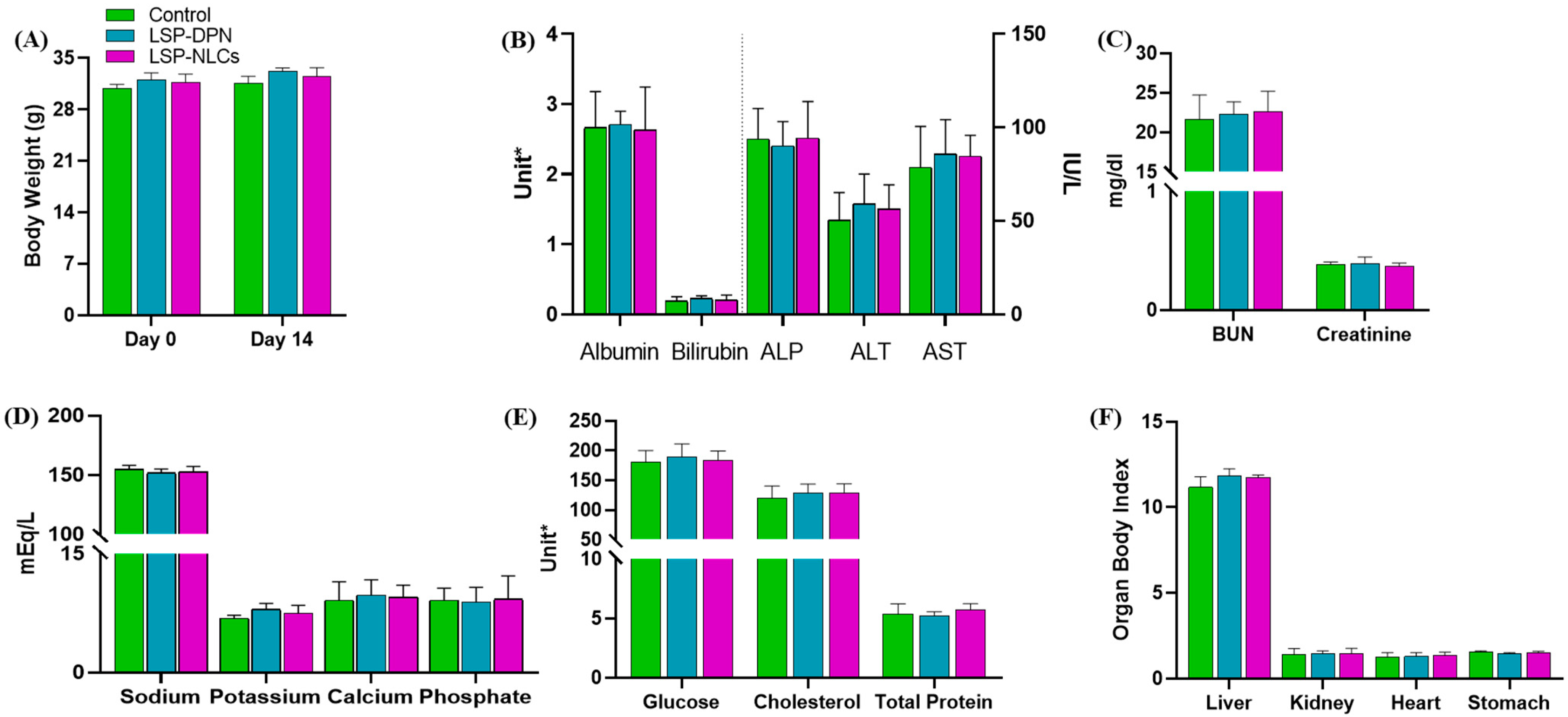
2.4.1. Body Weight Measurements, Observation of Clinical Signs and Food Consumption
2.4.2. Hematology Analysis
2.4.3. Serum Biochemistry
2.4.4. Organ to Body Ratio
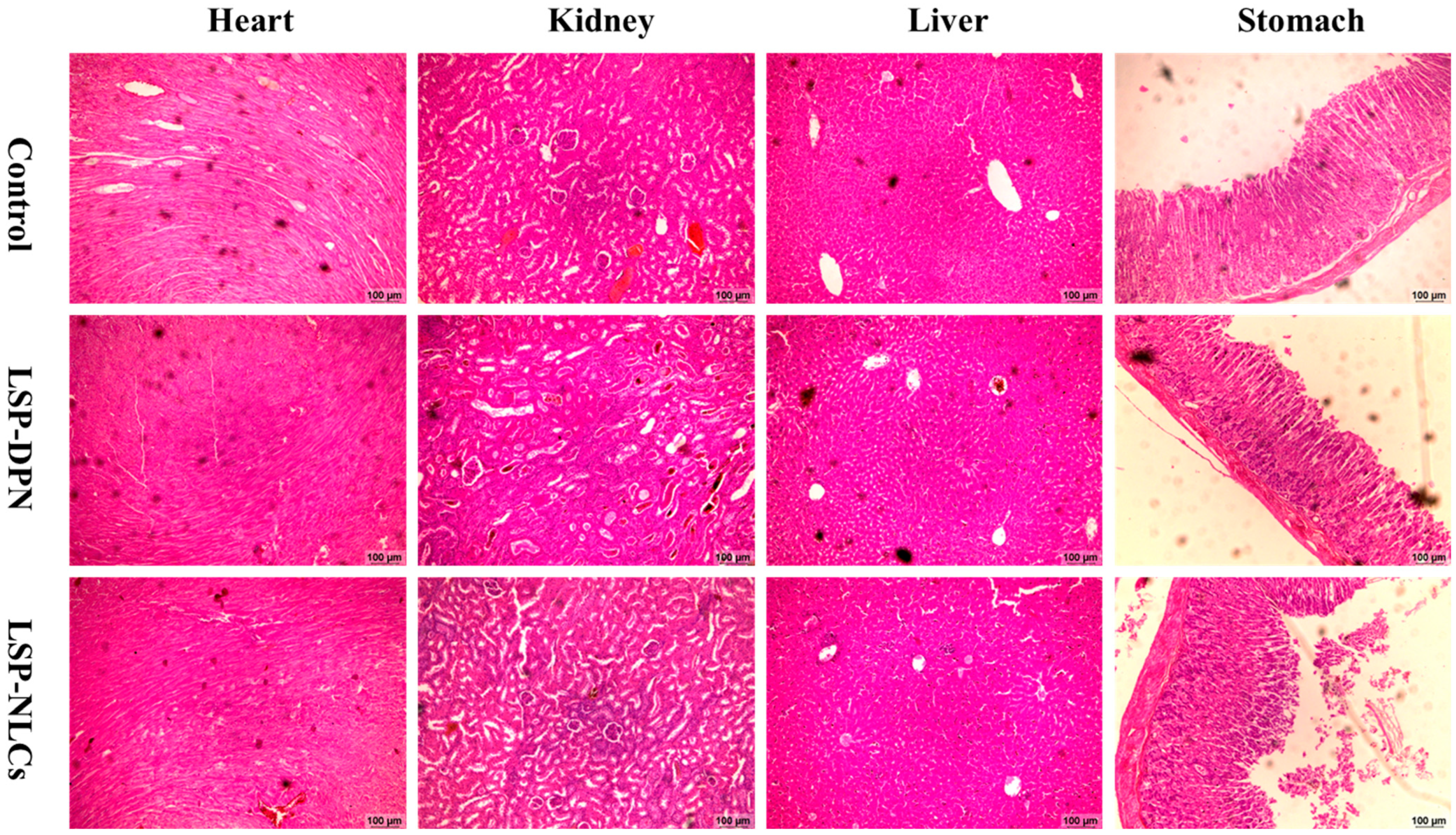
2.4.5. Histopathology of Vital Organs
3. Materials and Methods
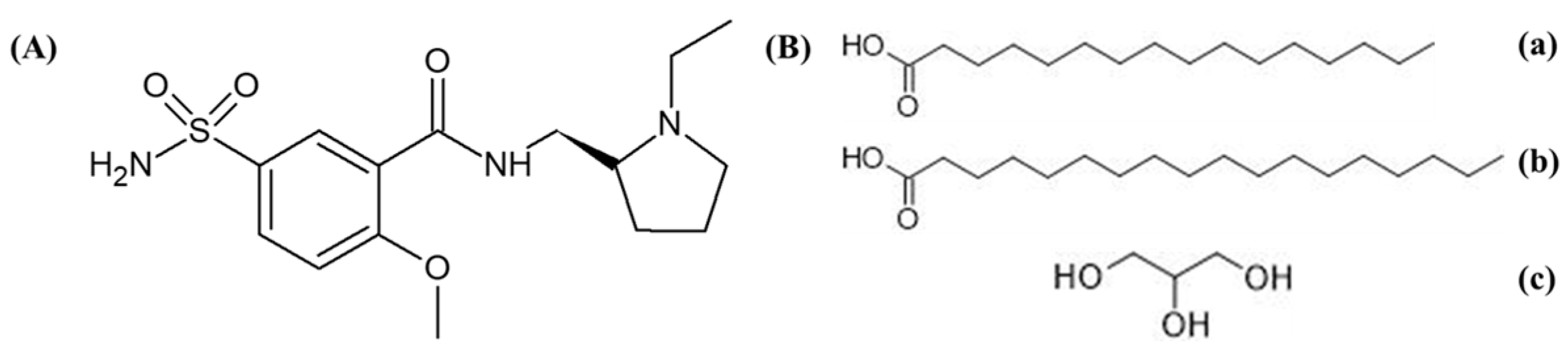
3.1. Materials
3.2. Preparation of LSP-NLCs
3.3. Particle Size, PDI and Zeta Potential Measurement
3.4. Entrapment Efficiency
3.5. In Vivo Studies
Experimental Animals
3.6. Biodistribution Study
3.7. Anti-Depressant and Anxiolytic Activity
3.7.1. Behavioral Studies
Forced Swim Test
Tail Suspension Test
Light and Dark Box Test
Open Field Test
3.8. Acute Oral Toxicity Study
3.8.1. Body Weight Measurements and Observation of Clinical Signs and Food Consumption
3.8.2. Hematology Analysis
3.8.3. Serum Biochemistry
3.8.4. Organ to Body Ratio
3.8.5. Histopathology of Vital Organs
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO and ILO Call for New Measures to Tackle Mental Health Issues at Work. Available online: https://www.who.int/news/item/28-09-2022-who-and-ilo-call-for-new-measures-to-tackle-mental-health-issues-at-work (accessed on 22 July 2023).
- Alam, M.; Najmi, A.K.; Ahmad, I.; Ahmad, F.J.; Akhtar, M.J.; Imam, S.S.; Akhtar, M. Formulation and evaluation of nano lipid formulation containing CNS acting drug: Molecular docking, in-vitro assessment and bioactivity detail in rats. Artif. Cells Nanomed. Biotechnol. 2018, 46, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Kalin, N.H. The Critical Relationship Between Anxiety and Depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Pandey, Y.R.; Kumar, S.; Gupta, B.K.; Ali, J.; Baboota, S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: Formulation, behavioural and biochemical estimation. Nanotechnology 2016, 27, 025102. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Sara, U.V.S.; Chauhan, I.; Gaur, P.K.; Singh, A.P.; Puri, D.; Ameeduzzafar. Solid lipid nanoparticles for nose to brain delivery of donepezil: Formulation, optimization by Box–Behnken design, in vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1838–1851. [Google Scholar] [CrossRef]
