Toxicity and Toxicokinetics of a Four-Week Repeated Gavage of Levamisole in Male Beagle Dogs: A Good Laboratory Practice Study
Abstract
1. Introduction
2. Results
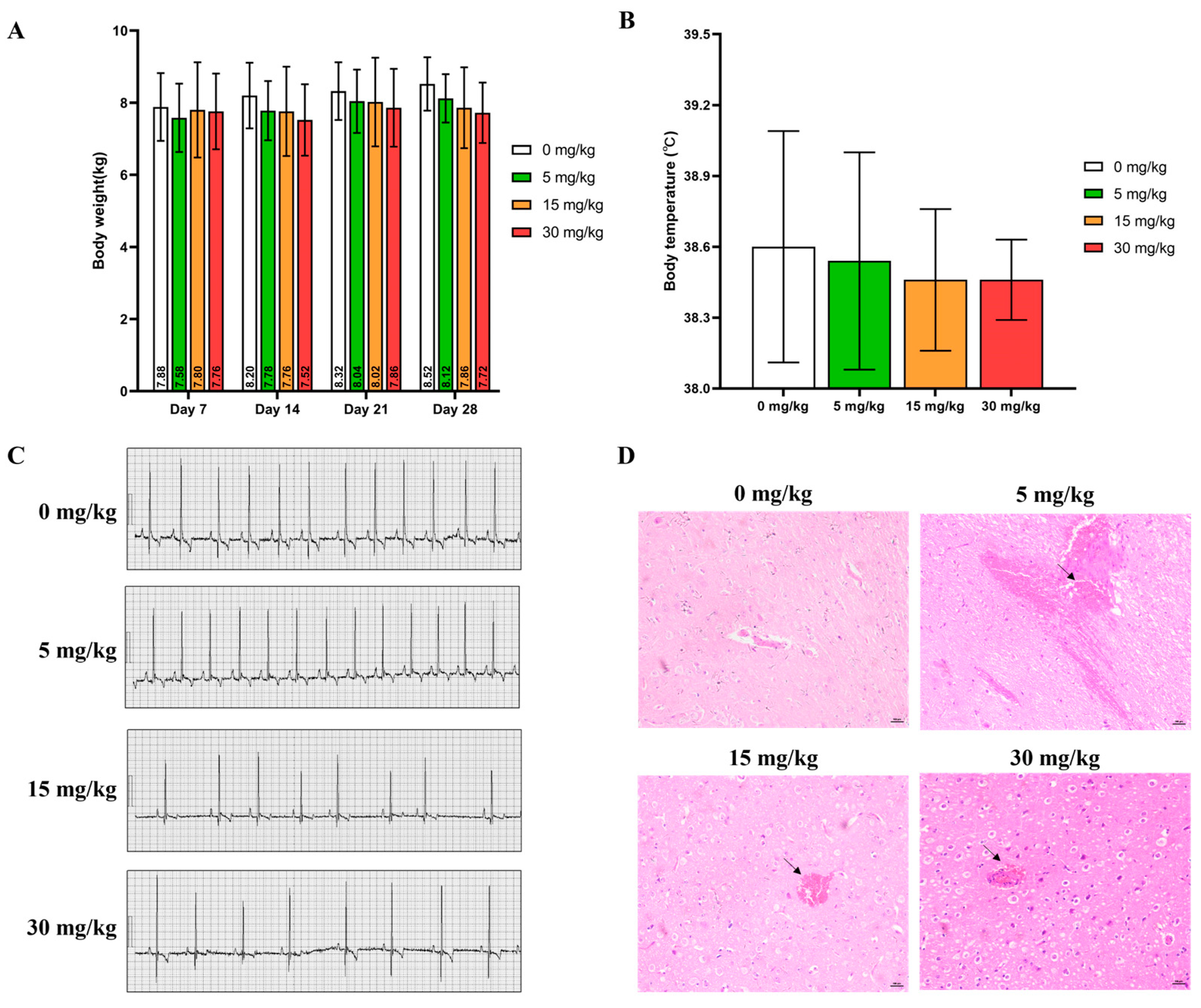
2.1. Clinical Observation
2.2. Hematological Examination
2.3. Blood Biochemical Indicators
2.4. Bone Marrow Examination
2.5. Gross Anatomy
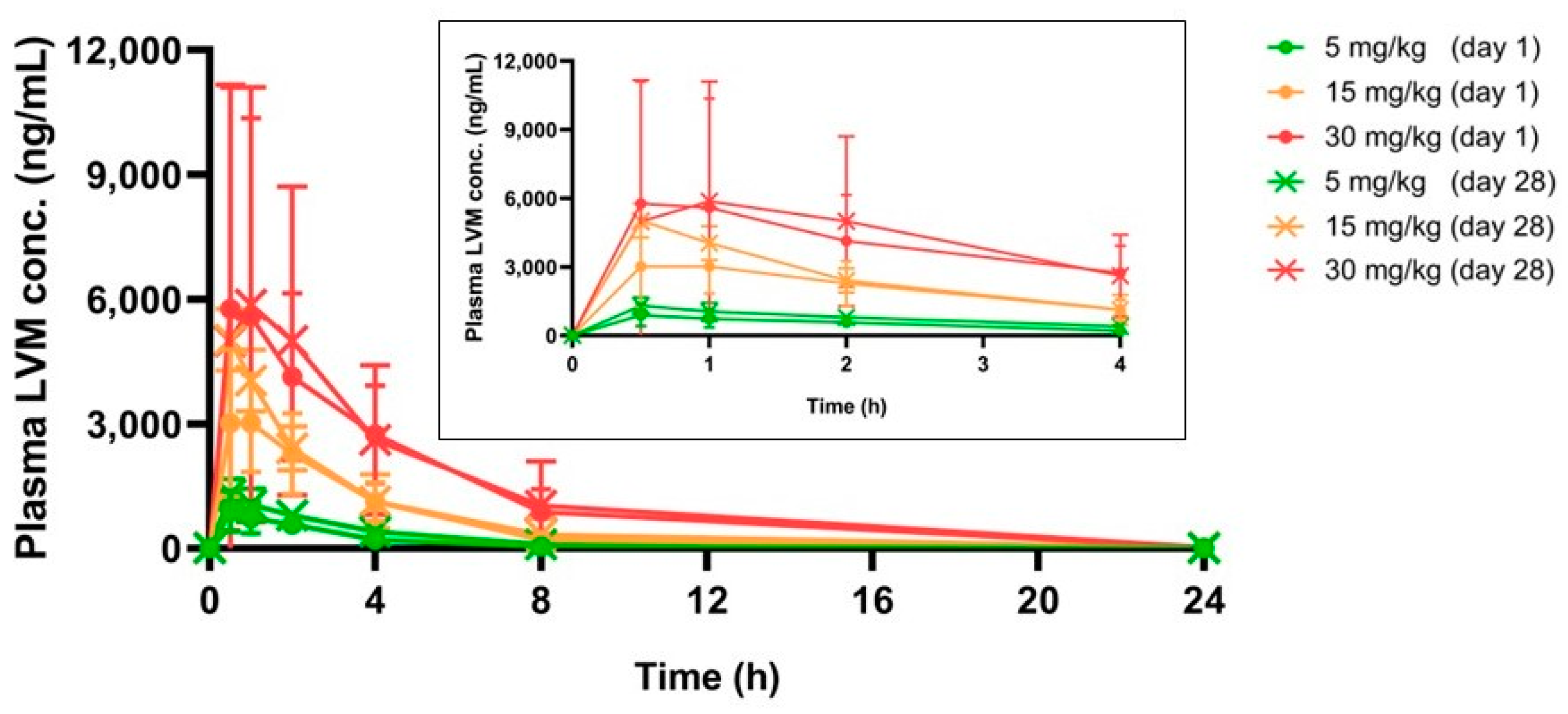
2.6. Toxicokinetics Study of LVM
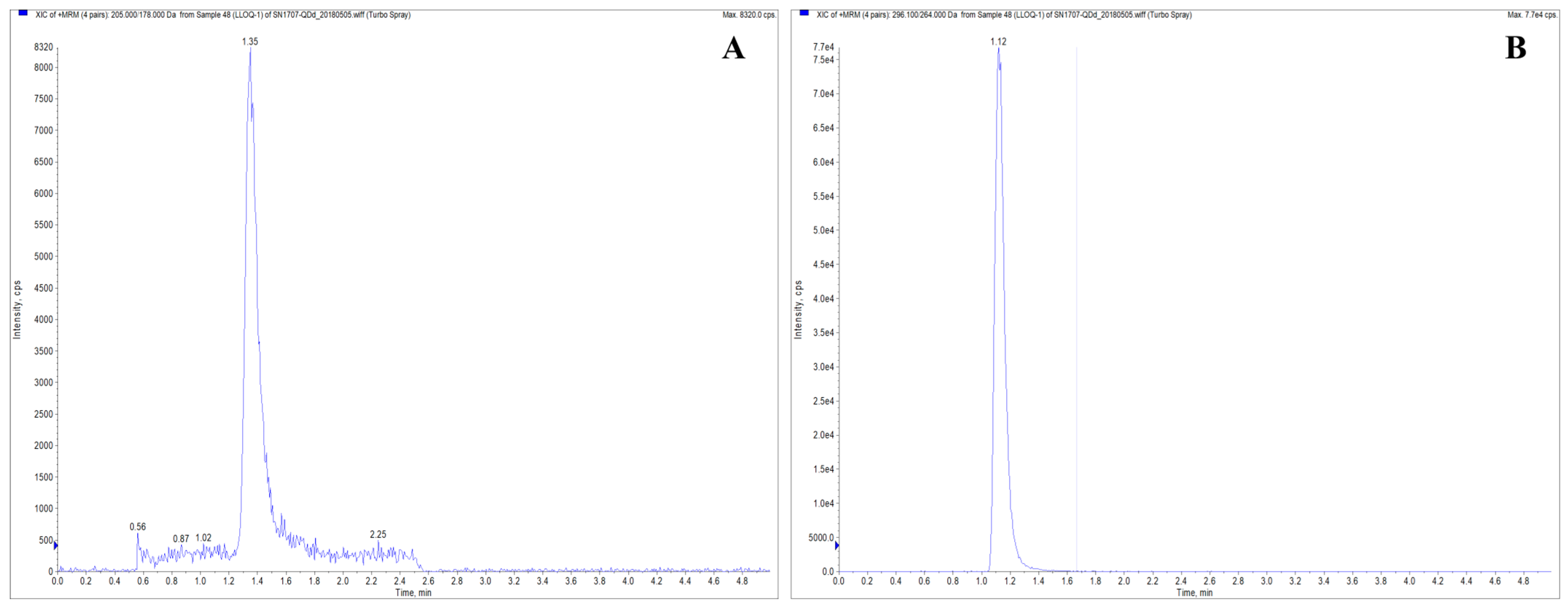
2.6.1. Method Validation
2.6.2. Pharmacokinetic Parameters
