Abstract
Novel analogs of quinoline and isoindoline containing various heterocycles, such as tetrazole, triazole, pyrazole, and pyridine, were synthesized and characterized using FT-IR, NMR, and mass spectroscopy, and their antioxidant and antidiabetic activities were investigated. The previously synthesized compound 1 was utilized in conjugation with ketone-bearing tetrazole and isoindoline-1,3-dione to synthesize Schiff’s bases 2 and 3. Furthermore, hydrazide 1 was treated with aryledines to provide pyrazoles 4a–c. Compound 5 was obtained by treating 1 with potassium thiocyanate, which was then cyclized in a basic solution to afford triazole 6. On the other hand, pyridine derivatives 7a–d and 8a–d were synthesized using 2-(4-acetylphenyl)isoindoline-1,3-dione via a one-pot condensation reaction with aryl aldehydes and active methylene compounds. From the antioxidant and antidiabetic studies, compound 7d showed significant antioxidant activity with an EC50 = 0.65, 0.52, and 0.93 mM in the free radical scavenging assays (DPPH, ABTS, and superoxide anion radicals). It also displayed noteworthy inhibitory activity against both enzymes α-glycosidase (IC50: 0.07 mM) and α-amylase (0.21 mM) compared to acarbose (0.09 mM α-glycosidase and 0.25 mM for α-amylase), and higher than in the other compounds. During in silico assays, compound 7d exhibited favorable binding affinities towards both α-glycosidase (−10.9 kcal/mol) and α-amylase (−9.0 kcal/mol) compared to acarbose (−8.6 kcal/mol for α-glycosidase and −6.0 kcal/mol for α-amylase). The stability of 7d was demonstrated by molecular dynamics simulations and estimations of the binding free energy throughout the simulation session (100 ns).
1. Introduction
Diabetes mellitus, often known as type 2 diabetes mellitus (T2DM), is a common metabolic condition characterized by elevated blood sugar levels [1,2,3]. This is due to the destruction of specialized cells (islets of Langerhans) that produce insufficient insulin in patients with T2DM [3,4,5,6]. T2DM is linked to the carbohydrate digestion enzymes α-glycosidase and α-amylase. They speed up the release of monosaccharides by hydrolyzing the α-(1→4) bonds between oligosaccharides. Blood sugar levels rise as a result, increasing the risk of developing T2DM [7,8]. While α-amylase is predominantly produced in the pancreas and released into the small intestine, α-glycosidase is present in the small intestine’s epithelial cells [9]. As carbohydrates undergo digestion in the small intestine, these enzymes are one of the main causes of postprandial hyperglycemia [9]. Inhibiting α-glycosidase and α-amylase is therefore considered one of the most effective strategies for treating T2DM. A variety of chemotherapeutics are used to reduce the enzyme activity of α-glycosidase and α-amylase. However, most of these oral hypoglycemic medications’ side effects involve gastrointestinal issues, affecting over 50% of patients [9,10]. Unabsorbed carbohydrates ferment, releasing gas in the colon, leading to cramps, diarrhea, abdominal bloating, and increased flatulence. Acute or persistent gastrointestinal issues should not be treated with these chemotherapy drugs. Additionally, reports indicate they are associated with hepatic dysfunction and renal impairment [9,10]. Thus, understanding the negative side of these drugs helps in the development of more efficient chemotherapeutics [11].
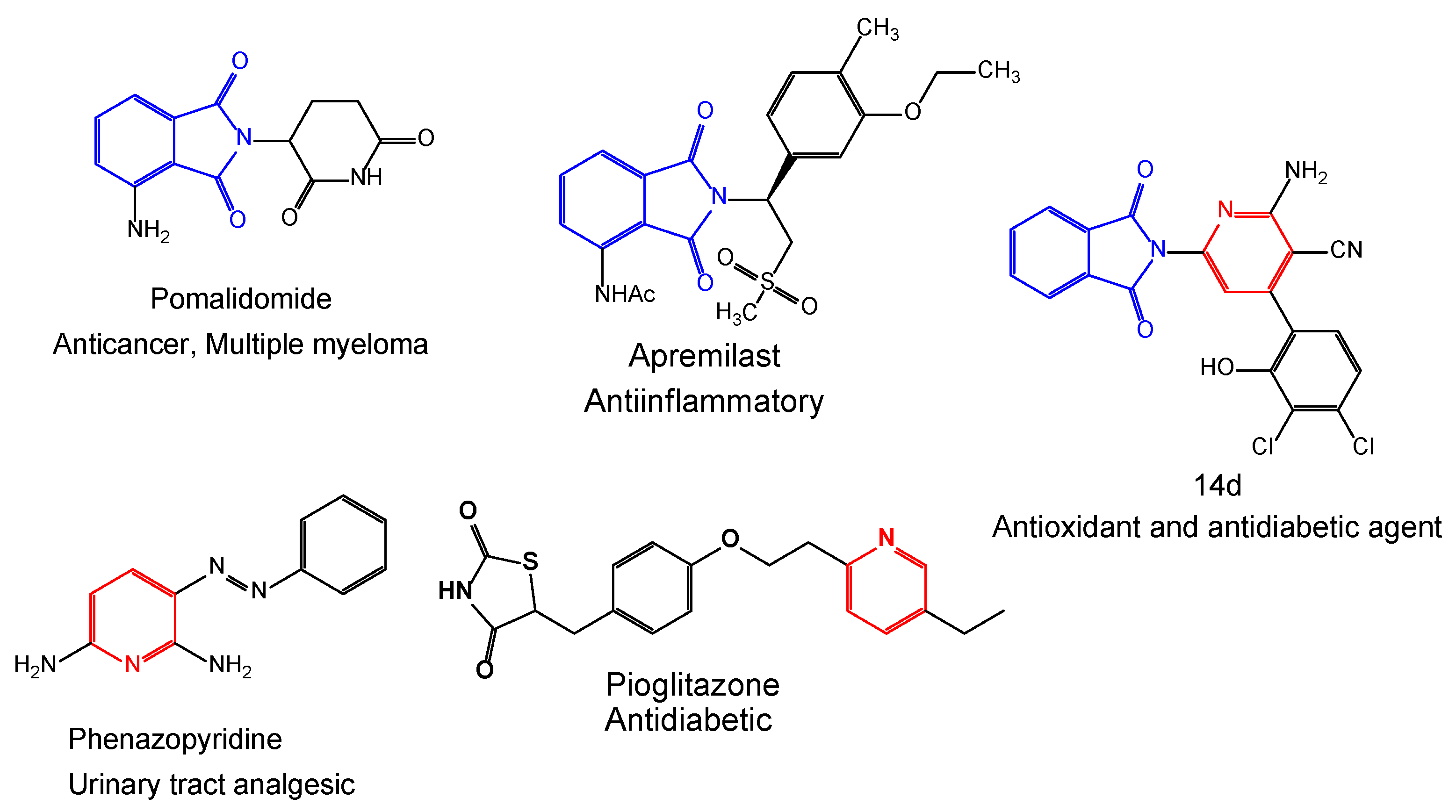
The improvement of useful and novel construction approaches for synthesizing polycyclic-containing heterocyclic molecules has assigned a wide-ranging field of pharmaceutical chemistry [12]. Various attempts have been focused on utilizing hydrazide as a unique synthon for synthesizing five- and six-membered heterocyclic rings, and their great biological applications [13,14,15,16,17,18,19]. Many heterocyclic compounds possessing nitrogen and oxygen exhibit various pharmacological and biological activities involving antimicrobial, antitubercular, antitumor, anti-inflammatory, antileishmanial, serine-protease-inhibitory, and antiviral activity (Figure 1) [16,17,18,19,20].
Figure 1.
Representative examples of biologically active drugs containing isoindoline and pyridine moieties.
For instance, investigations have showcased the antimicrobial potential of certain nitrogen- and oxygen-containing heterocycles. One study reported the synthesis and evaluation of novel 1,2,4-triazole derivatives, revealing potent antibacterial effects against both Gram-positive and Gram-negative bacterial strains [21]. In the realm of antitubercular activity, another study observed the promising inhibitory outcomes of pyrazole derivatives against Mycobacterium tuberculosis [22].
Moreover, heterocyclic compounds have been investigated for their antitumor properties. In a separate study, the effectiveness of indole-fused oxazole derivatives as potent inhibitors of cell proliferation, with an effectiveness surpassing 75%, was highlighted against various cancer cell lines [23]. In the field of anti-inflammatory agents, a study revealed the noteworthy anti-inflammatory activity of pyrimidine derivatives, achieved by obstructing proinflammatory mediators [24]. Furthermore, these compounds have demonstrated efficacy against infectious diseases. Research conducted by de Oliveira et al. (2020) revealed that thiazole derivatives displayed potent antileishmanial activity (>80%) against Leishmania infantum, the causative agent of visceral leishmaniasis [25].
Additionally, heterocyclic compounds have been explored for their potential in inhibiting serine proteases and antiviral properties. A recent study reported that a series of 2,5-diketopiperazines demonstrated significant serine protease inhibitory activity (>77%), which could have implications for antiviral drug development [26]. These examples highlight the significant contribution of heterocyclic compounds containing nitrogen and oxygen in various pharmacological and biological activities, underscoring their potential as a rich source of drug leads for diverse therapeutic applications.
In addition, enaminonitriles and α-aminonitriles are versatile substances that have received great attention as precursor groups for the construction of novel heterocyclic compounds, such as azoles and azines [27,28,29,30,31]. Our research group has earlier synthesized some azole and azine compounds, such as triazole, pyridine, piperazine, and morpholine derivatives, that have anticancer and antimicrobial activities [32,33,34,35].
In the current study, we are focusing on synthesizing new hybrid chemical structures containing N-heterocycles by combining various rings into one framework to produce polycyclic molecules. Our goal is to achieve antioxidant and antidiabetic activities using these new structures, since there have been no insights into the antidiabetic properties of these molecules. We are targeting both carbohydrate-digesting enzymes (α-glycosidase and α-amylase) using both in vitro and in silico approaches. This study contributes to the discovery of quinoline and isoindoline–polycyclic compounds as potential dual inhibitors of both α-glycosidase and α-amylase enzymes.
2. Results and Discussion
2.1. Chemistry
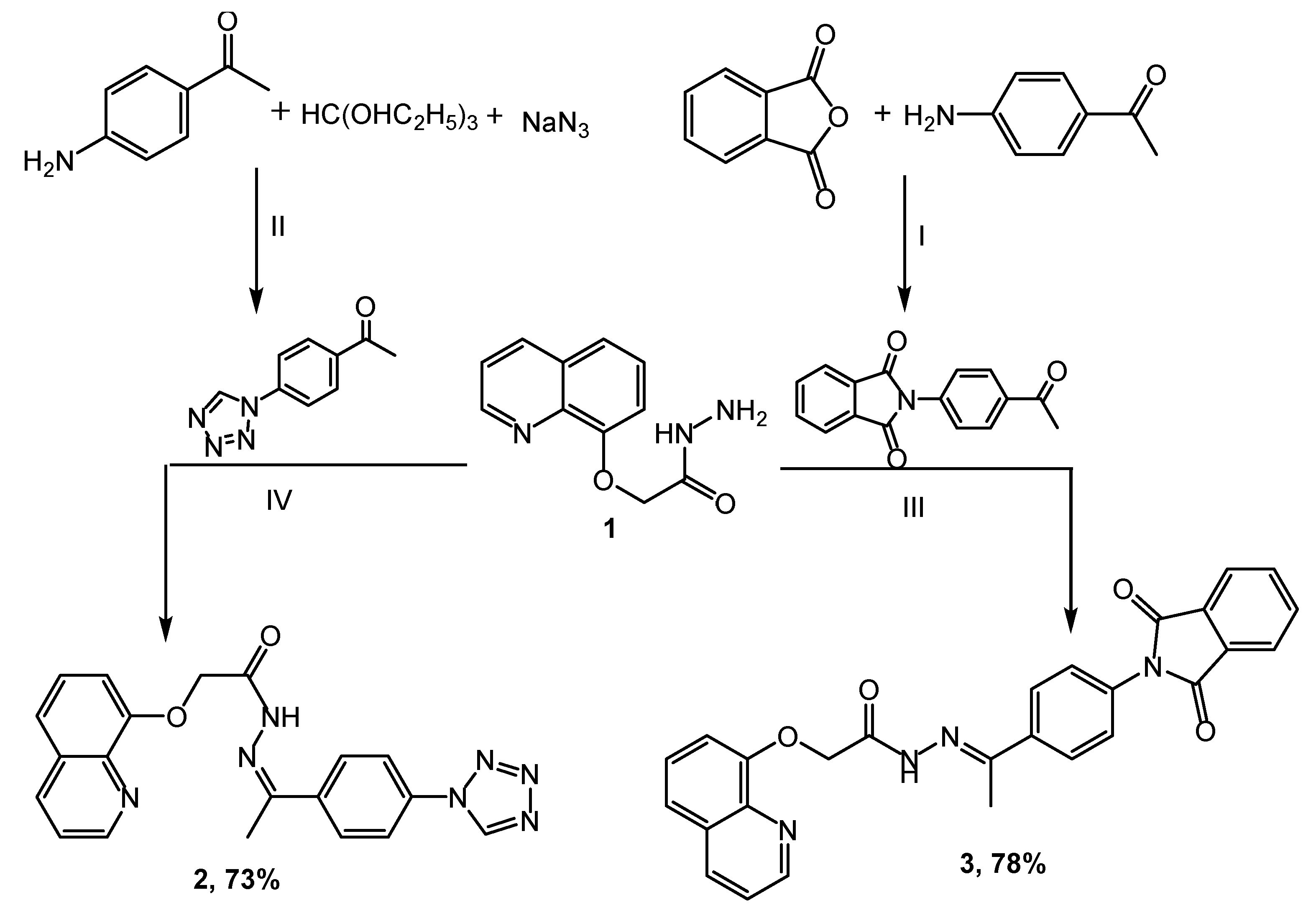
The novel heterocyclic scaffolds, such as tetrazole, triazole, pyrazole, and pyridine, were synthesized as shown in Scheme 1, Scheme 2 and Scheme 3. Quinolinyl hydrazide 1 was synthesized by stirring quinoline ester and hydrazine hydrate in ethanol using the ultrasound irradiation method for 0.5 h [36,37,38]. The key intermediate 1 was used for synthesizing some heterocyclic moieties. The condensation reaction of hydrazide 1 with 1-(4-(1H-tetrazol-1-yl)phenyl)ethanone in acetic acid provides a 73% yield of Schiff base 2. Substrate 3 was achieved by coupling hydrazide 1 with 2-(4-acetylphenyl)isoindoline-1,3-dione in a 78% yield, and the reaction was catalyzed using an amount of glacial acetic acid (Scheme 1).
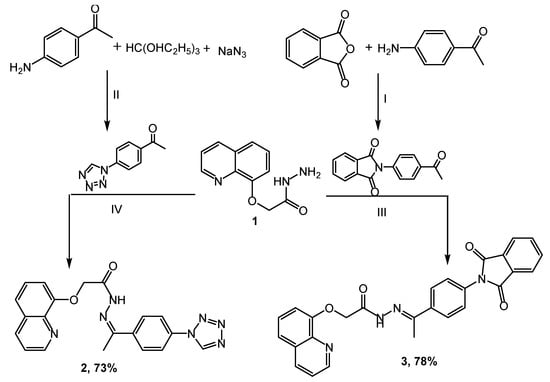
Scheme 1.
Synthesis of compounds (2,3): Reagents and condition: (I) Acetone, reflux for 2 h; (II) NH4Cl, DMF, reflux for 5 h; (III) AcOH, EtOH, reflux for 8 h; (IV) AcOH, EtOH, reflux for 6 h.
