Metformin in Gestational Diabetes Mellitus: To Use or Not to Use, That Is the Question
Abstract
:1. Introduction
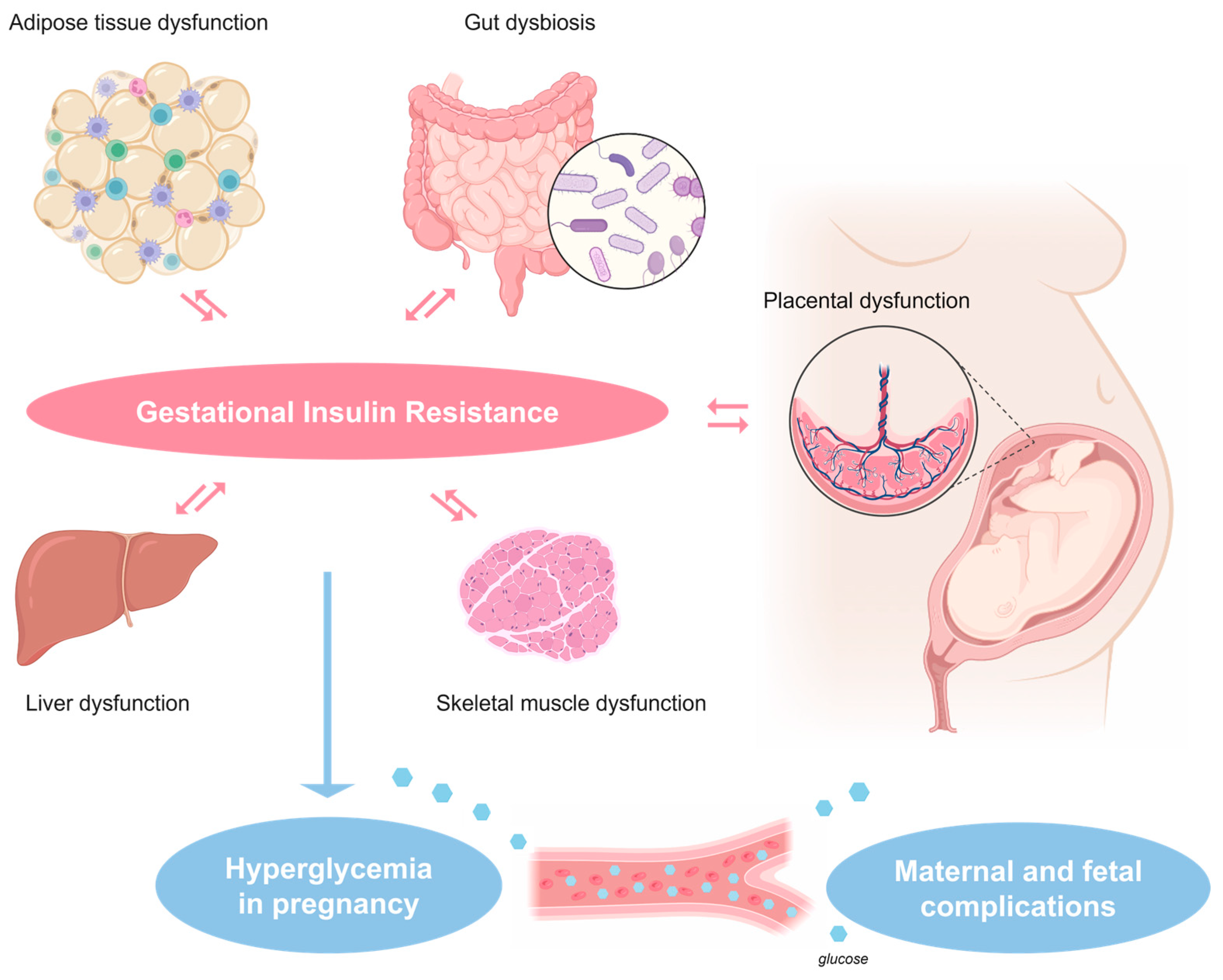
2. Pathophysiology of Gestational Insulin Resistance and Gestational Diabetes Mellitus (GDM)
3. Metformin
4. Treatment Options for GDM
5. Benefits of Metformin Use in Pregnancies Complicated by GDM
6. Drawbacks of Metformin Use in Pregnancies Complicated by GDM
7. Why and How the Guidelines on Metformin Use for GDM Differ
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas 10th Edition. 2021. Available online: https://www.diabetesatlas.org (accessed on 29 July 2023).
- Mirabelli, M.; Chiefari, E.; Tocci, V.; Greco, E.; Foti, D.P.; Brunetti, A. Gestational diabetes: Implications for fetal growth, intervention timing, and treatment options. Curr. Opin. Pharmacol. 2021, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Capula, C.; Chiefari, E.; Vero, A.; Arcidiacono, B.; Iiritano, S.; Puccio, L.; Pullano, V.; Foti, D.; Brunetti, A.; Vero, R. Gestational diabetes mellitus: Screening and outcomes in southern italian pregnant women. ISRN Endocrinol. 2013, 2013, 387495. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Tocci, V.; Donnici, A.; Giuliano, S.; Sarnelli, P.; Salatino, A.; Greco, M.; Puccio, L.; Chiefari, E.; Foti, D.P.; et al. Maternal Preconception Body Mass Index Overtakes Age as a Risk Factor for Gestational Diabetes Mellitus. J. Clin. Med. 2023, 12, 2830. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.M.; Vellinga, A.; Harreiter, J.; Simmons, D.; Desoye, G.; Corcoy, R.; Adelantado, J.M.; Devlieger, R.; Van Assche, A.; Galjaard, S.; et al. Epidemiology of gestational diabetes mellitus according to IADPSG/WHO 2013 criteria among obese pregnant women in Europe. Diabetologia 2017, 60, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Hillier, T.A.; Pedula, K.L.; Ogasawara, K.K.; Vesco, K.K.; Oshiro, C.E.S.; Lubarsky, S.L.; Van Marter, J. A Pragmatic, Randomized Clinical Trial of Gestational Diabetes Screening. N. Engl. J. Med. 2021, 384, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.D.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Ferrara, A.; Hedderson, M.M.; Quesenberry, C.P.; Selby, J.V. Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the carpenter and coustan plasma glucose thresholds. Diabetes Care 2002, 25, 1625–1630. [Google Scholar] [CrossRef]
- National Institutes of Health. National Institutes of Health consensus development conference statement: Diagnosing gestational diabetes mellitus, March 4–6, 2013. Obstet. Gynecol. 2013, 122 Pt 1, 358–369. [Google Scholar] [CrossRef]
- Brown, F.M.; Wyckoff, J. Application of One-Step IADPSG Versus Two-Step Diagnostic Criteria for Gestational Diabetes in the Real World: Impact on Health Services, Clinical Care, and Outcomes. Curr. Diabetes Rep. 2017, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Beardsall, K.; Amanda, L.; Ogilvy-Stuart, A.L. Developmental physiology of carbohydrate metabolism and the pancreas. In Maternal-Fetal and Neonatal Endocrinology; Kovacs, C.S., Deal, C.L., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 587–597. ISBN 9780128148235. [Google Scholar] [CrossRef]
- Bozzetti, P.; Ferrari, M.M.; Marconi, A.M.; Ferrazzi, E.; Pardi, G.; Makowski, E.L.; Battaglia, F.C. The relationship of maternal and fetal glucose concentrations in the human from midgestation until term. Metabolism 1988, 37, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Lain, K.Y.; Catalano, P.M. Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 2007, 50, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Alonso-Magdalena, P.; Soriano, S.; Ropero, A.B.; Quesada, I. The role of oestrogens in the adaptation of islets to insulin resistance. J. Physiol. 2009, 587 Pt 21, 5031–5037. [Google Scholar] [CrossRef] [PubMed]
- Auffret, J.; Freemark, M.; Carré, N.; Mathieu, Y.; Tourrel-Cuzin, C.; Lombès, M.; Movassat, J.; Binart, N. Defective prolactin signaling impairs pancreatic β-cell development during the perinatal period. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1309–E1318. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M. Trying to understand gestational diabetes. Diabet. Med. 2014, 31, 273–281. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007, 30 (Suppl. S2), S112–S119. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Chiefari, E.; Foryst-Ludwig, A.; Currò, G.; Navarra, G.; Brunetti, F.S.; Mirabelli, M.; Corigliano, D.M.; Kintscher, U.; Britti, D.; et al. Obesity-related hypoxia via miR-128 decreases insulin-receptor expression in human and mouse adipose tissue promoting systemic insulin resistance. EBioMedicine 2020, 59, 102912. [Google Scholar] [CrossRef]
- Chiefari, E.; Mirabelli, M.; La Vignera, S.; Tanyolaç, S.; Foti, D.P.; Aversa, A.; Brunetti, A. Insulin Resistance and Cancer: In Search for a Causal Link. Int. J. Mol. Sci. 2021, 22, 11137. [Google Scholar] [CrossRef]
- Friedman, J.E.; Ishizuka, T.; Shao, J.; Huston, L.; Highman, T.; Catalano, P. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes 1999, 48, 1807–1814. [Google Scholar] [CrossRef]
- Greco, M.; Mirabelli, M.; Tocci, V.; Mamula, Y.; Salatino, A.; Brunetti, F.S.; Dragone, F.; Sicilia, L.; Tripolino, O.; Chiefari, E.; et al. Prothymosin-Alpha, a Novel and Sensitive Biomarker of the Inflammatory and Insulin-Resistant Statuses of Obese Individuals: A Pilot Study Involving Humans. Endocrines 2023, 4, 427–436. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Pantham, P.; Aye, I.L.; Powell, T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhou, L.; Novák, P.; Shi, X.; Lin, C.B.; Zhu, X.; Yin, K. Metabolic Dysfunction in the Regulation of the NLRP3 Inflammasome Activation: A Potential Target for Diabetic Nephropathy. J. Diabetes Res. 2022, 2022, 2193768. [Google Scholar] [CrossRef] [PubMed]
