Abstract
Despite the possibility of postoperative pain occurrence, in some patients, vitreoretinal surgeries (VRSs) require performance of general anesthesia (GA). The administration of intraoperative intravenous rescue opioid analgesics (IROA) during GA constitutes a risk of perioperative adverse events. The Adequacy of Anesthesia (AoA) concept consists of an entropy electroencephalogram to guide the depth of GA and surgical pleth index (SPI) to optimize the titration of IROA. Preemptive analgesia (PA) using cyclooxygenase-3 (COX-3) inhibitors is added to GA to minimize the demand for IROA and reduce postoperative pain. The current analysis evaluated the advantage of PA using COX-3 inhibitors added to GA with AoA-guided administration of IROA on the rate of postoperative pain and hemodynamic stability in patients undergoing VRS. A total of 165 patients undergoing VRS were randomly allocated to receive either GA with AoA-guided IROA administration with intravenous paracetamol/metamizole or with preemptive paracetamol or metamizole. Preemptive paracetamol resulted in a reduction in the IROA requirement; both preemptive metamizole/paracetamol resulted in a reduced rate of postoperative pain as compared to metamizole alone. We recommend using intraoperative AOA-guided IROA administration during VRS to ensure hemodynamic stability alongside PA using both paracetamol/metamizole to reduce postoperative pain.
1. Introduction
Ophthalmic surgery has witnessed a major transformation from performance of vitreoretinal surgeries (VRSs) traditionally under general anesthesia (GA) in the 1990s to utility of a wide variety of regional anesthesia (RA) techniques in the present century [1]. Nevertheless, there are contraindications to perform RA alone, including antiplatelet therapy, predicted impaired cooperation with the operator during long-lasting procedures due to neurological deficits, and danger of respiratory disorders during pars plana vitrectomy (PPV) under RA with monitored anesthesia care in elderly patients, which still imposes the necessity of VRS performance under GA [2,3]. Although attempts have been made to perform VRS under RA in patients using oral anticoagulants [4], it entails the administration of intraoperative rescue opioid analgesia (IROA) in the case of an increased heart rate and blood pressure, which are signs of inadequate analgesia. The utility of IROA has been identified as an independent risk factor for postoperative nausea and vomiting (PONV) in the first 48 h after VRS [4], and therefore RA techniques are employed to reduce the dose of IROA. The addition of different preoperative, preventive RA techniques to GA in patients undergoing VRS also reduces the IROA dose [5,6], which in turn decreases the rate of PONV, leading to efficient postoperative analgesia and diminishing the rate of the oculocardiac reflex (OCR), although it was not completely eliminated [7].
Metamizole and paracetamol (acetaminophen) are the two most widely used non-opioid analgesics, possessing both a central (inhibition of cyclooxygenase-3, COX-3) and a peripheral mechanism of action [8,9,10,11,12], which have proven their analgesic efficacy in the treatment of postoperative pain with a single dose administered as preemptive analgesia [13,14,15,16,17,18], in reducing the incidence of PONV [13,14] and a quite good safety profile [11,19,20,21] compared to standard nonsteroidal anti-inflammatory drugs (NSAIDs), in terms of safety for the upper intestinal tract and kidneys in patients with an increased risk of stomach or renal problems or other contraindications for standard NSAIDs [22,23].
Surgical painful (nociceptive) afferent stimulation in the operation field during GA in the case of insufficient intraoperative analgesia leads to the stress hormone release, which triggers an increase in heart rate and arterial blood pressure, whereas the administration of IROA attenuates this effect. On the one hand, an inadequate dosing may be responsible for intolerable postoperative pain perception (IPPP) [24] in the mechanism of central sensitization [25]; on the other hand, an excessive dosage may lead to hemodynamic instability with dangerous bradycardia and hypotension as well as an increased requirement for intravenous fluids.
Hence, appropriate titration of IROA constitutes a major challenge in response to observed fluctuations of abovementioned hemodynamic parameters accompanied by anesthesiologic intuition, in view of the fact that insufficient intraoperative analgesia may not necessarily be reflected in tachycardia and hypertension as volatile anesthetics tend to blunt the hemodynamic response to nociceptive stimulation [26], especially in the population of diabetics and the elderly.
Intraoperative monitoring of analgesia efficacy is crucial and is therefore gaining increasing popularity in daily anesthesiological practice as control of the intraoperative efficacy of IROA administration during GA, reflecting the nociception/antinociception balance [27], was proven to be more effective compared to IROA administration, based on observation of hemodynamic changes in reaction to painful stimuli intraoperatively [28,29].
The Adequacy of Anesthesia (AoA) concept is based on monitoring the depth of GA detected from a forehead sensor using an entropy electroencephalogram (Response Entropy, RE; State Entropy, SE) and the surgical pleth index (SPI) derived from a finger photoplethysmography signal, both of which do not require complex preoperative preparations [30].
Observance of the SE value within the range of 40–60 as a result of proper administration of the hypnotic GA component, reflecting the proper suppression of the limbic system, alongside observance of the increase in the SPI value on the monitor (0—no painful stimulation, 100—maximum painful stimulation) after a painful stimulus and returning to the baseline level after the IROA bolus, makes the monitoring with AoA guidance easy [31]. The reliability of variations in SPI values in response to nociceptive stimulation was proven to correlate with serum opioid concentration [32] and reduce the requirement for total cumulative IROA during GA [33]. The employment of AoA guidance for IROA titration also proved its utility in decreasing postoperative pain perception expressed on the numerical rating scale (NRS) compared to standard practice in patients undergoing lumbar discectomy [34].
To the best of our knowledge, so far, no study has been carried out in order to investigate the potential effect of combined preemptive analgesia (PA) using two different cyclooxygenase type 3 inhibitors together in patients undergoing VRS under GA with AoA guidance on the incidence of postoperative pain and hemodynamic stability. Therefore, the aim of the current study was to assess the utility of AoA guidance of GA in patients undergoing VRS with combined PA using both paracetamol and metamizole or one of them.
2. Results
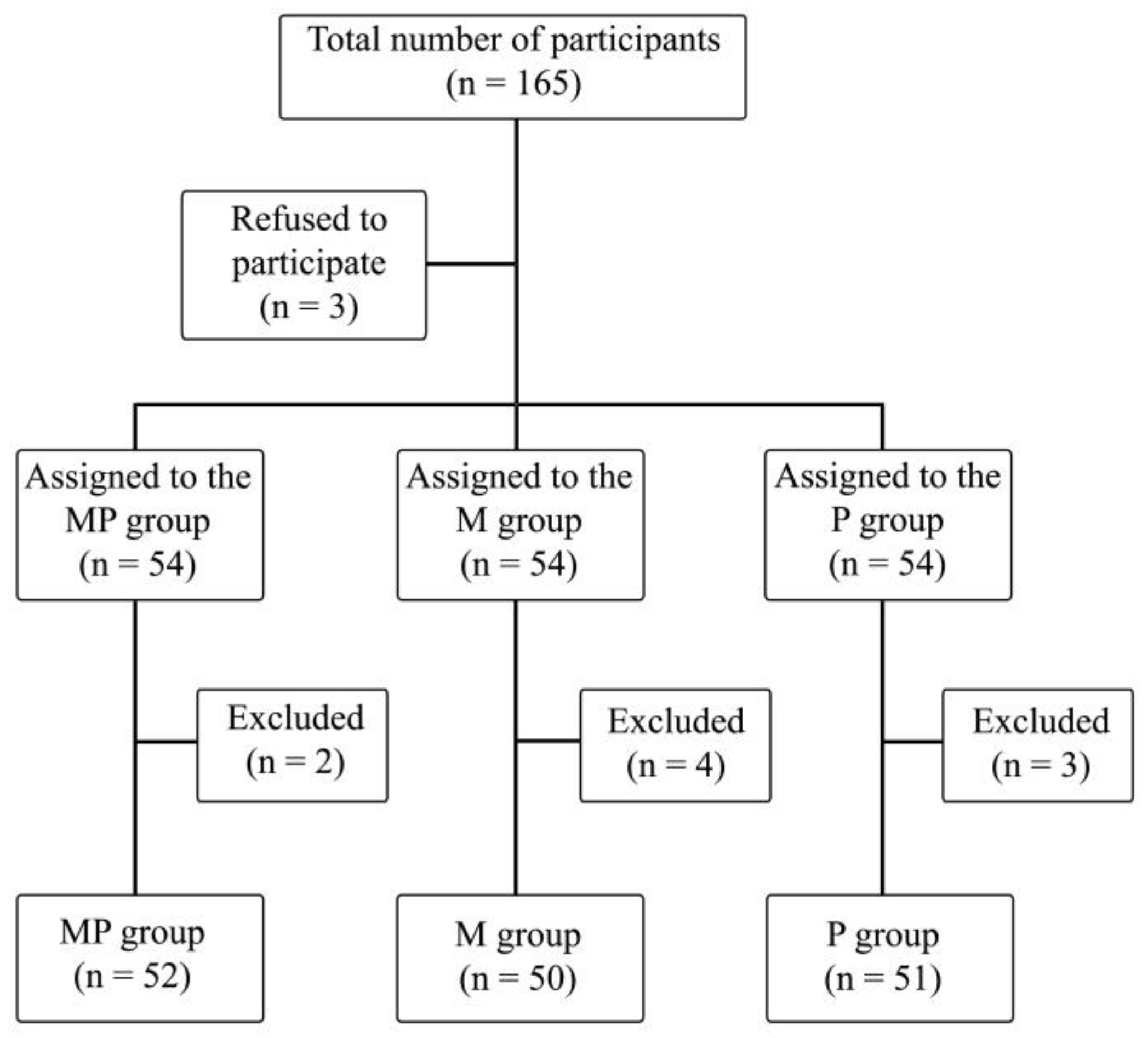
The study included 165 patients, of which 9 patients were excluded due to technical problems with intraoperative SPI measurement, postoperative arousal leading to inability of observation during stage 5, or inability of declaring postoperative pain perception. A total of 153 patients’ data were ultimately analyzed (Figure 1).