- Vitorino, C.; Silva, S.; Gouveia, F.; Bicker, J.; Falcão, A.; Fortuna, A. QbD-driven development of intranasal lipid nanoparticles for depression treatment. Eur. J. Pharm. Biopharm. 2020, 153, 106–120. [Google Scholar] [CrossRef]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K.; Bhatnagar, A. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int. J. Pharm. 2014, 470, 99–106. [Google Scholar] [CrossRef]
- Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. [Google Scholar] [CrossRef]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef]
- Cunha, S.; Almeida, H.; Amaral, M.H.; Lobo, S.J.M.; Silva, A.C. Intranasal Lipid Nanoparticles for the Treatment of Neurodegenerative Diseases. Curr. Pharm. Des. 2017, 23, 6553–6562. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int. J. Pharm. 2002, 242, 377–379. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Lee, Y.-B. Improvement and validation of a liquid chromatographic method for the determination of levosulpiride in human serum and urine. J. Chromatogr. B 2003, 796, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, S.S.; Bhatia, N.M.; Bhatia, M.S. Implications of formulation design on lipid-based nanostructured carrier system for drug delivery to brain. Drug Deliv. 2016, 23, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, R.; Khan, M.I.; Akhtar, M.F.; Madni, A.; Sohail, M.F.; Saleem, A.; Irshad, K.; Sharif, A.; Rana, M. Development and in-vitro evaluation of chitosan and glyceryl monostearate based matrix lipid polymer hybrid nanoparticles (LPHNPs) for oral delivery of itraconazole. Heliyon 2023, 9, e14281. [Google Scholar] [CrossRef]
