Humoral and Cellular Immune Response after Three Doses of Sinopharm [Vero Cell]-Inactivated COVID-19 Vaccine in Combination with SARS-CoV-2 Infection Leads to Hybrid Immunity
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Participant Selection and Serum Collection
4.3. SARS-CoV-2 Serological Analyses
4.4. T-Cell Response
4.5. Statistical Analysis
4.6. SARS-CoV-2 Serological Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Participant self-questionnaire
- History of previous SARS-CoV-2 infections with the date on which the symptoms appeared or the date of the last positive PCR result;
- History of vaccination (date and name of the 1st, 2nd and 3rd dose of SARS-CoV-2 vaccine);
- Diseases of the cardiovascular system (hypertension, ischemic heart disease, heart failure, heart valve diseases, myocarditis, endocarditis, pericarditis, deep vein thrombosis, etc.);
- Diseases of the endocrine system (diabetes mellitus, metabolic syndrome, hyperthyroidism, hypothyroidism, Cushing’s syndrome, etc.);
- Diseases of the nervous system (cerebrovascular diseases, stroke, epilepsy, multiple sclerosis, polyneuropathy, neuroborreliosis, etc.).
- Liver diseases (hepatitis B, hepatitis C, cirrhosis, etc.);
- Autoimmune diseases (systemic lupus, rheumatoid arthritis, systemic sclerosis, etc.);
- Pulmonary diseases (asthma, COPD, emphysema, pulmonary hypertension, etc.);
- Kidney diseases (hypertensive nephropathy, diabetic nephropathy, hydronephrosis, chronic renal insufficiency, etc.);
- The presence of allergic reactions (atopy, allergic dermatitis, allergic rhinitis, allergic asthma, etc.);