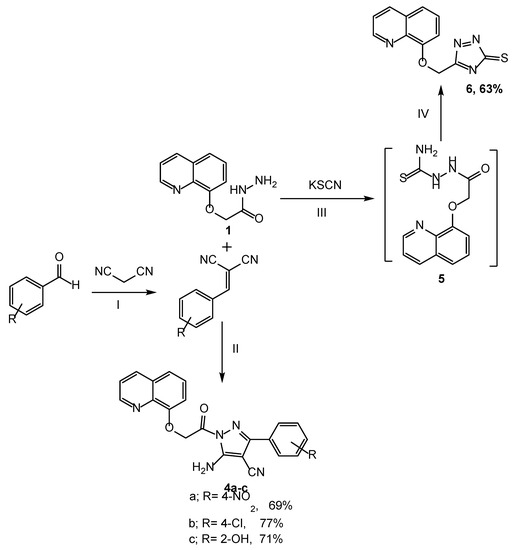
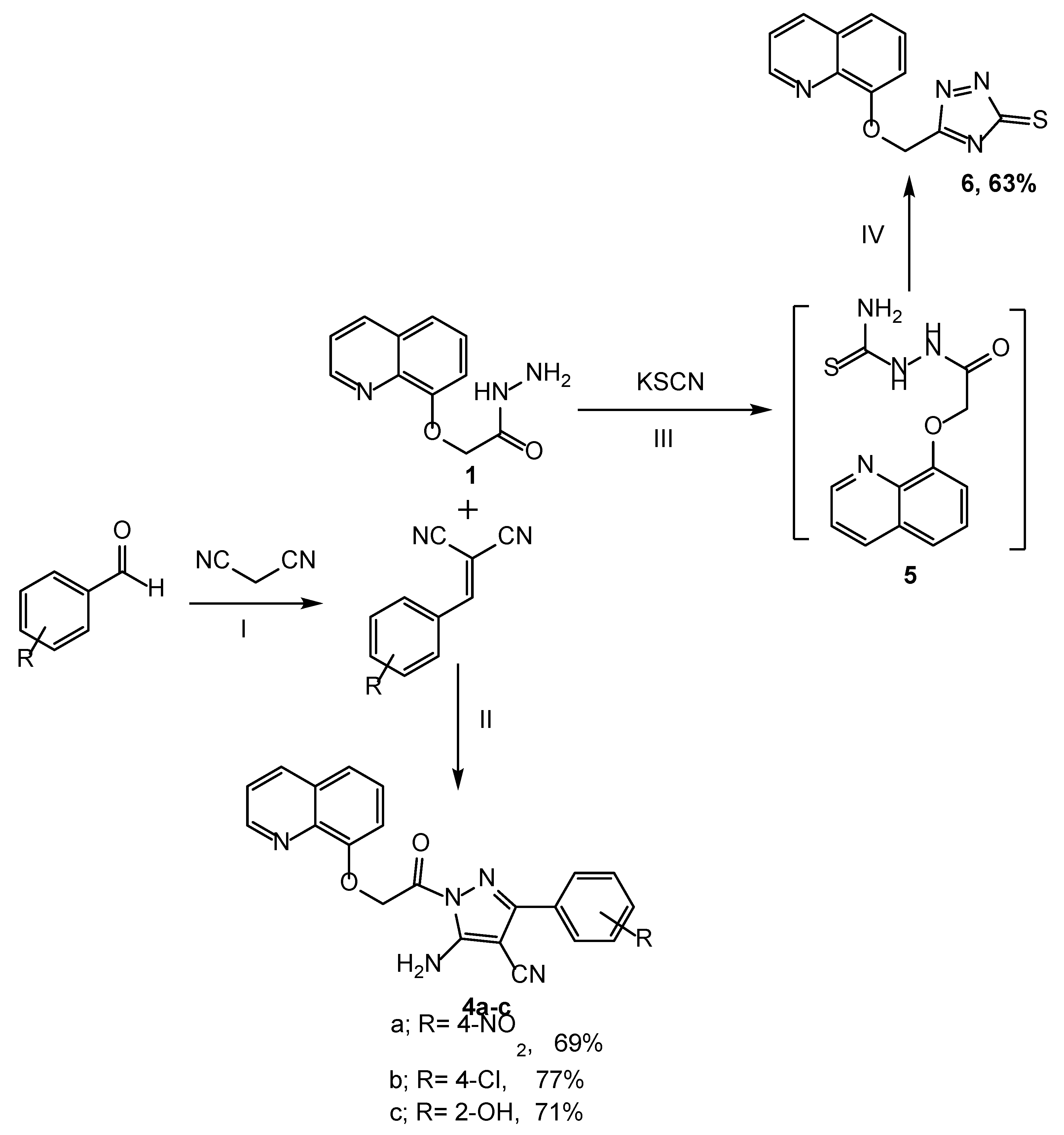
Scheme 2.
Synthesis of compounds (4a–c, 6): Reagents and condition: (I) NH4OAc, ethanol, reflux for 2 h; (II) Propanol, reflux for 9 h; (III) Ethanol: HCl, potassium thiocyanate, reflux for 10 h; (IV) NaOH (6N), reflux for 2 h.
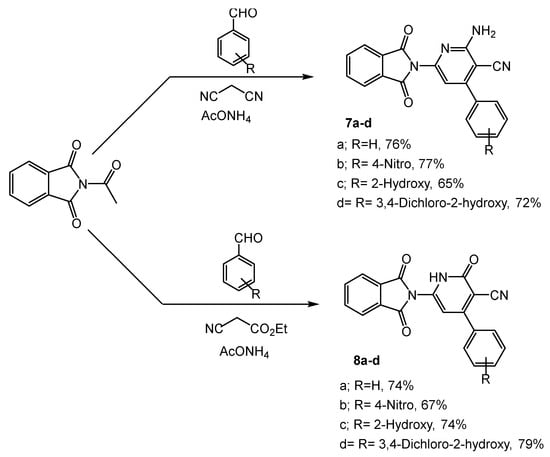
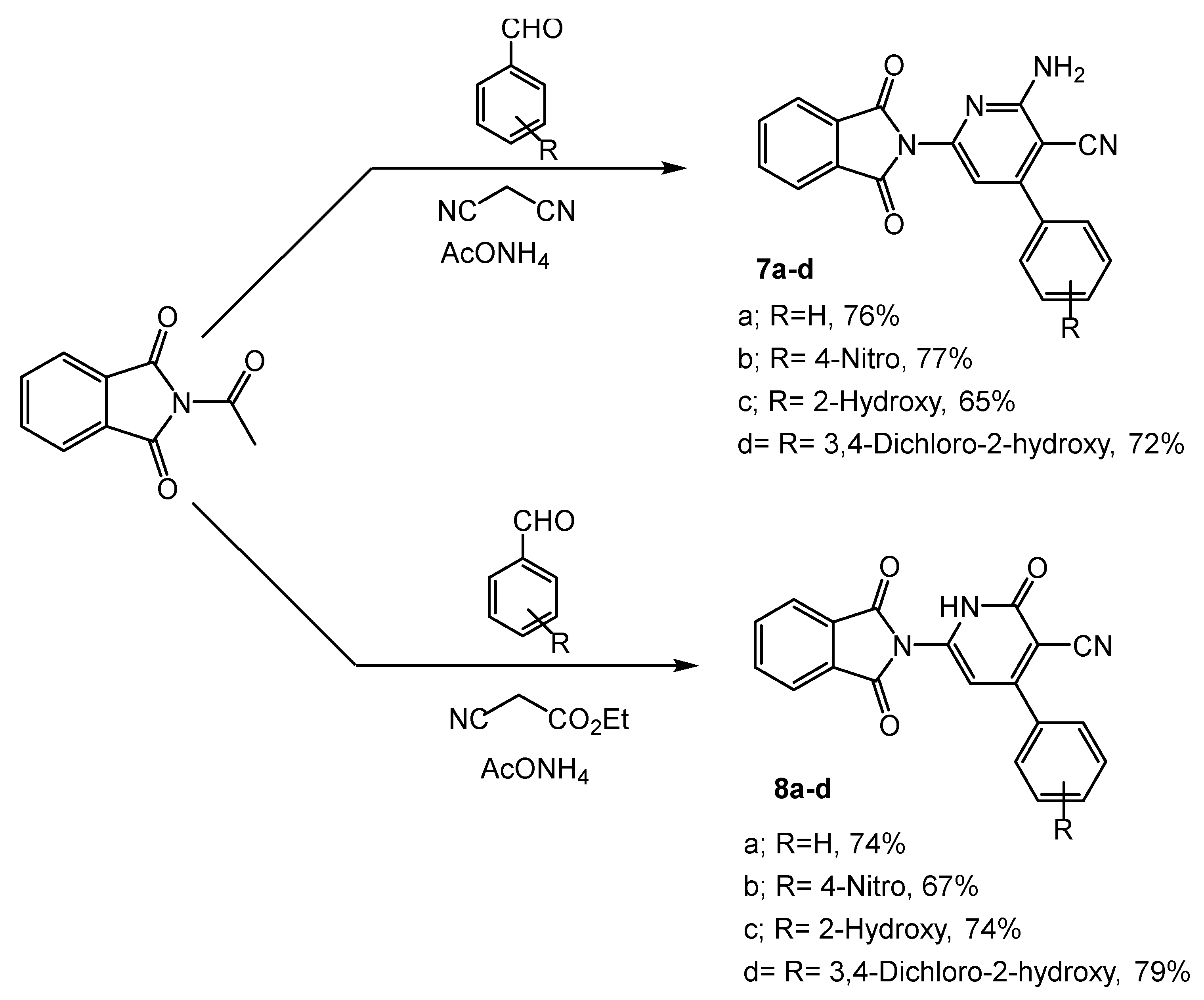
Scheme 3.
Synthesis of pyridine compounds (7a–d, 8a–d): Reagents and condition: EtOH, reflux for 14 h.
In Scheme 2, pyrazoles 4a–c were obtained in 69–77% yields through the treatment of compound 1 with substituted aryledines in propanol for 9 h. Also, the reactions of 1 with potassium thiocyanate afforded compound 5, which was conveniently cyclized using a 10% NaOH solution to produce a new derivative of triazole 6 in a 63% yield. The synthesis was accordance with other fused systems such as pyridine–imidazoles [37].
On the other hand, 2-(4-acetylphenyl)isoindoline-1,3-dione was used as an intermediate for the synthesis of pyridine derivatives 7a–d (65–77%), and 8a–d (67–79%) through a one-pot condensation reaction with aryl aldehydes and some active methylene, such as malononitrile and ethyl cyanoacetate in the presence of ammonium acetate (Scheme 3).
The chemical structures of the target molecules were confirmed based on their IR, NMR, and mass spectral data. The IR spectra of compound 2 have the bands 1673 and 3186 cm−1 allocated to C=O and NH, respectively. The 1H NMR spectra of 2 presented a singlet signal at δ 2.66 assigned to CH3 protons, as well as the signal of the NH proton, which appeared at 10.31 ppm. The bands at 3437 and 1678 cm−1 in compound 3 correspond to amino and carbonyl groups, respectively. Compound 3 was confirmed in 1H NMR by disappearing NH2 protons and by increasing the eight aromatic protons to the earlier protons at 7.26–8.54 ppm.
Furthermore, the functional groups of compounds 4a–c were distinguished by the IR spectrum. The bonds at 3332 and 3255 cm−1 were assigned to the vibrations of NH2. Two new stretching modes were observed at 1687 and 1613, corresponding to the C=O and C=N groups, respectively, whereas compound 6 was distinguished by a band of the thiol group at 825 cm−1. The 1H NMR of compounds 4a–c showed the peak of the new NH2 protons at δ 7.20–7.49 and the disappearance of the NH proton, while the signal of NH2 disappeared and was revealed to form the triazole derivative 6. The structure of derivatives 7a–d and 8a–d was confirmed in the IR spectrum through the appearance of NH2 peaks of 7a–d at 3335 and 3283 cm−1, and the NH absorption peaks of 8a–d at 3340 and 3250 cm−1. The C=O stretching modes of 7a–d were predicted by calculations between 1638 and 1671 cm−1 and at 1674–1680 cm−1 for 8a–d. Also, the appearance of typical bands at δ 7.27–7.50 and 9.16–9.34 corresponded to the protons of NH2 in 7a–d and NH in 8a–d, respectively. In addition, an increased number of aromatic protons obviously indicated the formation of the respective compounds. Furthermore, the structures of the target compounds were also evidently confirmed by mass spectrum.
2.2. Biological Assays
2.2.1. Antioxidant Activity
The ability of the samples to scavenge free radicals was evaluated using a range of in vitro tests, including DPPH, ABTS, and superoxide anion radicals, with BHA utilized as a positive control [39,40,41]. Table 1 summarizes the results reported as EC50 values (mg of tests per mL), demonstrating that the compounds were comparatively greater (p < 0.05) than the standard (positive control) in radical scavenging activities. In all the assays used in this study, 7d was more effective than the other compounds tested. In general, 7d exhibited higher free radical scavenging activity in three antioxidant assays. The results revealed that 7d possesses a strong antioxidant ability and is significantly similar (p < 0.05) compared to the positive control [42]. These results are in accordance with the previous results obtained by the authors for the phytocompounds caffeic acid and syringic acid [43], and synthesized novel phenyl-pyrano-thiazol-2-one derivatives [44].

Table 1.
Antioxidant activity of test samples.
2.2.2. Inhibitory Effects on Yeast α-Glycosidase and α-Amylase
In vitro α-glycosidase inhibitory investigations showed that 7d had a higher potential inhibitory effect than the other tested compounds. The IC50 value was found to be 0.07 mM, while the acarbose examined under the same conditions had an IC50 value of 0.07 mM. In terms of the IC50 values, it is clear that the tested 7d inhibited the yeast α-glycosidase strongly, and was considerably similar (p < 0.05) to acarbose and greater than other substituents (Table 2). Moreover, similar tests were carried out to reveal if the synthesized molecules inhibited α-amylase, another important carbohydrate-hydrolyzing enzyme. Table 2 outlines the 50% inhibition of α-amylase by the test molecules. Results showed that 7d (IC50: 0.21 mM) had the best inhibitory activity when compared to the rest of the compounds. The inhibitory effect of the compounds on α-amylase (based on the IC50 values) was comparatively similar (p < 0.05) to the standard drug acarbose (IC50: 0.25 mM).

Table 2.
Inhibitory activities of test samples against α-amylase and α-glycosidase enzymes.
2.2.3. Kinetic Analysis of α-Glycosidase and α-Amylase Inhibition
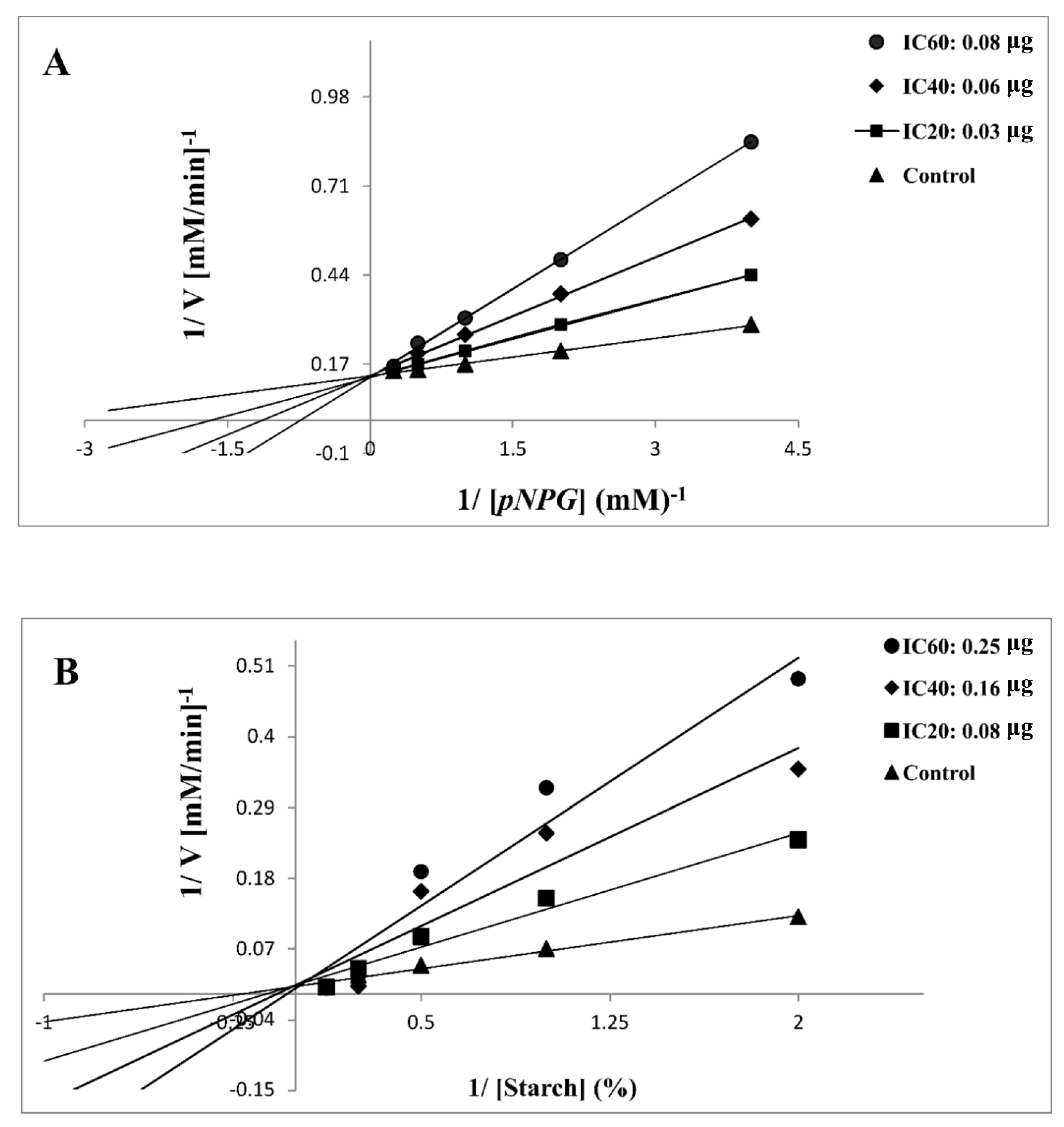
In this study, 7d was selected for further kinetic inhibition experiments against yeast α-glycosidase and α-amylase, since it exhibited remarkable inhibitory activity. For the kinetic studies, α-glycosidase and α-amylase were incubated with designated concentrations of the substrate pNPG and starch, respectively, in the absence (control) or presence of IC20-, IC40-, and IC60-inhibitory concentrations of 7d. The mode of inhibition, [Km], and [Vmax] values were determined by graphical means using Lineweaver–Burk plots. Except for the varied slopes and x-intercepts, the LB plots revealed that the intersecting point for the different concentrations of 7d against α-glycosidase (Figure 2A) and α-amylase (Figure 2B) arose from the same y-intercept as the uninhibited enzyme. With the increasing concentrations of 7d, both the slope and the vertical axis intercept increased, while the horizontal axis intercept (−1/Km) also increased. The kinetic results established that the maximum velocity (Vmax) of the 7d reaction (with increasing concentrations) remained constant and was catalyzed by α-glycosidase and α-amylase (Table 3). These results indicated that the mechanism of α-glycosidase and α-amylase inhibition was reversible, corresponding to the classical pattern of competitive inhibition [42,43,44]. The inhibitory constant (Ki), determined from Dixon plots for α-glycosidase and α-amylase, was 0.47 and 0.75 mg, respectively, of 7d, as shown in Table 3.