- Salunkhe, S.S.; Bhatia, N.M.; Thorat, J.D.; Choudhari, P.B.; Bhatia, M.S. Formulation, development and evaluation of ibuprofen loaded nanoemulsion prepared by nanoprecipitation technique: Use of factorial design approach as a tool of optimization methodology. J. Pharm. Investig. 2014, 44, 273–290. [Google Scholar] [CrossRef]
- Jacobs, C.; Müller, R.H. Production and Characterization of a Budesonide Nanosuspension for Pulmonary Administration. Pharm. Res. 2002, 19, 189–194. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, F.; Pan, X.; Zhou, T.; Liu, X.; Zheng, Z.; Luo, L.; Zhang, Y. Investigation of the transport and absorption of Angelica sinensis polysaccharide through gastrointestinal tract both in vitro and in vivo. Drug Deliv. 2017, 24, 1360–1371. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Wang, M.; Jing, G.; Zhu, R.; Wang, S. Antidepressant effects of curcumin and HU-211 coencapsulated solid lipid nanoparticles against corticosterone-induced cellular and animal models of major depression. Int. J. Nanomed. 2016, 11, 4975–4990. [Google Scholar] [CrossRef] [PubMed]
- Mallipeddi, R.; Rohan, L.C. Progress in antiretroviral drug delivery using nanotechnology. Int. J. Nanomed. 2010, 5, 533–547. [Google Scholar]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid lipid nanoparticles for targeted brain drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Ito, S.; Zhang, X.; Ito, H.; Zhang, M.-R.; Suzuki, H.; Maeda, K.; Kusuhara, H.; Suhara, T.; Sugiyama, Y. Possible Role of Organic Cation Transporters in the Distribution of [11C]Sulpiride, a Dopamine D2 Receptor Antagonist. J. Pharm. Sci. 2017, 106, 2558–2565. [Google Scholar] [CrossRef]
- Ford, C.P. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef]
- Singh, S.; Kamal, S.S.; Sharma, A.; Kaur, D.; Katual, M.K.; Kumar, R. Formulation and in-vitro evaluation of solid lipid nanoparticles containing Levosulpiride. Open Nanomed. J. 2017, 4, 17–29. [Google Scholar] [CrossRef]