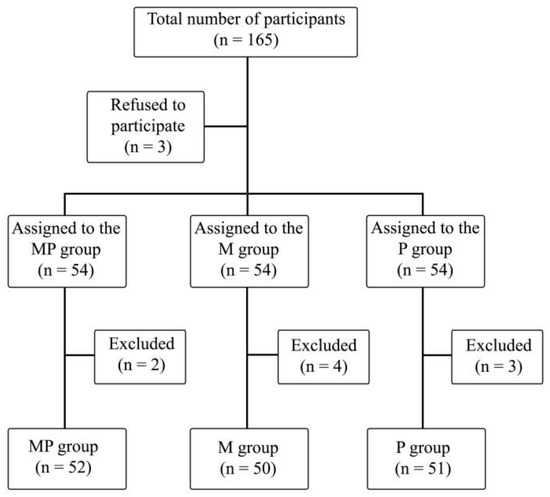
Figure 1.
Randomization graph. PM—paracetamol/metamizole group; M—metamizole group; P—paracetamol group.
Table 1 shows the anthropometric data of the patients. Significant differences were noted for height, weight, and BMI.

Table 1.
Anthropometric data in studied groups of patients.
We analyzed the frequency of surgical maneuvers during the procedure (Table 2) among the groups to ensure the homogeneity of the groups according to intraoperative nociception received by patients, which could induce the severity of postoperative pain. There was a statistically significant difference in the case of gas–liquid exchange, which occurred less frequently in the PM group compared to the M group. Significant differences were also noted for silicon oil injection between the PM and P groups.

Table 2.
The frequency of surgical maneuvers (numbers and percentages) during the procedure.
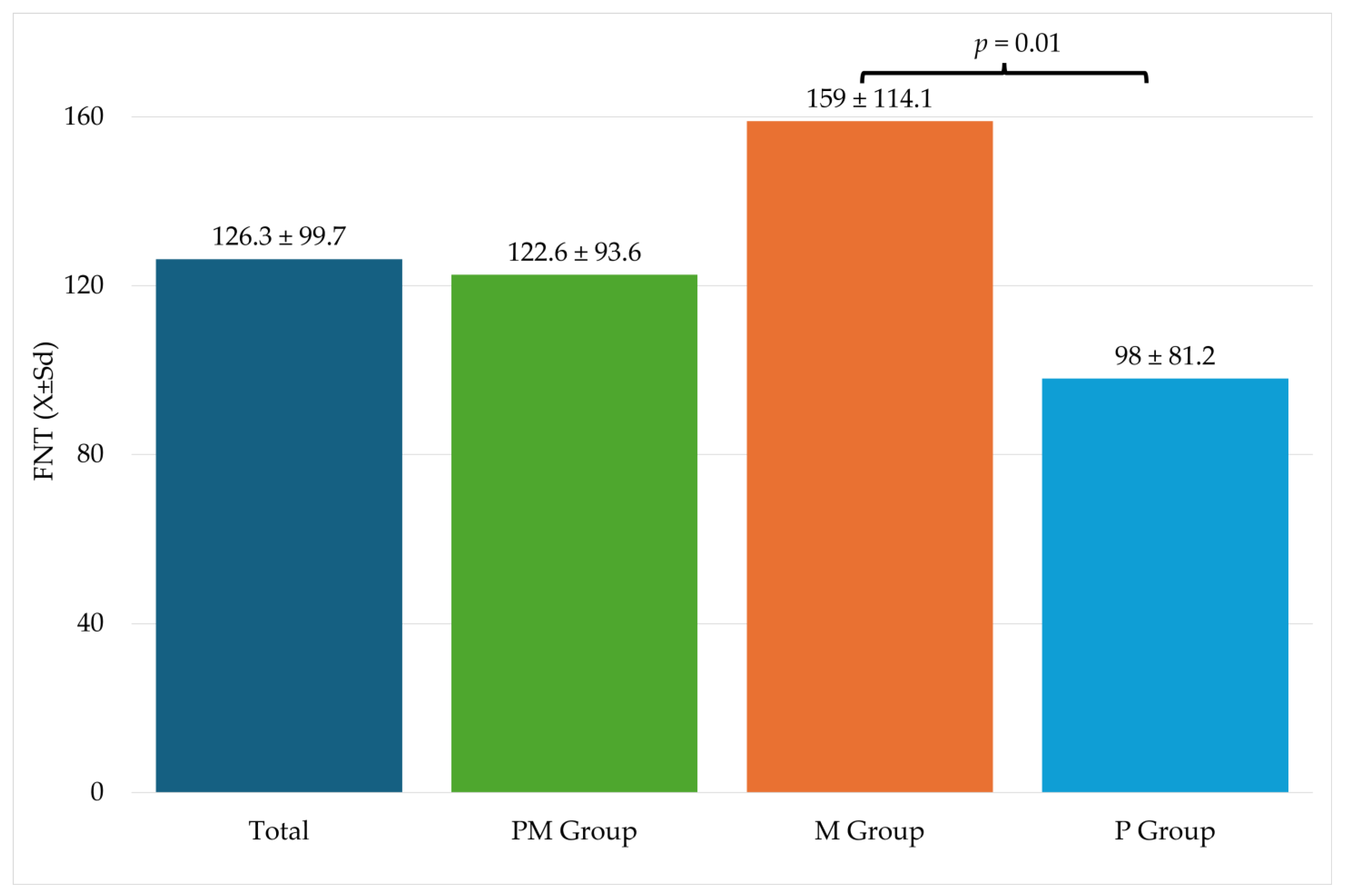
Table 3 shows information related to the procedure. Significantly higher FNT values were recorded in the M group compared to the P group (Figure 2).

Table 3.
Characteristics of the performed surgery.