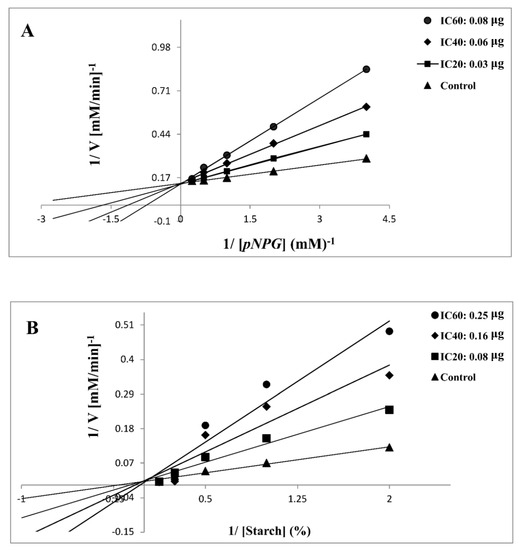
Figure 2.
Lineweaver–Burk plot of substrate-dependent enzyme kinetics on the inhibition of α-glycosidase (A) and α-amylase (B) activity by 7d.

Table 3.
Enzyme kinetics of α-glycosidase and α-amylase by 7d.
2.3. Computational Assays
2.3.1. Molecular Docking Simulation
To understand the interaction of the protein–ligand complex, the docking study was performed for the quinoline and isoindoline–polycyclic compounds (1,2,3,4a–4c,6,7a–7d, and 8a–8d) with their respective target proteins, α-glycosidase and α-amylase, along with the control drug acarbose. Out of 15 compounds docked, compound 7d had the best binding affinity, the highest total number of nonbonded interactions, and the highest number of hydrogen bonds. Compound 7d was chosen for additional in silico analysis because it met the aforementioned criteria for both pharmacological targets, in contrast to acarbose and other quinoline and isoindoline–polycyclic compounds, and the current work was meant to find a dual inhibitor. Results from the molecular docking of quinoline and isoindoline–polycyclic compounds against the target enzymes α-glycosidase and α-amylase have been provided in Table 4.

Table 4.
Molecular docking results of quinoline and isoindoline–polycyclic compounds with their respective target proteins.
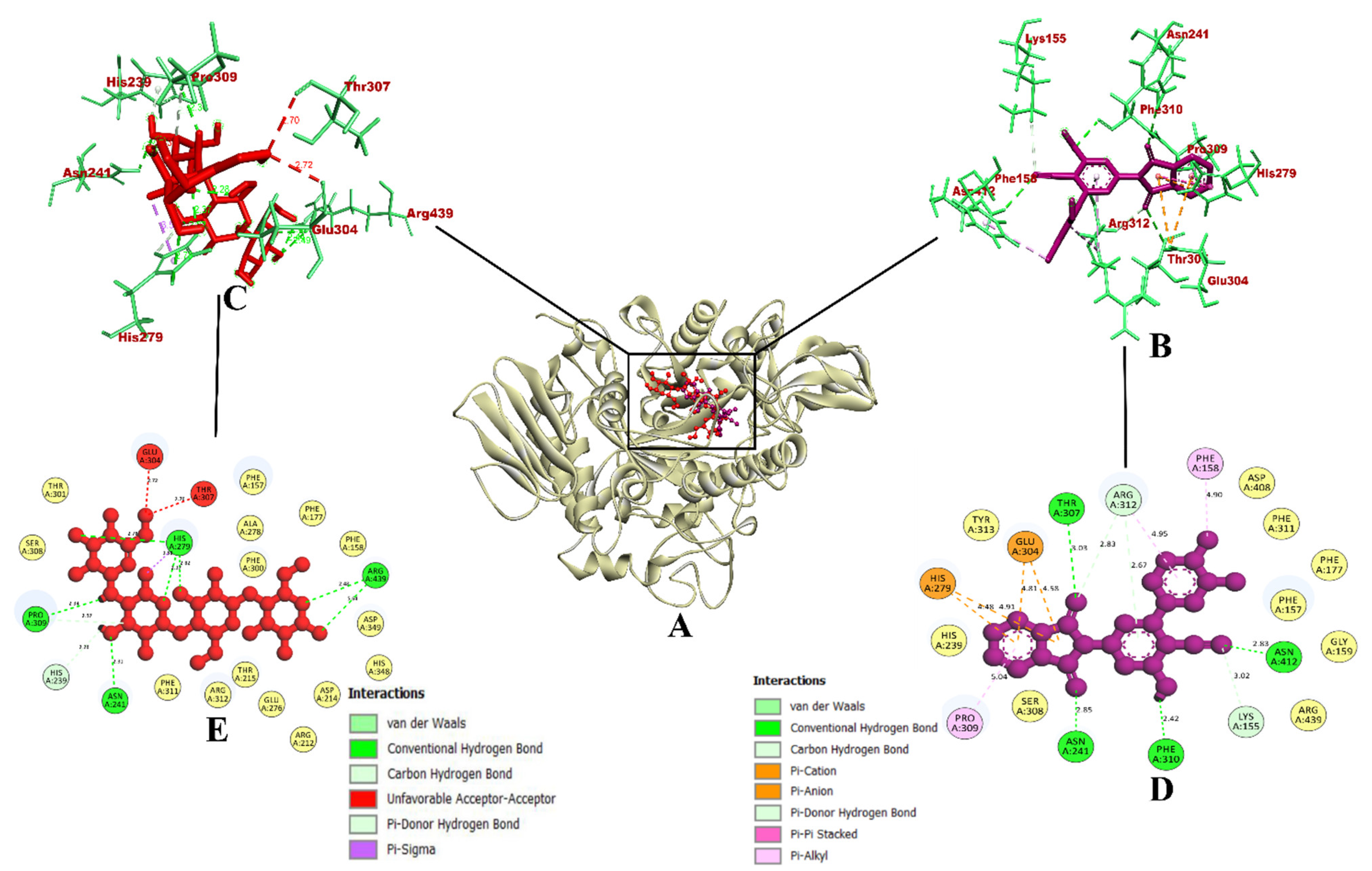
The molecular interaction of compound 7d with protein α-glycosidase had a total of 15 intermolecular interactions, including the formation of 7 hydrogen bonds. The hydrogen bonds were Asn214 (2.85 Å), Thr307 (3.03 Å), Asn412 (2.83 Å), Phe310 (2.42 Å), Lys155 (3.02 Å), Arg312 (2.83 Å), and Arg312 (2.67 Å). In addition, five electrostatic bonds were formed with His279 (4.91 Å), His279 (4.47 Å), Glu304 (4.53 Å), Glu304 (4.58 Å), and Glu304 (4.80 Å). Also, three pi-alkyl bonds with Phe158 (4.90 Å), Arg312 (4.95 Å), and Pro309 (5.04Å) were formed. With these binding interactions in the inhibitor binding site, compound 7d was predicted to have a binding affinity of −10.9 kcal/mol. The acarbose drug had a binding affinity of −8.6 kcal/mol. It had formed a total of nine intermolecular interactions, out of which eight were found to be hydrogen bonds, including Pro309 (2.39 Å), His239 (2.28 Å), Asn241 (2.93 Å), His279 (2.27 Å, 2.82 Å, and 2.42 Å), and Arg439 (2.48 Å and 2.68 Å). It also formed an electrostatic bond with Arg439 (3.58 Å). Even though it had eight hydrogen bonds, the binding affinity was found to be lower than compound 7d.
The binding interactions were similar and the docking was accurate according to a previous study by the authors [45], in which the molecules quercetin and catechin were docked into the same homology-built model of α-glycosidase. The results were also in accordance with a study where the phytocompounds caffeic acid, syringic acid [43], and rutin [46] from jackfruit flour were evaluated for their α-glycosidase-inhibitory activity using the same homology-built model for in silico studies, and yeast α-glycosidase for in vitro studies. For synthesized chemical compounds, a similar pattern of results was obtained in a study where pyrazoline-embedded 1,2,3-triazole derivatives were evaluated for their α-glycosidase-inhibitory activity using the same model in vitro and in silico [47]. Furthermore, another study also depicted the 1-(4-(Methoxy(phenyl)methyl)-2-methylphenoxy)butan-2-one derivative as a single crystal α-glycosidase inhibitor molecule [48]. Since all of these depict the similar binding interactions of their reported potential hit compounds in the same inhibitor binding site of the same homology-built model of α-glycosidase, compound 7d could potentially act as an inhibitor of α-glycosidase. The visualization of the binding interactions of compound 7d and acarbose with α-glycosidase in 3D is presented in Figure 3.
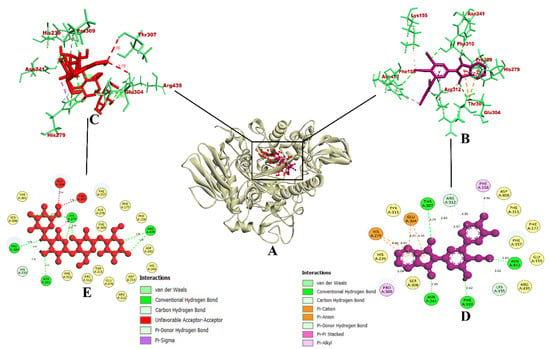
Figure 3.
(A) Interaction of 7d compound in the binding site of protein α-glycosidase; (B) Representation of the 3D structure of 7d interaction with protein; (C) Representation of the 3D structure of the acarbose interaction with protein; (D,E) A 2D representation of compound 7d and acarbose with bonded and nonbonded residues, along with their respective distances.
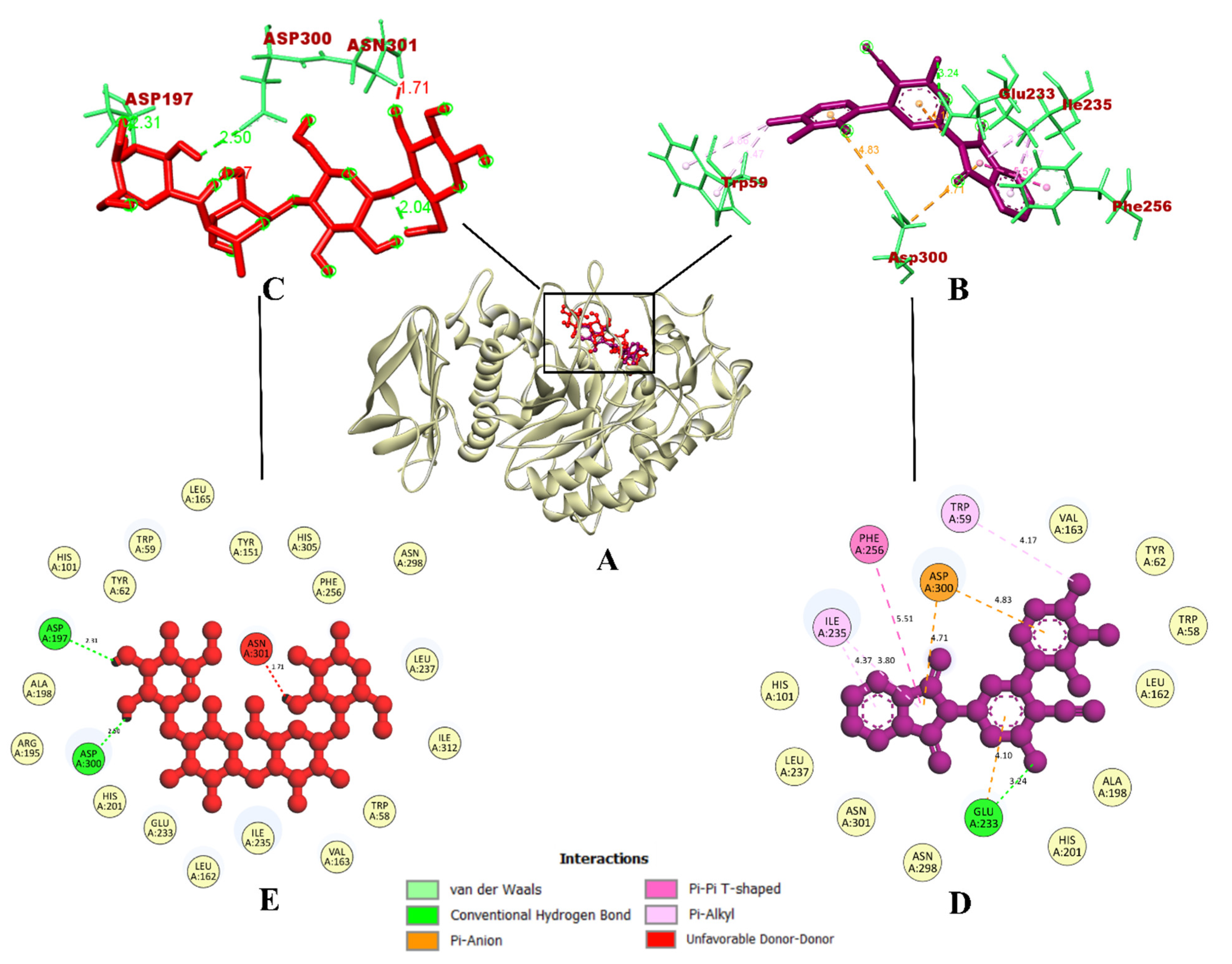
On the other hand, compound 7d interacted with protein α-amylase, forming a total of seven intermolecular interactions. Among these, one hydrogen bond was established with Glu233 (3.24 Å). Additionally, a pi-pi bond with Phe256 (5.51 Å), three pi-alkyl bonds with Trp59 (4.17 Å), and Ile235 (4.37 Å and 3.80 Å) were formed. Further, two electrostatic pi-anion bonds with Asp300 (4.71 and 4.83 Å) were also formed. These binding interactions within the inhibitor binding site led to the prediction of a binding affinity of −9.0 kcal/mol for compound 7d. In contrast, the control drug acarbose was predicted to have a binding affinity of −6.0 kcal/mol. It had formed a total of four intermolecular interactions, which were hydrogen bonds formed with Pro332 (2.54 Å), Arg398 (2.44 Å), Tyr333 (2.58 Å), and Trp280 (1.99 Å).
Despite having five hydrogen bonds, it was observed that compound 7d had a better binding affinity. The binding interactions of compound 7d were similar and the docking was accurate, according to a previous study [41], where phenolic compounds such as caffeic acid and syringic acid were found to inhibit the same α-amylase protein model (PDB ID: 1DHK). In the case of the synthesized compounds, fluorinated 2,3-disubstituted thiazolidinone–pyrazoles [49], and (2-chloro-6-(trifluoromethyl) benzyloxy) arylidene)-based rhodanine and rhodanine-acetic-acid derivatives [50] were reported as potential hit compounds for the α-amylase protein model (PDB ID: 1DHK). By virtue of the similar binding interactions of their reported potential hit compounds in the same inhibitor binding site of the α-amylase protein, compound 7d could act as a potential inhibitor. The visualization of the binding interactions of compound 7d and acarbose with α-amylase in 3D has been provided in Figure 4. Since compound 7d was found with the highest binding efficiency compared to all of the quinoline and isoindoline–polycyclic compounds and acarbose, the compound could act as a potential inhibitor of both α-glycosidase and α-amylase proteins.
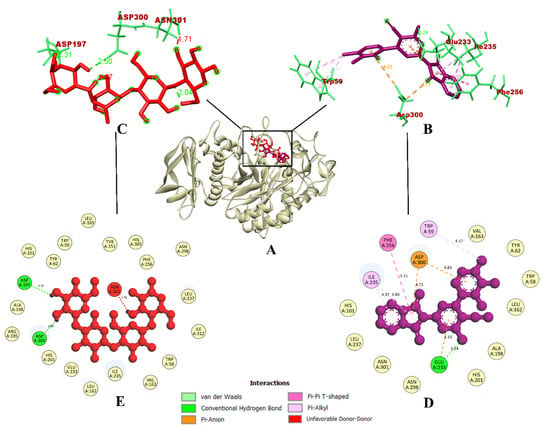
Figure 4.
(A) Interaction of 7d compound in the binding site of protein α-amylase; (B) Representation of the 3D structure of the 7d interaction with protein; (C) Representation of the 3D structure of the acarbose interaction with protein; (D,E) A 2D representation of compound 7d and acarbose with bonded and nonbonded residues, along with their respective distances.