- Primary and secondary immunodeficiencies (yes or no, and which);
- Severe diseases of the hematopoietic system (yes or no, and which);
- Oncological diseases (yes or no, and which);
- Pregnancy and breastfeeding status.
Appendix B
| Commercial ELISA Name | Purpose of Detection | Reference Values | Automated System | |
|---|---|---|---|---|
| anti-SARS-CoV-2 neutralizing antibodies–NA | Novel coronavirus SARS-CoV-2 Neutralizing Antibody Detection Kit (ELISA) Shanghai GeneoDx Biotech Co, Ltd. | neutralizing antibodies (NA) | <79 U/mL: negative ≥79 to <81 U/mL: borderline ≥81 U/mL: positive | DYNEX DS2®, Dynex Technologies |
| anti-SARS-CoV-2 IgG S1 | Anti-SARS-CoV-2 QuantiVac ELISA (IgG), EUROIMMUN AG, Lübeck, Germany | IgG antibodies against S1 (including RBD) | <8 RU/mL: negative ≥8 to <11 RU/mL: borderline ≥11 RU/mL: positive | EuroImmun I Analyzer |
| anti-SARS-CoV-2 IgM N | Anti-SARS-CoV-2 NCP ELISA (IgM), EUROIMMUN AG, Lübeck, Germany | IgM antibodies against the nucleocapsid protein (N) | Ratio < 0.8: negative Ratio ≥ 0.8 to <1.1: borderline Ratio ≥ 1.1: positive | EuroImmun I Analyzer |
| anti-SARS-CoV-2 IgG N | EIA COVID-19 NP IgG, TestLine Clinical Diagnostics | IgG antibodies against the nucleocapsid protein (N) | <18 U/mL: negative ≥8 to <22 U/mL: borderline ≥22 U/mL: positive | DYNEX DS2®, Dynex Technologies |
| anti-SARS-CoV-2 IgM RBD | EIA COVID-19 RBD IgM, TestLine Clinical Diagnostics | IgM antibodies against the RBD domain | <18 U/mL: negative ≥8 to <22 U/mL: borderline ≥22 U/mL: positive | DYNEX DS2®, Dynex Technologies |
References