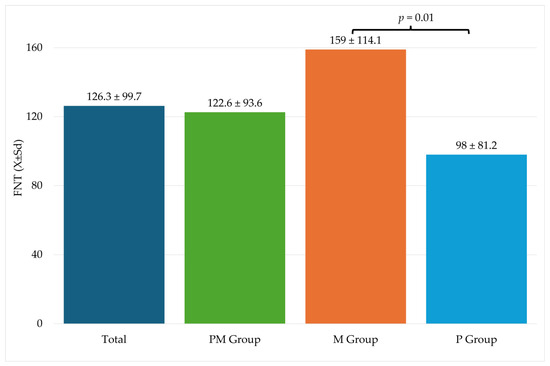
Figure 2.
Fentanyl use during surgery. FNT—fentanyl; M—metamizole group; P—paracetamol group; Sd—standard deviation.
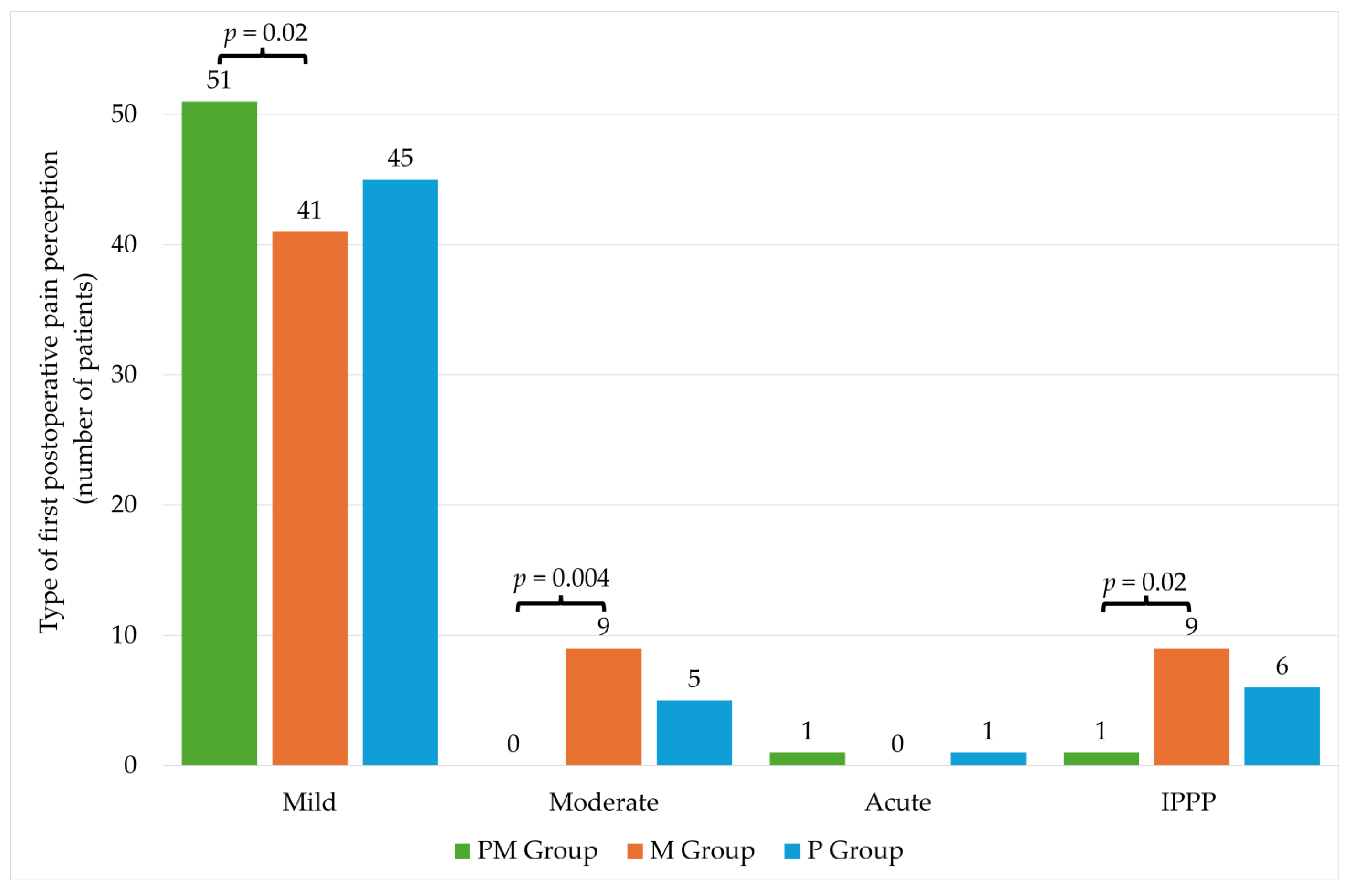
There were no statistically significant differences in individual groups in the level of pain intensity on the NRS scale (Table 4). The significantly higher percentage of patients with mild pain was observed in the PM group compared to the M group. The opposite situation was observed in the case of moderate pain, where a significantly higher percentage occurred in the M group. No significant differences were noted for acute pain. In the case of IPPP, there were differences between PM and M groups (Figure 3).

Table 4.
Assessment of the occurrence of postoperative pain in the examined groups.
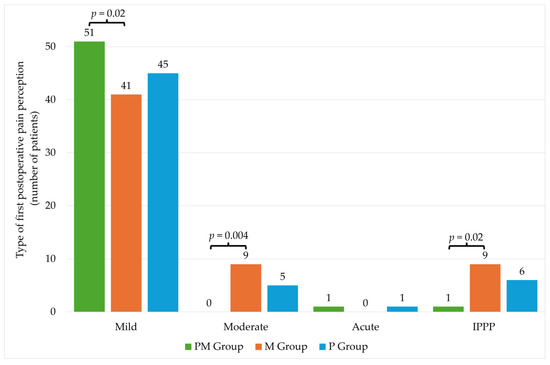
Figure 3.
Type of first postoperative pain perception. PM—paracetamol/metamizole group; M—metamizole group; P—paracetamol group; IPPP—intolerable postoperative pain perception.
Table 5 shows hemodynamic changes during individual stages of anesthesia. Significant differences were noted only during stage 4 between groups M and P in the case of mean SAP and mean MAP. These values were higher in the P group.

Table 5.
Hemodynamic changes in patients with intraoperative pain perception during certain stages of anesthesia.
Table 6 shows hemodynamic fluctuations. In stage 3, significantly higher levels of min SAP and min MAP were recorded in the PM group compared to the M group. In stage 4, max HR was significantly higher in the PM group compared to the M group. Significant differences were also noted for min SAP, which achieved the highest value in the P group. Moreover, in the case of min DAP, the highest value was also recorded in the P group. In stage 5, significant differences were recorded only in the case of max SPI. Its value was significantly higher in the PM group compared to the M group.

Table 6.
Comparison of hemodynamic fluctuations of monitored patients’ parameters at the same stage between studied groups.
3. Discussion
The current study analysis covering the utility of PA using paracetamol and metamizole in patients undergoing VRS revealed the incidence of IPPP in 16 out of 153 patients (10.46%) despite the allocation to groups. In a group of patients receiving combined PA using metamizole and paracetamol, only 1 out of 53 patients (1.88%) experienced postoperative pain of a type other than mild, whereas the utility of preemptive metamizole resulted in an incidence of IPPP in 9 out of 50 patients (18%) and that of preemptive paracetamol resulted in an IPPP incidence in 6 out of 50 patients (12%), which appeared to be statistically significant (p < 0.05). Only in one patient from the PM group was another postoperative pain type other than mild observed, which seems to be quite an achievement in view of the fact that the majority of patients received noxious surgical maneuvers like endolaser treatment and indentation, which we had already proved in our other study from this field [35].
The impact of the burgeoning geriatric and diabetic population has increased requirements for VRS, whereas concomitant diseases, anti-platelet therapy, and risk of globe perforation due to impaired cooperation with the operator during a long-lasting procedure may possibly exclude performance of VRS under RA with monitored anesthesia care. Therefore, the necessity of employment of the GA regimen entailing the use of IROA encourages us to seek PA modalities, which were reported to diminish the requirement for IROA and as a result reduce the presence of perioperative adverse events like postoperative pain, PONV, and OCR, similarly to RA techniques added to GA as PA.