- Bonde, L.; Vilsbøll, T.; Nielsen, T.; Bagger, J.I.; Svare, J.A.; Holst, J.J.; Larsen, S.; Knop, F.K. Reduced postprandial GLP-1 responses in women with gestational diabetes mellitus. Diabetes Obes. Metab. 2013, 15, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Tocci, V.; Caroleo, P.; Giuliano, S.; Greco, E.; Luque, R.M.; Puccio, L.; Foti, D.P.; Aversa, A.; et al. Clinical Effectiveness and Safety of Once-Weekly GLP-1 Receptor Agonist Dulaglutide as Add-On to Metformin or Metformin Plus Insulin Secretagogues in Obesity and Type 2 Diabetes. J. Clin. Med. 2021, 10, 985. [Google Scholar] [CrossRef]
- Purrello, F.; Gullo, D.; Brunetti, A.; Buscema, M.; Italia, S.; Goldfine, I.D.; Vigneri, R. Direct effects of biguanides on glucose utilization in vitro. Metabolism 1987, 36, 774–776. [Google Scholar] [CrossRef]
- Benzi, L.; Trischitta, V.; Ciccarone, A.; Cecchetti, P.; Brunetti, A.; Squatrito, S.; Marchetti, P.; Vigneri, R.; Navalesi, R. Improvement with metformin in insulin internalization and processing in monocytes from NIDDM patients. Diabetes 1990, 39, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Luft, D.; Schmülling, R.M.; Eggstein, M. Lactic acidosis in biguanide-treated diabetics: A review of 330 cases. Diabetologia 1978, 14, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Asif, S.; Bennett, J.; Marakkath, B. Metformin-associated Lactic Acidosis: An Unexpected Scenario. Cureus 2019, 11, e4397. [Google Scholar] [CrossRef] [PubMed]
- Rajasurya, V.; Anjum, H.; Surani, S. Metformin Use and Metformin-associated Lactic Acidosis in Intensive Care Unit Patients with Diabetes. Cureus 2019, 11, e4739. [Google Scholar] [CrossRef] [PubMed]
- Nabrdalik, K.; Skonieczna-Żydecka, K.; Irlik, K.; Hendel, M.; Kwiendacz, H.; Łoniewski, I.; Januszkiewicz, K.; Gumprecht, J.; Lip, G.Y.H. Gastrointestinal adverse events of metformin treatment in patients with type 2 diabetes mellitus: A systematic review, meta-analysis and meta-regression of randomized controlled trials. Front. Endocrinol. 2022, 13, 975912. [Google Scholar] [CrossRef] [PubMed]
- Fruehwald-Schultes, B.; Kern, W.; Oltmanns, K.M.; Sopke, S.; Toschek, B.; Born, J.; Fehm, H.L.; Peters, A. Metformin does not adversely affect hormonal and symptomatic responses to recurrent hypoglycemia. J. Clin. Endocrinol. Metab. 2001, 86, 4187–4192. [Google Scholar] [CrossRef]
- Petrie, J.R.; Rossing, P.R.; Campbell, I.W. Metformin and cardiorenal outcomes in diabetes: A reappraisal. Diabetes Obes. Metab. 2020, 22, 904–915. [Google Scholar] [CrossRef]
- Vial, G.; Detaille, D.; Guigas, B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Weinberg, S.E.; Hamanaka, R.B.; Soberanes, S.; Sullivan, L.B.; Anso, E.; Glasauer, A.; Dufour, E.; Mutlu, G.M.; Budigner, G.S.; et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 2014, 3, e02242. [Google Scholar] [CrossRef]
- Owe, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348 Pt 3, 607–614. [Google Scholar]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006, 574 Pt 1, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.W.; Hughey, C.C.; Lantier, L.; Sundelin, E.I.; Peggie, M.; Zeqiraj, E.; Sicheri, F.; Jessen, N.; Wasserman, D.H.; Sakamoto, K. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 2018, 24, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Bahne, E.; Sun, E.W.L.; Young, R.L.; Hansen, M.; Sonne, D.P.; Hansen, J.S.; Rohde, U.; Liou, A.P.; Jackson, M.L.; de Fontgalland, D.; et al. Metformin-induced glucagon-like peptide-1 secretion contributes to the actions of metformin in type 2 diabetes. JCI Insight 2018, 3, e93936. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, O.; Deschênes-Simard, X.; St-Germain, E.; Igelmann, S.; Huot, G.; Cadar, A.E.; Bourdeau, V.; Pollak, M.N.; Ferbeyre, G. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 2013, 12, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Ouslimani, N.; Peynet, J.; Bonnefont-Rousselot, D.; Thérond, P.; Legrand, A.; Beaudeux, J.L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 2005, 54, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Sambe, T.; Mason, R.P.; Dawoud, H.; Bhatt, D.L.; Malinski, T. Metformin treatment decreases nitroxidative stress, restores nitric oxide bioavailability and endothelial function beyond glucose control. Biomed. Pharmacother. 2018, 98, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Castello, R.; Negri, C.; Tosi, F.; Perrone, F.; Caputo, M.; Zanolin, E.; Muggeo, M. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: A randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J. Clin. Endocrinol. Metab. 2000, 85, 139–146. [Google Scholar] [CrossRef]
- Morley, L.C.; Tang, T.M.H.; Balen, A.H. Metformin Therapy for the Management of Infertility in Women with Polycystic Ovary Syndrome: Scientific Impact Paper No. 13. BJOG 2017, 124, e306–e313. [Google Scholar] [CrossRef]
- Ling, S.; Tian, Y.; Zhang, H.; Jia, K.; Feng, T.; Sun, D.; Gao, Z.; Xu, F.; Hou, Z.; Li, Y.; et al. Metformin reverses multidrug resistance in human hepatocellular carcinoma Bel-7402/5-fluorouracil cells. Mol. Med. Rep. 2014, 10, 2891–2897. [Google Scholar] [CrossRef]