2.3.2. Molecular Dynamics Simulation
Simulation studies analyze the dynamic behavior of protein–ligand complexes in a solvated environment [51,52]. They measure the protein–ligand complex’s root-mean-square deviation (RMSD), radius of gyration (Rg), solvent-accessible surface area (SASA), ligand RMSD, hydrogen bonds, and variation of the secondary-structure pattern between the protein and their complexes [53]. The RMSD of the protein–ligand complex reflects its stability and indicates the presence of a ligand in the binding pocket. The Rg measures the varied masses to the RMS distances considering the central axis of rotation.
This study analyzes the protein’s structural changes during simulation, including its shape, folding, and capability at each time step along the entire trajectory. The RMSF focuses on the protein structural regions that deviate the most/least from the mean. The SASA examines the formation of the area around the hydrophobic cores between the protein–ligand complexes and analyzes the ligand hydrogen bonds over the total simulation period [54]. The intermolecular hydrogen bonds between the ligands and their respective proteins are also considered and plotted during the analysis [55]. In this paper, six simulations were conducted for 100 ns with the native protein alone and in complex with the representative compounds (compound 7d and acarbose).
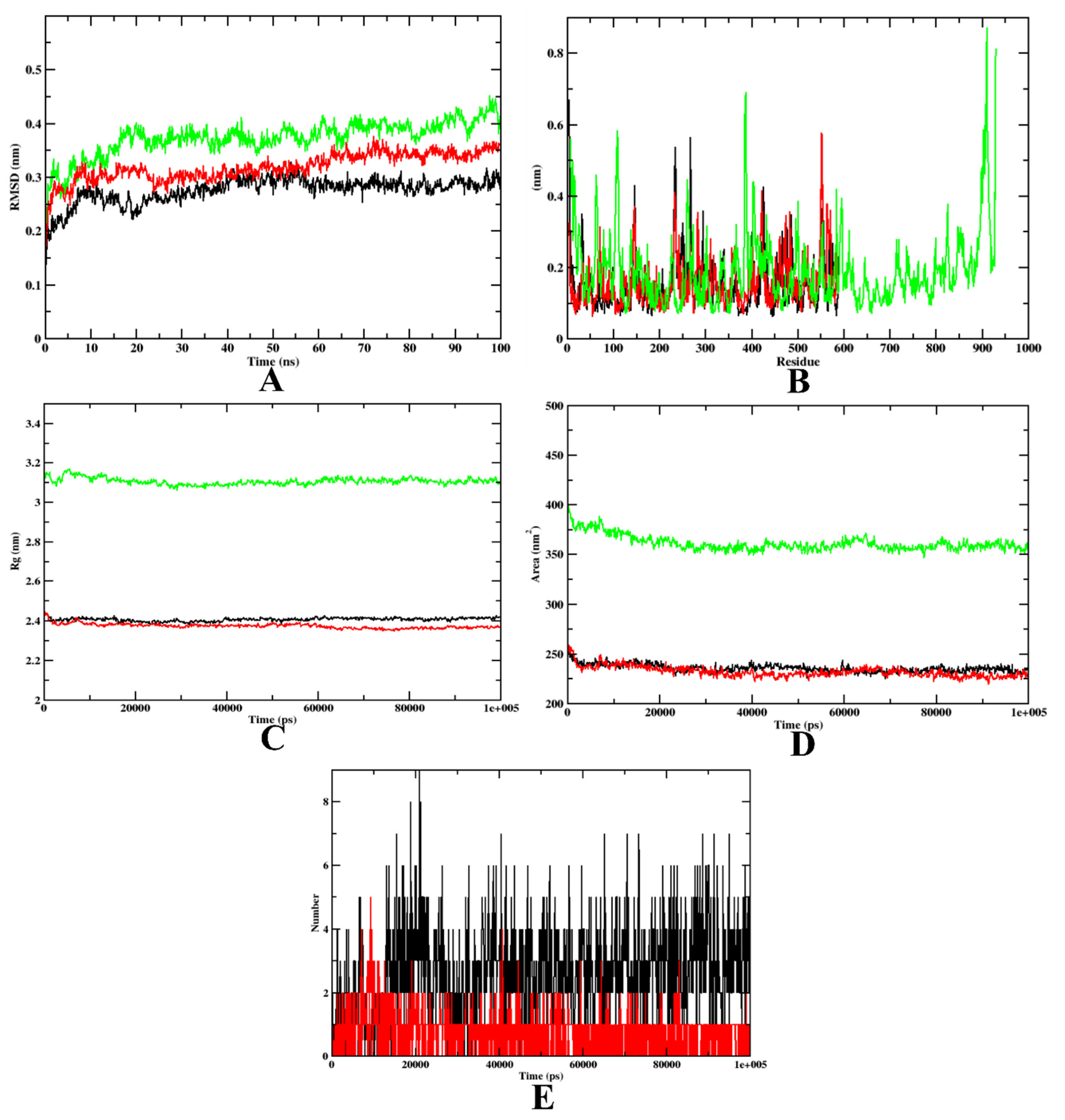
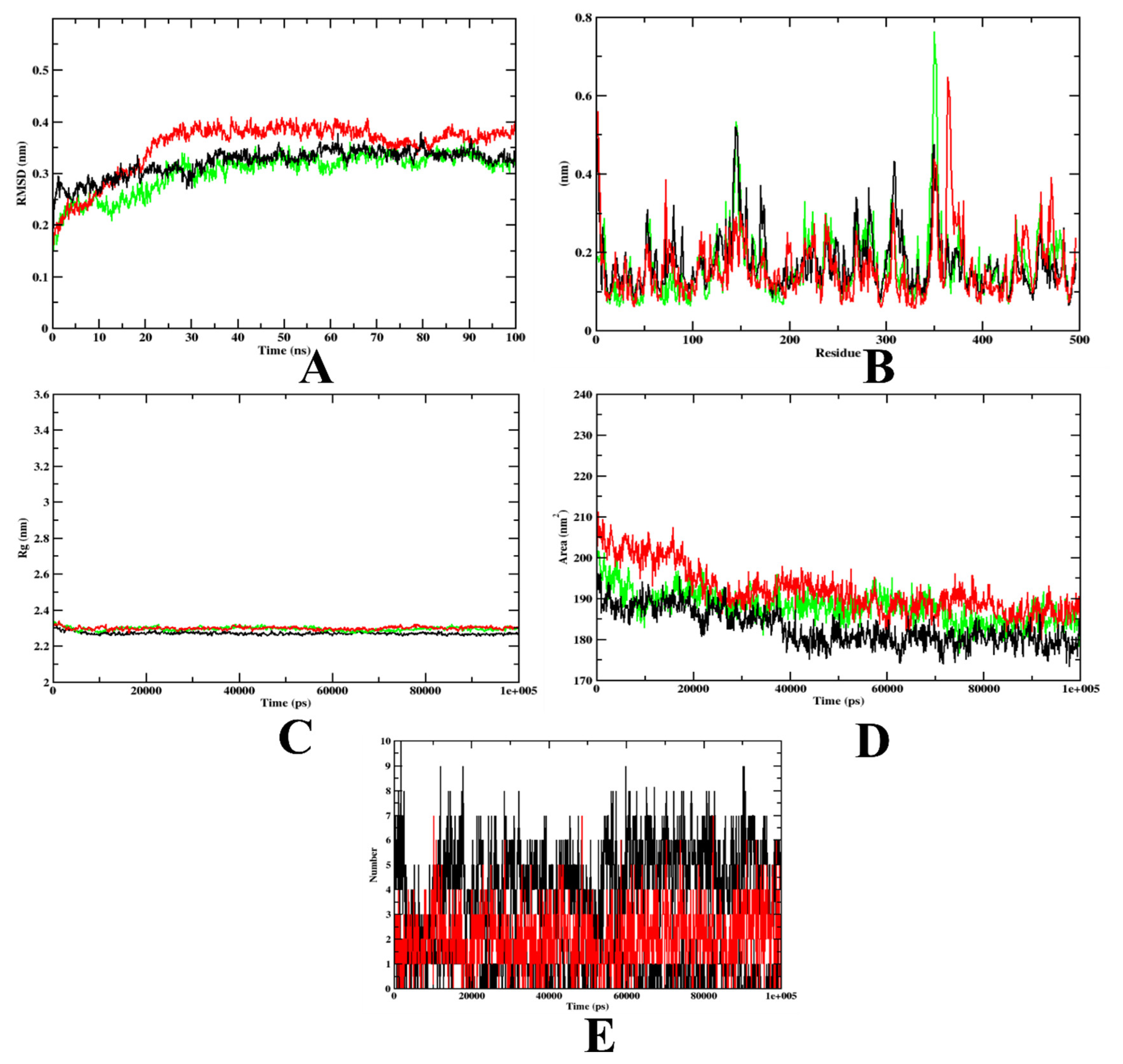
In the case of α-glycosidase, the RMSD plot indicates that apoprotein became stable at 20 ns, yet compound 7d and the acarbose-bound protein complexes were stable after 10 ns. These plots indicate that the compounds did not leave the protein up to 100 ns of time, and were stable inside the inhibitor-binding pocket (Figure 5A). The RMSF plots showed that the binding of acarbose and 7d to the protein was energetically favorable, causing no alterations in the stability. However, the protein–7d complex was found with lesser fluctuations than the protein–acarbose complex, indicating a comparatively higher stability (Figure 5B). The Rg plots also indicate that the protein–7d complex was compact throughout the simulation (Figure 5C). Further, the SASA plots show a significant decrease in the surface area of the inhibitor-binding site. This is due to the accumulation of the same by acarbose and 7d (Figure 5D). Therefore, it can be concluded that 7d could occupy the inhibitor-binding site with more stability. Finally, based on the hydrogen plot, 7d-α-glycosidase had a maximum number of nine hydrogen bonds and acarbose-α-glycosidase had five hydrogen bonds (Figure 5E). The MD simulation snapshots at different time intervals, such as 20, 40, 60, 80, and 100 ns, to assess the stability of 7d at the binding site of α-glycosidase, were extracted (Supplementary Figure S1). Compound 7d was found to be stable throughout the simulation period. By virtue of the MD trajectory analysis, both compound 7d and acarbose were stable inside the inhibitor-binding site of the α-glycosidase protein. Table 5 details the MD trajectories obtained.
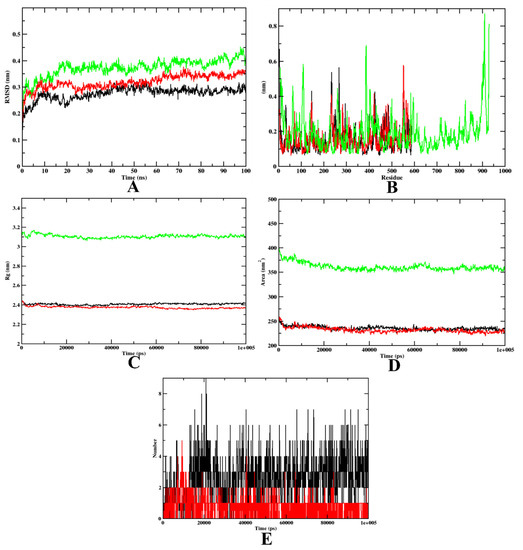
Figure 5.
A plot of the molecular dynamics simulations trajectories obtained after 100 ns for 7d and acarbose bound with α-glycosidase protein. (A) RMSD; (B) RMSF; (C) Rg; (D) SASA; (E) Ligand hydrogen bonds; Green: α-glycosidase apoprotein; Black: α-glycosidase–7d complex; Red: α-glycosidase–acarbose complex (negative control).

Table 5.
MD trajectory values for α-glycosidase complexed with acarbose and 7d.
The outcomes of our MD simulation are in accordance with a previous study, which reported caffeic acid and syringic acid as potential lead inhibitors of the α-glycosidase protein (using the same homology-built protein model) [45]. Both syringic acid and caffeic acid have been reported to penetrate the inhibitor-binding site of the α-glycosidase protein. Since compound 7d, syringic acid, and caffeic acid’s binding interactions and MD outcomes are on par, compound 7d could act as a potential inhibitor of α-glycosidase. Our MD results are also in accordance with a study where phenyl-pyrano-thiazol-2-one derivatives were validated using an MD simulation [44]. The MD outcomes of compound 7d were also found to be on par with a study where a 1-(4-(Methoxy(phenyl)methyl)-2-methylphenoxy)butan-2-one derivative was used as a single crystal α-glycosidase inhibitor molecule [48]. Both studies used the same homology-built protein model of α-glycosidase in their MD simulation studies. Therefore, compound 7d could be considered a potential inhibitor of α-glycosidase.
In the case of α-amylase, based on the RMSD graph prediction, the 7d–α-amylase complex showed better stability when compared to the acarbose complex. The predicted value of 7d–α-amylase was found to be stable after 20 ns. The protein backbone almost showed a similar pattern to the 7d–α-amylase complex. However, for the protein–acarbose complex, it was 35 ns. The RMSD plots show that both acarbose and 7d were stable inside the inhibitor-binding pocket of the protein (Figure 6A). On the other hand, the RMSF value of all the complexes and the protein backbone was almost similar, with a fluctuation at the terminal and loop regions (Figure 6B). The Rg value of all complexes shows that both acarbose and 7d-bound complexes were compact throughout the simulation (Figure 6C). The decreased SASA values over the simulation period indicate the significant occupation of the inhibitor-binding-site area by the compounds 7d and acarbose (Figure 6D). In addition, the 7d–α-amylase complex has a maximum of 10 hydrogen bonds, yet the acarbose–α-amylase has a maximum of 7 hydrogen bonds. These outcomes depict the stability and firmness of the 7d–protein complex over the acarbose-bound complex (Figure 6E). The MD simulation snapshots at different time intervals, such as 20, 40, 60, 80, and 100 ns, to assess the stability of 7d at the binding site of α-amylase, were extracted (Supplementary Figure S2). Compound 7d was found to be stable throughout the simulation period.
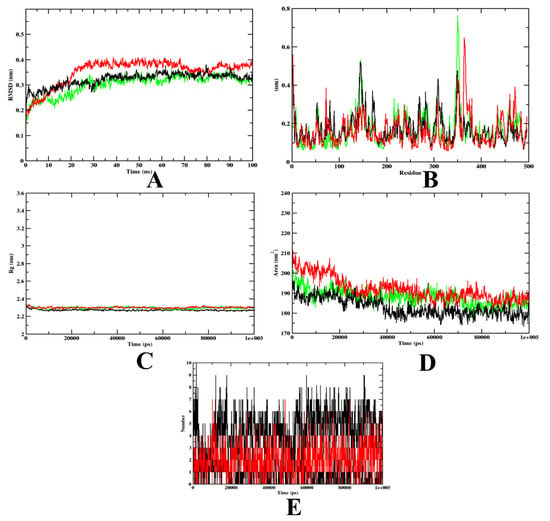
Figure 6.
A plot of the molecular dynamics simulation trajectories obtained after 100 ns for 7d and acarbose bound with the α-amylase protein. (A) RMSD; (B) RMSF; (C) Rg; (D) SASA; (E) Ligand hydrogen bonds; Green: α-amylase apoprotein; Black: α-amylase–7d complex; Red: α-amylase–acarbose complex (negative control).
Based on all the MD plot evaluations, it can be predicted that the 7d–α-glycosidase and 7d–α-amylase showed better stability and flexibility. Table 6 depicts the MD trajectory values obtained for the simulation run of 7d and acarbose complexed with α-amylase. The results of our MD simulation are in accordance with a previous study, which reported fluorinated 2,3-disubstituted thiazolidinone–pyrazoles as the potential lead inhibitors of the α-amylase protein [49]. Since the reported compounds penetrate the inhibitor-binding site, compound 7d could act as a potential inhibitor of α-amylase. Our MD results are also in accordance with caffeic acid and syringic acid cefoperazone as potential lead inhibitors of the α-amylase protein [46]. The stability of compound 7d inside the inhibitor-binding site of the α-amylase protein was hence proven in comparison with the published literature.

Table 6.
MD trajectory values for α-amylase complexed with acarbose and 7d.
2.4. Binding Free Energy Calculations
The free binding energy was calculated using the MD trajectories using the MMPBSA method to evaluate the energy formed during complex formation. The summary of the free binding energy is given in Table 7, along with various energy terms, electrostatic energy, van der Waals energy, polar energy, SASA energy, and binding energy. According to the prediction, the binding energies of the 7d–α-glycosidase complex, at −186.186 kJ/mol, are better in comparison with the acarbose–α-glycosidase complex, at −137.894 kJ/mol. Also, the residue-wise energy decomposition analysis also depicts that compound 7d had a higher binding energy compared to acarbose in the case of both α-glycosidase (Supplementary Figure S3) and α-amylase (Supplementary Figure S4). It is known that the more negative the value, the higher the stability. The overall calculation depicts that the van der Waals is more superlative than the other energy formed. Thus, based on the evaluation, the 7d complex showed better stability than the control complex. The study result had a similar pattern to previous studies that have performed binding free energy calculations for α-glycosidase [43,44,45,46,47,48] and α-amylase [43,49].

Table 7.
Binding free energy calculations of 7d and their controls with α-glycosidase proteins.
