Combination Treatment with Liposomal Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells
Abstract
1. Introduction
2. Results
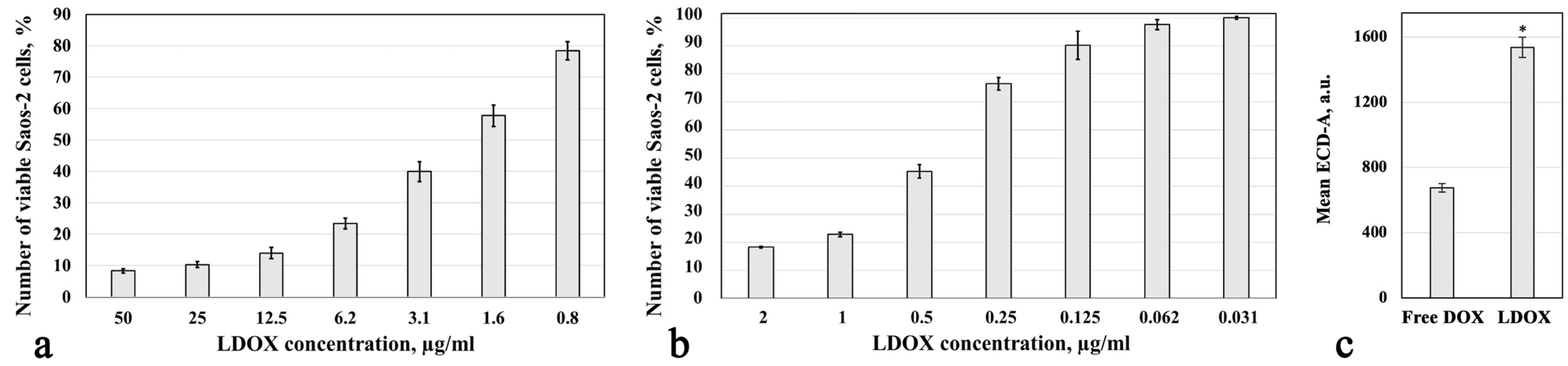
2.1. Cytotoxic Response and Drug Accumulation
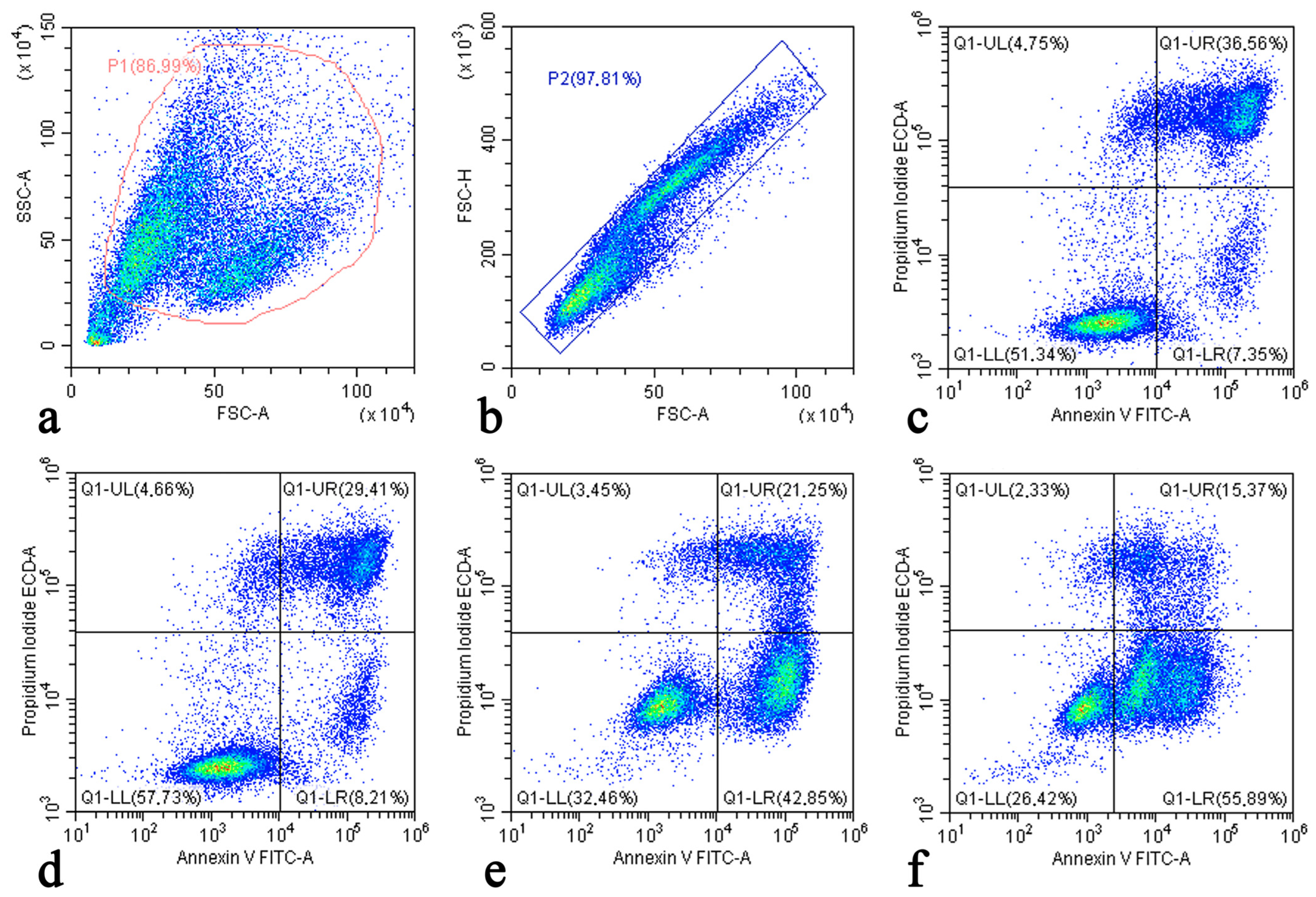
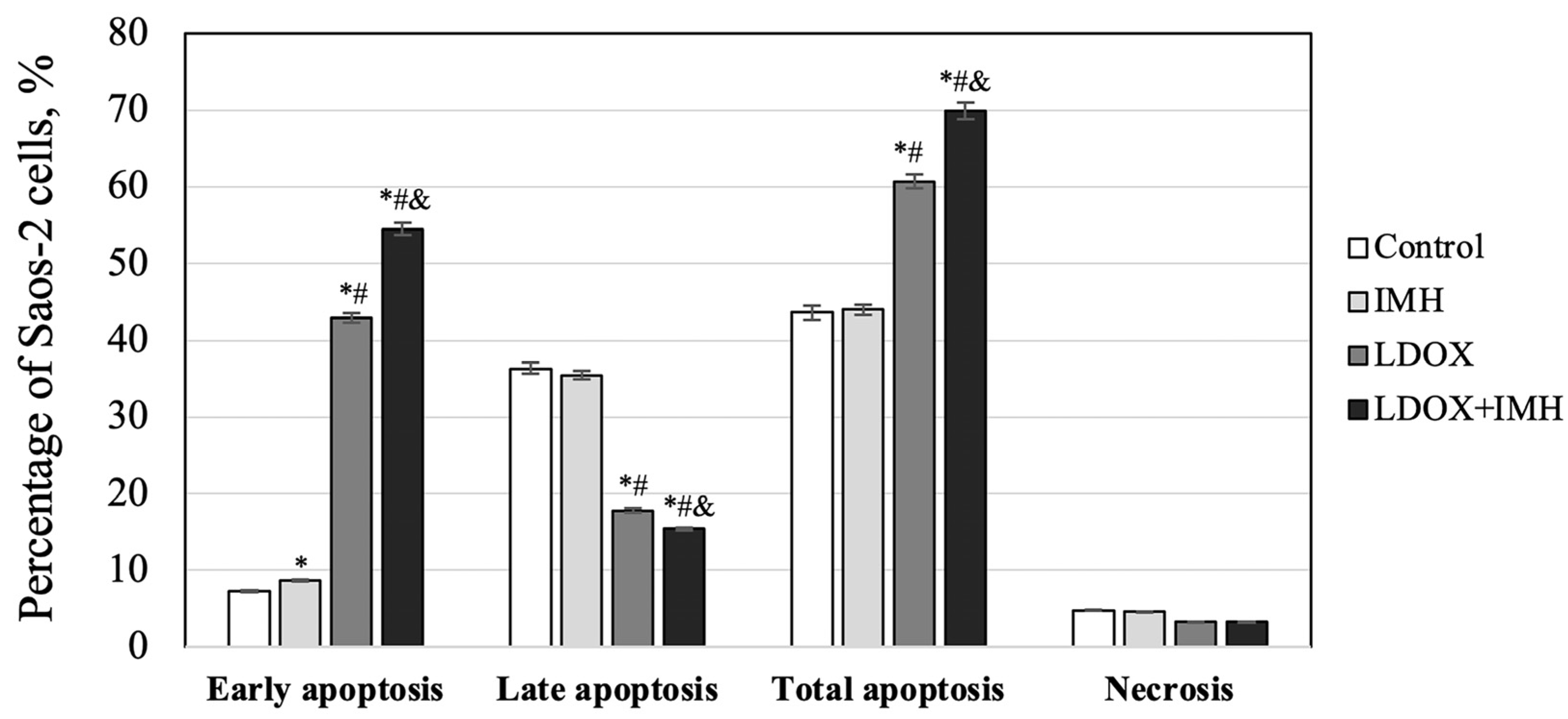
2.2. Apoptosis and Necrosis Detection
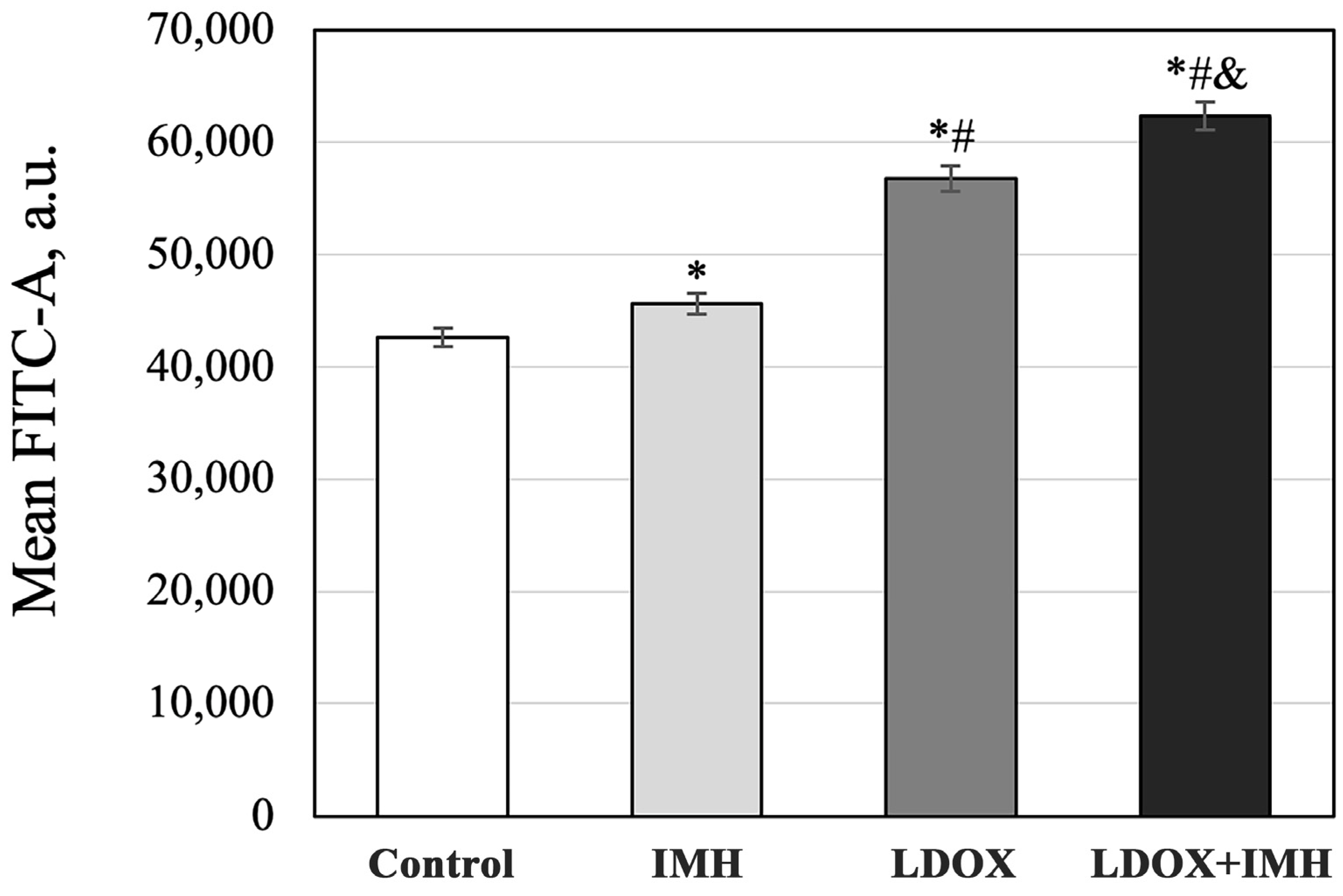
2.3. Reactive Oxygen Species Measurements
2.4. Bax Expression
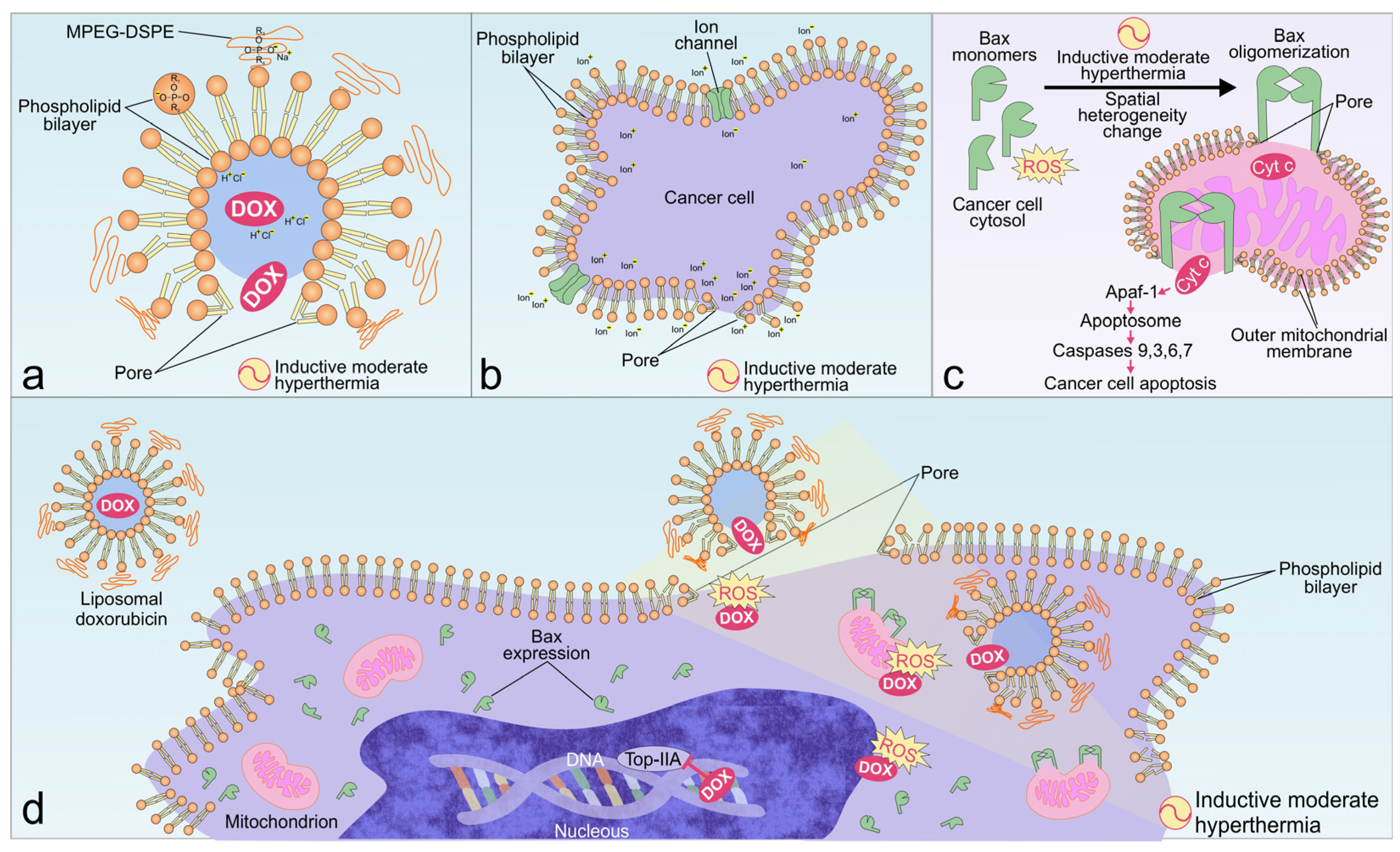
3. Discussion
4. Materials and Methods
4.1. Cell Culture
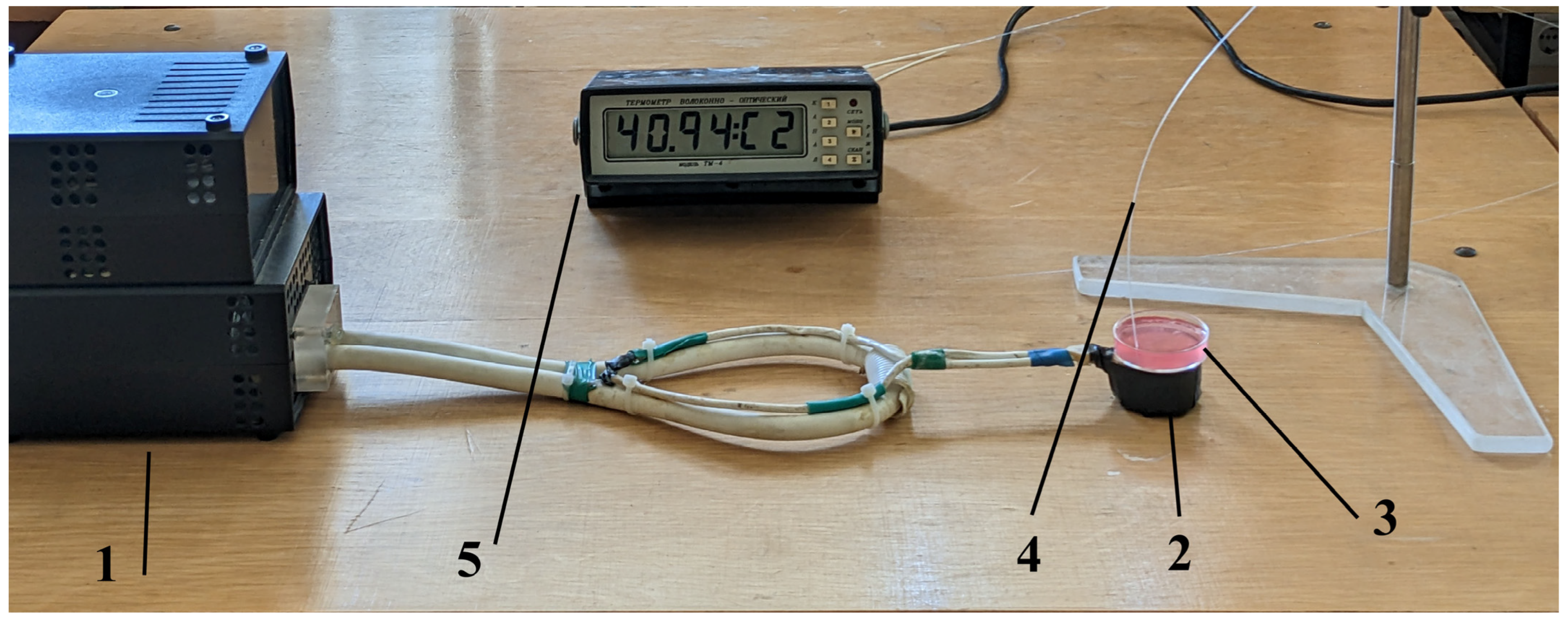
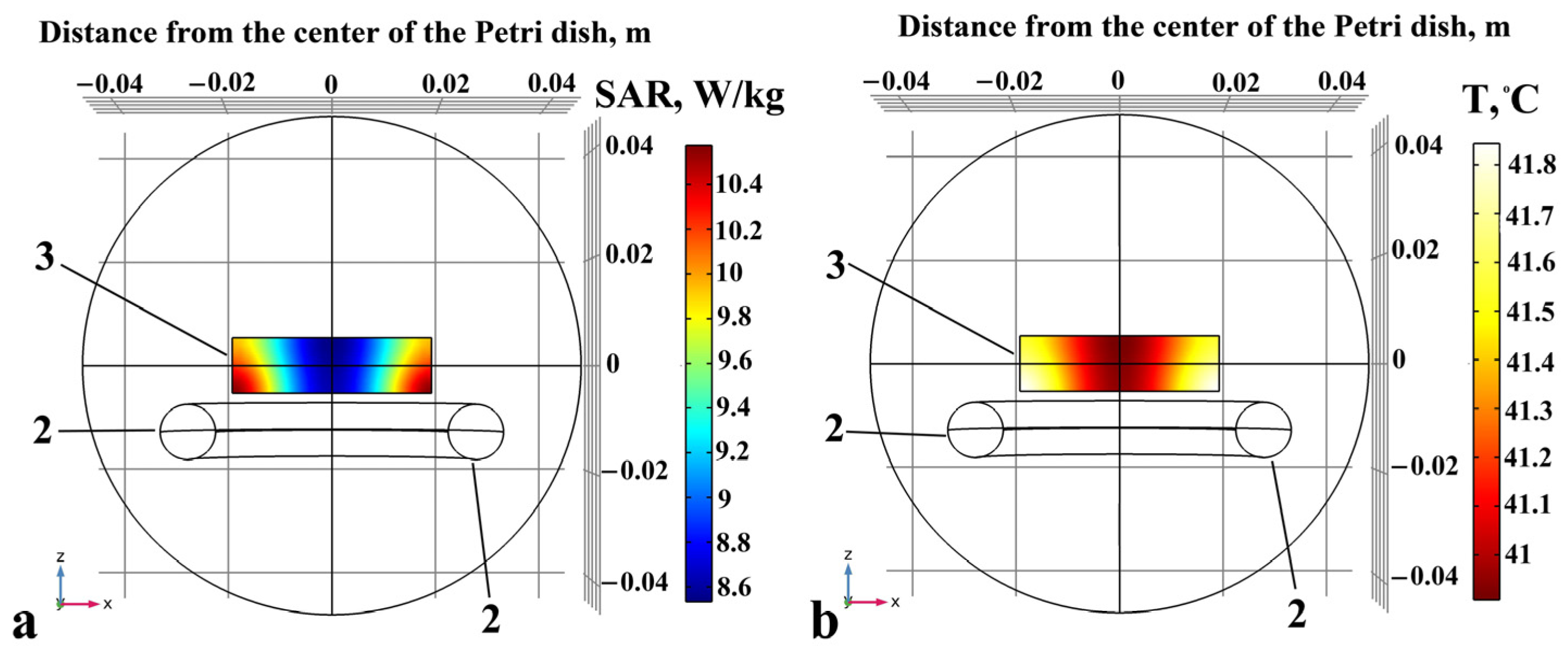
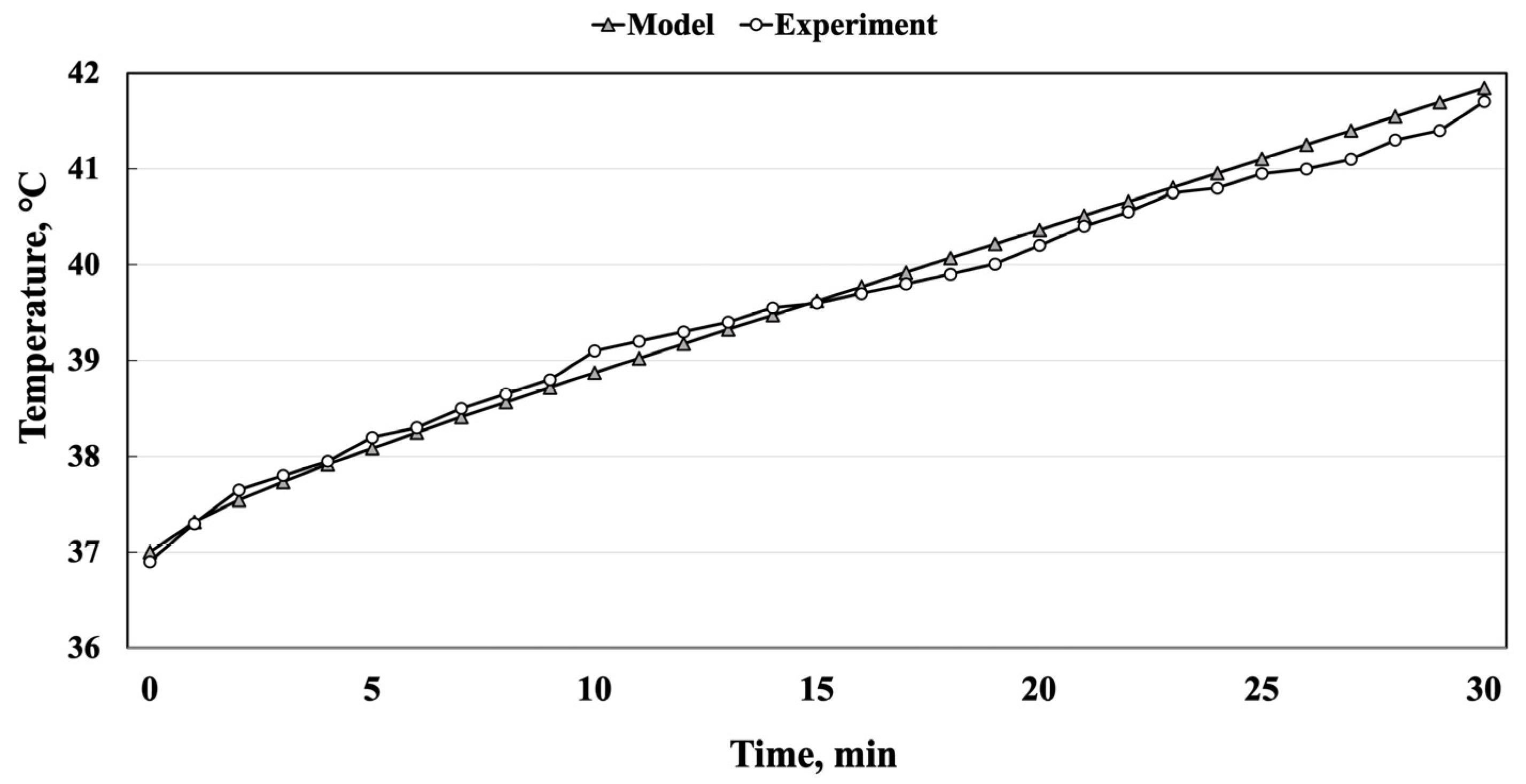
4.2. Inductive Moderate Hyperthermia
4.3. Cell Viability Assays
4.4. Flow Cytometric Assessment of Drug Accumulation
4.5. Flow Cytometric Assessment of Apoptosis and Necrosis
4.6. Flow Cytometric Detection of Reactive Oxygen Species
4.7. Immunocytochemical Assay
4.8. Image Analysis
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Picci, P. Osteosarcoma (osteogenic sarcoma). Orphanet J. Rare Dis. 2007, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A comprehensive review. Sicot J. 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Valery, P.C.; Laversanne, M.; Bray, F. Bone cancer incidence by morphological subtype: A global assessment. Cancer Causes Control. 2015, 26, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, R.; Hogendoorn, P.C.W. Epidemiology of primary bone tumors and economical aspects of bone metastases. In Bone Sarcomas and Bone Metastases—From Bench to Bedside, 3rd ed.; Heymann, D., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 17–23. [Google Scholar] [CrossRef]
- Showalter, A.; Limaye, A.; Oyer, J.L.; Igarashi, R.; Kittipatarin, C.; Copik, A.J.; Khaled, A.R. Cytokines in immunogenic cell death: Applications for cancer immunotherapy. Cytokine 2017, 97, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Susa, M.; Iyer, A.K.; Ryu, K.; Hornicek, F.J.; Mankin, H.; Amiji, M.M.; Duan, Z. Doxorubicin loaded polymeric nanoparticulate delivery system to overcome drug resistance in osteosarcoma. BMC Cancer 2009, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wan, S.; Peng, X.; Zhao, M.; Li, S.; Pu, Y.; He, B. Human serum albumin-based doxorubicin prodrug nanoparticles with tumor pH-responsive aggregation-enhanced retention and reduced cardiotoxicity. J. Mater. Chem. B 2020, 8, 3939–3948. [Google Scholar] [CrossRef]
- Gabizon, A.A.; Patil, Y.; La-Beck, N.M. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist. Updat. 2016, 29, 90–106. [Google Scholar] [CrossRef]
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia treatment as a promising anti-cancer strategy: Therapeutic targets, perspective mechanisms and synergistic combinations in experimental approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Orel, V.B.; Papazoglou, A.S.; Tsagkaris, C.; Moysidis, D.V.; Papadakos, S.; Galkin, O.Y.; Orel, V.E.; Syvak, L.A. Nanotherapy based on magneto-mechanochemical modulation of tumor redox state. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1868. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Iwao, Y. Albumin Nanoparticles. In Albumin in Medicine; Otagiri, M., Chuang, V., Eds.; Springer: Singapore, 2016; pp. 91–100. [Google Scholar] [CrossRef]