- Sohail, M.F.; Hussain, S.Z.; Saeed, H.; Javed, I.; Sarwar, H.S.; Nadhman, A.; Huma, Z.E.; Rehman, M.; Jahan, S.; Hussain, I.; et al. Polymeric nanocapsules embedded with ultra-small silver nanoclusters for synergistic pharmacology and improved oral delivery of Docetaxel. Sci. Rep. 2018, 8, 13304. [Google Scholar] [CrossRef]
- Singh, T.; Sinha, N.; Singh, A. Biochemical and histopathological effects on liver due to acute oral toxicity of aqueous leaf extract of Ecliptaalba on female Swiss albino mice. Indian J. Pharmacol. 2013, 45, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Arif, S.T.; Zaman, S.u.; Khan, M.A.; Tabish, T.A.; Sohail, M.F.; Arshad, R.; Kim, J.-K.; Zeb, A. Augmented Oral Bioavailability and Prokinetic Activity of Levosulpiride Delivered in Nanostructured Lipid Carriers. Pharmaceutics 2022, 14, 2347. [Google Scholar] [CrossRef]
- Khan, M.A.; Ansari, M.M.; Arif, S.T.; Raza, A.; Choi, H.-I.; Lim, C.-W.; Noh, H.-Y.; Noh, J.-S.; Akram, S.; Nawaz, H.A.; et al. Eplerenone nanocrystals engineered by controlled crystallization for enhanced oral bioavailability. Drug Deliv. 2021, 28, 2510–2524. [Google Scholar] [CrossRef]
- Sabzichi, M.; Mohammadian, J.; Mohammadi, M.; Jahanfar, F.; Movassagh Pour, A.A.; Hamishehkar, H.; Ostad-Rahimi, A. Vitamin D-Loaded Nanostructured Lipid Carrier (NLC): A New Strategy for Enhancing Efficacy of Doxorubicin in Breast Cancer Treatment. Nutr. Cancer 2017, 69, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Jangra, A.; Kwatra, M.; Singh, T.; Pant, R.; Kushwah, P.; Sharma, Y.; Saroha, B.; Datusalia, A.K.; Bezbaruah, B.K. Piperine Augments the Protective Effect of Curcumin Against Lipopolysaccharide-Induced Neurobehavioral and Neurochemical Deficits in Mice. Inflammation 2016, 39, 1025–1038. [Google Scholar] [CrossRef]
- Es-safi, I.; Mechchate, H.; Amaghnouje, A.; Elbouzidi, A.; Bouhrim, M.; Bencheikh, N.; Hano, C.; Bousta, D. Assessment of Antidepressant-like, Anxiolytic Effects and Impact on Memory of Pimpinella anisum L. Total Extract on Swiss Albino Mice. Plants 2021, 10, 1573. [Google Scholar] [CrossRef]
