Effect of Repeated Administration of ɣ-Valerolactone (GVL) and GHB in the Mouse: Neuroadaptive Changes of the GHB and GABAergic System
Abstract
:1. Introduction
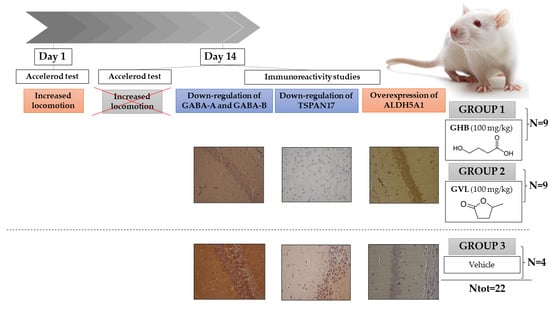
2. Results
2.1. Motor Activity Assessment
2.2. Immunohistochemistry
2.2.1. TSPAN17
2.2.2. ALDH5A1
2.2.3. GABA-A and GABA-B
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drug Preparation and Animal Dose Determination
4.3. Motor Activity Assessment
4.4. Laboratory Protocol
4.5. Immunohistochemistry Analysis
4.6. Data and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maitre, M. The Gamma-Hydroxybutyrate Signalling System in Brain: Organization and Functional Implications. Prog. Neurobiol. 1997, 51, 337–361. [Google Scholar] [CrossRef]
- Busardò, F.P.; Jones, A.W. GHB Pharmacology and Toxicology: Acute Intoxication, Concentrations in Blood and Urine in Forensic Cases and Treatment of the Withdrawal Syndrome. Curr. Neuropharmacol. 2015, 13, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, W.N.; Bessman, S.P. Gamma-Hydroxybutyrate in Mammalian Brain. Reversible Oxidation by Lactic Dehydrogenase. J. Biol. Chem. 1964, 239, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Nutt, D.J. Gamma Hydroxy Butyrate Abuse and Dependency. J. Psychopharmacol. 2005, 19, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Berto, R.; Viel, G.; Montagnese, S.; Raduazzo, D.I.; Ferrara, S.D.; Dauvilliers, Y. Narcolepsy and Effectiveness of Gamma-Hydroxybutyrate (GHB): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sleep Med. Rev. 2012, 16, 431–443. [Google Scholar] [CrossRef]
- Marinetti, L.; Montgomery, M.A. The Use of GHB to Facilitate Sexual Assault. Forensic Sci. Rev. 2010, 22, 41–59. [Google Scholar]
- Carter, L.P.; Chen, W.; Wu, H.; Mehta, A.K.; Hernandez, R.J.; Ticku, M.K.; Coop, A.; Koek, W.; France, C.P. Comparison of the Behavioral Effects of Gamma-Hydroxybutyric Acid (GHB) and Its 4-Methyl-Substituted Analog, Gamma-Hydroxyvaleric Acid (GHV). Drug Alcohol Depend. 2005, 78, 91–99. [Google Scholar] [CrossRef]
- Johansen, S.S.; Windberg, C.N. Simultaneous Determination of γ-Hydroxybutyrate (GHB) and Its Analogues (GBL, 1.4-BD, GVL) in Whole Blood and Urine by Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Anal. Toxicol. 2011, 35, 8–14. [Google Scholar] [CrossRef]
- Marinetti, L.J.; Leavell, B.J.; Jones, C.M.; Hepler, B.R.; Isenschmid, D.S.; Commissaris, R.L. Gamma Butyrolactone (GBL) and Gamma Valerolactone (GVL): Similarities and Differences in Their Effects on the Acoustic Startle Reflex and the Conditioned Enhancement of Startle in the Rat. Pharmacol. Biochem. Behav. 2012, 101, 602–608. [Google Scholar] [CrossRef]
- Camuto, C.; Arfè, R.; Tirri, M.; de la Torre, X.; Mazzarino, M.; Marti, M.; De-Giorgio, F.; Botrè, F. Urinary Excretion and Effects on Visual Placing Response in Mice of Gamma-Valero-Lactone, an Alternative to Gamma-hydroxy-Butyrate for Drug-Facilitated Sexual Assault. Emerg. Trends Drugs Addict. Health 2022, 2, 100028. [Google Scholar] [CrossRef]
- Andresen-Streichert, H.; Jungen, H.; Gehl, A.; Müller, A.; Iwersen-Bergmann, S. Uptake of Gamma-Valerolactone--Detection of Gamma-Hydroxyvaleric Acid in Human Urine Samples. J. Anal. Toxicol. 2013, 37, 250–254. [Google Scholar] [CrossRef]