- Kang, K.W.; Song, M.G. Organic Nanomaterials: Liposomes, Albumin, Dendrimer, Polymeric Nanoparticles. In Radionanomedicine. Biological and Medical Physics, Biomedical Engineering; Lee, D., Ed.; Springer: Cham, Switzerland, 2018; pp. 105–123. [Google Scholar] [CrossRef]
- Young, R.J.; Natukunda, A.; Litière, S.; Woll, P.J.; Wardelmann, E.; van der Graaf, W.T. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: Pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur. J. Cancer 2014, 50, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Cheng, Z.; Zhao, K.; Chen, Y.; Zhang, A.; Gan, W.; Zhang, Y. Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics. J. Nanobiotechnol. 2023, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yu, B.; Li, D.; Tian, Y.; Liu, Y.; Jiang, J. Recent advances in nanoplatforms for the treatment of osteosarcoma. Front. Oncol. 2022, 12, 805978. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, D.N.; Tardi, P.G.; Mayer, L.D.; Bally, M.B. A comparison of liposomal formulations of doxorubicin with drug administered in free form: Changing toxicity profiles. Drug Saf. 2001, 24, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Trucco, M.M.; Meyer, C.F.; Thornton, K.A.; Shah, P.; Chen, A.R.; Wilky, B.A.; Carrera-Haro, M.A.; Boyer, L.C.; Ferreira, M.F.; Shafique, U.; et al. A phase II study of temsirolimus and liposomal doxorubicin for patients with recurrent and refractory bone and soft tissue sarcomas. Clin. Sarcoma Res. 2018, 8, 21. [Google Scholar] [CrossRef]
- Zabielska-Koczywąs, K.; Lechowski, R. The use of liposomes and nanoparticles as drug delivery systems to improve cancer treatment in dogs and cats. Molecules 2017, 22, 2167. [Google Scholar] [CrossRef]
- Chidiac, T.; Budd, G.T.; Pelley, R.; Sandstrom, K.; McLain, D.; Elson, P.; Crownover, R.; Marks, K.; Muschler, G.; Joyce, M.; et al. Phase II trial of liposomal doxorubicin (Doxil) in advanced soft tissue sarcomas. Investig. New Drugs 2000, 18, 253–259. [Google Scholar] [CrossRef]
- Giordano, F.; Lenna, S.; Rampado, R.; Brozovich, A.; Hirase, T.; Tognon, M.G.; Martini, F.; Agostini, M.; Yustein, J.T.; Taraballi, F. Nanodelivery systems face challenges and limitations in bone diseases management. Adv. Ther. 2021, 4, 2100152. [Google Scholar] [CrossRef]