In patients with a contraindication for NSAIDs, pain management is challenging; therefore, metamizole and paracetamol are extensively used as an alternative to classic NSAIDs and opioids in postoperative pain management [22]. As part of a multimodal concept after minor-to-intermediate surgery, both paracetamol and metamizole, when administered intravenously, were proven to possess equivalent analgesic efficacy, being widely employed to achieve satisfactory improvement in postoperative pain therapy [23,36,37], although there are conflicting data in terms of the influence of their use on the IROA sparring effect and reduction in IROA-dosing-related adverse events, like nausea and vomiting, mainly due to a difference in anesthetic regimens [36,38,39].
Paracetamol is widely used in the management of acute and chronic pain [19], and its analgesic efficacy as a preemptive analgesia has already been well-recognized in patients undergoing lumbar discectomy [40], total abdominal hysterectomy [41], lower limb surgery [42], and cholecystectomy [43], whereas metamizole was successfully used in the management of pain after laparoscopic operations [44].
In the course of the current study, no adverse events connected with metamizole or paracetamol administration were observed; nevertheless, their utility is not free from potential side effects [45,46]. Metamizole is a controversial drug reported to incidentally induce agranulocytosis [23,47,48,49], anaphylaxis [50], Kounis syndrome (coincidental occurrence of allergic reaction and acute coronary syndrome secondary to vasospasm) [51]. Paracetamol, on the other hand, can have side effects such as tachycardia, hypertension, hepatotoxicity, and nephrotoxicity [52], and its use for pain control has been discussed in some studies [53].
Despite potential drawbacks, their analgesic efficacy was also utilized for the reduction in postoperative pain perception in ophthalmic surgeries, as pain intensity after a vitrectomy with scleral buckling has been reported to be the most severe [54]. De Araújo et al. used 1 g of metamizole in an intravenous infusion 40 min before panretinal photocoagulation and recorded significant reduction in the pain perception associated with the procedure as compared to the placebo control group [55]. Landwehr et al. compared analgesic potency of intravenous paracetamol and intravenous metamizole both in a dose of 1 g and proved their similar efficacy for postoperative analgesia after retinal surgery compared to the control group [56]. Interestingly, metamizole was proven to also act via substance P and the cannabinoid system [57,58] with the involvement of a possible higher reduction in IROA compared to that observed in our previous study [59] and IROA-related postoperative adverse events compared to that in our additional report [60]. In this study, we used 2.5 g of metamizole as PA and observed the incidence of IPPP in 18% of our patients in the metamizole group, whereas in our previous study, we used 1 g of metamizole and observed IPPP in 22.9% of patients [59]. Increasing the dose of metamizole used as PA to 2.5 g in a single dose with the subsequent reduction in its postoperative dosing to a 5 g daily cumulative dose did not lead to proportional reduction in IPPP and we find it interesting but questionable also due to the fact that the IROA dose in this study was 159 ± 114.1 mcg in the metamizole group compared to 165.7 ± 116.8 mcg in the previous study [59].
In the case of paracetamol, Sadrolsadat et al. investigated its influence when administered either as PA preoperatively or upon emergence from GA compared to the control group in patients undergoing PPV. They found pain scores to be lower in both the paracetamol groups, compared with the control at recovery [61].
Numerous abovementioned studies prove that the addition of intravenous PA to GA may reduce the demand for IROA administration, which was proven in the current study in the case of paracetamol contrary to metamizole and combined PA using both paracetamol and metamizole. Interestingly, a statistically significant difference of demand for IROA using FNT between groups M and P was observed (likewise in our previous study in a smaller group of patients) [59]. Interestingly, an inexplicably higher demand for IROA was noted in the M group compared to the PM group, although not statistically significant, similar to that between the M group and control group without PA in the previous study [59]. In a study performed by Ledowski et al. [62], the variations of the SPI value after surgical stimulation and redressing the value down to a baseline level after a bolus of rescue FNT enabled them to monitor the efficacy of IROA titration; therefore, it was also assumed that AoA guidance of IROA administration in our study helped monitor the effectiveness of every single rescue bolus of FNT in the case of insufficient analgesia produced by intravenous PA.
Employment of similar anesthetic modality in the current study analysis also revealed that IROA cannot be eliminated completely, even if the use of SPI in guidance of IROA administration in the case of intraoperative afferent nociceptive stimulation due to incomplete efficacy of PA using intravenous preemptive analgesia with either paracetamol or metamizole or combined PA using both of them was employed. Therefore, the use of AoA-guided IROA administration during GA, whenever the patients experienced afferent intraoperative nociceptive stimulation (any incident of intraoperative ∆SPI > 15 compared to SPI value before the start of VRS in stage 2 constituting an indication for ROA administration), was observed to produce more efficient suppression of central sensitization in the case of insufficient intravenous PA using paracetamol or metamizole detected using SPI monitoring.
The employment of AoA guidance of GA for VRS resulted in the achievement of hemodynamic stability, because in the majority of cases, no statistically significant differences were observed between analyzed parameters between studied groups in terms of SAP, MAP, DAP, and HR. In no case was the intraoperative use of vasopressors needed, which for some authors is included in the intraoperative hypotension classification [63]. Several statistically significant differences were of no clinical value as the values of hemodynamic parameters did not meet criteria of hypotension understood as blood pressure values’ difference of more than 30% from the baseline values [64] that might have resulted in multiorgan failure, leading to heart attack or stroke [65] or conversely malignant hypertension with risk of pulmonary edema.
There are several limitations of the current analysis. First of all, the perception of postoperative pain is a subjective phenomenon, which is burdensome in quantification [66]. We did not analyze the rate of postoperative pain after discharge from PACU to the DoL on purpose as the project involved monitoring NRS as well as SPI values in stage 5, whereas patients’ arousal (changing position in bed, cough, etc.) markedly interferes in SPI monitoring [67]; therefore, such comparison has no clinical value [68]. The rate of incidence of PONV, OCR, and oculo-emetic reflex will be analyzed separately (likewise in our previous study in this field) [60]. Similarly, intraoperative pain perception during certain stages of VRS will also be separately analyzed. Second, 9 of 165 patients were excluded from the final analysis due to technical problems with intraoperative SPI measurement, postoperative arousal leading to inability of observation during stage 5, or inability of declaring postoperative pain perception, which may have proportionally influenced the final results. Finally, we intentionally did not study the groups without AoA guidance, as the current study design is based on the previous study findings with a different PA regimen under AoA guidance compared to AoA guidance of IROA administration alone, with the intention of investigating the utility of combined paracetamol/metamizole compared to their separate use under AoA guidance. An anesthesia regimen including monitoring the depth of anesthesia, regardless of the technique used, and monitoring the nociception/antinociception balance, regardless of whether SPI, antinociception index, or pupillometry of the nociception level was applied, is becoming a global standard; therefore, the administration of IROA based on observing changes in hemodynamic values alongside anesthesiological intuition may no longer be justified in light of the availability of current medical technology and thus raises ethical concerns.
4. Materials and Methods
4.1. Patients
Patients who were scheduled for elective VRS in the Department of Ophthalmology of the 5th Regional Hospital in Sosnowiec, Poland, and met the inclusion criteria were asked to participate in the study. No previous surgical procedures, no history of increased intraocular pressure, and no treatment with antiglaucoma drugs were the inclusion criteria. Exclusion criteria involved factors affecting the anatomy or function of the posterior segment of the eye that predispose to glaucoma, including penetrating ocular trauma, uveitis, scleral buckling, retinal vein occlusion. One hundred and sixty-five patients with American Society of Anaesthesiologists (ASA) score I–III were enrolled after obtaining written informed consent. Then, randomization was performed by opening sealed envelopes. In compliance with the Helsinki Declaration, ethical approval for this study (KNW/0022/KB1/122/17) was provided by the Ethical Committee of Medical University of Silesia on 5 December 2017 (Chairman Ph.D., MD Bogusław Okopień). The project was registered in the Clinical Trial Registry (Silesian MUKOAiIT7, NCT03389243). Data were collected between 15 January 2018 and 16 December 2022.