- Messineo, S.; Arcidiacono, B.; Corigliano, D.M.; Foti, D.P.; Castano, J.P.; Luque, R.M.; Brunetti, A. Metformin inhibits vistatin gene expression via HIF1 in PC3 prostate cancer cells: A potential role for visfatin as a non-invasive biomarker. Abstract #1226. Diabetologia 2017, 60, S564–S565. [Google Scholar]
- Greco, M.; Chiefari, E.; Accattato, F.; Corigliano, D.M.; Arcidiacono, B.; Mirabelli, M.; Liguori, R.; Brunetti, F.S.; Pullano, S.A.; Scorcia, V.; et al. MicroRNA-1281 as a Novel Circulating Biomarker in Patients with Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Mirabelli, M.; Chiefari, E.; Greco, M.; Di Vito, A.; Bonapace, G.; Brunetti, F.S.; Crocerossa, F.; Epstein, A.L.; Foti, D.P.; et al. The anticancer effects of Metformin in the male germ tumor SEM-1 cell line are mediated by HMGA1. Front. Endocrinol. 2022, 13, 1051988. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, E.; Foti, D.P.; Sgarra, R.; Pegoraro, S.; Arcidiacono, B.; Brunetti, F.S.; Greco, M.; Manfioletti, G.; Brunetti, A. Transcriptional Regulation of Glucose Metabolism: The Emerging Role of the HMGA1 Chromatin Factor. Front. Endocrinol. 2018, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, A.; Foti, D.; Goldfine, I.D. Identification of unique nuclear regulatory proteins for the insulin receptor gene, which appear during myocyte and adipocyte differentiation. J. Clin. Investig. 1993, 92, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, A.; Brunetti, L.; Foti, D.; Accili, D.; Goldfine, I.D. Human diabetes associated with defects in nuclear regulatory proteins for the insulin receptor gene. J. Clin. Investig. 1996, 97, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Dunwoodie, S.L. The role of hypoxia in development of the Mammalian embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Piscitelli, S.; Passaro, F.; Russo, T. HMGA Proteins in Stemness and Differentiation of Embryonic and Adult Stem Cells. Int. J. Mol. Sci. 2020, 21, 362. [Google Scholar] [CrossRef]
- Swenson, K.S.; Wang, D.; Jones, A.K.; Nash, M.J.; O’Rourke, R.; Takahashi, D.L.; Kievit, P.; Hennebold, J.D.; Aagaard, K.M.; Friedman, J.E.; et al. Metformin Disrupts Signaling and Metabolism in Fetal Hepatocytes. Diabetes 2023, 72, db230089. [Google Scholar] [CrossRef]
- Iiritano, S.; Chiefari, E.; Ventura, V.; Arcidiacono, B.; Possidente, K.; Nocera, A.; Nevolo, M.T.; Fedele, M.; Greco, A.; Greco, M.; et al. The HMGA1-IGF-I/IGFBP system: A novel pathway for modulating glucose uptake. Mol. Endocrinol. 2012, 26, 1578–1589. [Google Scholar] [CrossRef]
- Murthi, P.; Pinar, A.A.; Dimitriadis, E.; Samuel, C.S. Inflammasomes-A Molecular Link for Altered Immunoregulation and Inflammation Mediated Vascular Dysfunction in Preeclampsia. Int. J. Mol. Sci. 2020, 21, 1406. [Google Scholar] [CrossRef]
- Stødle, G.S.; Silva, G.B.; Tangerås, L.H.; Gierman, L.M.; Nervik, I.; Dahlberg, U.E.; Sun, C.; Aune, M.H.; Thomsen, L.C.V.; Bjørge, L.; et al. Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts. Clin. Exp. Immunol. 2018, 193, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Vangrieken, P.; Al-Nasiry, S.; Bast, A.; Leermakers, P.A.; Tulen, C.B.M.; Schiffers, P.M.H.; van Schooten, F.J.; Remels, A.H.V. Placental Mitochondrial Abnormalities in Preeclampsia. Reprod. Sci. 2021, 28, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Zhong, Y.; Li, Q.; Wu, M.; Yang, L.; Liu, X.; Zou, L. Metformin Corrects Glucose Metabolism Reprogramming and NLRP3 Inflammasome-Induced Pyroptosis via Inhibiting the TLR4/NF-κB/PFKFB3 Signaling in Trophoblasts: Implication for a Potential Therapy of Preeclampsia. Oxidative Med. Cell Longev. 2021, 2021, 1806344. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Robinson, I.G.; Pantaleão, L.C.; Armstrong, J.L.; Thackray, B.D.; Holzner, L.M.W.; Knapton, A.E.; Virtue, S.; Jenkins, B.; Koulman, A.; et al. The metabolic response of human trophoblasts derived from term placentas to metformin. Diabetologia 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef] [PubMed]
- Major, C.A.; Henry, M.J.; De Veciana, M.; Morgan, M.A. The effects of carbohydrate restriction in patients with diet-controlled gestational diabetes. Obstet. Gynecol. 1998, 91, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Kapur, K.; Kapur, A.; Hod, M. Nutrition Management of Gestational Diabetes Mellitus. Ann. Nutr. Metab. 2021, 76, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, N.; Balducci, S.; Lencioni, C.; Bertolotto, A.; Tumminia, A.; Dodesini, A.R.; Pintaudi, B.; Marcone, T.; Vitacolonna, E.; Napoli, A. Review of general suggestions on physical activity to prevent and treat gestational and pre-existing diabetes during pregnancy and in postpartum. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 115–126. [Google Scholar] [CrossRef]
- Riddle, M.C. Oral pharmacologic management of type 2 diabetes. Am. Fam. Physician 1999, 60, 2613–2620. [Google Scholar]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Vanky, E.; Zahlsen, K.; Spigset, O.; Carlsen, S.M. Placental passage of metformin in women with polycystic ovary syndrome. Fertil. Steril. 2005, 83, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, A.; Fernandez-Twinn, D.S.; Blackmore, H.L.; Ashmore, T.J.; Heaton, R.A.; Jenkins, B.; Koulman, A.; Hargreaves, I.P.; Aiken, C.E.; Ozanne, S.E. Maternal but not fetoplacental health can be improved by metformin in a murine diet-induced model of maternal obesity and glucose intolerance. J. Physiol. 2022, 600, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Wei, D.; Loeken, M.R. Lack of metformin effect on mouse embryo AMPK activity: Implications for metformin treatment during pregnancy. Diabetes Metab Res. Rev. 2014, 30, 23–30. [Google Scholar] [CrossRef]