- Al-Jamal, T.; Kostarelos, K. Mild hyperthermia accelerates doxorubicin clearance from tumour-extravasated temperature-sensitive liposomes. Nanotheranostics 2022, 6, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.M.; Vujaskovic, Z.; Yuan, F.; Needham, D.; Dewhirst, M.W. Hyperthermia mediated liposomal drug delivery. Int. J. Hyperth. 2006, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Shimose, S.; Sugita, T.; Nitta, Y.; Kubo, T.; Ikuta, Y.; Murakami, T. Effect of thermosensitive liposomal doxorubicin with hyperthermia on primary tumor and lung metastases in hamster osteosarcoma. Int. J. Oncol. 2001, 19, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Piazena, H.; Notter, M.; Thomsen, A.R.; Grosu, A.L.; Scholkmann, F.; Pockley, A.G.; Multhoff, G. From localized mild hyperthermia to improved tumor oxygenation: Physiological mechanisms critically involved in oncologic thermo-radio-immunotherapy. Cancers 2023, 15, 1394. [Google Scholar] [CrossRef] [PubMed]
- Rotundo, S.; Brizi, D.; Flori, A.; Giovannetti, G.; Menichetti, L.; Monorchio, A. Shaping and focusing magnetic field in the human body: State-of-the art and promising technologies. Sensors 2022, 22, 5132. [Google Scholar] [CrossRef] [PubMed]
- Wust, P.; Stein, U.; Ghadjar, P. Non-thermal membrane effects of electromagnetic fields and therapeutic applications in oncology. Int. J. Hyperth. 2021, 38, 715–731. [Google Scholar] [CrossRef]
- Martino, C.F.; Belchenko, D.; Ferguson, V.; Nielsen-Preiss, S.; Qi, H.J. The effects of pulsed electromagnetic fields on the cellular activity of SaOS-2 cells. Bioelectromagnetics 2008, 29, 125–132. [Google Scholar] [CrossRef]
- Cmoch, A.; Podszywalow-Bartnicka, P.; Palczewska, M.; Piwocka, K.; Groves, P.; Pikula, S. Stimulators of mineralization limit the invasive phenotype of human osteosarcoma cells by a mechanism involving impaired invadopodia formation. PLoS ONE 2014, 9, e109938. [Google Scholar] [CrossRef]
- Barnes, F.; Greenebaum, B. Role of radical pairs and feedback in weak radio frequency field effects on biological systems. Environ. Res. 2018, 163, 165–170. [Google Scholar] [CrossRef]
- Klimanov, M.Y.; Syvak, L.A.; Orel, V.E.; Lavryk, G.V.; Tarasenko, T.Y.; Orel, V.B.; Rykhalskyi, A.Y.; Stegnii, V.V.; Nesterenko, A.O. Efficacy of combined regional inductive moderate hyperthermia and chemotherapy in patients with multiple liver metastases from breast cancer. Technol. Cancer Res. Treat. 2018, 17, 1533033818806003. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Smolanka, I.; Orel, V.E.; Syvak, L.; Golovko, T.; Dosenko, I.; Lyashenko, A.; Smolanka, I.; Dasyukevich, O.; Tarasenko, T.; et al. Efficacy of combination neoadjuvant chemotherapy and regional inductive moderate hyperthermia in the treatment of patients with locally advanced breast cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820963599. [Google Scholar] [CrossRef]
- Schlemmer, M.; Wendtner, C.M.; Lindner, L.; Abdel-Rahman, S.; Hiddemann, W.; Issels, R.D. Thermochemotherapy in patients with extremity high-risk soft tissue sarcomas (HR-STS). Int. J. Hyperth. 2010, 26, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Orel, V.E.; Kudryavets, Y.I.; Satz, S.; Bezdenezhnih, N.A.; Danko, M.L.; Khranovskaya, N.N.; Romanov, A.V.; Dzyatkovskaya, N.N.; Burlaka, A.P. Mechanochemically activated doxorubicin nanoparticles in combination with 40 MHz frequency irradiation on A-549 lung carcinoma cells. Drug Deliv. 2005, 12, 171–178. [Google Scholar] [CrossRef]