Exclusion criteria were pregnancy, drug or alcohol abuse, history of neurological disease or neurosurgical operation that would impair entropy electroencephalogram monitoring and history of pulmonary disease, history of allergy to or abuse of metamizole or paracetamol, acute or chronic pain, signs predicting difficult laryngeal mask placement, cardiac arrhythmia in ECG that might impair SPI monitoring.
Patients were randomly allocated into three groups. In group M, patients received GA anesthesia in addition to PA using a single dose of 2.5 g of metamizole (Pyralgin at 0.5 g/mL, 5 mL solution; Polpharma S.A., Gdansk, Poland) in 100 milliliters of a saline solution 30 min before arrival to the operating room. In group P, patients received GA anesthesia in addition to PA using a single dose of 1 g of acetaminophen (Paracetamol Kabi at 10 mg/mL, solution of 100 mL; Fresenius Kabi, Błonie, Poland) in 100 milliliters of a saline solution 30 min before arrival to the operating room. In group PM, patients received GA in addition to combined abovementioned PA techniques in the P and M group, according to the latest change in the guidelines for postoperative pain management [69].
The day before VRS during the preoperative visit, patients were informed about the possibility of postoperative pain. They were trained on the use of the Numeric Pain Rating Scale (NRS) regarding how to report pain. On a scale from 0 to 10, 0 indicated no pain and 10 indicated the worst pain imaginable.
4.2. Anesthesia Technique
Regardless of group allocation, patients were kept fasted for at least 12 h and before the induction of anesthesia started, on the day of surgery, all patients received medication in a dose of 3.75–7.5 mg/kg of midazolam (Dormicum, Roche, Warsaw, Poland) in accordance with age and body weight [70]. Directly before surgery, the patients were preoxygenated for approximately 5 min with 100% oxygen and intravenously 10 mL/kg body weight of an Optylite solution was infused. The preoxygenation time was intended to ensure patient safety and avoid any potential risk. Anesthesia was induced intravenously with fentanyl at 1 mcg/kg body weight, and Propofol (Propofol 1% Fresenius, Cairo, Egypt) at a single dose of 2.5 mg/kg body weight. After loss of consciousness, the patients in all groups were paralyzed with a standard intravenous dose of 0.6 mg/kg rocuronium (Esmeron, Fresenius, Błonie, Poland) and after 45 s, the laryngeal mask (LMA) was placed. CO2 was maintained at 35–37 mmHg etCO2. After LMA placement, before operation started, sevoflurane concentration was maintained at the level around 40–45 State Entropy.
Throughout anesthesia induction and surgery, standard monitoring procedures were utilized and close attention was paid to vital parameters such as the non-invasive arterial pressure (NIBP), heart rate (HR), standard electrocardiography (ECG), arterial blood saturation (SaO2), fraction of inspired oxygen in the gas mixture (FiO2), fraction of inspired sevoflurane (FiAA), fraction of expired sevoflurane (FeAA), exhaled carbon dioxide concentration (etCO2), minimal alveolar concentration of sevoflurane (MAC). The depth of anesthesia was monitored with the entropy electroencephalogram (State and Response Entropy), intraoperative analgesia was guided with the surgical pleth index (SPI), and muscle relaxation was maintained using NMT monitoring (Carescape B650, GE, Helsinki, Finland).
4.2.1. Stage 1
On admission to the operating theater, the sensor of the entropy electroencephalogram (RE, SE) on the patients’ forehead, the pulse oximeter (for SPI measurement) on the contralateral finger to venous access and NIBP cuff on the right arm, and standard ECG on the patients’ back were placed according to the manufacturer’s suggestions, and the first values were recorded.
4.2.2. Stage 2
In order to calculate the mean SPI value during stage 2, SPI values were taken into account starting from 5 min after laryngeal mask placement to the beginning of the sterilization of the orbit to allow the calibration of the SPI sensor.
4.2.3. Stage 3—Intraoperative
The SPI score was monitored online and recorded with a sampling frequency of 1 min. When the SPI value reached ∆SPI > 15 points above the mean SPI value in stage 2, a rescue dose of 1 mcg/kg body weight of FNT was administered intravenously every 5 min until the SPI value decreased to the value of mean SPI recorded in stage 2. The time duration of VRS was counted from the speculum installation to the speculum removal.
We assumed that the initial dose of FNT of 1 mcg per kilogram of body weight would produce sufficient analgesia before the installation of the speculum. In addition, in 2013, Gruenewald et al. [27] proposed ∆SPI > 10 or an absolute SPI value > 50 as a predictor of inadequate analgesia. In other studies, only absolute value ∆SPI > 50 became an indication for rescue analgesia [71]. In the methodology of the current study, a compromising protocol of ∆SPI > 15, compared to the calculated baseline during stage 2, lasting at least one minute as an indicator for rescue analgesia was adopted to avoid a potentially hazardous overdosage of FNT as a result of the potential miscalculation of the SPI score due to its variations.
VRSs were performed by the same ophthalmic surgeon (A.L.-B.) with over 10 years of experience in VRS, performing over 400 vitreoretinal procedures a year. Eye globe preparation included three or four 23-gauge ports. Initial core vitrectomy was followed by peripheral vitreous removal with scleral indentation. The removal of epiretinal membranes and/or internal limiting membrane peeling was the next surgical step. If needed, 4 sorts of tamponades were applied: temporary heavy perfluorocarbon liquids, air, SF6, or silicon oil. All retinal brakes or degenerations were treated with laser photocoagulation.
The incidence of intraoperative OCR was recorded, which is typically identified in the case of a rapid decrease in heart rate by 20% from the baseline during ocular manipulations. In the case of the presence of OCR, the surgeon was asked to stop stimulation in the surgical field, and intravenous atropine was administered intravenously in a single dose of 0.5 milligrams when the hemodynamic manifestation of OCR did not withdraw.
4.2.4. Stage 4—Emergence from GA
All the patients were monitored further (SPI, HR, SAP, MAP, DAP, SaO2) by the anesthesiological team performing GA.
4.2.5. Stage 5—Postoperative
In the post-anesthesia care unit (PACU), all the patients were monitored further (SPI, HR, SAP, MAP, DAP, SaO2) by the anesthesiological team blinded to the patients’ group allocation. Along with postoperative hemodynamic parameters, the presence of adverse effects such as nausea and vomiting (PONV), level of sedation, and allergic reactions was monitored for each patient at the same time as pain assessment for 24 h. In the case of PONV, ondansetron (Ondansetron Accord, Accord Healthcare Limited, Harrow, UK) in a single intravenous dose of 4 mg was administered intravenously. The Optylite solution at 5 milliliters/kg body weight was infused in the case of MAP < 65 mmHg. The patients received oxygen at 3 L/min via a nasal cannula. The patients were questioned in terms of their perception of pain intensity using the Numeric Pain Rating Scale (NRS) ranging from 0 (no pain) to 10 (maximum pain) every 10 min. In the case of pain perception NRS > 3, a standard dose of NSAID to meet each patient’s individual needs, like dexketoprofen (Dexak, Berlin-Chemie Menarini, Berlin, Germany) or ibuprofen (Ibuprofen B.Braun Melsunngen, Germany), alongside paracetamol or metamizole, was administered according to contemporary guidelines of acute pain treatment issued by the Polish Society of Anaesthesiologists [24]. SPI values were monitored online and mean SPI values were recorded with a sampling time of 1 min (trends in software provided by producer). NRS and SPI values were recorded for acute pain (NRS 7–10), average pain (NRS 4–6), and mild pain (NRS 0–3) perception intervals. The patients were observed and monitored in the PACU for at least 30 min until transfer from the PACU to Department of Ophthalmology. The monitoring and data recording were then ceased with the exception of the incidence of PONV, which was postoperatively recorded during the first 24 h and each time anti-emetic ondansetron (Ondansetron Accord at 2 mg/mL, 2 mL solution, Accord Healthcare Limited, Great Britain) was administered intravenously in a dose of 4 milligrams.