- Borgen, L.A.; Okerholm, R.A.; Lai, A.; Scharf, M.B. The Pharmacokinetics of Sodium Oxybate Oral Solution Following Acute and Chronic Administration to Narcoleptic Patients. J. Clin. Pharmacol. 2004, 44, 253–257. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Committee on Drug Dependence; WHO: Geneva, Switzerland, 2012; pp. 1–26. [Google Scholar]
- Madah-Amiri, D.; Myrmel, L.; Brattebø, G. Intoxication with GHB/GBL: Characteristics and Trends from Ambulance-Attended Overdoses. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 98. [Google Scholar] [CrossRef] [PubMed]
- Miró, Ò.; Galicia, M.; Dargan, P.; Dines, A.M.; Giraudon, I.; Heyerdahl, F.; Hovda, K.E.; Yates, C.; Wood, D.M.; Liakoni, E.; et al. Intoxication by Gamma Hydroxybutyrate and Related Analogues: Clinical Characteristics and Comparison between Pure Intoxication and That Combined with Other Substances of Abuse. Toxicol. Lett. 2017, 277, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.G.T.; Chan, K.F.Y.; Gibson, K.M.; Snead, O.C. Gamma-Hydroxybutyric Acid: Neurobiology and Toxicology of a Recreational Drug. Toxicol. Rev. 2004, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.M.; van Noorden, M.S.; Franzek, E.; Dijkstra, B.A.G.; Loonen, A.J.M.; De Jong, C.A.J. The Neurobiological Mechanisms of Gamma-Hydroxybutyrate Dependence and Withdrawal and Their Clinical Relevance: A Review. Neuropsychobiology 2016, 73, 65–80. [Google Scholar] [CrossRef]
- Cash, C.D.; Hechler, V.; Mersel, M.; Maitre, M. Kinetic Characterisation and Solubilisation of Gamma-Hydroxybutyrate Receptors from Rat Brain. Neurosci. Lett. 1996, 209, 25–28. [Google Scholar] [CrossRef]
- Kemmel, V.; Miehe, M.; Roussel, G.; Taleb, O.; Nail-Boucherie, K.; Marchand, C.; Stutz, C.; Andriamampandry, C.; Aunis, D.; Maitre, M. Immunohistochemical Localization of a GHB Receptor-like Protein Isolated from Rat Brain. J. Comp. Neurol. 2006, 498, 508–524. [Google Scholar] [CrossRef]
- Bay, T.; Eghorn, L.F.; Klein, A.B.; Wellendorph, P. GHB Receptor Targets in the CNS: Focus on High-Affinity Binding Sites. Biochem. Pharmacol. 2014, 87, 220–228. [Google Scholar] [CrossRef]
- Kaupmann, K.; Cryan, J.F.; Wellendorph, P.; Mombereau, C.; Sansig, G.; Klebs, K.; Schmutz, M.; Froestl, W.; van der Putten, H.; Mosbacher, J.; et al. Specific Gamma-Hydroxybutyrate-Binding Sites but Loss of Pharmacological Effects of Gamma-Hydroxybutyrate in GABA(B)(1)-Deficient Mice. Eur. J. Neurosci. 2003, 18, 2722–2730. [Google Scholar] [CrossRef]
- Schuler, V.; Lüscher, C.; Blanchet, C.; Klix, N.; Sansig, G.; Klebs, K.; Schmutz, M.; Heid, J.; Gentry, C.; Urban, L.; et al. Epilepsy, Hyperalgesia, Impaired Memory, and Loss of Pre- and Postsynaptic GABA(B) Responses in Mice Lacking GABA(B(1)). Neuron 2001, 31, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Ossato, A.; Vigolo, A.; Trapella, C.; Seri, C.; Rimondo, C.; Serpelloni, G.; Marti, M. JWH-018 Impairs Sensorimotor Functions in Mice. Neuroscience 2015, 300, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Maitre, M.; Andriamampandry, C.; Kemmel, V.; Schmidt, C.; Hodé, Y.; Hechler, V.; Gobaille, S. Gamma-Hydroxybutyric Acid as a Signaling Molecule in Brain. Alcohol 2000, 20, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Borgen, L.A.; Okerholm, R.; Morrison, D.; Lai, A. The Influence of Gender and Food on the Pharmacokinetics of Sodium Oxybate Oral Solution in Healthy Subjects. J. Clin. Pharmacol. 2003, 43, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.; Lowe, P.; Symonds, A. The Possible Influence of Micro-Organisms and Putrefaction in the Production of GHB in Post-Mortem Biological Fluid. Forensic Sci. Int. 2004, 139, 183–190. [Google Scholar] [CrossRef]