- Nguyen, L.; Chan, S.Y.; Teo, A.K.K. Metformin from mother to unborn child—Are there unwarranted effects? EBioMedicine 2018, 35, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Cassina, M.; Donà, M.; Di Gianantonio, E.; Litta, P.; Clementi, M. First-trimester exposure to metformin and risk of birth defects: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Given, J.E.; Loane, M.; Garne, E.; Addor, M.C.; Bakker, M.; Bertaut-Nativel, B.; Gatt, M.; Klungsoyr, K.; Lelong, N.; Morgan, M.; et al. Metformin exposure in first trimester of pregnancy and risk of all or specific congenital anomalies: Exploratory case-control study. BMJ 2018, 361, k2477. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ma, J.; Tang, J.; Hu, D.; Zhang, W.; Zhao, X. Comparative Efficacy and Safety of Metformin, Glyburide, and Insulin in Treating Gestational Diabetes Mellitus: A Meta-Analysis. J. Diabetes Res. 2019, 2019, 9804708. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Q.; Xu, G.X.; Teng, X.Y.; Xu, J.W.; Tang, L.F.; Feng, C.; Rao, J.P.; Jin, M.; Wang, L.Q. Glycemic control and neonatal outcomes in women with gestational diabetes mellitus treated using glyburide, metformin, or insulin: A pairwise and network meta-analysis. BMC Endocr. Disord. 2021, 21, 199. [Google Scholar] [CrossRef]
- Capula, C.; Chiefari, E.; Borelli, M.; Oliverio, R.; Vero, A.; Foti, D.; Puccio, L.; Vero, R.; Brunetti, A. A new predictive tool for the early risk assessment of gestational diabetes mellitus. Prim. Care Diabetes 2016, 10, 315–323. [Google Scholar] [CrossRef]
- Chiswick, C.; Reynolds, R.M.; Denison, F.; Drake, A.J.; Forbes, S.; Newby, D.E.; Walker, B.R.; Quenby, S.; Wray, S.; Weeks, A.; et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 778–786. [Google Scholar] [CrossRef]
- Vanky, E.; Stridsklev, S.; Heimstad, R.; Romundstad, P.; Skogøy, K.; Kleggetveit, O.; Hjelle, S.; von Brandis, P.; Eikeland, T.; Flo, K.; et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: A randomized, controlled multicenter study. J. Clin. Endocrinol. Metab. 2010, 95, E448–E455. [Google Scholar] [CrossRef] [PubMed]
- Løvvik, T.S.; Carlsen, S.M.; Salvesen, Ø.; Steffensen, B.; Bixo, M.; Gómez-Real, F.; Lønnebotn, M.; Hestvold, K.V.; Zabielska, R.; Hirschberg, A.L.; et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.; McKinley, M.C.; McNally, R.; Graham, U.; Watson, C.J.; Lyons, T.J.; McClements, L. Risk of pre-eclampsia in women taking metformin: A systematic review and meta-analysis. Diabet Med. 2018, 35, 160–172. [Google Scholar] [CrossRef]
- Anness, A.R.; Baldo, A.; Webb, D.R.; Khalil, A.; Robinson, T.G.; Mousa, H.A. Effect of metformin on biomarkers of placental- mediated disease: A systematic review and meta-analysis. Placenta 2021, 107, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cluver, C.A.; Hiscock, R.; Decloedt, E.H.; Hall, D.R.; Schell, S.; Mol, B.W.; Brownfoot, F.; Kaitu’u-Lino, T.J.; Walker, S.P.; Tong, S. Use of metformin to prolong gestation in preterm pre-eclampsia: Randomised, double blind, placebo controlled trial. BMJ 2021, 374, n2103. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Ozanne, S.E.; Aiken, C.E. Impact of metformin treatment during pregnancy on maternal outcomes: A systematic review/meta-analysis. Sci. Rep. 2021, 11, 9240. [Google Scholar] [CrossRef] [PubMed]
- Kunasegaran, T.; Balasubramaniam, V.R.M.T.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biology 2021, 10, 1027. [Google Scholar] [CrossRef]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Scarpello, J.H.; Hodgson, E.; Howlett, H.C. Effect of metformin on bile salt circulation and intestinal motility in type 2 diabetes mellitus. Diabet. Med. 1998, 15, 651–656. [Google Scholar] [CrossRef]
- Preiss, D.; Dawed, A.; Welsh, P.; Heggie, A.; Jones, A.G.; Dekker, J.; Koivula, R.; Hansen, T.H.; Stewart, C.; Holman, R.R.; et al. Sustained influence of metformin therapy on circulating glucagon-like peptide-1 levels in individuals with and without type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 356–363. [Google Scholar] [CrossRef]
- Molina-Vega, M.; Picón-César, M.J.; Gutiérrez-Repiso, C.; Fernández-Valero, A.; Lima-Rubio, F.; González-Romero, S.; Moreno-Indias, I.; Tinahones, F.J. Metformin action over gut microbiota is related to weight and glycemic control in gestational diabetes mellitus: A randomized trial. Biomed. Pharmacother. 2022, 145, 112465. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Wu, K.L.H.; Lee, W.C.; Leu, S.; Chan, J.Y.H. Prenatal Metformin Therapy Attenuates Hypertension of Developmental Origin in Male Adult Offspring Exposed to Maternal High-Fructose and Post-Weaning High-Fat Diets. Int. J. Mol. Sci. 2018, 19, 1066. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Cui, J.; Hu, S.; Wang, R.; Li, H.; Sun, B. Maternal Treatment with Metformin Persistently Ameliorates High-Fat Diet-Induced Metabolic Symptoms and Modulates Gut Microbiota in Rat Offspring. Nutrients 2022, 14, 3612. [Google Scholar] [CrossRef]
- Ponzo, V.; Ferrocino, I.; Zarovska, A.; Amenta, M.B.; Leone, F.; Monzeglio, C.; Rosato, R.; Pellegrini, M.; Gambino, R.; Cassader, M.; et al. The microbiota composition of the offspring of patients with gestational diabetes mellitus (GDM). PLoS ONE 2019, 14, e0226545. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Nie, Y.; Shao, R.; Duan, S.; Jiang, Y.; Wang, M.; Xing, Z.; Sun, Q.; Liu, X.; Xu, W. Diversified gut microbiota in newborns of mothers with gestational diabetes mellitus. PLoS ONE 2018, 13, e0205695. [Google Scholar] [CrossRef] [PubMed]