4.3. Statistical Analysis
Statistical calculations were performed using STATISTICA 13.3 (StatSoft, Kraków, Poland). Quantitative data were characterized using the mean and standard deviation as well as the median and interquartile range. Qualitative data were presented using percentages. Normality of distribution was verified with the Shapiro–Wilk test. Due to the lack of normal distribution, a quantitative data analysis was performed using the Kruskal–Wallis test and Dunn’s post hoc test. The chi-square test was used to analyze qualitative data. Statistical significance was set at the level p < 0.05.
Sample Size Calculation
We took into account the time of onset results of a previous study with 35 patients per group. The aim was to detect a significant difference in the time of onset of anesthesia among the paracetamol, metamizole, and paracetamol- and metamizole-alone groups. The calculation was based on a one-way ANOVA test conducted with α = 0.05 and a power of 80%, and the sample size was calculated as 55 patients per group.
5. Conclusions
In conclusion, contrary to numerous studies proving efficacy of PA using COX-3 inhibitors, our analysis proved an advantage of the intravenous infusion of combined PA using paracetamol and metamizole before VRS under AoA guidance in terms of incidence of postoperative pain perception. In addition, preemptive paracetamol administration diminished the requirement for intravenous FNT.
Utility of preemptive analgesia using both paracetamol and metamizole seems to be justified in terms of the observed tendency for improved postoperative analgesia, whereas paracetamol alone tends to reduce intraoperative demand for IROA.
Author Contributions
Conceptualization, M.J.S.; methodology, M.J.S. and A.L.-B.; software, N.Z.; formal analysis, M.J.S. and N.Z.; investigation, M.J.S., A.L.-B. and K.M.; data curation, M.J.S. and K.M.; writing—original draft preparation, M.J.S.; writing—review and editing, M.J.S. and N.Z.; visualization, N.Z.; supervision, M.J.S.; project administration, B.O.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Medical University of Silesia, grant number: KNW-1-183/N/9/K.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Medical University of Silesia (KNW/0022/KB1/122/17; 5 December 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Acknowledgments
The authors sincerely thank Teresa Paczyńska (anesthetic nurse assisting for every GA for PPV) at Regional Hospital no. 5 in Sosnowiec for her enthusiastic co-operation with the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sallam, A.A.B.; Donachie, P.H.J.; Williamson, T.H.; Sparrow, J.M.; Johnston, R.L. The Royal College of Ophthalmologists’ National Ophthalmology Database Study of Vitreoretinal Surgery: Report 5, Anaesthetic Techniques. Br. J. Ophthalmol. 2016, 100, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Licina, A.; Sidhu, S.; Xie, J.; Wan, C. Local versus General Anaesthesia for Adults Undergoing Pars Plana Vitrectomy Surgery. Cochrane Database Syst. Rev. 2016, 9, CD009936. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.F.; He, X.; Nirwan, R.S.; Sridhar, J.; Kuriyan, A.E. Perioperative Management of Anticoagulants in Ocular Surgeries. Int. Ophthalmol. Clin. 2020, 60, 3–15. [Google Scholar] [CrossRef]
- Andonegui, J.; Capdevila, F.; Zubicoa, A.; Ibáñez, B. Randomised Controlled Trial on Vitreoretinal Surgery with and without Oral Anticoagulants: Surgical Complications, Visual Results and Perioperative Thromboembolic Events. Trials 2019, 20, 677. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, K.; Boost, K.A.; Wolf, A.; Kampik, A.; Schaumberger, M.; Haritoglou, C. A 23-gauge pars plana vitrectomy after induction of general anesthesia: Effect of additional retrobulbar anesthesia on postoperative pain. Ophthalmologe 2014, 111, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Lambat, S.P.; Somani, S.S.; Nangia, P.V.; Nangia, V.B. Surgical Exposure for Vitrectomy in Retinopathy of Prematurity. Indian J. Ophthalmol. 2023, 71, 3569–3570. [Google Scholar] [CrossRef]
- Schönfeld, C.-L.; Hierneis, S.; Kampik, A. Preemptive Analgesia with Ropivacaine for Pars Plana Vitrectomy: Randomized Controlled Trial on Efficacy and Required Dose. Retina 2012, 32, 912–917. [Google Scholar] [CrossRef]
- Ohashi, N.; Kohno, T. Analgesic Effect of Acetaminophen: A Review of Known and Novel Mechanisms of Action. Front. Pharmacol. 2020, 11, 580289. [Google Scholar] [CrossRef]
- Freo, U. Paracetamol for Multimodal Analgesia. Pain Manag. 2022, 12, 737–750. [Google Scholar] [CrossRef]
- Topuz, R.D.; Gündüz, Ö.; Karadağ, Ç.H.; Ulugöl, A. Non-Opioid Analgesics and the Endocannabinoid System. Balk. Med. J. 2020, 37, 309–315. [Google Scholar] [CrossRef]
- Silva, F.; Costa, G.; Veiga, F.; Cardoso, C.; Paiva-Santos, A.C. Parenteral Ready-to-Use Fixed-Dose Combinations Including NSAIDs with Paracetamol or Metamizole for Multimodal Analgesia—Approved Products and Challenges. Pharmaceuticals 2023, 16, 1084. [Google Scholar] [CrossRef] [PubMed]
- Collares, E.F.; Troncon, L.E.A. Effects of Dipyrone on the Digestive Tract. Braz. J. Med. Biol. Res. 2019, 52, e8103. [Google Scholar] [CrossRef]
- Doleman, B.; Read, D.; Lund, J.N.; Williams, J.P. Preventive Acetaminophen Reduces Postoperative Opioid Consumption, Vomiting, and Pain Scores After Surgery: Systematic Review and Meta-Analysis. Reg. Anesth. Pain Med. 2015, 40, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Turan, A.; Souza, K.; Pergolizzi, J.; Hornuss, C. Intravenous Acetaminophen Reduces Postoperative Nausea and Vomiting: A Systematic Review and Meta-Analysis. Pain 2013, 154, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Stangler, M.I.S.; Lubianca, J.P.N.; Lubianca, J.N.; Lubianca Neto, J.F. Dipyrone as Pre-Emptive Measure in Postoperative Analgesia after Tonsillectomy in Children: A Systematic Review. Braz. J. Otorhinolaryngol. 2021, 87, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, E.; Dinçer, E.; Turan, G.; Özgültekin, A. Postoperative Analgesic Efficacy of Preemptive and Postoperative Lornoxicam or Tramadol in Lumbar Disc Surgery. Turk. J. Anaesthesiol. Reanim. 2019, 47, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Sierżantowicz, R.; Lewko, J.; Bitiucka, D.; Lewko, K.; Misiak, B.; Ładny, J.R. Evaluation of Pain Management after Surgery: An Observational Study. Medicina 2020, 56, 65. [Google Scholar] [CrossRef]
- Alshehri, A.A. Comparative Evaluation of Postoperative Pain Scores and Opioid Consumption in Septorhinoplasty After Administration of Single-Dose Preemptive Paracetamol and Ibuprofen: A Randomized Controlled Trial. Int. Arch. Otorhinolaryngol. 2023, 27, e471–e477. [Google Scholar] [CrossRef]
- Freo, U.; Ruocco, C.; Valerio, A.; Scagnol, I.; Nisoli, E. Paracetamol: A Review of Guideline Recommendations. J. Clin. Med. 2021, 10, 3420. [Google Scholar] [CrossRef]