2.5. Physicochemical and ADMET Properties
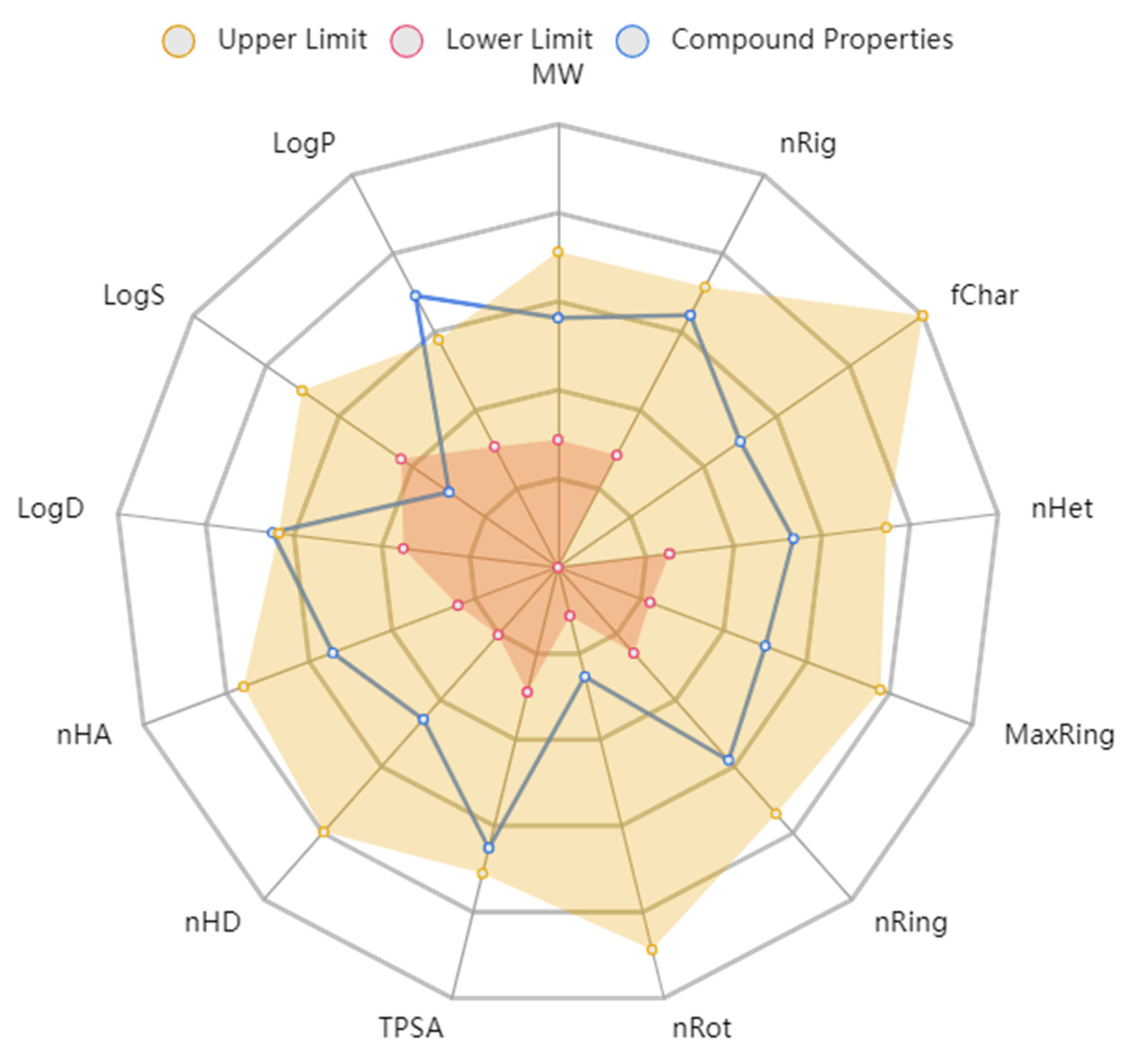
The physicochemical properties depict that compound 7d would not violate the Lipinski rule of five and the Pfizer rule (Table 8). The compound tends to be within the boundaries of the ideal compound to be given as an oral agent (Figure 7). The molecular weight and density of the compound are below the upper limit and can be digested by the body. The other structural properties, such as hydrogen donors, hydrogen acceptors, rotatable bonds, flexibility, and the topological polar surface area, also show that compound 7d could be given as an oral drug.

Table 8.
Physicochemical properties of compound 7d.
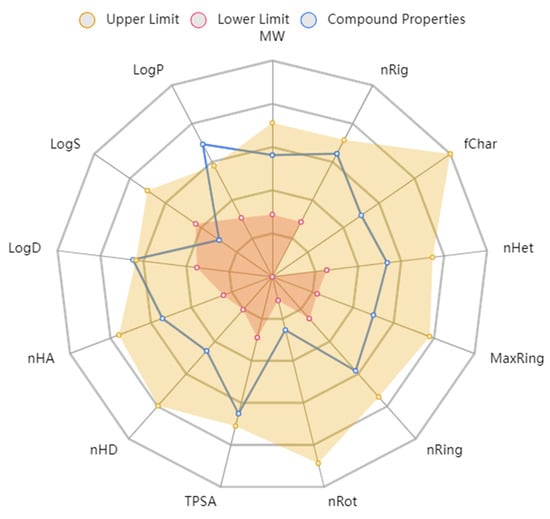
Figure 7.
ADMET radar calculation of compound 7d.
In the case of the ADMET properties, compound 7d was predicted to have good absorption values with reference to Caco-2 and MDCK cell lines. It was not considered a Pgp inhibitor. The volume distribution score of the compound was good and indicates the appropriate distribution in the human body. The compound would not inhibit any cytochrome P enzymes and would not cross the blood–brain barrier. It is also predicted with a clearance value of 15 mL/kg/min, indicating its clean exit from the human body after inducing the pharmacological effect. Furthermore, the AMES toxicity, carcinogenicity, and acute toxicity rule parameters predicted that compound 7d is not considered toxic to the human body. These results indicate that compound 7d could be used for further in vivo and clinical trial studies. These results are in accordance with our previous studies, where caffeic acid, syringic acid, and rutin were considered suitable for oral consumption [43,46].
2.6. Structure–Activity Relationship (SAR)
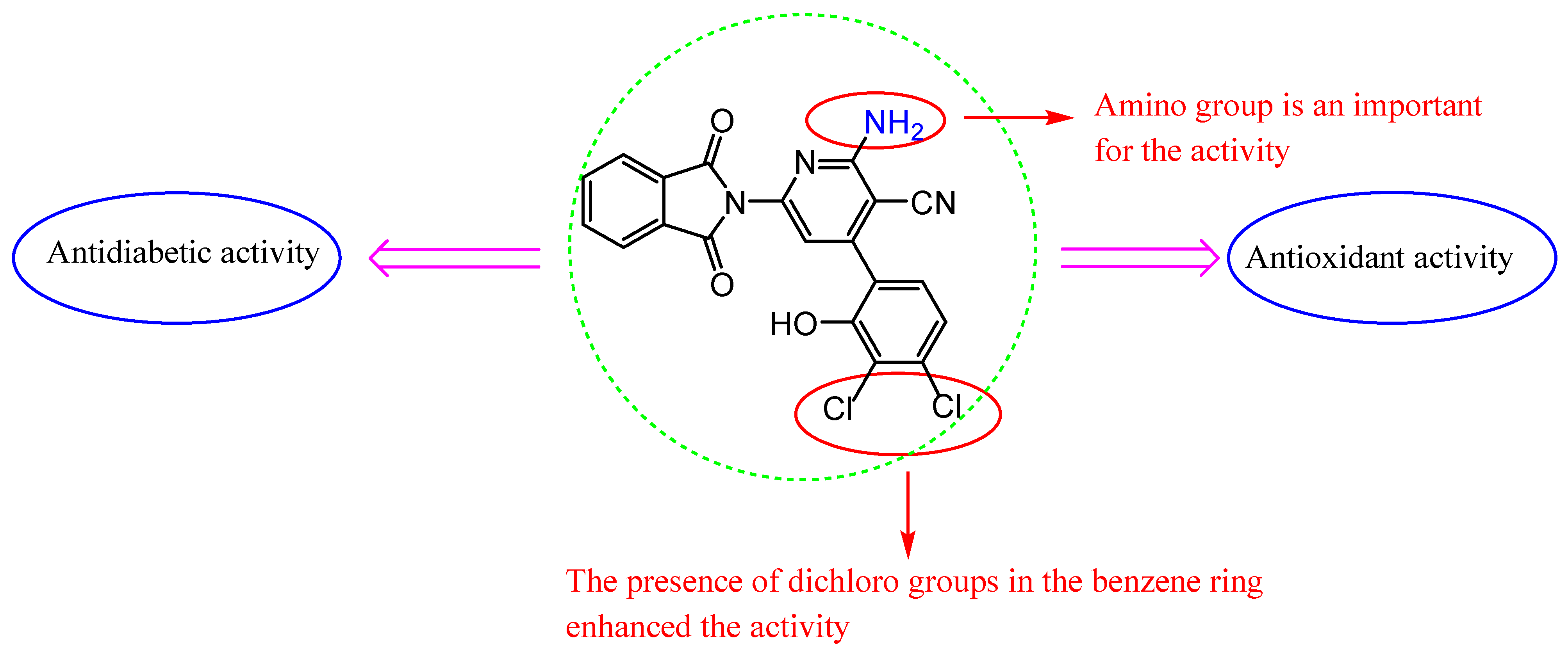
We discuss the SAR based on changes in the chemical structures and antioxidant and antidiabetic results of these molecules (Figure 8). The molecules varied in activity according to the heterocyclic ring and substituents. We found that the coupling of isoindoline to the quinoline moiety in Schiff base 3 showed promising antioxidant activity and less antidiabetic activity than 2 with a tetrazole ring. In common, isoindoline–pyridine derivatives showed higher activity than other heterocyclic rings, and the attached amino group on position 2 of pyridine showed the best activity compared to the oxo group in the same position.
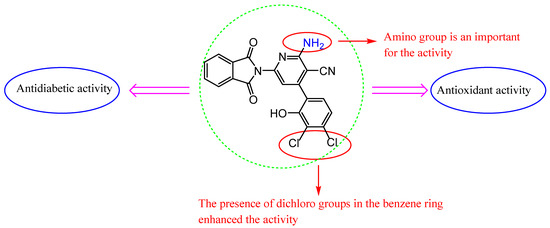
Figure 8.
SAR of compounds 7d.
In addition, the presence of electron-withdrawing chloro groups at the 3,4 positions of the 2-hydroxy phenyl 7d appear to increase the inhibition of antioxidant activities more than compound 7c, which has no chloro substituents, and more than the standard (BHA) in DPPH, ABTS, and superoxide anion radical assays. Furthermore, compound 7d inhibited the activity of both enzymes (α-glycosidase and α-amylase) significantly. Observing the structure of the compounds that displayed high antioxidant and antidiabetic activities, we can realize that compound 7d carrying the chloro groups at the 3,4 positions of the 2-hydroxy phenyl is the most effective compound.
3. Materials and Methods
3.1. Reagents and Instrumentation
The melting points of the prepared compounds were determined on a Gallenkamp electronic apparatus. TLC was utilized to monitor the reactions’ progress and purity. The FT-IR spectra were recorded utilizing KBr discs on the FT-IR Jasco 4100 infrared spectrophotometer (λ, cm−1). NMR spectra were performed on the Bruker DRX Spectrometer (1H, 400 MHz and 13C, 100 MHz) in CDCl3 and DMSO-d6 using TMS as the internal standard. Mass spectra (m/z, %) were recorded on an Agilent Model 8890 spectrometer. Elemental analyses were determined utilizing the LECO Truspec Micro Analyzer (LECO, St. Joseph, MI, USA).
3.2. Synthesis
3.2.1. Synthesis of 2-(Quinolin-8-yloxy)acetohydrazide 1
To a solution of ethyl 2-(quinolin-8-yloxy)acetate (7 g, 30 mmol) in 40 mL ethanol, NH2NH2.H2O (1.5 g, 30 mmol) was added and stirred for 0.5 h at RT using ultrasound irradiation. A formed white precipitate was washed with a little amount of water, filtered, then crystallized from ethanol to give 1. White powder, yield 94%; mp. 137–139 °C Lit. 138 °C [36].
3.2.2. Synthesis of 1-(4-(1H-Tetrazol-1-yl)phenyl)ethanone
Triethyl orthoformate (1.48 g, 10 mmol), was dropped to a solution of 1-(4-aminophenyl)ethanone (1.35 g, 10 mmol) and NaN3 (1.3 g, 20 mmol) in 30 mL DMF, and refluxed at 100 °C for 5 h. Then, the mixture was mixed with ice-cold water and the obtained yellow solid was recrystallized from acetic acid/ethanol to give 1-(4-(1H-tetrazol-1-yl)phenyl)ethanone in pure form. Yellow solid, mp. 172–174 °C. Lit. 172–175 °C [37].
3.2.3. Synthesis of 8-(2-((2-(1-(4-(1H-Tetrazol-1-yl)phenyl)ethylidene)hydrazinyl)oxy)-2-oxoethoxy)quinoline 2
Hydrazide 1 (2 g, 13 mmol) was dissolved in 20 mL ethanol, and 1-(4-(1H-tetrazol-1-yl)phenyl)ethanone (2.4 g, 13 mmol) in 10 mL ethanol was added and stirred with the mixture for 15 min, 5 drops of AcOH was added, and then refluxed for 6 h. The mixture was cooled in the refrigerator and the formed precipitate was recrystallized from ethanol.
Brown solid; yield: 73%; m.p. 194–196 °C. FT-IR: 3186 (NH), 2998 (CH2), 1673 (C=O), and 1620 (C=N). 1H NMR (400 MHz, CDCl3); δ 2.46 (s, 3H, CH3), 4.85 (s, 2H, CH2), 7.22–8.22 (m, 11H, Ar-H) and 10.24 (s, 1H, NH); 13C NMR (100 MHz, CDCl3); δ 36.3, 64.5, 118.2, 120.4, 122.8 128.5, 128.7, 129.3, 129.9, 130.6, 131.5, 132.1, 132.7, 135.1, 138.8, 147.2, 150.5, and 172.6. MS: 404 (M + 1); Anal. Calcd for C20H17N7O3: C, 59.55; H, 4.25; N, 24.31; Found: C, 59.51; H, 4.06; N, 24.25%.
3.2.4. Synthesis of 2-(4-Acetylphenyl)isoindoline-1,3-dione
Phthalic anhydride (2 g, 13 mmol) was dissolved in 35 mL acetone, and p-aminoacetophenone (1.76 g, 13 mmol) in 15 mL acetone was added dropwise to the solution, allowed to stir for 10 min, and refluxed at 60 °C for 2 h. Then, the solvent was removed and the crude product was crystallized from ethanol as a white product. Yield 91%, m.p 186–188. Lit. = 189–191 °C [38].
3.2.5. Synthesis of N’-(1-(4-(1,3-Dioxoisoindolin-2-yl)phenyl)ethylidene)-2-(quinolin-8-yloxy)acetohydrazide 3
A solution of 1 (2 g, 13 mmol) and compound 2-(4-acetylphenyl)isoindoline-1,3-dione (3.5 g, 13 mmol) in 40 mL ethanol was refluxed for 8 h in the presence of a few drops of AcOH. The solution was allowed to stay in the refrigerator overnight. The produced colorless crystalline recrystallized from ethanol.
Colorless crystals; yield: 78%; m.p. 211–213 °C; FT-IR: 3437, 3332, 3227 (NH), 2213 (CN), 1678, 1626 (C=O), 1596 (C=N), and 1275 (CO). 1H NMR (400 MHz, CDCl3); δ 2.45 (s, 3H, CH3), 5.32 (s, 2H, CH2), 7.26–8.55 (m, 14H, Ar-H), and 9.00 (s, 1H, NH); 13C NMR (100 MHz, CDCl3); δ 37.3, 66.9, 124.4, 125.8, 128.8, 129.3, 129.8, 135.2, 135. 7, 136.9, 141.7, 145.4, 149.9, 168.9, and 171.5; MS: 461 (M + 1); Anal. Calcd for C27H20N4O4: C, 69.82; H, 4.34; N, 12.06; Found: C, 69.76; H, 4.28; N, 12.02%.
3.2.6. Synthesis of 5-Amino-3-(4-substitutedphenyl)-1-(2-(quinolin-8-yloxy)acetyl)-1H-pyrazole-4-carbonitrile 4a–c
Substituted aryledines: 4-nitro, 4-chloro, 2-hydroxy (2-(4-substitued-benzylidene)malononitrile (2.15 mmol), and compound 1 (0.5 g, 2.15 mmol) in 20 mL of propanol were refluxed for 9 h. After cooling, the precipitate appeared and was cleaned with distilled water, and recrystallized from ethanol.
5-Amino-3-(4-nitrophenyl)-1-(2-(quinolin-8-yloxy)acetyl)-1H-pyrazole-4-carbonitrile 4a.
Pale yellow powder; yield: 69%; m.p. 157–159 °C. FT-IR: 3332, 3255 (NH2), 2988 (CH2), 2214 (CN), 1687 (C=O), 1613 (C=N), and 1156 (C-O); 1H NMR (400 MHz, CDCl3); δ 4.62 (s, 2H, CH2), 6.69 (s, 2H, NH2), and 6.50–7.31 (m, 10H, Ar-H); 13C NMR (100 MHz, CDCl3); δ 69.2, 112.2, 120.5, 126.8, 127.9, 129.3, 132.0, 132.7, 133.7, 139.1, 140.3, 145.6, 147.2, 147.4, 152.5, 159.1, and 165.8; MS: 415 (M + 1); Anal. Calcd for C21H14N6O4: C, 60.87; H, 3.41; N, 20.28; Found: C, 60.80; H, 3.27; N, 20.19%.