- Rodolaki, K.; Pergialiotis, V.; Iakovidou, N.; Boutsikou, T.; Iliodromiti, Z.; Kanaka-Gantenbein, C. The impact of maternal diabetes on the future health and neurodevelopment of the offspring: A review of the evidence. Front. Endocrinol. 2023, 14, 1125628. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, J.M.; Garrett, A.J.; Schneider, L.A.; Hodyl, N.A.; Goldsworthy, M.R.; Coat, S.; Rowan, J.A.; Hague, W.M.; Pitcher, J.B. Reduced Cortical Excitability, Neuroplasticity, and Salivary Cortisol in 11-13-Year-Old Children Born to Women with Gestational Diabetes Mellitus. EBioMedicine 2018, 31, 143–149. [Google Scholar] [CrossRef]
- Lynch, K.M.; Alves, J.M.; Chow, T.; Clark, K.A.; Luo, S.; Toga, A.W.; Xiang, A.H.; Page, K.A. Selective morphological and volumetric alterations in the hippocampus of children exposed in utero to gestational diabetes mellitus. Hum. Brain Mapp. 2021, 42, 2583–2592. [Google Scholar] [CrossRef]
- Wouldes, T.A.; Battin, M.; Coat, S.; Rush, E.C.; Hague, W.M.; Rowan, J.A. Neurodevelopmental outcome at 2 years in offspring of women randomised to metformin or insulin treatment for gestational diabetes. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F488–F493. [Google Scholar] [CrossRef]
- Landi, S.N.; Radke, S.; Engel, S.M.; Boggess, K.; Stürmer, T.; Howe, A.S.; Funk, M.J. Association of Long-term Child Growth and Developmental Outcomes with Metformin vs Insulin Treatment for Gestational Diabetes. JAMA Pediatr. 2019, 173, 160–168. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Aiken, C.E.; Ozanne, S.E. Comparative impact of pharmacological treatments for gestational diabetes on neonatal anthropometry independent of maternal glycaemic control: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003126. [Google Scholar] [CrossRef] [PubMed]
- Salomäki, H.; Vähätalo, L.H.; Laurila, K.; Jäppinen, N.T.; Penttinen, A.M.; Ailanen, L.; Ilyasizadeh, J.; Pesonen, U.; Koulu, M. Prenatal metformin exposure in mice programs the metabolic phenotype of the offspring during a high fat diet at adulthood. PLoS ONE 2013, 8, e56594. [Google Scholar] [CrossRef]
- Schoonejans, J.M.; Blackmore, H.L.; Ashmore, T.J.; Aiken, C.E.; Fernandez-Twinn, D.S.; Ozanne, S.E. Maternal Metformin Intervention during Obese Glucose-Intolerant Pregnancy Affects Adiposity in Young Adult Mouse Offspring in a Sex-Specific Manner. Int. J. Mol. Sci. 2021, 22, 8104. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.C.; Khoueiry, R.; Quanico, J.; Acloque, H.; Guerquin, M.J.; Bertoldo, M.J.; Chevaleyre, C.; Ramé, C.; Fournier, I.; Salzet, M.; et al. In Utero Exposure to Metformin Reduces the Fertility of Male Offspring in Adulthood. Front. Endocrinol. 2021, 12, 750145. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, P.; Battista, S.; Barchi, M.; Di Agostino, S.; Pierantoni, G.M.; Fedele, M.; Chiariotti, L.; Tramontano, D.; Fusco, A. HMGA1 and HMGA2 protein expression in mouse spermatogenesis. Oncogene 2002, 21, 3644–3650. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P.K.; Page, D.C. Germ cell determination and the developmental origin of germ cell tumors. Development 2021, 148, dev198150. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Hague, W.M.; Gao, W.; Battin, M.R.; Moore, M.P. MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N. Engl. J. Med. 2008, 358, 2003–2015. [Google Scholar] [CrossRef]
- Rowan, J.A.; Rush, E.C.; Plank, L.D.; Lu, J.; Obolonkin, V.; Coat, S.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diabetes Res. Care 2018, 6, e000456. [Google Scholar] [CrossRef]
- Hanem, L.G.E.; Salvesen, Ø.; Juliusson, P.B.; Carlsen, S.M.; Nossum, M.C.F.; Vaage, M.Ø.; Ødegård, R.; Vanky, E. Intrauterine metformin exposure and offspring cardiometabolic risk factors (PedMet study): A 5–10 year follow-up of the PregMet randomised controlled trial. Lancet Child Adolesc. Health 2019, 3, 166–174. [Google Scholar] [CrossRef]
- Dodd, J.M.; Grivell, R.M.; Deussen, A.R.; Hague, W.M. Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes. Cochrane Database Syst. Rev. 2018, 7, CD010564. [Google Scholar] [CrossRef]
- Hughes, R.C.; Gardiner, S.J.; Begg, E.J.; Zhang, M. Effect of pregnancy on the pharmacokinetics of metformin. Diabet. Med. 2006, 23, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Espnes, K.A.; Hønnås, A.; Løvvik, T.S.; Gundersen, P.O.M.; Naavik, A.; Skogvoll, E.; Westin, A.A.; Spigset, O.; Vanky, E. Metformin serum concentrations during pregnancy and post partum—A clinical study in patients with polycystic ovary syndrome. Basic Clin. Pharmacol. Toxicol. 2022, 130, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Leoni, M.; Caprio, M.; Fabbri, A. Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World J. Diabetes 2021, 12, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- De Jager, J.; Kooy, A.; Lehert, P.; Wulffelé, M.G.; van der Kolk, J.; Bets, D.; Verburg, J.; Donker, A.J.; Stehouwer, C.D. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: Randomised placebo controlled trial. BMJ 2010, 340, c2181. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148 (Suppl. S4), 1995S–2027S. [Google Scholar] [CrossRef] [PubMed]
- Hariz, A.; Bhattacharya, P.T. Megaloblastic Anemia. [Updated 3 April 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537254/ (accessed on 29 July 2023).