- Liao, J.-C.; Tsai, J.-C.; Liu, C.-Y.; Huang, H.-C.; Wu, L.-Y.; Peng, W.-H. Antidepressant-like activity of turmerone in behavioral despair tests in mice. BMC Complement. Altern. Med. 2013, 13, 299. [Google Scholar] [CrossRef]
- Saleem, U.; Ahmad, B.; Ahmad, M.; Erum, A.; Hussain, K.; Irfan Bukhari, N. Is folklore use of Euphorbia helioscopia devoid of toxic effects? Drug Chem. Toxicol. 2016, 39, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Tariq, I.; Ali, M.Y.; Sohail, M.F.; Amin, M.U.; Ali, S.; Bukhari, N.I.; Raza, A.; Pinnapireddy, S.R.; Schäfer, J.; Bakowsky, U. Lipodendriplexes mediated enhanced gene delivery: A cellular to pre-clinical investigation. Sci. Rep. 2020, 10, 21446. [Google Scholar] [CrossRef]
- Ali, M.Y.; Tariq, I.; Farhan Sohail, M.; Amin, M.U.; Ali, S.; Pinnapireddy, S.R.; Ali, A.; Schäfer, J.; Bakowsky, U. Selective anti-ErbB3 aptamer modified sorafenib microparticles: In vitro and in vivo toxicity assessment. Eur. J. Pharm. Biopharm. 2019, 145, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, H.S.; Sohail, M.F.; Saljoughian, N.; Rehman, A.U.; Akhtar, S.; Nadhman, A.; Yasinzai, M.; Gendelman, H.E.; Satoskar, A.R.; Shahnaz, G. Design of mannosylated oral amphotericin B nanoformulation: Efficacy and safety in visceral leishmaniasis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.F.; Sarwar, H.S.; Javed, I.; Nadhman, A.; Hussain, S.Z.; Saeed, H.; Raza, A.; Irfan Bukhari, N.; Hussain, I.; Shahnaz, G. Cell to rodent: Toxicological profiling of folate grafted thiomer enveloped nanoliposomes. Toxicol. Res. 2017, 6, 814–821. [Google Scholar] [CrossRef]
- Ali, S.; Amin, M.U.; Tariq, I.; Sohail, M.F.; Ali, M.Y.; Preis, E.; Ambreen, G.; Pinnapireddy, S.R.; Jedelská, J.; Schäfer, J.; et al. Lipoparticles for Synergistic Chemo-Photodynamic Therapy to Ovarian Carcinoma Cells: In vitro and in vivo Assessments. Int. J. Nanomed. 2021, 16, 951–976. [Google Scholar] [CrossRef] [PubMed]


| Groups | Light Dark Box Model | Open Field Test | ||