5-Amino-3-(4-chlorophenyl)-1-(2-(quinolin-8-yloxy)acetyl)-1H-pyrazole-4-carbonitrile 4b.
Orange solid; yield: 77%; m.p. 163–165 °C. FT-IR: 3327, 3235 (NH2), 2956 (CH2), 2254 (CN), 1681 (C=O), 1601 (C=N), and 1166 (C-O); 1H NMR (400 MHz, CDCl3); δ 4.56 (s, 2H, CH2), 7.49 (s, 2H, NH2), and 6.93–8.10 (m, 10H, Ar-H); 13C NMR (100 MHz, CDCl3); δ 66.9, 124.4, 125.8, 128.8, 129.3, 129.8, 135.2, 135. 7, 136.8, 141.7, 145.4, 149.9, and 168.8; MS: 402 (M + 1); Anal. Calcd for C21H14ClN5O2: C, 62.46; H, 3.49; N, 17.34; Found: C, 62.35; H, 3.36; N, 17.18%.
5-Amino-3-(2-hydroxyphenyl)-1-(2-(quinolin-8-yloxy)acetyl)-1H-pyrazole-4-carbonitrile 4c.
Pale yellow solid; yield: 71%; m.p. 157–159 °C. FT-IR: 3413 (OH), 3334, 3252 (NH2), 2976 (CH2), 2227 (CN), 1684 (C=O), 1607 (C=N), and 1143 (C-O); 1H NMR (400 MHz, CDCl3); δ 5.14 (s, 2H, CH2), 7.22 (s, 2H, NH2), 6.78–8.64 (m, 10H, Ar-H), and 8.70 (s, 1H, OH); 13C NMR (100 MHz, CDCl3); δ 77.8, 115.2, 123.5, 127.1, 127.5, 128.0, 130.6, 132.9, 134.6, 138.7, 140.9, 142.2, 143.1, 143.6, 152.5, 155.1, and 167.4; MS: 386 (M + 1); Anal. Calcd for C21H15N5O3: C, 65.45; H, 3.92; N, 18.17; Found: C, 65.36; H, 4.03; N, 18.11%.
Synthesis of 2-(2-(quinolin-8-yloxy)acetyl)hydrazinecarbothioamide 5.
A solution of 2-(quinolin-8-yloxy)acetohydrazide (0.5 g, 2.15 mmol) in ethanol: HCl (20:10 mL) was stirred and potassium thiocyanate (0.21 g, 2.15 mmol) in 10 mL ethanol was added and heated in an oil bath for 10 h at 100 °C. The mixture was cooled and taken directly in situ for the further step without workup and crystallization.
3.2.7. Synthesis of 5-((Quinolin-8-yloxy)methyl)-3H-1,2,4-triazole-3-thione 6
The reaction mixture of compound 5 was heated under reflux with a solution of NaOH (15 mL, 6 N) for 2 h and mixed into ice. The mixture was neutralized with a few drops of hydrochloric acid. The product was recrystallized from ethanol: water (1:1) to give 6.
Yellow solid; yield: 63%; m.p. 187–189 °C. FT-IR: 2989 (CH2), 825 (C=S), and 1645 (C=N); 1H NMR (400 MHz, CDCl3); δ 4.71 (s, 2H, CH2) and 6.04–7.70 (m, 6H, Ar-H); 13C NMR (100 MHz, CDCl3); δ 67.0, 120.2, 124.4, 125.9, 128.8, 129.7, 130.5, 130.6, 132.9, 136.9, 145.7, and 165.4; MS: 257 (M + 1); Anal. Calcd. for C12H8N4OS: C, 56.24; H, 3.15; N, 21.86; Found: C, 56.16; H, 3.07; N, 21.75%.
3.2.8. Synthesis of 2-Amino-6-(1,3-dioxoisoindolin-2-yl)-4-subsstitued-phenyl-1,2-dihydropyridine-3-carbonitrile 7a–d
A mixture of aldehydes (benzaldehyde, paranitro benzaldehyde, salicylaldehyde, and 3,4-dichloro salicylaldehyde) (20 mmol), 7 (20 mmol), ammonium acetate (60 mmol), and malononitrile (20 mmol) in ethanol (40 mL) was heated under reflux for 14 h. After cooling, the resulting solid was recrystallized from ethanol to afford compounds 7a–d.
2-Amino-6-(1,3-dioxoisoindolin-2-yl)-4-phenylnicotinonitrile 7a.
Yellow crystals; yield: 76%; m.p. 167–169 °C. FT-IR: 3335, 3283 (NH2), 2996, 2925 (CH), 2194 (CN), 1668, 1638 (C=O), 1593 (C=N), and 1236 (C-O); 1H NMR (400 MHz, CDCl3); δ 7.37–8.73 (m, 10H, Ar-H), and 7.50 (s, 2H, NH2); 13C NMR (100 MHz, CDCl3); δ 108.9, 111.1, 124.3, 125.8, 128.4, 128.9, 129.1, 129.9, 129.6, 136.8, 141.8, 146.9, 150.1, 161.9, and 166.9; MS: 341 (M + 1); Anal. Calcd. for C20H12N4O2: C, 70.58; H, 3.55; N, 16.46; Found: C, 70.31; H, 3.48; N, 16.29%.
2-Amino-6-(1,3-dioxoisoindolin-2-yl)-4-(4-nitrophenyl)nicotinonitrile 7b.
Brown powder; yield: 77%; m.p. 153–155 °C. FT-IR: 3356, 3292 (NH2), 3012, 2962 (CH), 2176 (CN), 1673,1641 (C=O), 1602 (C=N), and 1214 (C-O); 1H NMR (400 MHz, CDCl3); δ 6.92–8.43 (m, 9H, Ar-H), and 7.27 (s, 2H, NH2); 13C NMR (100 MHz, CDCl3); δ 117.7, 118.9, 125.1, 128.2, 129.3, 129.6, 129.9, 133.8, 134.6, 137.4, 138.2, 155.2, 157.1, 158.4, 161.2, 162.8, and 165.3, MS: 386 (M + 1); Anal. Calcd. for C20H11N5O4: C, 62.34; H, 2.88; N, 18.17; Found: C, 62.19; H, 2.70; N, 18.04%.
2-Amino-6-(1,3-dioxoisoindolin-2-yl)-4-(2-hydroxyphenyl)nicotinonitrile 7c.
Pale brown powder; yield: 65%; m.p. 160–162 °C. FT-IR: 3442 (OH), 3317, 3270 (NH2), 2993, 2920 (CH), 2188 (CN), 1671,1644 (C=O), 1607 (C=N), and 1218 (C-O); 1H NMR (400 MHz, CDCl3); δ 6.94–8.14 (m, 9H, Ar-H), 7.39 (s, 2H, NH2), and 8.42 (s, 1H, OH); 13C NMR (100 MHz, CDCl3); δ 113.7, 116.8, 119.3, 123.9, 124.0, 127.5, 128.3, 131.9, 132.0, 135.3, 136.2, 152.3, 153.7, 160.7, 162.7, 164.4, and 167.1. MS: 357 (M + 1); Anal. Calcd. For C20H12N4O3: C, 67.41; H, 3.39; N, 15.72; Found: C, 67.25; H, 3.27; N, 15.58%.
2-Amino-4-(3,4-dichloro-2-hydroxyphenyl)-6-(1,3-dioxoisoindolin-2-yl)nicotinonitrile 7d.
Yellow powder; yield: 72%; m.p. 171–173 °C. FT-IR: 3425 (OH), 3323, 3280 (NH2), 3021, 2944 (CH), 2168 (CN), 1668,1646 (C=O), 1590 (C=N), and 1222 (C-O); 1H NMR (400 MHz, CDCl3); δ 6.90–8.35 (m, 7H, Ar-H), 7.31 (s, 2H, NH2), and 8.68 (s, 1H, OH); 13C NMR (100 MHz, CDCl3); δ 112.9, 114.3, 116.5, 122.8, 123.8, 124.5, 126.8, 127.8, 128.6, 129.4, 130.7, 131.9, 135.2, 135.3, 143.4, 151.2, 162.8, and 167.4. MS: 425 (M + 1); Anal. Calcd. For C20H10Cl2N4O3: C, 56.49; H, 2.37; N, 13.18; Found: C, 56.31; H, 2.25; N, 13.04%.
3.2.9. Synthesis of 6-(1,3-Dioxoisoindolin-2-yl)-2-oxo-4-substituted-phenyl-1,2-dihydropyridine-3-carbonitriles 8a–d
A mixture of aldehydes (benzaldehyde, paranitro benzaldehyde, salicylaldehyde, and 3,4-dichloro salicylaldehyde) (20 mmol), 2-acetylisoindoline-1,3-dione (20 mmol), ethyl cyanoacetate (20 mmol), and ammonium acetate (80 mmol) in ethanol (40 mL) was refluxed for 14 h. The resulting precipitate was recrystallized from ethanol to give derivatives 8a–d.
6-(1,3-Dioxoisoindolin-2-yl)-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile 8a.
Brown crystals; yield: 74%; m.p. 216–218 °C. FT-IR: 3250 (NH), 3098, 2909 (CH), 2217 (CN), 1674 (C=O), and 1595 (C=N); 1H NMR (400 MHz, CDCl3); δ 6.92–8.41 (m, 10H, Ar-H) and 9.22 (s, 1H, NH); 13C NMR (100 MHz, CDCl3); δ 102.8, 105.8, 106.3, 114.5, 124.1, 125.7, 128.7, 129.3, 129.8, 136.9, 141.5, 146.5, 150.2, 160.5, and 170.9; MS: 382 (M + 1); Anal. Calcd. For C20H11N3O3: C, 70.38; H, 3.25; N, 12.31; Found: C, 70.12; H, 3.08; N, 12.17%.
6-(1,3-Dioxoisoindolin-2-yl)-4-(4-nitrophenyl)-2-oxo-1,2-dihydropyridine-3-carbonitrile 8b.
Orange powder; yield: 67%; m.p. 221–223 °C. FT-IR: 3277 (NH), 3091, 2903 (CH), 2223 (CN), 1674 (C=O), and 1590 (C=N); 1H NMR (400 MHz, CDCl3); δ 6.90–7.51 (m, 9H, Ar-H) and 9.72 (s, 1H, NH); 13C NMR (100 MHz, CDCl3); δ 102.9, 104.3, 106.5, 112.8, 123.3, 124.5, 126.4, 128.1, 129.4, 130.7, 131.9, 135.2, 136.2, 145.2, 152.8, 167.0, and 167.4. MS: 387 (M + 1); Anal. Calcd. For C20H10N4O5: C, 62.18; H, 2.61; N, 14.50; Found: C, 62.02; H, 2.46; N, 14.31%.
6-(1,3-Dioxoisoindolin-2-yl)-4-(2-hydroxyphenyl)-2-oxo-1,2-dihydropyridine-3-carbonitrile 8c.
Brown powder; yield: 74%; m.p. 205–207 °C. FT-IR: 3427 (OH), 3260 (NH), 3089, 2917 (CH), 2224 (CN), 1678 (C=O), and 1593 (C=N); 1H NMR (400 MHz, CDCl3); δ 6.73–8.22 (m, 9H, Ar-H), 8.87 (s, 1H, OH), and 9.30 (s, 1H, NH); 13C NMR (100 MHz, CDCl3); δ 113.3, 113.8, 114.9, 120.6, 123.2, 123.3, 126.5, 127.4, 129.7, 130.6, 134.3, 134.8, 135.7, 156.4, 159.1, 164.6, and 166.8. MS: 357 (M + 1); Anal. Calcd. For C20H11N3O4: C, 67.23; H, 3.10; N, 11.76; Found: C, 66.92; H, 2.81; N, 11.74%.
4-(3,4-Dichloro-2-hydroxyphenyl)-6-(1,3-dioxoisoindolin-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile 8d.
Dark brown powder; yield: 79%; m.p. 233–235 °C. FT-IR: 3446 (OH), 3255 (NH), 3090, 2921 (CH), 2214 (CN), 1680 (C=O), and 1597 (C=N); 1H NMR (400 MHz, CDCl3); δ 6.62–8.23 (m, 7H, Ar-H), 9.24 (s, 1H, OH), and 9.34 (s, 1H, NH); 13C NMR (100 MHz, CDCl3); δ 112.1, 113.4, 116.8, 121.1, 123.2, 124.5, 126.7, 128.2, 130.3, 131.4, 133.2, 134.5, 137.4, 152.3, 157.7, 161.9, and 164.7. MS: 426 (M + 1); Anal. Calcd. For C20H9Cl2N3O4: C, 56.36; H, 2.13; N, 9.86; Found: C, 56.15; H, 2.04; N, 9.34%.
3.3. Biological Assays
3.3.1. Antioxidant Assays
The DPPH free radical, ABTS cation radical, and superoxide anion radical scavenging approaches were used in the antioxidant activity of the present study. The capacity for the radical scavenging was represented by EC50 values [56]. The EC50 values for the aforementioned antioxidant activities show the proportion of free, cation, and anion radicals that the tested samples were able to scavenge at 50% [57,58]. Every sample was observed three times. Butylated hydroxyanisole (BHA) is the positive control drug in this evaluation of antioxidant activity [59].
3.3.2. Inhibition of α-Amylase and α-Glycosidase
α-Amylase (EC 3.2.1.1, type-VI B porcine pancreatic α-amylase) and α-glycosidase (EC 3.2.1.20, type-1 α-glycosidase) inhibition was tested using soluble starch (1%) and pNPG, respectively, according to the modified method described by Ramu [60,61], with acarbose as a positive control. Inhibitory activity of α-amylase and α-glycosidase was expressed as percent inhibition, calculated using the following formula.
Inhibition (%) = (A control − A sample)/A control × 100
The IC50 values were obtained from a graph that correlated the percentage of the inhibition of each sample with its concentration. Each experiment included three replicates, along with appropriate blanks. The concentration needed to reduce α-glycosidase activity by 50% under the specified assay conditions was referred to as the IC50 [62,63,64].
3.3.3. Kinetics of α-Glycosidase and α-Amylase Inhibition
The enzyme kinetics of the compounds’ inhibition of the glycosidase activity were examined using different concentrations of the substrate against the IC20, IC40, and IC60 inhibitory concentrations of the compounds, as previously reported [42,65,66,67]. The type of inhibition, Km, and Vmax were calculated using a double-reciprocal plot [68] of the substrate concentration and velocity (1/V against 1/[pNPG]). The inhibitory constant (Ki) was also determined using the Dixon plot [69].
3.4. Computational Studies
3.4.1. Molecular Docking Simulation
The homology model of α-glycosidase was built from the Saccharomyces cerevisiae (yeast) α-glycosidase MAL-32 sequence obtained from UniProt (UniProt ID: P38158), using the SWISS-MODEL (https://swissmodel.expasy.org/) (accessed on: 16 June 2023). Validation of the protein model was completed in previous studies and was found to be stable with the X-ray crystal structure of S. cerevisiae isomaltase (PDB ID: 3AXH), showing a 72% identical and 84% similar sequence [45,46], whereas the X-ray crystal structure of porcine pancreatic α-amylase (PDB ID: 1DHK) was retrieved from the RCSB PDB database. Since human α-glycosidase is not yet characterized, the authors had to use a S. cerevisiae/yeast model in both in vitro and in silico studies [70,71,72]. The 2D structures of the quinoline and isoindoline–polycyclic compounds were drawn and 3D optimized using ACD ChemSketch. Acarbose was chosen as the control drug for the study both in vitro and in silico, and its structure was retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) (accessed on: 16 June 2023).
According to previous works [73,74], both the protein and ligand structures were constructed for molecular docking simulation using AutoDock Tools 1.5.6. Water and heteroatoms were removed from the protein structures. Polar hydrogens, on the other hand, were used to stabilize it. Using Kollmann-united charges and Gasteiger charges, the energy of the protein and ligand structures was reduced. Prior to obtaining the completed protein and ligand structures in PDBQT format for the molecular docking simulation, all atoms were assigned an AutoDock 4 atom type after energy reduction [75].
The selection of the binding site for α-glycosidase was performed according to a previous study [39], whereas for α-amylase, it was performed using literature analysis [49]. Using AutoDock Tools 1.5.6, the binding pocket of the proteins was set in the grid box. Details of the grid boxes for both α-glycosidase and α-amylase have been given in Table 9.

Table 9.
The position of the grid boxes placed on the binding pocket of the respective target proteins.
The compounds’ molecular docking was accomplished using command-line software known as AutoDock Vina 1.1.2. It perturbs and allocates ligands into the target site using the Broyden–Fletcher–Goldfarb–Shanno (BGFS) algorithm, and it analyzes the scoring function of each ligand conformation [76]. Because of the huge number of torsions induced during ligand synthesis, ligands were treated as flexible throughout the docking simulation, whereas the protein was assumed to be rigid. However, ligand molecules were permitted 10 degrees of freedom. The initial binding posture with a zero root-mean-square deviation (RMSD) of atomic positions is regarded to be extremely genuine [77].