- Amicrone, M.; Aves, M.J.; Isidoro, J.; Zé-Zé, L.; Durate, S.; Vieira, R.; Guimar, R.; Gpmes, J.P.; Gordo, I. Mutation rate of SARS-CoV-2 and emergence of mutators during experimental evolution. Evol. Med. Public Health 2022, 10, 141–142. [Google Scholar] [CrossRef]
- Dearlove, B.; Lewitus, E.; Bai, H.; Rolland, M.A. SARS-CoV-2 vaccine candidate would likely match all currently circulating variants. Proc. Natl. Acad. Sci. USA 2020, 117, 23652–23662. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Bruel, T.; Grzelak, L.; Guivel-Benhassine, F.; Staropoli, I.; Porrot, F.; Planchais, C.; Buchrieser, J.; Rajah, M.M.; Bishop, E.; et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralising antibodies. Nat. Med. 2021, 27, 917–924. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Moustsen-Helms, I.R.; Schelde, A.B.; Gram, M.A.; Emborg, H.D.; Nielsen, J.; Hansen, C.H.; Andersen, M.A.; Meaidi, M.; Wohlfahrt, J.; et al. Vaccine effectiveness against SARS-CoV-2 reinfection during periods of Alpha, Delta, or Omicron dominance: A Danish nationwide study. PLoS Med. 2022, 19, e1004037. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Breathnach, A.S.; Riley, P.A.; Cotter, M.P.; Houston, A.C.; Habibi, M.S.; Planche, T.D. Prior COVID-19 significantly reduces the risk of subsequent infection, but reinfections are seen after eight months. J. Infect. 2021, 82, e11–e12. [Google Scholar] [CrossRef]
- Wang, B.X. Susceptibility and prognosis of COVID-19 patients with cardiovascular disease. Open Heart 2020, 7, e001310. [Google Scholar] [CrossRef]