- Dabbagh, A.; Mahmoodian, R.; Abdullah, B.J.; Abdullah, H.; Hamdi, M.; Abu Kasim, N.H. Low-melting-point polymeric nanoshells for thermal-triggered drug release under hyperthermia condition. Int. J. Hyperth. 2015, 31, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Bala, V.-M.; Lampropoulou, D.I.; Grammatikaki, S.; Kouloulias, V.; Lagopati, N.; Aravantinos, G.; Gazouli, M. Nanoparticle-Mediated Hyperthermia and Cytotoxicity Mechanisms in Cancer. Int. J. Mol. Sci. 2024, 25, 296. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; ten Hagen, T.L.; Hossann, M.; Süss, R.; van Rhoon, G.C.; Eggermont, A.M.; Haemmerich, D.; Koning, G.A. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J. Control. Release 2013, 168, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Maswadeh, H.; Khan, A.; Alorainy, M.S.; Al-Wabel, N.A.; Demetzos, C. In vitro and in vivo activity of thermosensitive liposomes loaded with doxorubicin and cisplatin. Drug Dev. Ind. Pharm. 2022, 48, 158–168. [Google Scholar] [CrossRef]
- Lokerse, W.J.; Bolkestein, M.; ten Hagen, T.L.; de Jong, M.; Eggermont, A.M.; Grüll, H.; Koning, G.A. Investigation of particle accumulation, chemosensitivity and thermosensitivity for effective solid tumor therapy using thermosensitive liposomes and hyperthermia. Theranostics 2016, 6, 1717–1731. [Google Scholar] [CrossRef]
- Morita, K.; Zywietz, F.; Kakinuma, K.; Tanaka, R.; Katoh, M. Efficacy of doxorubicin thermosensitive liposomes (40 degrees C) and local hyperthermia on rat rhabdomyosarcoma. Oncol. Rep. 2008, 20, 365–372. [Google Scholar] [CrossRef]
- Liu, P.; Xu, L.X.; Zhang, A. Enhanced efficacy of anti-tumor liposomal doxorubicin by hyperthermia. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 4354–4357. [Google Scholar] [CrossRef]
- Silverman, L.; Barenholz, Y. In vitro experiments showing enhanced release of doxorubicin from Doxil® in the presence of ammonia may explain drug release at tumor site. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Haghiralsadat, F.; Amoabediny, G.; Naderinezhad, S.; Nazmi, K.; De Boer, J.P.; Zandieh-Doulabi, B.; Forouzanfar, T.; Marco, N. Helder EphA2 targeted doxorubicin-nanoliposomes for osteosarcoma treatment. Pharm. Res. 2017, 34, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Kullenberg, F.; Degerstedt, O.; Calitz, C.; Pavlović, N.; Balgoma, N.; Gråsjö, K.; Sjögren, E.; Hedeland, M.; Heindryckx, F.; Lennernäs, H. In vitro cell toxicity and intracellular uptake of doxorubicin exposed as a solution or liposomes: Implications for treatment of hepatocellular carcinoma. Cells 2021, 10, 1717. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Cornelius, V.R.; Plummer, C.J.; Levitt, G.; Verrill, M.; Canney, P.; Jones, A. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, M.; Lukovic, D.; Zlabinger, K.; Spannbauer, A.; Gugerell, A.; Pavo, N.; Traxler, D.; Pils, D.; Maurer, G.; Jakab, A.; et al. Liposomal doxorubicin attenuates cardiotoxicity via induction of interferon-related DNA damage resistance. Cardiovasc. Res. 2020, 116, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lan, J.; Li, Z.; Zeng, R.; Wang, Y.; Zhen, L.; Jin, H.; Ding, Y.; Zhang, T. A novel diosgenin-based liposome delivery system combined with doxorubicin for liver cancer therapy. Pharmaceutics 2022, 14, 1685. [Google Scholar] [CrossRef] [PubMed]
- Krishan, A.; Ganapathi, R.N.; Israel, M. Effect of adriamycin and analogs on the nuclear fluorescence of propidium iodide-stained cells. Cancer Res. 1978, 38, 3656–3662. [Google Scholar]
- He, H.; Ni, J.; Huang, J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol. Lett. 2014, 7, 1352–1362. [Google Scholar] [CrossRef]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar]
- Staropoli, N.; Ciliberto, D.; Botta, C.; Fiorillo, L.; Grimaldi, A.; Lama, S.; Caraglia, M.; Salvino, A.; Tassone, P.; Tagliaferri, P. Pegylated liposomal doxorubicin in the management of ovarian cancer: A systematic review and metaanalysis of randomized trials. Cancer Biol. Ther. 2014, 15, 707–720. [Google Scholar] [CrossRef]
- Liburdy, R.P. Electromagnetic Fields and Biomembranes. In Bioelectrodynamics and Biocommunication; Ho, M.-W., Popp, F.-A., Warnke, U., Eds.; World Scientific: Singapore, 1994; pp. 159–193. [Google Scholar]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A review on electroporation-based intracellular delivery. Molecules 2018, 23, 3044. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Levitt, B.B. Cellular and molecular effects of non-ionizing electromagnetic fields. Rev. Environ. Health 2023. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Macleod, K.; Abra, R.M.; Huang, A.H.; Hahn, G.M. Hyperthermia induces doxorubicin release from long-circulating liposomes and enhances their anti-tumor efficacy. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W.; Lee, C.T.; Ashcraft, K.A. The future of biology in driving the field of hyperthermia. Int. J. Hyperth. 2016, 32, 4–13. [Google Scholar] [CrossRef]
- Bennink, R.J.; van den Hoff, M.J.; van Hemert, F.J.; de Bruin, K.M.; Spijkerboer, A.L.; Vanderheyden, J.L.; Steinmetz, N.; van Eck-Smit, B.L. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J. Nucl. Med. 2004, 45, 842–848. [Google Scholar]
- Zhao, J.; Zhang, N.; Ma, X.; Li, M.; Feng, H. The dual role of ferroptosis in anthracycline-based chemotherapy includes reducing resistance and increasing toxicity. Cell Death Discov. 2023, 9, 184. [Google Scholar] [CrossRef]
- Orel, V.E.; Krotevych, M.; Dasyukevich, O.; Rykhalskyi, O.; Syvak, L.; Tsvir, H.; Tsvir, D.; Garmanchuk, L.; Orel, V.B.; Sheina, I.; et al. Effects induced by a 50 Hz electromagnetic field and doxorubicin on Walker-256 carcinosarcoma growth and hepatic redox state in rats. Electromagn. Biol. Med. 2021, 40, 475–487. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Woynarowska, B.A.; Woynarowski, J.M. Preferential targeting of apoptosis in tumor versus normal cells. Biochim. Biophys. Acta 2002, 1587, 309–317. [Google Scholar] [CrossRef]