Furthermore, they have the highest binding affinity of any position, indicating that the binding is more effective. Biovia Discovery Studio Visualizer 2021, an open-source visualizing GUI program, was used to complete the visualization of the molecular docking simulation. Binding affinity, total number of bonds, and respective hydrogen bonds were used to estimate the amount of ligand interaction [78].
3.4.2. Molecular Dynamics Simulation
Docked complexes of α-glycosidase and α-amylase complexed with 7d and acarbose were used for the molecular dynamics (MD) simulation. According to a previous study [79], the MD simulation was undertaken using the biomolecular software program GROMACS-2018.1. GROMACS is a comprehensive software program for modeling Newtonian equations of motion or performing molecular dynamics on systems involving hundreds to millions of particles. It is designed particularly for biological compounds having several complicated binding connections, such as proteins, lipids, and nucleic acids. The software excels at calculating nonbonded interactions, which are frequently regarded as the most critical in simulations [80]. The SwissParam server was utilized to construct the ligand topology, and the CHARMM36 forcefield was used to approximate the ligand structures. On the other hand, protein structure was added with the CHARMM36 forcefield using the pdb2gmx module. The next stage was to perform 5000 steps of energy reduction in a vacuum using the steepest descent technique [81].
Each protein complex was separated from the box’s borders by a distance of ten. The solvent was integrated into the TIP3P water model with the appropriate amount of Na+ and Cl- counterions to maintain the required 0.15 M salt content. Six simulations were performed for 100 ns at a 310 K temperature and 1 bar pressure. The trajectory analysis of the RMSD, RMSF, Rg, SASA, and ligand hydrogen bond parameters was performed, and the results were plotted in graphical form using XMGRACE v1.0, a GUI-based software used for plotting MD simulation results [79,80,81].
3.4.3. Binding Free Energy Calculations
The MD simulation results for α-glycosidase and α-amylase complexed with 7d and acarbose were used to calculate binding free energy using the Molecular Mechanics/Poisson–Boltzmann Surface Area (MM-PBSA) approach. It is another use of molecular dynamics simulations and thermodynamics to determine the amount of ligand binding to the protein. The binding free energy for each ligand–protein combination was calculated using the g_mmpbsa v1.2 software with the MmPbSaStat.py script and the GROMACS 2018.1 trajectories as the input [47,48,49,50]. The binding free energy is calculated using three components in the g_mmpbsa program: molecular mechanical energy, polar and apolar solvation energies, and molecular mechanical energy. The calculation is performed utilizing the MD trajectories from the last 50 ns to determine G with dt 1000 frames. It is evaluated using molecular mechanical energy and polar–apolar solvation energies. Equations (1) and (2), used to calculate the free binding energy, are given below [51,52].
ΔGBinding = GComplex − (GProtein + GLigand)
ΔG = ΔEMM + ΔGSolvation − TΔS = ΔE(Bonded+non-bonded) + ΔG(Polar+non-polar) – TΔS
GBinding: binding free energy; GComplex: total free energy of the protein–ligand complex; GProtein and GLigand: total free energies of the isolated protein and ligand in a solvent, respectively; ΔG: standard free energy; ΔEMM: average molecular mechanics potential energy in a vacuum; GSolvation: solvation energy; ΔE: the total energy of bonded as well as nonbonded interactions; ΔS: change in the entropy of the system upon ligand binding; T: temperature in Kelvin [53,54,55].
3.4.4. Physicochemical and ADMET Properties
The physicochemical and biological absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties of the selected compound were performed using the ADMETlab 2.0 server (https://admetmesh.scbdd.com/) (accessed on: 16 June 2023). The SMILES format of compound 7d was obtained and subjected to the calculation of both the abovementioned properties. ADMETlab 2.0 is designed to provide a systematic evaluation of the ADMET properties, as well as physicochemical properties and medicinal chemistry friendliness. It incorporates updated functional modules, predictive models, explanations, and an improved user interface. By leveraging these enhancements, ADMETlab 2.0 aims to assist medicinal chemists in accelerating the drug research and development process [73,74].
3.5. Statistical Analyses
The experiments were carried out in triplicate, and the findings were reported as mean ± SE. The EC50 values were calculated using the Graph Pad PRISM software (version 4.03) and were considered statistically significant if the ‘p’ values were 0.05 or below. Results were subjected to a one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test, and the mean comparisons were performed by Duncan’s multiple range test using SPSS (version 21.0, Chicago, IL, USA).
4. Conclusions
In conclusion, we have successfully synthesized a series of polycyclic compounds containing different heterocycles, such as tetrazole, triazole, pyrazole, and pyridine. These compounds were prepared using various synthetic approaches, including one-pot three-component condensation reactions. The researchers also demonstrated the synthesis of Schiff bases and pyrazoles through specific reactions. In the antidiabetic assays, one compound, namely 7d, stood out as a potential lead candidate. Compound 7d showed superior inhibition of both α-glycosidase (IC50: 0.07 mM) and α-amylase (0.21 mM) compared to the standard acarbose, indicating its potential as an effective antidiabetic agent. It also showed the potent antioxidant activity of EC50 = 0.65, 0.52, and 0.93 mM during the free radical scavenging assays (DPPH, ABTS, and superoxide anion radicals). Molecular docking (α-glycosidase: −10.9 kcal/mol and α-amylase: −9.0 kcal/mol) and dynamics simulations further confirmed the binding interactions and stability of 7d with the target enzymes, providing valuable insights into its mode of action. Additionally, physicochemical and ADMET studies were conducted, which revealed that compound 7d exhibited no toxic effects. This finding suggests that 7d could be considered for further development as an oral antidiabetic medication. To summarize, the combination of in silico and in vitro studies has yielded promising results for compound 7d as a potential antidiabetic agent. Its efficient inhibition of key enzymes, stable binding interactions, lack of toxicity, and oral availability make it a promising candidate for further investigation during in vivo and clinical trials, with the need for prior dose-dependent in vivo toxicity experiments. These findings open up new possibilities for the development of novel therapeutics in the field of diabetes treatment. However, further studies and validations are required to fully assess the safety and efficacy of compound 7d before it can be considered for human trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph16091222/s1, Figure S1: MD simulation of compound 7d with α-glucosidase, Figure S2: MD simulation of compound 7d with α-amylase, Figure S3: The interactive amino acid residues decomposition analysis of α-glucosidase with compound 7d and acarbose, and Figure S4: The interactive amino acid residues decomposition analysis of α-amylase with compound 7d and acarbose; Figure S5: 1 H NMR of compound 3; 13C NMR of compound 3; MS of compound 3; 1H NMR of compound 4b; 13C NMR of compound 4b; MS of compound 4b; 1H NMR of compound 6; 13C NMR of compound 6; MS of compound 6; 1H NMR of compound 7a; 13C NMR of compound 7a; MS of compound 7a; 1H NMR of compound 8a; 13C NMR of compound 8a; MS of compound 8a.
Author Contributions
M.A.-G.: Methodology, software, formal analysis, data curation, and writing—original draft. O.A.: Formal analysis and writing an original draft. A.-B.A.-O.: Formal analysis, writing an original draft, and editing. N.A.Y.A.: writing, editing, and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia through project no. IFKSUOR3-491-1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in the article and the Supplementary Materials.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research work through project no. IFKSUOR3-491-1. They are also thankful to Taibah University for research facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huseynova, M.; Taslimi, P.; Medjidov, A.; Farzaliyev, V.; Aliyeva, M.; Gondolova, G.; Şahin, O.; Yalçın, B.; Sujayev, A.; Orman, E.B.; et al. Synthesis, characterization, crystal structure, electrochemical studies and biological evaluation of metal complexes with thiosemicarbazone of glyoxylic acid. Polyhedron 2018, 155, 25–33. [Google Scholar] [CrossRef]
- Gülçin, I.; Scozzafava, A.; Supuran, C.T.; Koksal, Z.; Turkan, F.; Çetinkaya, S.; Bingöl, Z.; Huyut, Z.; Alwasel, S.H. Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase isoenzymes. J. Enzym. Inhibit. Med. Chem. 2016, 31, 1698–1702. [Google Scholar] [CrossRef]
- Bursal, E.; Aras, A.; Kılıç, Ö.; Taslimi, P.; Gören, A.C.; Gülçin, İ. Phytochemical content, antioxidant activity, and enzyme inhibition effect of Salvia eriophora Boiss. & Kotschy against acetylcholinesterase, α-amylase, butyrylcholinesterase, and α-glycosidase enzymes’. J. Food Biochem. 2019, 43, e12776. [Google Scholar] [CrossRef]
- Patil, S.M.; Shirahatti, P.S.; Ramu, R. Azadirachta indica A. Juss (neem) against diabetes mellitus: A critical review on its phytochemistry, pharmacology, and toxicology. J. Pharm. Pharmacol. 2022, 74, 681–710. [Google Scholar] [CrossRef] [PubMed]
- Ramu, R.; Shirahatti, P.S.; Deepika, T.H.; Bajpe, S.N.; Sreepathi, N.; Patil, S.M.; Prasad, N. Investigating Musa paradisiaca (Var. Nanjangud rasa bale) pseudostem in preventing hyperglycemia along with improvement of diabetic complications. J. Appl. Biol. Biotechnol. 2022, 10, 56–65. [Google Scholar] [CrossRef]
- Maradesha, T.; Martiz, R.M.; Patil, S.M.; Prasad, A.; Babakr, A.T.; Silina, E.; Stupin, V.; Achar, R.R.; Ramu, R. Integrated network pharmacology and molecular modeling approach for the discovery of novel potential MAPK3 inhibitors from whole green jackfruit flour targeting obesity-linked diabetes mellitus. PLoS ONE 2023, 18, e0280847. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Storr, S.J.; Royle, L.; Chapman, C.J.; Hamid, U.M.; Robertson, J.F.; Murray, A.; Dwek, R.A.; Rudd, P.M. The O-linked glycosylation of secretory/shed MUC1 from an advanced breast cancer patient’s serum. Glycobiology 2008, 18, 456–462. [Google Scholar] [CrossRef]
- Patil, S.M.; Martiz, R.M.; Satish, A.M.; Shbeer, A.M.; Ageel, M.; Al-Ghorbani, M.; Parameswaran, S.; Ramu, R. Discovery of novel coumarin derivatives as potential dual inhibitors against α-glucosidase and α-amylase for the management of post-prandial hyperglycemia via molecular modelling approaches. Molecules 2022, 27, 3888. [Google Scholar] [CrossRef] [PubMed]
- Simsek, E.; Lu, X.; Ouzounov, S.; Block, T.M.; Mehta, A.S. α-Glucosidase inhibitors have a prolonged antiviral effect against hepatitis B virus through the sustained inhibition of the large and middle envelope glycoproteins. Antivir. Chem. Chemother. 2006, 17, 259–267. [Google Scholar] [CrossRef]
- Ramu, R.; Patil, S.M. A perspective on the effective conduction of functional-based coaching program on diabetic Indonesian communities. Oman Med. J. 2021, 36, e281. [Google Scholar] [CrossRef]
- Cabrele, C.; Reiser, O. The modern face of synthetic heterocyclic chemistry. J. Org. Chem. 2016, 81, 10109–10125. [Google Scholar] [CrossRef]
- Hosseini, H.; Bayat, M. Cyanoacetohydrazides in Synthesis of Heterocyclic Compounds. Top. Curr. Chem. 2018, 376, 40. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghorbani, M.; Pavankumar, G.S.; Naveen, P.; Shamanth Neralagundi, H.G.; Prabhakar, B.T.; Khanum, S.A. Synthesis and an angiolytic role of novel piperazine-benzothiozole analogues on neovascularisation, a chief tumoral parameter in neoplastic development. Bioorg. Chem. 2016, 65, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Sarshira, E.M.; Hamada, N.M.; Moghazi, Y.M.; Abdelrahman, M.M. Synthesis and biological evaluation of some heterocyclic compounds from salicylic acid hydrazide. J. Heter. Chem. 2016, 53, 1970–1982. [Google Scholar] [CrossRef]
- Grande, F.; Yamada, R.; Cao, X.; Aiello, F.; Garofalo, A.; Neamati, N. Synthesis and biological evaluation of novel hydrazide based cytotoxic agents. Exp. Opin. Investig. Drugs 2009, 18, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lei, L.; Liu, Z.; Wang, H.; Meng, Q. Design, Synthesis, and Biological Evaluation of Novel Nitrogen Heterocycle-Containing Ursolic Acid Analogs as Antitumor Agents. Molecules 2019, 24, 877. [Google Scholar] [CrossRef]
- Kella, C.R.; Balachandran, C.; Arun, Y.; Kaliyappan, E.; Mahalingam, S.M.; Ignacimuthu, S.; Arumugam, N.; Almansour, A.I.; Kumar, R.S.; Perumal, P.T. A novel class of 1,4-disubstituted 1,2,3-triazoles: Regioselective synthesis, antimicrobial activity and molecular docking studies. Arab. J. Chem. 2020, 13, 9047–9057. [Google Scholar] [CrossRef]
- Kalkhambkar, R.G.; Kulkarni, G.M.; Kamanavalli, C.M.; Premkumar, N.; Asdaq, S.M.B.; Sun, C.M. Synthesis and biological activities of some new fluorinated coumarins and 1-aza coumarins. Eur. J. Med. Chem. 2008, 10, 2178–2188. [Google Scholar] [CrossRef]
- Al-Ghorbani, M.; Gouda, M.A.; Baashen, M.; Alharbi, O.; Almalki, F.A.; Ranganatha, L.V. Piperazine heterocycles as potential anticancer agents: A review. Pharm. Chem. J. 2022, 56, 1. [Google Scholar] [CrossRef]
- Patil, B.S.; Krishnamurthy, G.; Shashikumar, N.D.; Lokesh, M.R.; Bhojya Naik, H.S. Synthesis and Antimicrobial Activity of Some [1,2,4]-Triazole Derivatives. J. Chem. 2012, 2013, e462594. [Google Scholar] [CrossRef]
- Sharshira, E.M.; Hamada, N.M.M. Synthesis and Antimicrobial Evaluation of Some Pyrazole Derivatives. Molecules 2012, 17, 4962–4971. [Google Scholar] [CrossRef]
- Vengurlekar, S.; Sharma, R.; Trivedi, P. Synthesis, Antifungal Evaluation and Molecular Docking Studies on 2-Thioxoimidazolidin-4-One Derivatives. Med. Chem. 2013, 9, 459–473. [Google Scholar] [CrossRef]
- Rashid, H.; Martines, M.A.U.; Duarte, A.P.; Jorge, J.; Rasool, S.; Muhammad, R.; Ahmad, N.; Umar, M.N. Research Developments in the Syntheses, Anti-Inflammatory Activities and Structure–Activity Relationships of Pyrimidines. RSC Adv. 2021, 11, 6060–6098. [Google Scholar] [CrossRef]
- De Oliveira, V.V.G.; de Souza, M.A.A.; Cavalcanti, R.R.M.; de Oliveira Cardoso, M.V.; Leite, A.C.L.; da Silva Junior, V.A.; de Figueiredo, R.C.B.Q. Study of in Vitro Biological Activity of Thiazoles on Leishmania (Leishmania) Infantum. J. Glob. Antimicrob. Resist. 2020, 22, 414–421. [Google Scholar] [CrossRef]
- Winyakul, C.; Phutdhawong, W.; Tamdee, P.; Sirirak, J.; Taechowisan, T.; Phutdhawong, W.S. 2,5-Diketopiperazine Derivatives as Potential Anti-Influenza (H5N2) Agents: Synthesis, Biological Evaluation, and Molecular Docking Study. Molecules 2022, 27, 4200. [Google Scholar] [CrossRef]