- Puig-Domingo, M.; Marazuela, M.; Giustina, A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine 2020, 68, 2–5. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Nagelkerke, N.; Ayoub, H.H.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Al-Kanaani, Z.; et al. Duration of immune protection of SARS-CoV-2 natural infection against reinfection. J. Travel Med. 2022, 29, taac109. [Google Scholar] [CrossRef]
- Pušnik, J.; Monzon-Posadas, W.O.; Zorn, J.; Peters, K.; Baum, M.; Proksch, H.; Schlüter, B.C.; Alter, G.; Menting, T.; Streeck, H. SARS-CoV-2 humoral and cellular immunity following different combinations of vaccination and breakthrough infection. Nat. Commun. 2023, 14, 572. [Google Scholar] [CrossRef]
- Scudellari, M. How the Coronavirus infects our cells. Nature 2021, 595, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Vicky, B.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, J.; Cheng, X.; Yu, L.; Zhang, L.; Wang, W.; Ni, L.; Wei, C.; Huang, Y.; Cheng, Z. Reduced numbers of T cells and B cells correlates with persistent SARS-CoV-2 presence in non-severe COVID-19 patients. Sci. Rep. 2020, 10, 17718. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.-F.; Xu, W.; Liu, S.-W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Tilocca, B.; Soggiu, A.; Sanguinetti, M.; Musella, V.; Britti, D.; Bonizzi, L.; Urbani, A.; Roncada, P. Comparative computational analysis of SARS-CoV-2 nucleocapsid protein epitopes in taxonomically related coronaviruses. Microbes Infect. 2020, 22, 188–194. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Zhang, Y.; Scheuermann, R.H.; Peters, B.; Sette, A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe 2020, 27, 671–680. [Google Scholar] [CrossRef]