- Grimmig, T.; Moll, E.-M.; Kloos, K.; Thumm, R.; Moench, R.; Callies, S.; Kreckel, J.; Vetterlein, M.; Pelz, J.; Polat, B.; et al. Upregulated heat shock proteins after hyperthermic chemotherapy point to induced cell survival mechanisms in affected tumor cells from peritoneal carcinomatosis. Cancer Growth Metastasis 2017, 10, 1179064417730559. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Chandna, S. Putative partners in Bax mediated cytochrome-c release: ANT, CypD, VDAC or none of them? Mitochondrion 2009, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buccellato, L.J.; Tso, M.; Akinci, O.I.; Chandel, N.S.; Budinger, G.R.S. Reactive oxygen species are required for hyperoxia-induced Bax activation and cell death in alveolar epithelial cells. J. Biol. Chem. 2004, 279, 6753–6760. [Google Scholar] [CrossRef] [PubMed]
- Wernig, F.; Xu, Q. Mechanical stress-induced apoptosis in the cardiovascular system. Prog. Biophys. Mol. Biol. 2002, 78, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Willgohs, E.; Nguyen, T.N.; Khan, E.M.; Goldkorn, T. Monte Carlo simulation of cell death signaling predicts large cell-to-cell stochastic fluctuations through the type 2 pathway of apoptosis. Biophys. J. 2008, 95, 3559–3562. [Google Scholar] [CrossRef]
- Skommer, J.; Brittain, T.; Raychaudhuri, S. Bcl-2 inhibits apoptosis by increasing the time-to-death and intrinsic cell-to-cell variations in the mitochondrial pathway of cell death. Apoptosis 2010, 15, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.Y.; Tsai, C.J.; Lan, Y.J.; Chiang, Y.W. The role of conformational heterogeneity in regulating the apoptotic activity of BAX protein. Phys. Chem. Chem. Phys. 2017, 19, 9584–9591. [Google Scholar] [CrossRef]
- Gnann, C.; Cesnik, A.J.; Lundberg, E. Illuminating non-genetic cellular heterogeneity with imaging-based spatial proteomics. Trends Cancer. 2021, 7, 278–282. [Google Scholar] [CrossRef]
- Barnes, C.A.; Mishra, P.; Baber, J.L.; Strub, M.P.; Tjandra, N. Conformational heterogeneity in the activation mechanism of Bax. Structure 2017, 25, 1310–1316.e3. [Google Scholar] [CrossRef]
- Ibraheem, A.; Manteghi, M. Performance of electrically coupled loop antenna inside human body at different frequency bands. In Proceedings of the 2014 IEEE Antennas and Propagation Society International Symposium (APSURSI), Memphis, TN, USA, 6–11 July 2014; pp. 975–976. [Google Scholar] [CrossRef]
- Tofani, S. Magnetic fields and apoptosis: A possible mechanism. Electromagn. Biol. Med. 2022, 41, 293–303. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, B.A.; Markel, J.E.; Kleinerman, E.S. Osteosarcoma overview. Rheumatol. Ther. 2017, 4, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Provvedini, D.; Curran, D.; Catherwood, B.; Sussman, H.; Manolagas, S. Characterization of a human osteoblastic osteosarcoma cell line (SAOS-2) with high bone alkaline phosphatase activity. J. Bone Miner. Res. 1987, 2, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, J.; Wiesnerova, L.; Chocholata, P.; Kulda, V.; Landsmann, L.; Cedikova, M.; Kripnerova, M.; Eberlova, L.; Babuska, V. Human cells with osteogenic potential in bone tissue research. Biomed. Eng. Online 2023, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Dou, B.; Tan, H.; Feng, Y.; Wang, N.; Wang, D. Tumor microenvironment-driven non-cell-autonomous resistance to antineoplastic treatment. Mol. Cancer 2019, 18, 69. [Google Scholar] [CrossRef]
- Capes-Davis, A.; Freshney, R.I. Freshney’s Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 1–832. [Google Scholar]
- Orel, V.E.; Tselepi, M.; Mitrelias, T.; Zabolotny, M.; Krotevich, M.; Shevchenko, A.; Rykhalskyi, A.; Romanov, A.; Orel, V.B.; Burlaka, A.; et al. Nonlinear Magnetochemical effects in nanotherapy of Walker-256 carcinosarcoma. ACS Appl. Bio Mater. 2019, 2, 3954–3963. [Google Scholar] [CrossRef]
- Cano, M.E.; Gil-Villegas, A.; Sosa, M.A.; Villagómez, J.C.; Baffa, O. Computer simulation of magnetic properties of human blood. Chem. Phys. Lett. 2006, 432, 548–552. [Google Scholar] [CrossRef]
- Popovic, M.E.; Minceva, M. Thermodynamic properties of human tissues. Therm. Sci. 2020, 24, 4115–4133. [Google Scholar] [CrossRef]
- Zhbanov, A.; Yang, S. Effects of aggregation on blood sedimentation and conductivity. PLoS ONE 2015, 10, e0129337. [Google Scholar] [CrossRef]
- Bronzino, J.D. Biomedical Engineering Fundamental, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–1569. [Google Scholar]
- Lyra-Leite, D.M.; Copley, R.R.; Freeman, P.P.; Pongpamorn, P.; Shah, D.; McKenna, D.E.; Lenny, B.; Pinheiro, E.A.; Weddle, C.J.; Gharib, M.; et al. Nutritional requirements of human induced pluripotent stem cells. Stem Cell Rep. 2023, 18, 1371–1387. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 1997, 21, A.3B.1–A.3B.2. [Google Scholar] [CrossRef] [PubMed]
- Catitti, G.; De Fabritiis, S.; Brocco, D.; Simeone, P.; De Bellis, D.; Vespa, S.; Veschi, S.; De Lellis, L.; Tinari, N.; Verginelli, F.; et al. Flow cytometry detection of anthracycline-treated breast cancer cells: An optimized protocol. Curr. Issues Mol. Biol. 2022, 45, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Logue, S.; Elgendy, M.; Martin, S. Expression, purification and use of recombinant annexin V for the detection of apoptotic cells. Nat. Protoc. 2009, 4, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS Using Oxidized DCFDA and Flow-Cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef]
- Kuwana, T. Application of cryo-electron microscopy for investigation of Bax-induced pores in apoptosis. Nanotechnol. Rev. 2017, 6, 47–55. [Google Scholar] [CrossRef]
- Peyerl, F.W.; Dai, S.; Murphy, G.A.; Crawford, F.; White, J.; Marrack, P.; Kappler, J.W. Elucidation of some Bax conformational changes through crystallization of an antibody-peptide complex. Cell Death Differ. 2007, 14, 447–452. [Google Scholar] [CrossRef]