- Tan, E.; Braithwaite, I.; McKinlay, C.J.D.; Dalziel, S.R. Comparison of Acetaminophen (Paracetamol) with Ibuprofen for Treatment of Fever or Pain in Children Younger Than 2 Years. JAMA Netw. Open 2020, 3, e2022398. [Google Scholar] [CrossRef]
- Wertli, M.M.; Flury, J.S.; Streit, S.; Limacher, A.; Schuler, V.; Ferrante, A.-N.; Rimensberger, C.; Haschke, M. Efficacy of Metamizole versus Ibuprofen and a Short Educational Intervention versus Standard Care in Acute and Subacute Low Back Pain: A Study Protocol of a Randomised, Multicentre, Factorial Trial (EMISI Trial). BMJ Open 2021, 11, e048531. [Google Scholar] [CrossRef] [PubMed]
- Konijnenbelt-Peters, J.; van der Heijden, C.; Ekhart, C.; Bos, J.; Bruhn, J.; Kramers, C. Metamizole (Dipyrone) as an Alternative Agent in Postoperative Analgesia in Patients with Contraindications for Nonsteroidal Anti-Inflammatory Drugs. Pain Pract. 2017, 17, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pogatzki-Zahn, E.; Chandrasena, C.; Schug, S.A. Nonopioid Analgesics for Postoperative Pain Management. Curr. Opin. Anaesthesiol. 2014, 27, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Misiołek, H.; Cettler, M.; Woroń, J.; Wordliczek, J.; Dobrogowski, J.; Mayzner-Zawadzka, E. The 2014 Guidelines for Post-Operative Pain Management. Anaesthesiol. Intensive Ther. 2014, 46, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Yoon, S.-H.; Lee, H.-J. Practical Strategies for the Prevention and Management of Chronic Postsurgical Pain. Korean J. Pain 2023, 36, 149–162. [Google Scholar] [CrossRef]
- Bharti, N.; Chari, P.; Kumar, P. Effect of Sevoflurane versus Propofol-Based Anesthesia on the Hemodynamic Response and Recovery Characteristics in Patients Undergoing Microlaryngeal Surgery. Saudi J. Anaesth. 2012, 6, 380–384. [Google Scholar] [CrossRef]
- Gruenewald, M.; Ilies, C. Monitoring the Nociception-Anti-Nociception Balance. Best Pract. Res. Clin. Anaesthesiol. 2013, 27, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.J.; Lim, B.G.; Kim, Y.S.; Lee, M.; Kim, H. Usefulness of Surgical Pleth Index-Guided Analgesia during General Anesthesia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Int. Med. Res. 2018, 46, 4386–4398. [Google Scholar] [CrossRef]
- Choi, B.-M.; Shin, H.; Lee, J.-H.; Bang, J.-Y.; Lee, E.-K.; Noh, G.-J. Performance of the Surgical Pleth Index and Analgesia Nociception Index in Healthy Volunteers and Parturients. Front. Physiol. 2021, 12, 554026. [Google Scholar] [CrossRef]
- De jonckheere, J.; Bonhomme, V.; Jeanne, M.; Boselli, E.; Gruenewald, M.; Logier, R.; Richebé, P. Physiological Signal Processing for Individualized Anti-Nociception Management During General Anesthesia: A Review. Yearb. Med. Inform. 2015, 10, 95–101. [Google Scholar] [CrossRef]
- Ledowski, T. Objective Monitoring of Nociception: A Review of Current Commercial Solutions. Br. J. Anaesth. 2019, 123, e312–e321. [Google Scholar] [CrossRef]
- Ryu, K.; Song, K.; Kim, J.; Kim, E.; Kim, S.-H. Comparison of the Analgesic Properties of Sevoflurane and Desflurane Using Surgical Pleth Index at Equi-Minimum Alveolar Concentration. Int. J. Med. Sci. 2017, 14, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, I.; Göhner, A.; Crozier, T.A.; Hesjedal, B.; Wiese, C.H.; Popov, A.F.; Bauer, M.; Hinz, J.M. Surgical Pleth Index-Guided Remifentanil Administration Reduces Remifentanil and Propofol Consumption and Shortens Recovery Times in Outpatient Anaesthesia. Br. J. Anaesth. 2013, 110, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Upton, H.D.; Ludbrook, G.L.; Wing, A.; Sleigh, J.W. Intraoperative “Analgesia Nociception Index”-Guided Fentanyl Administration During Sevoflurane Anesthesia in Lumbar Discectomy and Laminectomy: A Randomized Clinical Trial. Anesth. Analg. 2017, 125, 81–90. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Niewiadomska, E.; Krawczyk, L.; Dobrowolski, D.; Grabarek, B.O.; Kawka, M.; Rejdak, R.; Szumera, I.; et al. Adequacy of Anaesthesia for Nociception Detection during Vitreoretinal Surgery. Life 2023, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Sener, M.; Kocum, A.; Caliskan, E.; Yilmaz, I.; Caylakli, F.; Aribogan, A. Administration of Paracetamol versus Dipyrone by Intravenous Patient-Controlled Analgesia for Postoperative Pain Relief in Children after Tonsillectomy. Braz. J. Anesthesiol. 2015, 65, 476–482. [Google Scholar] [CrossRef]
- Hearn, L.; Derry, S.; Moore, R.A. Single Dose Dipyrone (Metamizole) for Acute Postoperative Pain in Adults. Cochrane Database Syst. Rev. 2016, 4, CD011421. [Google Scholar] [CrossRef]
- McNicol, E.D.; Tzortzopoulou, A.; Cepeda, M.S.; Francia, M.B.D.; Farhat, T.; Schumann, R. Single-Dose Intravenous Paracetamol or Propacetamol for Prevention or Treatment of Postoperative Pain: A Systematic Review and Meta-Analysis. Br. J. Anaesth. 2011, 106, 764–775. [Google Scholar] [CrossRef]
- Korkmaz Dilmen, O.; Tunali, Y.; Cakmakkaya, O.S.; Yentur, E.; Tutuncu, A.C.; Tureci, E.; Bahar, M. Efficacy of Intravenous Paracetamol, Metamizol and Lornoxicam on Postoperative Pain and Morphine Consumption after Lumbar Disc Surgery. Eur. J. Anaesthesiol. 2010, 27, 428–432. [Google Scholar] [CrossRef]
- Yin, F.; Wang, X.-H.; Liu, F. Effect of Intravenous Paracetamol on Opioid Consumption in Multimodal Analgesia After Lumbar Disc Surgery: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2022, 13, 860106. [Google Scholar] [CrossRef]
- Ohnesorge, H.; Günther, V.; Grünewald, M.; Maass, N.; Alkatout, İ. Postoperative Pain Management in Obstetrics and Gynecology. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 287–297. [Google Scholar] [CrossRef]
- Khalili, G.; Janghorbani, M.; Saryazdi, H.; Emaminejad, A. Effect of Preemptive and Preventive Acetaminophen on Postoperative Pain Score: A Randomized, Double-Blind Trial of Patients Undergoing Lower Extremity Surgery. J. Clin. Anesth. 2013, 25, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Celep, B.; Çiçek, R.; Kalender, H.Ü.; Yılmaz, H. Comparing the Efficacy of Preemptive Intravenous Paracetamol on the Reducing Effect of Opioid Usage in Cholecystectomy. J. Res. Med. Sci. 2013, 18, 172–177. [Google Scholar] [PubMed]
- Stessel, B.; Boon, M.; Joosten, E.A.; Ory, J.-P.; Evers, S.; van Kuijk, S.M.J.; Dubois, J.; Hoofwijk, D.; Jamaer, L.; Buhre, W.F.F.A. Metamizole versus Ibuprofen at Home after Day Surgery: Study Protocol for a Randomised Controlled Trial. Trials 2016, 17, 471. [Google Scholar] [CrossRef]
- Kötter, T.; da Costa, B.R.; Fässler, M.; Blozik, E.; Linde, K.; Jüni, P.; Reichenbach, S.; Scherer, M. Metamizole-Associated Adverse Events: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0122918. [Google Scholar] [CrossRef]
- Marano, M.; Roversi, M.; Severini, F.; Memoli, C.; Musolino, A.; Pisani, M.; Cecchetti, C.; Villani, A. Adverse Drugs Reactions to Paracetamol and Ibuprofen in Children: A 5-Year Report from a Pediatric Poison Control Center in Italy. Ital. J. Pediatr. 2023, 49, 20. [Google Scholar] [CrossRef] [PubMed]