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. The impact of pre-existing crossreactive immunity on SARS-CoV-2 infection and vaccine responses. Nat. Rev. Immunol. 2023, 23, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.K.P.; Cohen, C.A.; Cheng, S.M.S.; Chen, C.; Kwok, K.-O.; Yiu, K.; Chan, T.-O.; Bull, M.; Ling, K.C.; Dai, Z.; et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology 2022, 27, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Premikha, M.; Chiew, C.J.; Wei, W.E.; Leo, Y.S.; Ong, B.; Lye, D.C.; Lee, V.J.; Tan, K.B. Comparative effectiveness of mRNA and inactivated whole virus vaccines against COVID-19 infection and severe disease in Singapore. Clin. Infect. Dis. 2022, 75, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Ella, R.; Reddy, S.; Blackwelder, W.; Potdar, V.; Yadav, P.; Sarangi, V.; Aileni, V.K.; Kanungo, S.; Rai, S.; Reddy, P.; et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): Interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet 2021, 398, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 209–2100. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Chen, S.; Guan, F.; Condotti, F.; Benlagha, K.; Chen, S.; Guan, F.; Candotti, F.; Benlagha, K.; Camara, N.O.S.; Herrada, A.A.; et al. The role of B cells in COVID-19 infection and vaccination. Front. Immunol. 2022, 13, 988536. [Google Scholar] [CrossRef]
- Misra, A.; Theel, E.S. Immunity to SARS-CoV-2: What do we know and should we be testing for it. J. Clin. Microbiol. 2022, 60, e00482-21. [Google Scholar] [CrossRef] [PubMed]