- Dhiman, P.; Pillai, R.R.; Wilson, A.B.; Premkumar, N.; Bharadwaj, B.; Ranjan, V.P.; Rajendiran, S. Cross-sectional association between vitamin B12 status and probable postpartum depression in Indian women. BMC Pregnancy Childbirth 2021, 21, 146. [Google Scholar] [CrossRef]
- Batalha, M.A.; Dos Reis Costa, P.N.; Ferreira, A.L.L.; Freitas-Costa, N.C.; Figueiredo, A.C.C.; Shahab-Ferdows, S.; Hampel, D.; Allen, L.H.; Pérez-Escamilla, R.; Kac, G. Maternal Mental Health in Late Pregnancy and Longitudinal Changes in Postpartum Serum Vitamin B-12, Homocysteine, and Milk B-12 Concentration Among Brazilian Women. Front. Nutr. 2022, 9, 923569. [Google Scholar] [CrossRef]
- Rupanagunta, G.P.; Nandave, M.; Rawat, D.; Upadhyay, J.; Rashid, S.; Ansari, M.N. Postpartum depression: Aetiology, pathogenesis and the role of nutrients and dietary supplements in prevention and management. Saudi Pharm. J. 2023, 31, 1274–1293. [Google Scholar] [CrossRef]
- Venkatramanan, S.; Armata, I.E.; Strupp, B.J.; Finkelstein, J.L. Vitamin B-12 and Cognition in Children. Adv. Nutr. 2016, 7, 879–888. [Google Scholar] [CrossRef]
- Bosch-Bayard, J.; Biscay, R.J.; Fernandez, T.; Otero, G.A.; Ricardo-Garcell, J.; Aubert-Vazquez, E.; Evans, A.C.; Harmony, T. EEG effective connectivity during the first year of life mirrors brain synaptogenesis, myelination, and early right hemisphere predominance. Neuroimage 2022, 252, 119035. [Google Scholar] [CrossRef] [PubMed]
- Gramer, G.; Hoffmann, G.F. Vitamin B12 Deficiency in Newborns and their Mothers-Novel Approaches to Early Detection, Treatment and Prevention of a Global Health Issue. Curr. Med. Sci. 2020, 40, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Gatford, K.L.; Houda, C.M.; Lu, Z.X.; Coat, S.; Baghurst, P.A.; Owens, J.A.; Sikaris, K.; Rowan, J.A.; Hague, W.M. Vitamin B12 and homocysteine status during pregnancy in the metformin in gestational diabetes trial: Responses to maternal metformin compared with insulin treatment. Diabetes Obes. Metab. 2013, 15, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Hale, T.W.; Kristensen, J.H.; Hackett, L.P.; Kohan, R.; Ilett, K.F. Transfer of metformin into human milk. Diabetologia 2002, 45, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S. Drugs and breastfeeding in women with diabetes. In Diabetes in Pregnancy; Robert, L., Ed.; Oxford Diabetes Library: Oxford, UK, 2012. [Google Scholar] [CrossRef]
- Mellitus, G.D. Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S254–S266. [Google Scholar] [CrossRef] [PubMed]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Cabero Roura, L.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S3), S173–S211. [Google Scholar] [CrossRef]
- Wender-Ożegowska, E.; Bomba-Opoń, D.; Brązert, J.; Celewicz, Z.; Czajkowski, K.; Gutaj, P.; Malinowska-Polubiec, A.; Zawiejska, A.; Wielgoś, M. Standards of Polish Society of Gynecologists and Obstetricians in management of women with diabetes. Ginekol. Pol. 2018, 89, 341–350. [Google Scholar] [CrossRef]
- Sciacca, L.; Bianchi, C.; Burlina, S.; Formoso, G.; Manicardi, E.; Sculli, M.A.; Resi, V. Position paper of the Italian Association of Medical Diabetologists (AMD), Italian Society of Diabetology (SID), and the Italian Study Group of Diabetes in pregnancy: Metformin use in pregnancy. Acta Diabetol. 2023, 60, 1421–1437. [Google Scholar] [CrossRef]
- Society of Maternal-Fetal Medicine (SMFM) Publications Committee. Electronic address: Pubs@smfm.org. SMFM Statement: Pharmacological treatment of gestational diabetes. Am. J. Obstet. Gynecol. 2018, 218, B2–B4. [Google Scholar] [CrossRef]
- Barbour, L.A.; Scifres, C.; Valent, A.M.; Friedman, J.E.; Buchanan, T.A.; Coustan, D.; Aagaard, K.; Thornburg, K.L.; Catalano, P.M.; Galan, H.L.; et al. A cautionary response to SMFM statement: Pharmacological treatment of gestational diabetes. Am. J. Obstet. Gynecol. 2018, 219, 367.e1–367.e7. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Rush, E.C.; Obolonkin, V.; Battin, M.; Wouldes, T.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition at 2 years of age. Diabetes Care 2011, 34, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Tew, M.P.; Tan, P.C.; Saaid, R.; Hong, J.G.S.; Omar, S.Z. Metformin in gestational diabetes mellitus: A double-blind placebo-controlled randomized trial. Int. J. Gynaecol. Obstet. 2022, 156, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S.; Donovan, L.E.; Zinman, B.; Sanchez, J.J.; Asztalos, E.; Ryan, E.A.; Fantus, I.G.; Hutton, E.; Armson, A.B.; Lipscombe, L.L.; et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): A multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Tertti, K.; Ekblad, U.; Koskinen, P.; Vahlberg, T.; Rönnemaa, T. Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes. Metab. 2013, 15, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Spaulonci, C.P.; Bernardes, L.S.; Trindade, T.C.; Zugaib, M.; Francisco, R.P. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am. J. Obstet. Gynecol. 2013, 209, 34.e1–34.e7. [Google Scholar] [CrossRef] [PubMed]