- Aizawa, M.; Ito, Y.; Fukuda, H. Roles of Gamma-Aminobutyric AcidB (GABA B) and Gamma-Hydroxybutyric Acid Receptors in Hippocampal Long-Term Potentiation and Pathogenesis of Absence Seizures. Biol. Pharm. Bull. 1997, 20, 1066–1070. [Google Scholar] [CrossRef]
- Farr, S.A.; Uezu, K.; Creonte, T.A.; Flood, J.F.; Morley, J.E. Modulation of Memory Processing in the Cingulate Cortex of Mice. Pharmacol. Biochem. Behav. 2000, 65, 363–368. [Google Scholar] [CrossRef]
- Arfè, R.; Bilel, S.; Tirri, M.; Frisoni, P.; Serpelloni, G.; Neri, M.; Boccuto, F.; Bernardi, T.; Foti, F.; De-Giorgio, F.; et al. Comparison of N-Methyl-2-Pyrrolidone (NMP) and the “Date Rape” Drug GHB: Behavioral Toxicology in the Mouse Model. Psychopharmacology 2021, 238, 2275–2295. [Google Scholar] [CrossRef]
- Frisoni, P.; Neri, M.; D’Errico, S.; Alfieri, L.; Bonuccelli, D.; Cingolani, M.; Di Paolo, M.; Gaudio, R.M.; Lestani, M.; Marti, M.; et al. Cytokine Storm and Histopathological Findings in 60 Cases of COVID-19-Related Death: From Viral Load Research to Immunohistochemical Quantification of Major Players IL-1β, IL-6, IL-15 and TNF-α. Forensic Sci. Med. Pathol. 2022, 18, 4–19. [Google Scholar] [CrossRef]








| Antibody | Concentration of Primary Antibody |
|---|---|
| ALDH5A1 (D-3) (sc-390754; Santa Cruz Biotechnology, Santa Cruz, California, U.S.A.) | 1:500 |
| TSPAN17 (ab180601; Abcam, Cambridge, U.K.) | 1:200 |
| GABA-A Receptor alpha 1 (ab33299; Abcam, Cambridge, U.K.) | 1:500 |
| GABA B Receptor 1 (ab55051; Abcam, Cambridge, U.K.) | 1:2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frisoni, P.; Corli, G.; Bilel, S.; Tirri, M.; Gasparini, L.C.; Alfieri, L.; Neri, M.; De-Giorgio, F.; Marti, M. Effect of Repeated Administration of ɣ-Valerolactone (GVL) and GHB in the Mouse: Neuroadaptive Changes of the GHB and GABAergic System. Pharmaceuticals 2023, 16, 1225. https://doi.org/10.3390/ph16091225
Frisoni P, Corli G, Bilel S, Tirri M, Gasparini LC, Alfieri L, Neri M, De-Giorgio F, Marti M. Effect of Repeated Administration of ɣ-Valerolactone (GVL) and GHB in the Mouse: Neuroadaptive Changes of the GHB and GABAergic System. Pharmaceuticals. 2023; 16(9):1225. https://doi.org/10.3390/ph16091225
Chicago/Turabian StyleFrisoni, Paolo, Giorgia Corli, Sabrine Bilel, Micaela Tirri, Laura Camilla Gasparini, Letizia Alfieri, Margherita Neri, Fabio De-Giorgio, and Matteo Marti. 2023. "Effect of Repeated Administration of ɣ-Valerolactone (GVL) and GHB in the Mouse: Neuroadaptive Changes of the GHB and GABAergic System" Pharmaceuticals 16, no. 9: 1225. https://doi.org/10.3390/ph16091225
APA StyleFrisoni, P., Corli, G., Bilel, S., Tirri, M., Gasparini, L. C., Alfieri, L., Neri, M., De-Giorgio, F., & Marti, M. (2023). Effect of Repeated Administration of ɣ-Valerolactone (GVL) and GHB in the Mouse: Neuroadaptive Changes of the GHB and GABAergic System. Pharmaceuticals, 16(9), 1225. https://doi.org/10.3390/ph16091225