- Detre, S. A “quickscore” method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. Clin. Pathol. 1995, 48, 876–878. [Google Scholar] [CrossRef]
- González-García, I.; Solé, R.V.; Costa, J. Metapopulation dynamics and spatial heterogeneity in cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 13085–13089. [Google Scholar] [CrossRef]
- Orel, V.E.; Ashykhmin, A.; Golovko, T.; Rykhalskyi, O.; Orel, V.B. Texture analysis of tumor and peritumoral tissues based on 18F-Fluorodeoxyglucose positron emission tomography/computed tomography hybrid imaging in patients with rectal cancer. J. Comput. Assist. Tomogr. 2021, 45, 820–828. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Huang, Y.; Shangguan, D.; Zhang, P. Single-cell profiling to explore immunological heterogeneity of tumor microenvironment in breast cancer. Front. Immunol. 2021, 12, 643692. [Google Scholar] [CrossRef] [PubMed]



| Experiment | Moran’s I, a.u. |
|---|---|
| Control | 0.40 ± 0.004 |
| IMH | 0.45 ± 0.004 * |
| LDOX | 0.53 ± 0.008 *# |
| LDOX plus IMH | 0.58 ± 0.005 *#& |
| Object | Parameter | Value |
|---|---|---|
| Medium | Density | 1018 (kg/m3) |
| Heat capacity at constant pressure | 3930 (J/(kg*K)) | |
| Thermal conductivity | 0.496 (W/(m*K)) | |
| Electrical conductivity | 1.24 (S/m) | |
| Relative permittivity | 1.0 | |
| Cancer cells | Density | 1090 (kg/m3) |
| Heat capacity at constant pressure | 3900 (J/(kg*K)) | |
| Thermal conductivity | 0.49 (W/(m*K)) | |
| Electrical conductivity | 1.5 (S/m) | |
| Relative permittivity | 55.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orel, V.E.; Diedkov, A.G.; Ostafiichuk, V.V.; Lykhova, O.O.; Kolesnyk, D.L.; Orel, V.B.; Dasyukevich, O.Y.; Rykhalskyi, O.Y.; Diedkov, S.A.; Prosvietova, A.B. Combination Treatment with Liposomal Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells. Pharmaceuticals 2024, 17, 133. https://doi.org/10.3390/ph17010133
Orel VE, Diedkov AG, Ostafiichuk VV, Lykhova OO, Kolesnyk DL, Orel VB, Dasyukevich OY, Rykhalskyi OY, Diedkov SA, Prosvietova AB. Combination Treatment with Liposomal Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells. Pharmaceuticals. 2024; 17(1):133. https://doi.org/10.3390/ph17010133
Chicago/Turabian StyleOrel, Valerii E., Anatoliy G. Diedkov, Vasyl V. Ostafiichuk, Oleksandra O. Lykhova, Denys L. Kolesnyk, Valerii B. Orel, Olga Yo. Dasyukevich, Oleksandr Yu. Rykhalskyi, Serhii A. Diedkov, and Anna B. Prosvietova. 2024. "Combination Treatment with Liposomal Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells" Pharmaceuticals 17, no. 1: 133. https://doi.org/10.3390/ph17010133
APA StyleOrel, V. E., Diedkov, A. G., Ostafiichuk, V. V., Lykhova, O. O., Kolesnyk, D. L., Orel, V. B., Dasyukevich, O. Y., Rykhalskyi, O. Y., Diedkov, S. A., & Prosvietova, A. B. (2024). Combination Treatment with Liposomal Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells. Pharmaceuticals, 17(1), 133. https://doi.org/10.3390/ph17010133