- Ruholamin, S.; Eshaghian, S.; Allame, Z. Neonatal outcomes in women with gestational diabetes mellitus treated with metformin in compare with insulin: A randomized clinical trial. J. Res. Med. Sci. 2014, 19, 970–975. [Google Scholar] [PubMed]
- Fornes, R.; Simin, J.; Nguyen, M.H.; Cruz, G.; Crisosto, N.; van der Schaaf, M.; Engstrand, L.; Brusselaers, N. Pregnancy, perinatal and childhood outcomes in women with and without polycystic ovary syndrome and metformin during pregnancy: A nationwide population-based study. Reprod. Biol. Endocrinol. 2022, 20, 30. [Google Scholar] [CrossRef]
- Tommasi, S.; Zheng, A.; Weninger, A.; Bates, S.E.; Li, X.A.; Wu, X.; Hollstein, M.; Besaratinia, A. Mammalian cells acquire epigenetic hallmarks of human cancer during immortalization. Nucleic Acids Res. 2013, 41, 182–195. [Google Scholar] [CrossRef]
- Deb, K.D.; Sarda, K. Human embryonic stem cells: Preclinical perspectives. J. Transl. Med. 2008, 6, 7. [Google Scholar] [CrossRef]
| Pharmacological Management: No (A) | Pharmacological Management: Yes (B) | Pharmacological Management: Not Reported (C) | |
|---|---|---|---|
| Maternal outcomes | |||
| Pre-eclampsia | 1.39 (0.99–1.96) | 1.24 (0.94–1.63) | 1.46 (1.21–1.78) * |
| Induction of labor | 1.33 (0.97–1.82) | 1.83 (0.02–136.80) | 1.88 (1.16–3.04) * |
| Instrumental delivery | 0.83 (0.02–41.94) * | 0.52 (0.20–1.33) | 1.10 (0.59–2.06) |
| Caesarean section | 1.16 (1.03–1.32) | 1.70 (0.99–2.90) | 1.38 (1.20–1.58) * |
| Shoulder dystocia | 1.26 (0.98–1.62) | 1.29 (0.87–1.92) | 1.48 (0.99–2.20) |
| Premature rupture of membranes | – | – | 1.13 (1.06–1.20) * |
| Postpartum hemorrhage | 1.07 (0.68–1.66) | – | 0.94 (0.75–1.17) |
| Neonatal outcomes | |||
| Stillbirth | 1.08 (0.38–3.11) | – | 0.78 (0.58–1.05) |
| Congenital malformation | 0.78 (0.36–1.70) | 1.62 (0.65–4.07) | 1.18 (1.10–1.26) * |
| Pre-term birth | 1.51 (1.26–1.80) * | 1.22 (0.99–1.50) | 1.51 (1.19–1.93) * |
| Respiratory distress syndrome | 1.38 (0.76–2.50) | 1.57 (1.19–2.08) * | 1.59 (0.89–2.83) |
| Low 1′ APGAR score | 1.43 (1.01–2.03) | 1.63 (0.88–3.02) | – |
| Low 5′ APGAR score | 1.11 (0.74–1.66) | 0.94 (0.70–1.27) | 1.12 (0.96–1.32) |
| Macrosomia | 1.70 (1.23–2.36) * | 1.56 (0.92–2.66) | 1.48 (1.13–1.95) |
| LGA birth | 1.57 (1.25–1.97) * | 1.61 (1.09–2.37) * | 1.42 (0.98–2.06) |
| Low birthweight | 1.40 (1.12–16.73) | 0.87 (0.53–1.43) | 0.94 (0.84–1.05) |
| SGA birth | 0.83 (0.55–1.23) | 0.77 (0.46–1.29) | 1.19 (0.67–2.11) |
| Neonatal hypoglycemia | 3.94 (0.37–42.05) | – | 11.71 (7.49–18.30) * |
| Neonatal jaundice | 1.33 (0.94–1.88) | 1.28 (1.02–1.62) * | – |
| Admission to NICU | 1.34 (0.86–2.09) | 2.29 (1.59–3.31) * | 2.28 (1.26–4.13) * |
| Society | First Line | Second Line |
|---|---|---|
| ACOG | Insulin | Metformin |
| ADA | Insulin | Metformin or glyburide |
| IDF | Insulin | Metformin |
| WHO | Insulin | Metformin |
| FIGO | Insulin | Metformin |
| PDA/PSGO | Insulin | None |
| DIPSI | Insulin | Metformin |
| SID | Insulin | None * |
| NICE | Metformin | Insulin |
| SMFM | Insulin or metformin | Glyburide |
| Study | Sample Size | Study Population Characteristics | Metformin Daily Dosage | Timing of Metformin Administration | Primary Study Outcome(s) |
|---|---|---|---|---|---|
| MIG [109], RCT | N = 751 women (N = 363, metformin group; N = 388, insulin group) | Singleton pregnant women diagnosed with GDM according to ADIPS criteria | 500 to 2500 mg (plus supplemental insulin, if needed) | Starting 20 to 33 weeks of gestation | Perinatal outcome: a composite of neonatal hypoglycemia, respiratory distress, need for phototherapy, birth trauma, 5-min Apgar score <7, or prematurity (RR 1.00, 95% CI 0.90–1.10, ns) |
| MIG TOFU [136], Post-RCT follow-up | N = 318 children (N = 154, children born to metformin-treated women; N = 164, children born to insulin-treated women) | Children (aged 2 years), born to mothers randomized in the MiG-trial | Children outcome: body composition measured with anthropometry, bioimpedance, and DXA (larger mid-upper arm circumferences, and larger subscapular and biceps skinfolds in the metformin-exposed group) | ||
| MIG TOFU [110], Post-RCT follow-up | N = 208 children (N = 103, children born to metformin-treated women; N = 105, children born to insulin-treated women) | Children (aged 7–9 years), born to mothers randomized in the MiG trial | Children outcome: body composition measured with anthropometry, bioimpedance, DXA, and MRI (larger measures of weight, arm and waist circumferences, waist-to-height ratio, BMI, triceps skinfold, fat mass and lean mass, abdominal fat volume in the metformin-exposed group from the Auckland cohort, no differences between groups in the Adelaide cohort) | ||