- Grygorian, L.; Pulendran, B. The immunology of SARS-CoV-2 infection and vaccines. Semin. Immunol. 2020, 50, 101422. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, L.; Czartoski, J.; Wan, Y.H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. A single mRNA immunisation boosts cross-variant neutralising antibodies elicited by SARS-CoV-2 infection. Science 2021, 372, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef] [PubMed]
- Ielapi, N.; Licastro, L.; Provenzano, M.; Andreucci, M.; Franciscis, S.; Serra, R. Cardiovascular disease as a biomarker for an increased risk of COVID-19 infection and related poor prognosis. Biomark. Med. 2020, 14, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Chung, M.K.; Zidar, D.A.; Bristow, M.R.; Cameron, S.J.; Chan, T.; Harding III, C.V.; Kwon, D.H.; Singh, T.; Tilton, J.C.; Tsai, E.J.; et al. COVID-19 and cardiovascular disease. Circ. Res. 2021, 128, 1214–1236. [Google Scholar] [CrossRef]
- Kuijpers, J.; Picavet, S.J.; Rond, L.; Zeeuw-Brouwer, M.; Rutkens, R.; Gijsbers, E.; Slits, I.; Engelfriet, P.; Buisman, A.; Verschuren, M. Potential determinants of antibody responses after vaccination against SARS-CoV-2 in older persons: The Doetinchem Cohort Study. Immun. Ageing 2023, 20, 57. [Google Scholar] [CrossRef]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Rubino, F.; Khunti, K.; Mingrone, G.; Hopkins, D.; Birkenfeld, A.L.; Boehm, B.; Amiel, S.; Holt, R.I.; Skyler, J.S.; et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 546–550. [Google Scholar] [CrossRef]
- Hinkle, E.D.; Wiersma, W.; Jurs, G.S. Applied Statistics for Behavioral Sciences, 2nd ed.; Houghton Mifflin Company: Boston, MA, USA, 1994. [Google Scholar]
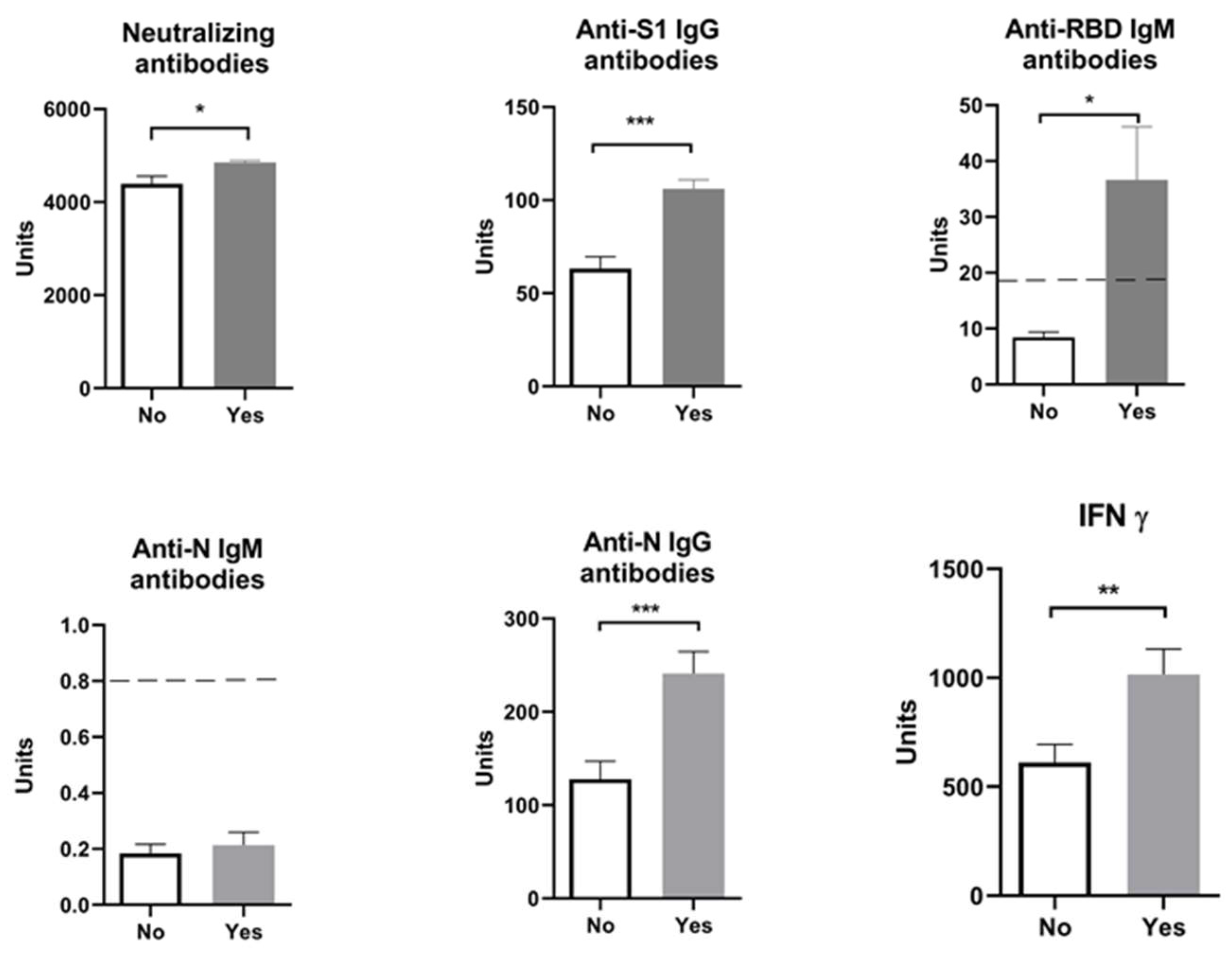
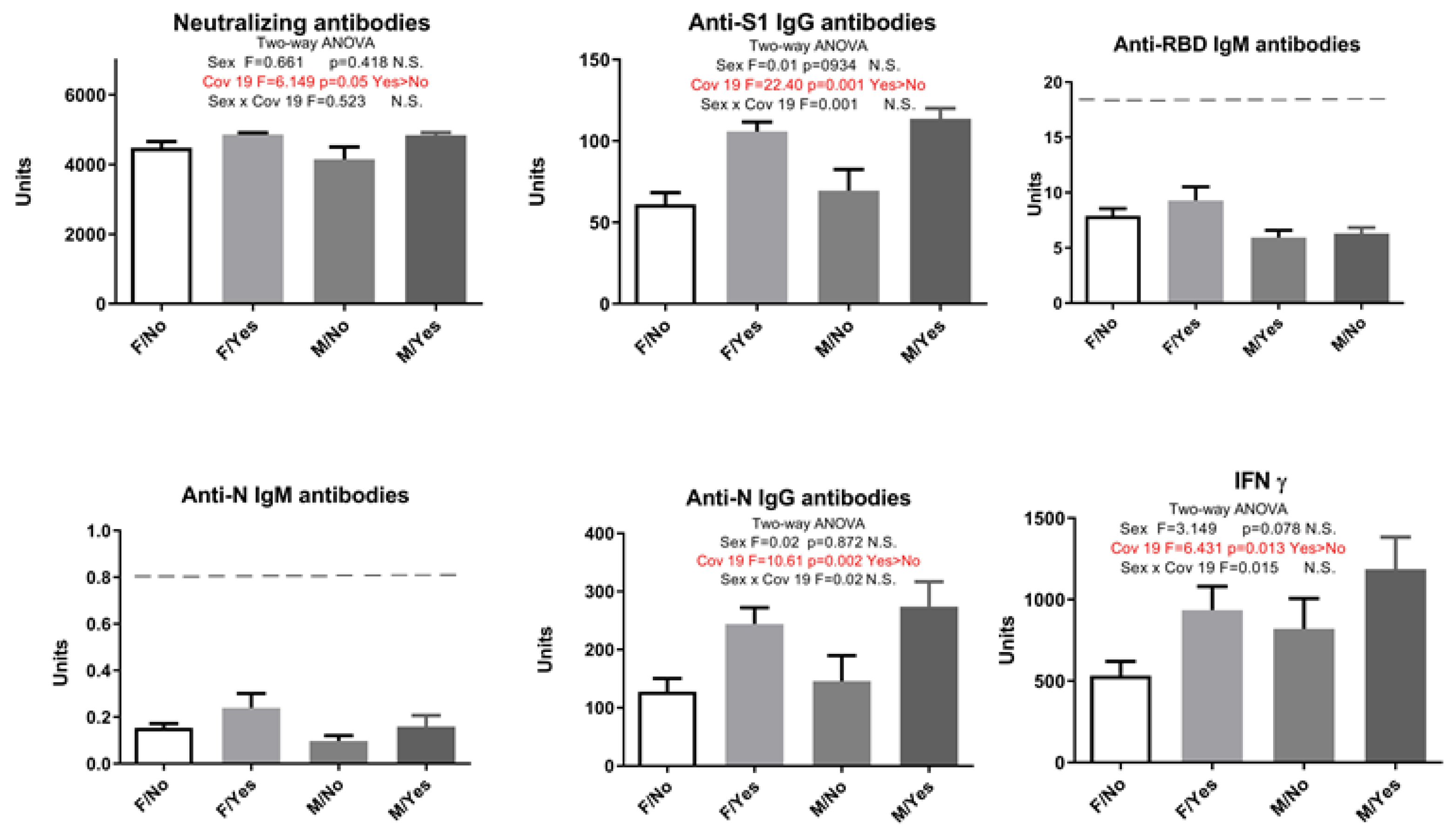