- Stammschulte, T.; Ludwig, W.-D.; Mühlbauer, B.; Bronder, E.; Gundert-Remy, U. Metamizole (Dipyrone)-Associated Agranulocytosis. An Analysis of German Spontaneous Reports 1990–2012. Eur. J. Clin. Pharmacol. 2015, 71, 1129–1138. [Google Scholar] [CrossRef]
- Andrade, S.; Bartels, D.B.; Lange, R.; Sandford, L.; Gurwitz, J. Safety of Metamizole: A Systematic Review of the Literature. J. Clin. Pharm. Ther. 2016, 41, 459–477. [Google Scholar] [CrossRef]
- Souki, M.A. Metamizole for Postoperative Pain Therapy. Eur. J. Anaesthesiol. 2016, 33, 785–786. [Google Scholar] [CrossRef]
- Blanca-López, N.; Pérez-Sánchez, N.; Agúndez, J.A.; García-Martin, E.; Torres, M.J.; Cornejo-García, J.A.; Perkins, J.R.; Miranda, M.A.; Andreu, I.; Mayorga, C.; et al. Allergic Reactions to Metamizole: Immediate and Delayed Responses. Int. Arch. Allergy Immunol. 2016, 169, 223–230. [Google Scholar] [CrossRef]
- Juste, J.F.M.; Garces, T.R.; Enguita, R.G.; Blasco, P.C.; Trallero, J.A. Cardiac Complications in a Metamizole-Induced Type I Kounis Syndrome. Braz. J. Anesthesiol. 2016, 66, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Delgado Nunes, V.; Buckner, S.; Latchem, S.; Constanti, M.; Miller, P.; Doherty, M.; Zhang, W.; Birrell, F.; Porcheret, M.; et al. Paracetamol: Not as Safe as We Thought? A Systematic Literature Review of Observational Studies. Ann. Rheum. Dis. 2016, 75, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Kimiaei Asadi, H.; Nikooseresht, M.; Noori, L.; Behnoud, F. The Effect of Administration of Ketamine and Paracetamol Versus Paracetamol Singly on Postoperative Pain, Nausea and Vomiting After Pediatric Adenotonsillectomy. Anesth. Pain Med. 2016, 6, e31210. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Alosi, P.; Reibaldi, M.; Longo, A.; Bonfiglio, V.; Avitabile, T.; Russo, A. Scleral Buckling: A Review of Clinical Aspects and Current Concepts. J. Clin. Med. 2022, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, R.B.; Zacharias, L.C.; de Azevedo, B.M.; Giusti, B.S.; Pretti, R.C.; Takahashi, W.Y.; Monteiro, M.L.R. Metamizole versus Placebo for Panretinal Photocoagulation Pain Control: A Prospective Double-Masked Randomized Controlled Study. Int. J. Retin. Vitr. 2015, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, S.; Kiencke, P.; Giesecke, T.; Eggert, D.; Thumann, G.; Kampe, S. A Comparison between IV Paracetamol and IV Metamizol for Postoperative Analgesia after Retinal Surgery. Curr. Med. Res. Opin. 2005, 21, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- de Souza, E.C.; Matos, D.M.; Viana, M.R.; Alvim, M.C.O.; Bonfante, H.L.; Pinto, A.F.; Nascimento, J.W.L. Evaluation of Hematological Alterations after Therapeutic Use of Dipyrone in Healthy Adults: A Prospective Study. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 385–390. [Google Scholar] [CrossRef]
- García Ramiro, M.; Alonso Guardo, L.; Matilla Álvarez, A.; Bartol Sevillano, R.; Vaquero Roncero, L.M.; Muriel Villoria, C. Eficacia de La Asociación Paracetamol-Metamizol vs. Paracetamol-Dexketoprofeno En Manejo de Dolor Agudo Postoperatorio. Rev. Soc. Española Dolor 2013, 20, 279–284. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Kawka, M.; Krawczyk, L.; Niewiadomska, E.; Dobrowolski, D.; Rejdak, R.; Król, S.; Żak, J.; et al. Preventive Analgesia, Hemodynamic Stability, and Pain in Vitreoretinal Surgery. Medicina 2021, 57, 262. [Google Scholar] [CrossRef]
- Pluta, A.; Stasiowski, M.J.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitrectomy under Adequacy of Anesthesia-An Additional Report. J. Clin. Med. 2021, 10, 4172. [Google Scholar] [CrossRef]
- Sadrolsadat, S.H.; Yousefshahi, F.; Ostadalipour, A.; Mohammadi, F.Z.; Makarem, J. Effect of Intravenous Acetaminophen on Postoperative Pain in Vitrectomy: A Randomized, Double-Blind, Clinical Trial. Anesth. Pain Med. 2017, 7, e13639. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T.; Pascoe, E.; Ang, B.; Schmarbeck, T.; Clarke, M.W.; Fuller, C.; Kapoor, V. Monitoring of Intra-Operative Nociception: Skin Conductance and Surgical Stress Index versus Stress Hormone Plasma Levels. Anaesthesia 2010, 65, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Südfeld, S.; Brechnitz, S.; Wagner, J.Y.; Reese, P.C.; Pinnschmidt, H.O.; Reuter, D.A.; Saugel, B. Post-Induction Hypotension and Early Intraoperative Hypotension Associated with General Anaesthesia. Br. J. Anaesth. 2017, 119, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Czajka, S.; Putowski, Z.; Krzych, Ł.J. Intraoperative Hypotension and Its Organ-Related Consequences in Hypertensive Subjects Undergoing Abdominal Surgery: A Cohort Study. Blood Press. 2021, 30, 348–358. [Google Scholar] [CrossRef]
- Gu, W.-J.; Hou, B.-L.; Kwong, J.S.W.; Tian, X.; Qian, Y.; Cui, Y.; Hao, J.; Li, J.-C.; Ma, Z.-L.; Gu, X.-P. Association between Intraoperative Hypotension and 30-Day Mortality, Major Adverse Cardiac Events, and Acute Kidney Injury after Non-Cardiac Surgery: A Meta-Analysis of Cohort Studies. Int. J. Cardiol. 2018, 258, 68–73. [Google Scholar] [CrossRef]
- Liu, R.; Gutiérrez, R.; Mather, R.V.; Stone, T.A.D.; Santa Cruz Mercado, L.A.; Bharadwaj, K.; Johnson, J.; Das, P.; Balanza, G.; Uwanaka, E.; et al. Development and Prospective Validation of Postoperative Pain Prediction from Preoperative EHR Data Using Attention-Based Set Embeddings. NPJ Digit. Med. 2023, 6, 209. [Google Scholar] [CrossRef]
- Ledowski, T.; Burke, J.; Hruby, J. Surgical Pleth Index: Prediction of Postoperative Pain and Influence of Arousal. Br. J. Anaesth. 2016, 117, 371–374. [Google Scholar] [CrossRef]
- Oh, S.K.; Won, Y.J.; Lim, B.G. Surgical Pleth Index Monitoring in Perioperative Pain Management: Usefulness and Limitations. Korean J. Anesthesiol. 2023. [Google Scholar] [CrossRef]
- Misiołek, H.; Zajączkowska, R.; Daszkiewicz, A.; Woroń, J.; Dobrogowski, J.; Wordliczek, J.; Owczuk, R. Postoperative Pain Management—2018 Consensus Statement of the Section of Regional Anaesthesia and Pain Therapy of the Polish Society of Anaesthesiology and Intensive Therapy, the Polish Society of Regional Anaesthesia and Pain Therapy, the Polish Association for the Study of Pain and the National Consultant in Anaesthesiology and Intensive Therapy. Anaesthesiol. Intensive Ther. 2018, 50, 173–199. [Google Scholar] [CrossRef]
- Owczuk, R. Guidelines for General Anaesthesia in the Elderly of the Committee on Quality and Safety in Anaesthesia, Polish Society of Anaesthesiology and Intensive Therapy. Anaesthesiol. Intensive Ther. 2013, 45, 57–61. [Google Scholar] [CrossRef]
- Ilies, C.; Ludwigs, J.; Gruenewald, M.; Thee, C.; Hanf, J.; Hanss, R.; Steinfath, M.; Bein, B. The Effect of Posture and Anaesthetic Technique on the Surgical Pleth Index. Anaesthesia 2012, 67, 508–513. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).