| ISRCTN10845466 [137], RCT | N = 106 women (N = 53, metformin group; N = 53, placebo group) | Singleton pregnant women diagnosed with GDM according to Malaysian national criteria | 1000 to 1500 mg (plus supplemental insulin, if needed) | Starting 16 to 30 weeks of gestation | Maternal outcome: change in A1c at 36 weeks of gestation (mean A1c increment +0.20% vs. +0.27%, ns) |
| MiTy [138], RCT | N = 502 women (N = 233, metformin group; N = 240, placebo group) | Singleton pregnant women with pregestational T2D | 2000 mg (as add-on to insulin) | Starting 6 to 22 weeks of gestation | Perinatal outcome: a composite of pregnancy loss, preterm birth, birth injury, moderate/severe respiratory distress, neonatal hypoglycemia, or neonatal intensive care unit admission longer than 24 h (RR 1.02, 95% CI 0.83–1.26, ns) |
| PregMet [83], RCT | N = 257 women (N= 136 metformin group, N = 240 placebo group) | Singleton pregnant women with PCOS | 2000 mg | Starting 5 to 12 weeks of gestation | Maternal outcomes: prevalence of pre-eclampsia (risk difference 3.7%, 95% CI −1.7–9.2, ns), GDM (risk difference 0.8%, 95% CI −8.6–10.2, ns), preterm delivery (risk difference −4.4%, 95% CI, −10.1–1.2, ns), and a composite of these three endpoints (risk difference 1.5%, 95% CI −8.9–11.3, ns) |
| PregMet 2 [84], RCT | N = 487 women (N= 244 metformin group, N = 243 placebo group) | Singleton pregnant women with PCOS | 2000 mg | Starting 6 to 12 weeks of gestation | Maternal outcomes: frequency of late miscarriage and preterm birth (5% vs. 10%, odds ratio 0.5, 95% CI 0.22–1.08, ns), incidence of GDM (25% vs. 24%, odds ratio 1.09, 95% CI 0.69–1.66, ns) |
| PedMet [111], Post-RCT follow-up | N = 141 children (N = 71, children born to metformin-treated women; N = 70, children born to placebo-treated women) | Children (aged 5–10 years), born to mothers randomized in the PregMet trial | Children outcomes: BMI Z score (difference in means +0.41, 95% CI 0.03–0.78) | ||
| NCT01240785 [139], RCT | N = 221 women (N= 111, metformin group; N = 110, insulin group) | Singleton pregnant women with GDM diagnosed according to Finnish national criteria | 1000 to 2000 mg (plus supplemental insulin, if needed) | Starting 22 to 34 weeks of gestation | Perinatal outcomes: birthweight expressed in grams (difference in means +15, 90% CI −121–89, ns), birthweight > 90 centile (RR 0.9, 95% CI 0.5–1.8, ns) |
| UMIN 000005393 [140], RCT | N = 94 women (N = 47, metformin group; N = 47, insulin group) | Singleton pregnant women with GDM diagnosed according to Carpenter-Coustan criteria | 1700 to 2550 mg | Starting 28 to 34 weeks of gestation | Maternal outcomes: gestational weight gain (mean Kg, 0.53 vs. 2.3), frequency of pre-eclampsia (21.7% vs. 15.2%, ns), preterm birth (10.9% vs. 10.9%, ns), caesarean section (71.7% vs. 65.2%, ns) |
| IRCT201306057841N4 [141], RCT | N = 119 women (N = 59, metformin group; N = 60, insulin group) | Singleton pregnant women with GDM diagnosed according to ADIPS criteria | 500 to 1500 mg (plus supplemental insulin, if needed) | Starting 25 to 35 weeks of gestation | Perinatal outcomes: caesarean section (74% vs. 70%, ns), neonatal hypoglycemia (ns), Apgar score (ns), birthweight (mean grams, 3176 vs. 3342, ns) |
| EMPOWaR [82], RCT | N = 449 (N = 226 metformin group, N = 223 placebo group) | Singleton pregnant women with obesity according to WHO criteria and without GDM | 500 to 2500 mg | Starting 12 to 16 weeks of gestation | Perinatal outcome: Z score of birthweight percentile (difference in means −0.029, 95% CI −0·217–0.158, ns) |
| Meta-Analysis Outcomes | Summary of Results |
|---|---|
| Maternal outcomes § |
|
| Neonatal outcomes ‡ |
|
| Children outcomes ‡ |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tocci, V.; Mirabelli, M.; Salatino, A.; Sicilia, L.; Giuliano, S.; Brunetti, F.S.; Chiefari, E.; De Sarro, G.; Foti, D.P.; Brunetti, A. Metformin in Gestational Diabetes Mellitus: To Use or Not to Use, That Is the Question. Pharmaceuticals 2023, 16, 1318. https://doi.org/10.3390/ph16091318
Tocci V, Mirabelli M, Salatino A, Sicilia L, Giuliano S, Brunetti FS, Chiefari E, De Sarro G, Foti DP, Brunetti A. Metformin in Gestational Diabetes Mellitus: To Use or Not to Use, That Is the Question. Pharmaceuticals. 2023; 16(9):1318. https://doi.org/10.3390/ph16091318
Chicago/Turabian StyleTocci, Vera, Maria Mirabelli, Alessandro Salatino, Luciana Sicilia, Stefania Giuliano, Francesco S. Brunetti, Eusebio Chiefari, Giovambattista De Sarro, Daniela P. Foti, and Antonio Brunetti. 2023. "Metformin in Gestational Diabetes Mellitus: To Use or Not to Use, That Is the Question" Pharmaceuticals 16, no. 9: 1318. https://doi.org/10.3390/ph16091318
APA StyleTocci, V., Mirabelli, M., Salatino, A., Sicilia, L., Giuliano, S., Brunetti, F. S., Chiefari, E., De Sarro, G., Foti, D. P., & Brunetti, A. (2023). Metformin in Gestational Diabetes Mellitus: To Use or Not to Use, That Is the Question. Pharmaceuticals, 16(9), 1318. https://doi.org/10.3390/ph16091318