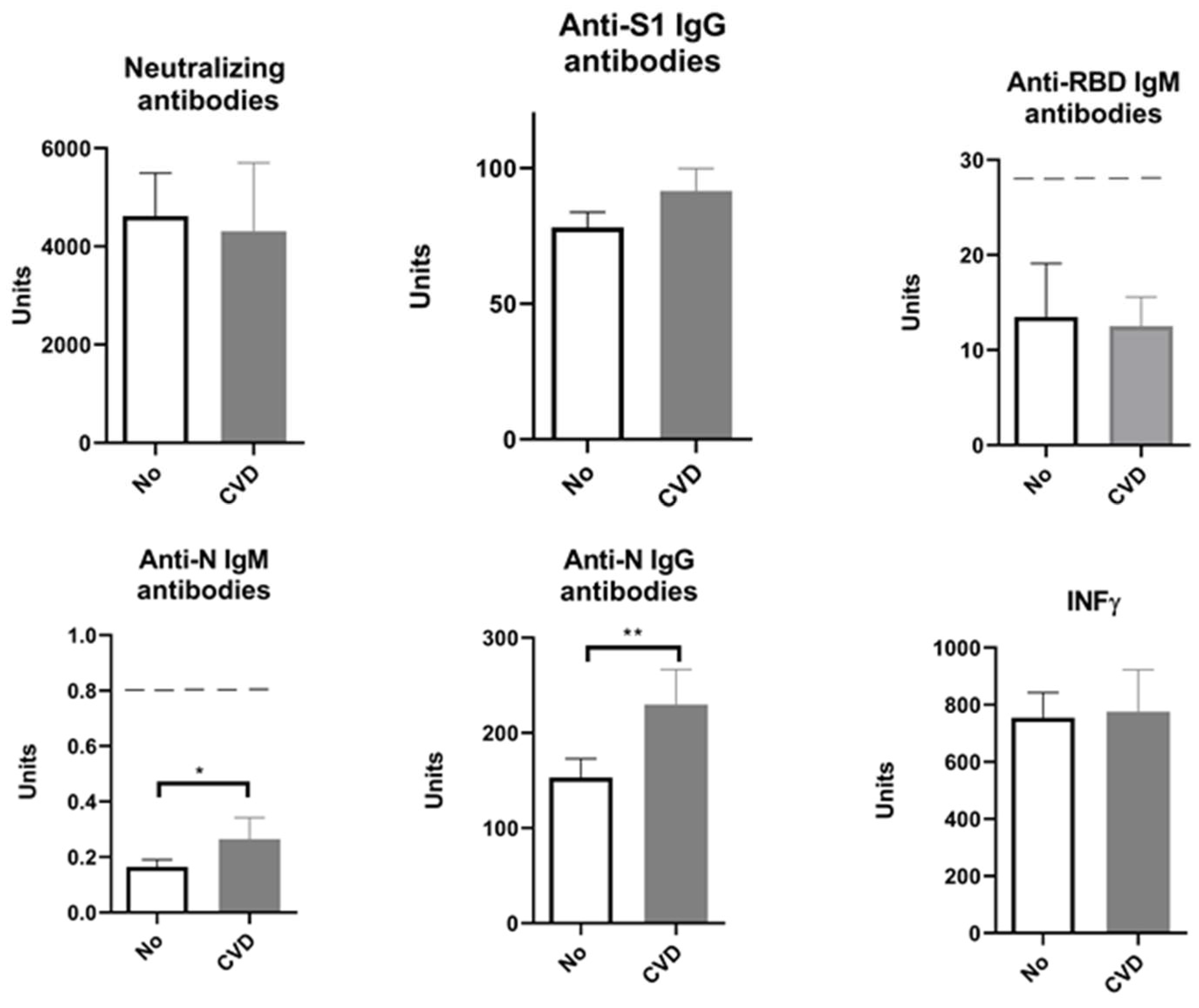

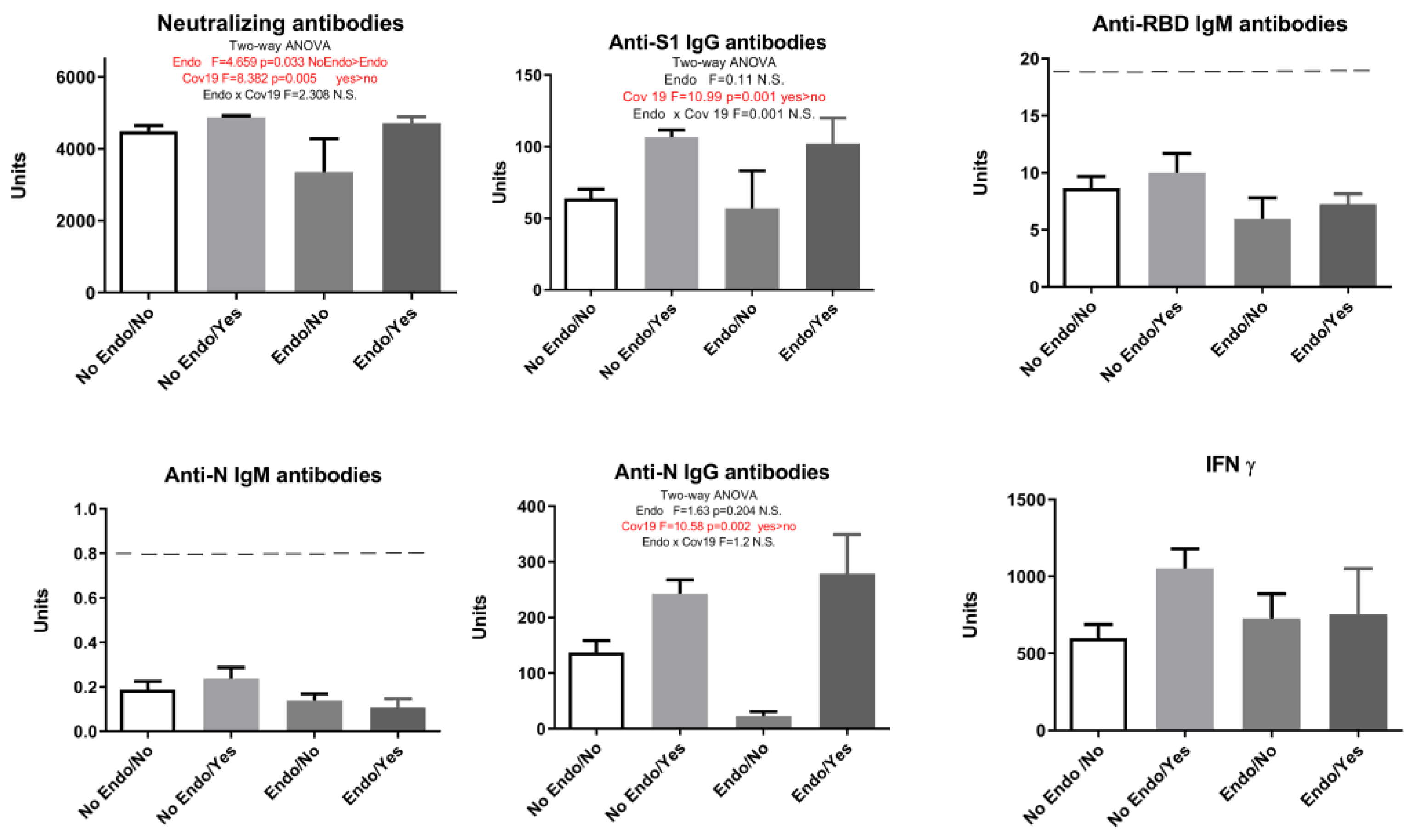
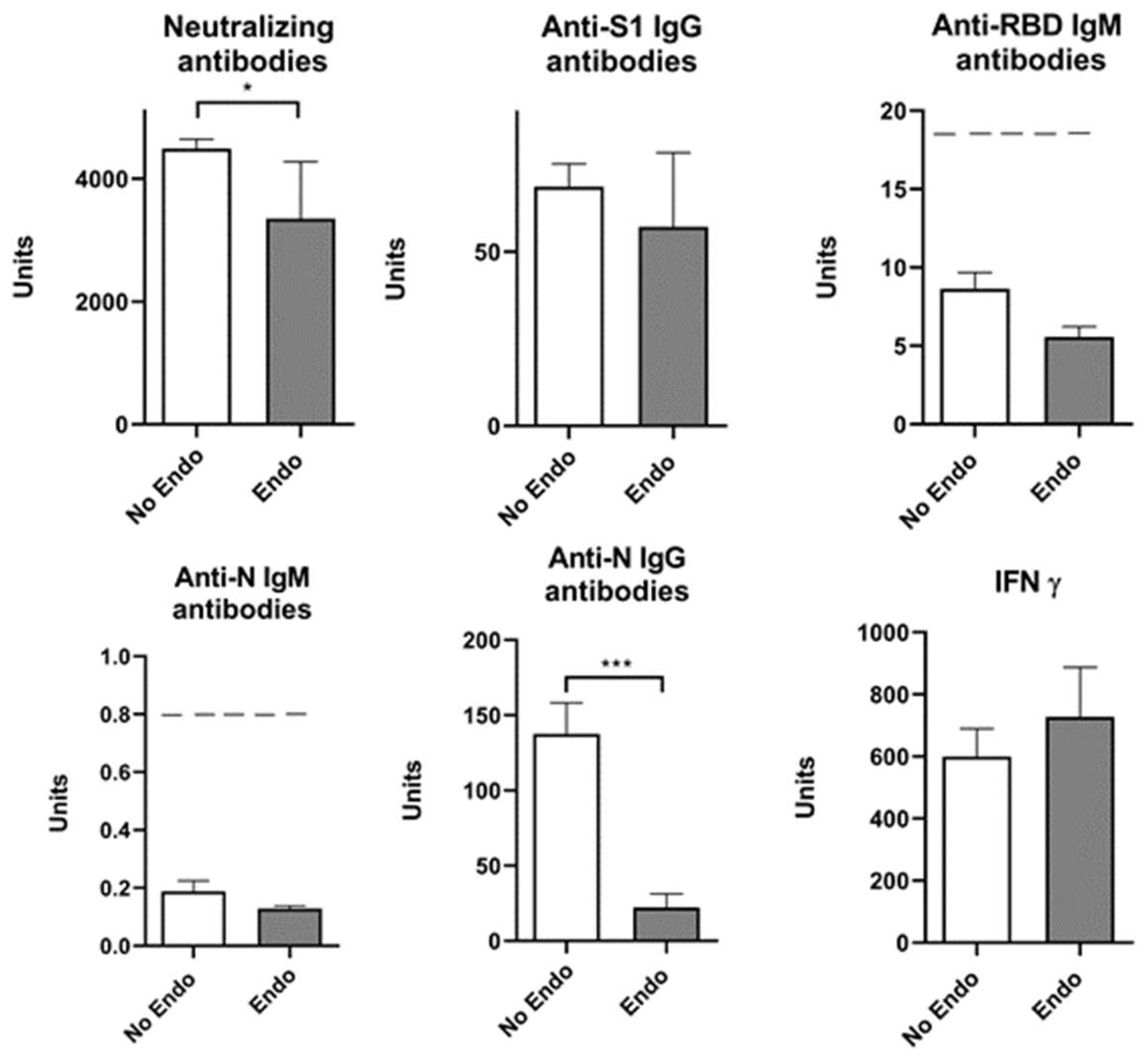

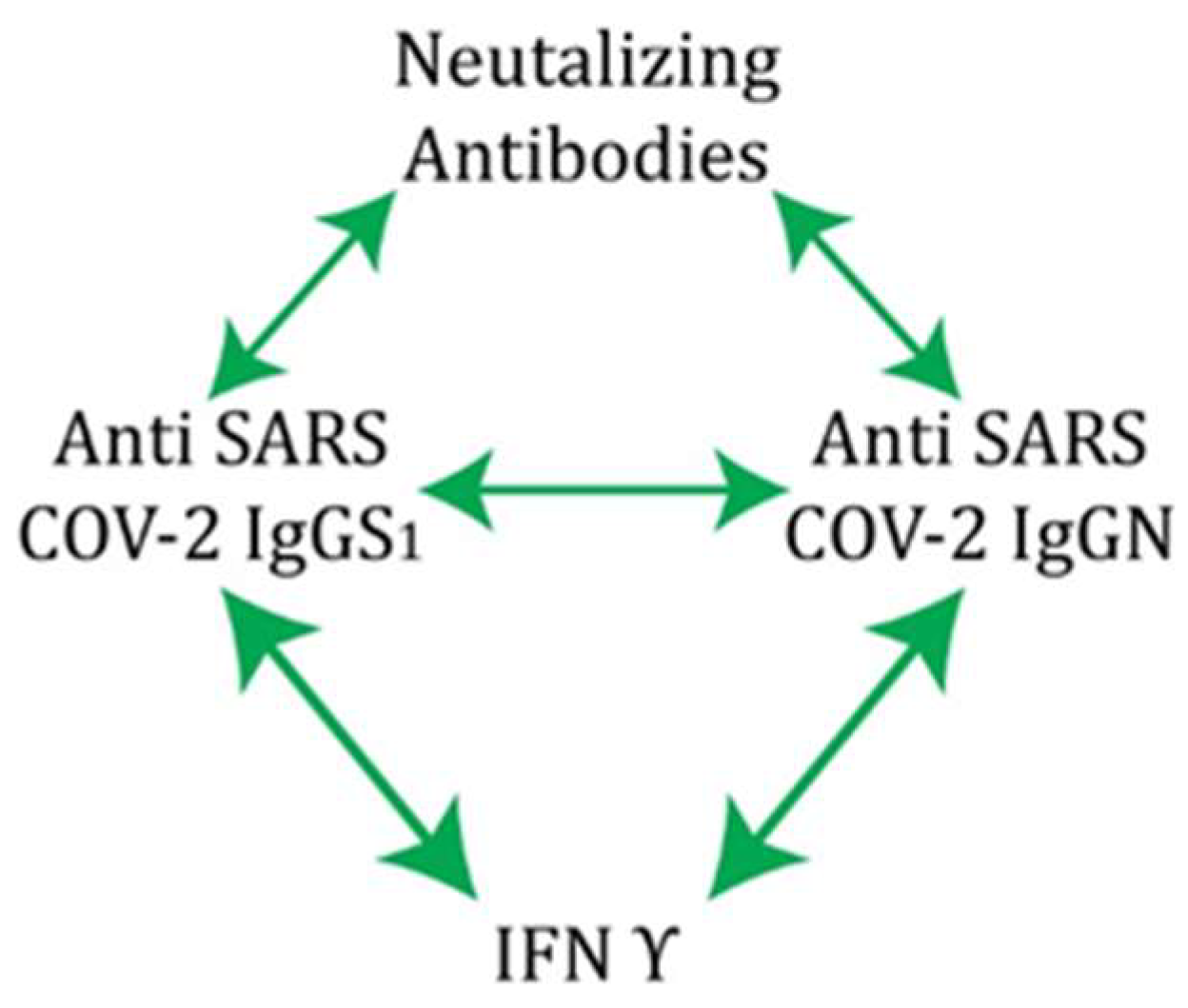
| Neutralizing Antibodies | Anti-S1 IgG Antibodies | Anti-RBD IgM Antibodies | Anti-N IgM Antibodies | Anti-N IgG Antibodies | IFN γ | |
|---|---|---|---|---|---|---|
| Neutralizing antibodies | ||||||
| anti-S1 IgG antibodies | 0.400 *** | |||||
| anti-RBD IgM antibodies | 0.0525 | 0.0923 | ||||
| anti-N IgM antibodies | 0.078 | 0.0002 | 0.098 | |||
| anti-N IgG antibodies | 0.240 *** | 0.666 *** | −0.099 | −0.039 | ||
| IFN γ | 0.142 | 0.392 *** | 0.196 | 0.0923 | 0.336 *** |
| Anamnestic Data | |||||
|---|---|---|---|---|---|
| Number of Participants | |||||
| Total number | 103 | ||||
| COVID-19 history | Yes | 43 | Before 3rd dose | 16 | |
| After 3rd dose | 27 | ||||
| No | 60 | ||||
| Sex | F | 75 | |||
| M | 28 | ||||
| Presence of cardiovascular diseases | Yes | 36 | Hypertension (n = 32) | ||
| Myocarditis (n = 1) | |||||
| Pericarditis (n = 1) | |||||
| Heart valve diseases (n = 2) | |||||
| No | 67 | ||||
| Presence of diseases of the nervous system | Yes | 1 | |||
| No | 102 | ||||
| Presence of endocrinological diseases | Yes | 11 | Diabetes mellitus (n = 3) | ||
| Thyroid gland diseases (n = 7) | |||||
| Pituitary gland diseases (n = 1) | |||||
| No | 92 | ||||
| Presence of liver diseases | Yes | 2 | |||
| No | 101 | ||||
| Presence of kidney diseases | Yes | 1 | |||
| No | 102 | ||||
| Presence of pulmonary diseases | Yes | 4 | |||
| No | 99 | ||||
| Presence of allergic reactions | Yes | 13 | |||
| No | 99 | ||||
| Presence of autoimmune diseases | Yes | 2 | |||