- Azzam, R.A.; Elsayed, R.E.; Elgemeie, G.H. Design and Synthesis of a New Class of Pyridine-Based N-Sulfonamides Exhibiting Antiviral, Antimicrobial, and Enzyme Inhibition Characteristics. ACS Omega 2020, 5, 26182–26194. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Abdelhamid, I.A.; Al-Matar, H.M.; Hilmy, N.M.; Elnagdi, M.H. Enamines as Precursors to Polyfunctional Heteroaromatic Compounds; a Decade of Development. Heterocyclic 2008, 75, 1849–1905. [Google Scholar] [CrossRef]
- Abdel-Motaleb, R.M.; Makhloof, A.M.A.S.; Ibrahim, H.M.; Elnagdi, M.H. Studies with azoles and benzoazoles: A novel simple approach for synthesis of 3-functionally substituted 3-acylindoles. J. Heterocycl. Chem. 2007, 44, 109–114. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Makhseed, S.; Abdel-Motaleb, R.M.; Makhlouf, A.M.; Elnagdi, M.H. Utility of cyanoacetamides as precursors to pyrazolo[3,4-d] pyrimidin-4-ones,2-aryl-6-substituted 1,2,3-triazolo[4,5-d]pyrimidines and pyrazolo[1,5-a]pyrimidine-3-carboxamides. Heterocyclic 2007, 71, 1951–1966. [Google Scholar] [CrossRef]
- Salaheldin, A.M. Enaminonitriles in Heterocyclic Synthesis: Novel Synthesis of 3-Aminopyrroles and Pyrrolo[3,2-d]pyrimidine Derivatives. Zeitsch. Naturfor. B 2008, 63, 564–570. [Google Scholar] [CrossRef]
- Al-Ghorbani, M.; Thirusangu, P.; Gurupadaswamy, H.D.; Girish, V.; Neralagundi, H.S.; Prabhakar, B.T.; Khanum, S.A. Synthesis and antiproliferative activity of benzophenone tagged pyridine analogues towards activation of caspase activated DNase mediated nuclear fragmentation in Dalton’s lymphoma. Bioorg. Chem. 2016, 65, 73–81. [Google Scholar] [CrossRef]
- Al-Ghorbani, M.; Gouda, M.A. Synthesis and in vitro anticancer activity of some novel cyclohepta[b]thiophene-3-carboxamides bearing pyrazole moiety. J. Heter. Chem. 2020, 57, 3213–3221. [Google Scholar] [CrossRef]
- Al-Ghorbani, M.; Thirusangu, P.; Gurupadaswamy, H.D.; Vigneshwaran, V.; Mohammed, Y.H.; Prabhakar, B.T.; Khanum, S.A. Synthesis of novel morpholine conjugated benzophenone analogues and evaluation of antagonistic role against neoplastic development. Bioorg. Chem. 2017, 71, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghorbani, M. Design, Synthesis of Some Quinoline-Heterocyclic Derivatives. Russ. J. Heter. Chem. 2022, 58, 1272–1279. [Google Scholar] [CrossRef]
- Peng, Y.; Song, G. Simultaneous microwave and ultrasound irradiation: A rapid synthesis of hydrazides. Green Chem. 2001, 3, 302–304. [Google Scholar] [CrossRef]
- Sonawane, H.R.; Vibhute, B.T.; Aghav, B.D.; Deore, J.V.; Patil, S.K. Versatile Applications of Transition Metal Incorporating Quinoline Schiff Base Metal Complexes: An Overview. Eur. J. Med. Chem. 2023, 258, 115549. [Google Scholar] [CrossRef]
- Gorobets, N.Y.; Yousefi, B.H.; Belaj, F.; Kappe, C.O. Rapid microwave-assisted solution phase synthesis of substituted 2-pyridone libraries. Tetrahedron 2004, 60, 8633–8644. [Google Scholar] [CrossRef]
- Güven, L.; Erturk, A.; Miloğlu, F.D.; Alwasel, S.; Gulcin, İ. Screening of Antiglaucoma, Antidiabetic, Anti-Alzheimer, and Antioxidant Activities of Astragalus alopecurus Pall—Analysis of Phenolics Profiles by LC-MS/MS. Pharmaceuticals 2023, 16, 659. [Google Scholar] [CrossRef] [PubMed]
- Karagecili, H.; İzol, E.; Kirecci, E.; Gulcin, İ. Determination of Antioxidant, Anti-Alzheimer, Antidiabetic, Antiglaucoma and Antimicrobial Effects of Zivzik Pomegranate (Punica granatum)—A Chemical Profiling by LC-MS/MS. Life 2023, 13, 735. [Google Scholar] [CrossRef]
- Karagecili, H.; Yılmaz, M.A.; Ertürk, A.; Kiziltas, H.; Güven, L.; Alwasel, S.H.; Gulcin, İ. Comprehensive Metabolite Profiling of Berdav Propolis Using LC-MS/MS: Determination of Antioxidant, Anticholinergic, Antiglaucoma, and Antidiabetic Effects. Molecules 2023, 28, 1739. [Google Scholar] [CrossRef]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Ranganatha, L.V.; Prasad, M.N. Inhibitory effect of banana (Musa sp. var. Nanjangud rasa bale) flower extract and its constituents Umbelliferone and Lupeol on α-glucosidase, aldose reductase and glycation at multiple stages. S. Afr. J. Bot. 2014, 95, 54–63. [Google Scholar] [CrossRef]
- Maradesha, T.; Patil, S.M.; Al-Mutairi, K.A.; Ramu, R.; Madhunapantula, S.V.; Alqadi, T. Inhibitory effect of polyphenols from the whole green jackfruit flour against α-glucosidase, α-amylase, aldose reductase and glycation at multiple stages and their interaction: Inhibition kinetics and molecular simulations. Molecules 2022, 27, 1888. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, S.; Nivetha, N.; Patil, S.M.; Martiz, R.M.; Ramu, R.; Sreenivasa, S.; Velmathi, S. One-pot three-component synthesis of novel phenyl-pyrano-thiazol-2-one derivatives and their anti-diabetic activity studies. Results Chem. 2022, 4, 100439. [Google Scholar] [CrossRef]
- Patil, S.M.; Martiz, R.M.; Ramu, R.; Shirahatti, P.S.; Prakash, A.; Kumar, B.P.; Kumar, N. Evaluation of flavonoids from banana pseudostem and flower (quercetin and catechin) as potent inhibitors of α-glucosidase: An in silico perspective. J. Biomol. Struct. Dyn. 2022, 40, 12491–12505. [Google Scholar] [CrossRef]
- Maradesha, T.; Patil, S.M.; Phanindra, B.; Achar, R.R.; Silina, E.; Stupin, V.; Ramu, R. Multiprotein Inhibitory Effect of Dietary Polyphenol Rutin from Whole Green Jackfruit Flour Targeting Different Stages of Diabetes Mellitus: Defining a Bio-Computational Stratagem. Separations 2022, 9, 262. [Google Scholar] [CrossRef]
- Banu, H.A.N.; Kalluraya, B.; Manju, N.; Ramu, R.; Patil, S.M.; Lokanatha Rai, K.M.; Kumar, N. Synthesis of Pyrazoline-Embedded 1,2,3-Triazole Derivatives via 1,3-Dipolar Cycloaddition Reactions with in vitro and in silico Studies. ChemistrySelect 2023, 8, e202203578. [Google Scholar] [CrossRef]
- Shivanna, C.; Patil, S.M.; Mallikarjunaswamy, M.; Ramu, R.; Akhileshwari, P.; Nagaraju, L.R.; Sridhar, M.A.; Khanum, S.A.; Ranganatha, L.; Silina, E.; et al. Synthesis, characterization, Hirschfeld surface analysis, crystal structure and molecular modeling studies of 1-(4-(Methoxy(phenyl)methyl)-2-methylphenoxy)butan-2-one derivative as a novel α-glucosidase inhibitor. Crystals 2022, 12, 960. [Google Scholar] [CrossRef]
- Casacchia, T.; Occhiuzzi, M.A.; Grande, F.; Rizzuti, B.; Granieri, M.C.; Rocca, C.; Gattuso, A.; Garofalo, A.; Angelone, T.; Statti, G. A pilot study on the nutraceutical properties of the Citrus hybrid Tacle® as a dietary source of polyphenols for supplementation in metabolic disorders. J. Funct. Foods 2019, 52, 370–381. [Google Scholar] [CrossRef]
- Kumar, V.; Ramu, R.; Shirahatti, P.S.; Kumari, V.C.; Sushma, P.; Mandal, S.P.; Patil, S.M. α-glucosidase; α-amylase inhibition; kinetics and docking studies of novel (2-chloro-6-(trifluoromethyl) benzyloxy) arylidene) based rhodanine and rhodanine acetic acid derivatives. ChemistrySelect 2021, 6, 9637–9644. [Google Scholar] [CrossRef]
- Kumar, V.; Shetty, P.; Arunodaya, H.S.; Chandra, K.S.; Ramu, R.; Patil, S.M.; Baliga, A.; Rai, V.M.; Shenoy, M.S.; Udupi, V.; et al. Potential fluorinated anti-MRSA thiazolidinone derivatives with antibacterial, antitubercular activity and molecular docking studies. Chem. Biodivers. 2022, 19, e202100532. [Google Scholar] [CrossRef] [PubMed]
- Simha, N.A.; Patil, S.M.; Chagalamari, A.; Satish, A.M.; Ramu, R. Protocol to identify multiple protein targets and therapeutic compounds using an in silico polypharmacological approach. STAR Protoc. 2023, 3, 102440. [Google Scholar] [CrossRef]
- Patil, S.M.; Phanindra, B.; Shirahatti, P.S.; Martiz, R.M.; Sajal, H.; Babakr, A.T.; Ramu, R. Computational approaches to define poncirin from Magnolia champaka leaves as a novel multi-target inhibitor of SARS-CoV-2. J. Biomol. Struct. Dyn. 2023, 10, 1–20. [Google Scholar] [CrossRef]
- Pushpa, V.H.; Jayanthi, M.K.; Rashmi, R.H.; Shivamurthy, V.K.N.; Patil, S.M.; Shirahatti, P.S.; Ramu, R. New insights on the phytochemical intervention for the treatment of neuropsychiatric disorders using the leaves of Michelia champaca: An in vivo and in silico approach. Pharm. Biol. 2022, 60, 1656–1668. [Google Scholar] [CrossRef]
- Pradeep, S.; Patil, S.M.; Dharmashekara, C.; Jain, A.; Ramu, R.; Shirahatti, P.S.; Mandal, S.P.; Reddy, P.; Srinivasa, C.; Patil, S.S.; et al. Molecular insights into the in silico discovery of corilagin from Terminalia chebula as a potential dual inhibitor of SARS-CoV-2 structural proteins. J. Biomol. Struct. Dyn. 2022, 10, 1–16. [Google Scholar] [CrossRef]
- Taha, M.; Salahuddin, M.; Rahim, F.; Imran, S.; Hussain, S.; Uddin, N.; Khan, K.M. New Quinoline Analogues: As Potential Diabetics Inhibitors and Molecular Docking Study. Polycycl. Arom. Comp. 2023, 1–23. [Google Scholar] [CrossRef]
- Rajendran, G.; Bhanu, D.; Aruchamy, B.; Ramani, P.; Pandurangan, N.; Bobba, K.N.; Oh, E.J.; Chung, H.Y.; Gangadaran, P.; Ahn, B.-C. Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry. Pharmaceuticals 2022, 15, 1250. [Google Scholar] [CrossRef]
- Aruchamy, B.; Drago, C.; Russo, V.; Pitari, G.M.; Ramani, P.; Aneesh, T.P.; Benny, S.; Vishnu, V. Imidazole-Pyridine Hybrids as Potent Anti-Cancer Agents. Eur. J. Pharm. Sci. 2023, 180, 106323. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.; Razab, R.; Junit, S.M.; Aziz, A.A. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale). Food Chem. 2008, 111, 38–44. [Google Scholar] [CrossRef]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Lakkapa, D.B.; Nagendra, M. Evaluation of Banana (Musa sp. var. Nanjangud Rasa bale) flower and pseudostem extracts on antimicrobial, cytotoxicity and thrombolytic activities. Int. J. Pharm. Pharm. Sci. 2015, 7, 136–140. [Google Scholar]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Lakkappa Dhananjaya, B.; MN, N.P. Assessment of In Vivo Antidiabetic Properties of Umbelliferone and Lupeol Constituents of Banana (Musa sp. var. Nanjangud Rasa Bale) Flower in Hyperglycaemic Rodent Model. PLoS ONE 2016, 11, e0151135. [Google Scholar] [CrossRef]
- Ramu, R.; Shirahatti, P.S.; Nayakavadi, S.; Zameer, V.R.F.; Dhananjaya, B.L.; Nagendra Prasad, M.N. The effect of a plant extract enriched in stigmasterol and β-sitosterol on glycaemic status and glucose metabolism in alloxan-induced diabetic rats. Food Funct. 2016, 7, 3999–4011. [Google Scholar] [CrossRef] [PubMed]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Prasad, M.N.N. Investigation of antihyperglycaemic activity of banana (Musa sp. var. Nanjangud rasa bale) pseudostem in normal and diabetic rats. J. Sci. Food Agric. 2015, 95, 165–173. [Google Scholar] [CrossRef]
- Mallikarjunaswamy, C.; Lakshmi Ranganatha, V.; Ramu, R.; Nagaraju, G.U. Facile microwave-assisted green synthesis of ZnO nanoparticles: Application to photodegradation, antibacterial and antioxidant. J. Mater. Sci. Mater. Electron. 2020, 31, 1004–1021. [Google Scholar] [CrossRef]
- Köksal, E.; Tohma, H.; Kılıç, Ö.; Alan, Y.; Aras, A.; Gülçin, İ.; Bursal, E. Assessment of Antimicrobial and Antioxidant Activities of Nepeta trachonitica: Analysis of Its Phenolic Compounds Using HPLC-MS/MS. Sci. Pharm. 2017, 85, 24. [Google Scholar] [CrossRef] [PubMed]
- Eruygur, N.; Koçyiğit, U.M.; Taslimi, P.; Ataş, M.; Tekin, M.; Gülçin, İ. Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extract. S. Afr. J. Bot. 2019, 120, 141–145. [Google Scholar] [CrossRef]
- Gülçin, I.; Bursal, E.; Sehitoğlu, M.H.; Bilsel, M.; Gören, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J. 1953, 55, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Martiz, R.M.; Patil, S.M.; Hombegowda, D.T.; Shbeer, A.M.; Alqadi, T.; Al-Ghorbani, M.; Ramu, R.; Prasad, A. Phyto-Computational Intervention of Diabetes Mellitus at Multiple Stages Using Isoeugenol from Ocimum tenuiflorum: A Combination of Pharmacokinetics and Molecular Modelling Approaches. Molecules 2022, 27, 6222. [Google Scholar] [CrossRef]
- Nivetha, N.; Martiz, R.M.; Patil, S.M.; Ramu, R.; Sreenivasa, S.; Velmathi, S. Benzodioxole grafted spirooxindole pyrrolidinyl derivatives: Synthesis, characterization, molecular docking and anti-diabetic activity. RSC Adv. 2022, 12, 24192–24207. [Google Scholar] [CrossRef] [PubMed]
- Shashank, P.M.; Prithvi, S.S.; Ramith, R. The pathogenicity of MERS-CoV, SARS-CoV and SARS-CoV-2: A comparative overview. Res. J. Biotech. 2021, 16, 182–192. [Google Scholar]
- Ammar, A.; Cavill, R.; Evelo, C.; Willighagen, E. PSnpBind: A database of mutated binding site protein–ligand complexes constructed using a multithreaded virtual screening workflow. J. Cheminform. 2022, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.P.; Singh, A.K.; Bansal, T.; Yadav, A.; Prajapati, K.S.; Shuaib, M.; Kumar, S. Identification of Natural Inhibitors Against SARS-CoV-2 Drugable Targets Using Molecular Docking, Molecular Dynamics Simulation, and MM-PBSA Approach. Front. Cell. Infect. Microbiol. 2021, 11, 730288. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Martiz, R.M.; Ramu, R.; Shirahatti, P.S.; Prakash, A.; Chandra, J.S.; Ranganatha, L.V. In silico identification of novel benzophenone-coumarin derivatives as SARS-CoV-2 RNAdependent RNA polymerase (RdRp) inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 13032–13048. [Google Scholar] [CrossRef] [PubMed]
- Sajal, H.; Patil, S.M.; Raj, R.; Shbeer, A.M.; Ageel, M.; Ramu, R. Computer-Aided Screening of Phytoconstituents from Ocimum tenuiflorum against Diabetes Mellitus Targeting DPP4 Inhibition: A Combination of Molecular Docking, Molecular Dynamics, and Pharmacokinetics Approaches. Molecules 2022, 27, 5133. [Google Scholar] [CrossRef] [PubMed]
- Martiz, R.M.; Patil, S.M.; Ramu, R.; Jayanthi, M.K.; Ashwini, P.; Ranganatha, L.V.; Khanum, S.A.; Silina, E.; Stupin, V.; Achar, R.R. Discovery of novel benzophenone integrated derivatives as anti-Alzheimer’s agents targeting presenilin-1 and presenilin-2 inhibition: A computational approach. PLoS ONE 2022, 17, e0265022. [Google Scholar] [CrossRef]
- Gurupadaswamy, H.D.; Ranganatha, V.L.; Ramu, R.; Patil, S.M.; Khanum, S.A. Competent synthesis of biaryl analogs via asymmetric Suzuki–Miyaura cross-coupling for the development of anti-inflammatory and analgesic agents. J. Iran. Chem. Soc. 2022, 19, 2421–2436. [Google Scholar] [CrossRef]
- Martiz, R.M.; Patil, S.M.; Abdulaziz, M.; Babalghith, A.; Al-Areefi, M.; Al-Ghorbani, M.; Kumar, J.M.; Prasad, A.; Nagalingaswamy, N.P.M.; Ramu, R. Defining the role of isoeugenol from Ocimum tenuiflorum against diabetes mellitus-linked Alzheimer’s disease through network pharmacology and computational methods. Molecules 2022, 27, 2398. [Google Scholar] [CrossRef]
- Bagewadi, Z.K.; Khan, T.Y.; Gangadharappa, B.; Kamalapurkar, A.; Shamsudeen, S.M.; Yaraguppi, D.A. Molecular dynamics and simulation analysis against superoxide dismutase (SOD) target of Micrococcus luteus with secondary metabolites from Bacillus licheniformis recognized by genome mining approach. Saudi J. Biol. Sci. 2023, 30, 103753. [Google Scholar] [CrossRef]
- Patil, S.M.; Manu, G.; Shivachandra, J.C.; Anil Kumar, K.M.; Vigneswaran, J.; Ramu, R.; Shirahatti, P.S.; Ranganatha, V.L. Computational screening of benzophenone integrated derivatives (BIDs) targeting the NACHT domain of the potential target NLRP3 inflammasome. Adv. Cancer Bio. Metastasis 2022, 5, 100056. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).