|---|---|---|---|---|
| Time Spent in Light Box | Number of Entries in the Lightbox | Number of Quadrant Invasion | Central Compartment Resting Time | |
| Positive control | 144 ± 73 | 5 ± 2 | 76 ± 19 | 11 ± 9 |
| Negative control | 52 ± 34 | 2 ± 2 | 19 ± 5 | 2 ± 2 |
| LSP dispersion | 76 ± 32 | 4 ± 1 | 44 ± 12 | 3 ± 2 |
| LSP-NLCs | 119 ± 10 * | 7 ± 2 * | 75 ± 22 ** | 6 ± 2 * |
| Group | WBC | RBC | HCT | Hb | MCV | MCH | PCV | Platelets |
|---|---|---|---|---|---|---|---|---|
| (×109/L) | (×1012/L) | % | (g/dL) | (fL) | (pg) | (%) | (×109/L) | |
| Control | 8.7 ± 2.3 | 8.4 ± 2.5 | 39.0 ± 2.9 | 14.5 ± 1.6 | 47.2 ± 3.1 | 14.4 ± 2.7 | 50.4 ± 7.4 | 762.3 ± 30.6 |
| LSP-DPN | 9.2 ± 2.5 | 9.4 ± 3.6 | 42.0 ± 2.5 | 15.1 ± 2.9 | 49.2 ± 1.9 | 15.0 ± 2.8 | 48.2 ± 5.1 | 755.1 ± 41.0 |
| LSP-NLCs | 8.9 ± 2.9 | 8.6 ± 2.8 | 40.3 ± 3.0 | 15.2 ± 2.2 | 48.8 ± 3.7 | 16.1 ± 2.4 | 48.8 ± 5.5 | 752.9 ± 52.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, S.T.; Khan, M.A.; Zaman, S.u.; Sarwar, H.S.; Raza, A.; Sarfraz, M.; Bin Jardan, Y.A.; Amin, M.U.; Sohail, M.F. Enhanced Antidepressant Activity of Nanostructured Lipid Carriers Containing Levosulpiride in Behavioral Despair Tests in Mice. Pharmaceuticals 2023, 16, 1220. https://doi.org/10.3390/ph16091220
Arif ST, Khan MA, Zaman Su, Sarwar HS, Raza A, Sarfraz M, Bin Jardan YA, Amin MU, Sohail MF. Enhanced Antidepressant Activity of Nanostructured Lipid Carriers Containing Levosulpiride in Behavioral Despair Tests in Mice. Pharmaceuticals. 2023; 16(9):1220. https://doi.org/10.3390/ph16091220
Chicago/Turabian StyleArif, Sadia Tabassam, Muhammad Ayub Khan, Shahiq uz Zaman, Hafiz Shoaib Sarwar, Abida Raza, Muhammad Sarfraz, Yousef A. Bin Jardan, Muhammad Umair Amin, and Muhammad Farhan Sohail. 2023. "Enhanced Antidepressant Activity of Nanostructured Lipid Carriers Containing Levosulpiride in Behavioral Despair Tests in Mice" Pharmaceuticals 16, no. 9: 1220. https://doi.org/10.3390/ph16091220