| No | 101 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukčević, M.; Šerović, K.; Despot, M.; Nikolić-Kokić, A.; Vujović, A.; Nikolić, M.; Blagojević, D.; Jovanović, T.; Despot, D. Humoral and Cellular Immune Response after Three Doses of Sinopharm [Vero Cell]-Inactivated COVID-19 Vaccine in Combination with SARS-CoV-2 Infection Leads to Hybrid Immunity. Pharmaceuticals 2024, 17, 122. https://doi.org/10.3390/ph17010122
Vukčević M, Šerović K, Despot M, Nikolić-Kokić A, Vujović A, Nikolić M, Blagojević D, Jovanović T, Despot D. Humoral and Cellular Immune Response after Three Doses of Sinopharm [Vero Cell]-Inactivated COVID-19 Vaccine in Combination with SARS-CoV-2 Infection Leads to Hybrid Immunity. Pharmaceuticals. 2024; 17(1):122. https://doi.org/10.3390/ph17010122
Chicago/Turabian StyleVukčević, Marija, Katarina Šerović, Mateja Despot, Aleksandra Nikolić-Kokić, Aleksandra Vujović, Milan Nikolić, Duško Blagojević, Tanja Jovanović, and Dragana Despot. 2024. "Humoral and Cellular Immune Response after Three Doses of Sinopharm [Vero Cell]-Inactivated COVID-19 Vaccine in Combination with SARS-CoV-2 Infection Leads to Hybrid Immunity" Pharmaceuticals 17, no. 1: 122. https://doi.org/10.3390/ph17010122
APA StyleVukčević, M., Šerović, K., Despot, M., Nikolić-Kokić, A., Vujović, A., Nikolić, M., Blagojević, D., Jovanović, T., & Despot, D. (2024). Humoral and Cellular Immune Response after Three Doses of Sinopharm [Vero Cell]-Inactivated COVID-19 Vaccine in Combination with SARS-CoV-2 Infection Leads to Hybrid Immunity. Pharmaceuticals, 17(1), 122. https://doi.org/10.3390/ph17010122