3. Discussion
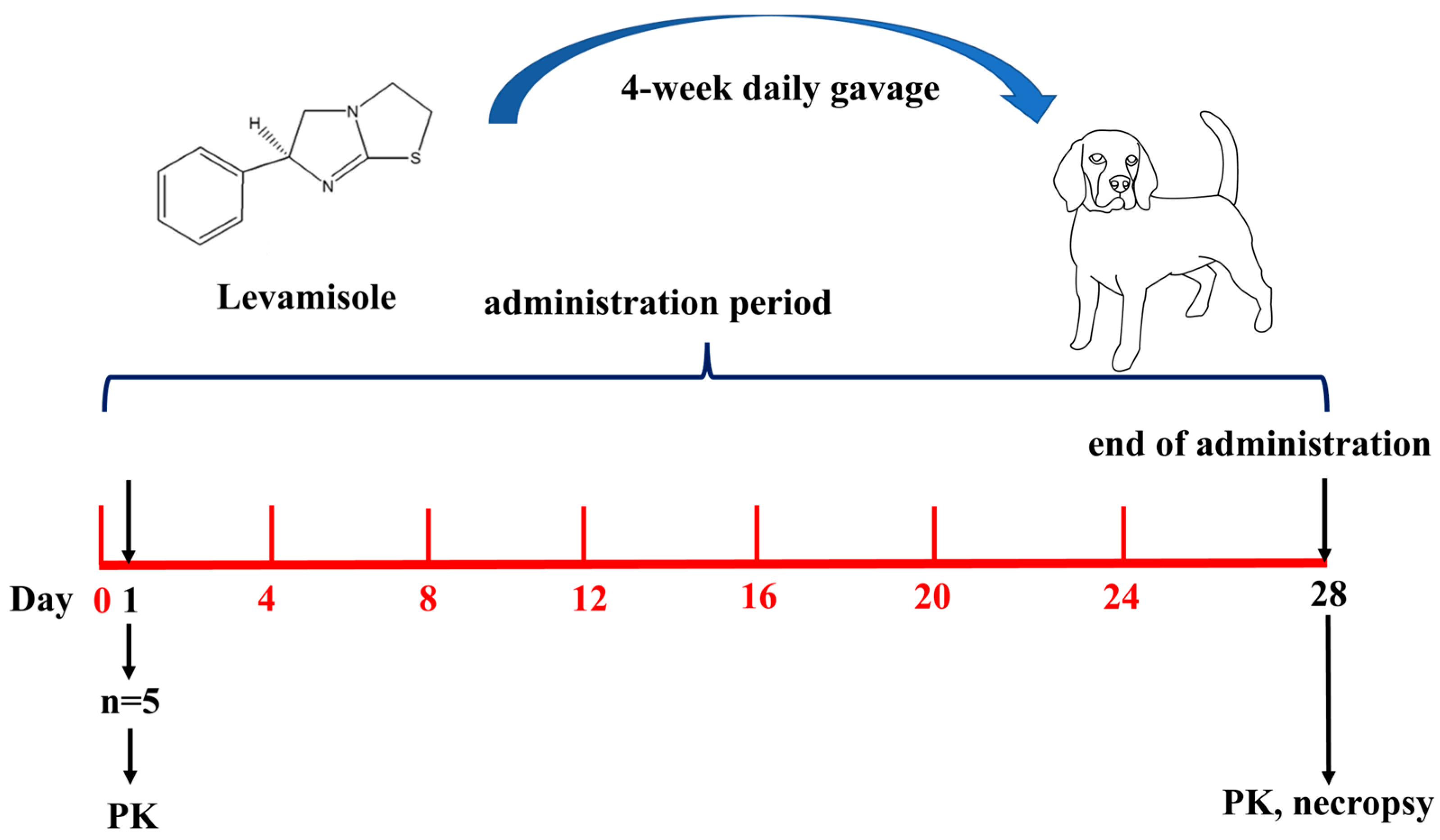
4. Materials and Methods
4.1. Chemicals
4.2. GLP Barrier Environment
4.3. Administration Regimen
4.4. Subchronic Oral Toxicity Study
4.4.1. Clinical Observations and Mortality
4.4.2. Body Weight
4.4.3. Food and Water Consumption
4.4.4. Body Temperature
4.4.5. Electrocardiogram
4.4.6. Ophthalmologic Examination
4.4.7. Hematology and Serum Biochemistry Indices
4.4.8. Bone Marrow Smear
4.4.9. Gross Necropsy
4.4.10. Organ/Body Weight Coefficient
4.4.11. Histological Study
4.5. Toxicokinetics Study
4.5.1. Blood Samples Collection
4.5.2. Sample Pretreatment
4.5.3. Analytical Method
4.5.4. Calibration Curve and Quality Control Samples
4.5.5. Method Validation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, X.; Wang, C.; Wang, W.; Shang, Y.; Li, Y.; Ni, J.; Chen, S.Z. Levamisole enhances DR4-independent apoptosis induced by TRAIL through inhibiting the activation of JNK in lung cancer. Life Sci. 2020, 257, 118034. [Google Scholar] [CrossRef]
- Niu, S.; Zhou, W.; Li, Y.; Huang, X. The signaling pathway of levamisole-sensitive-acetylcholine receptors involved in short-term forgetting of Caenorhabditis elegans. Mol. Genet. Genom. MGG 2022, 297, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Sadati, N.Y.; Youssefi, M.R.; Hosseinifard, S.M.; Tabari, M.A.; Giorgi, M. Pharmacokinetics and pharmacodynamics of single and multiple-dose levamisole in belugas (Huso huso): Main focus on immunity responses. Fish Shellfish. Immunol. 2021, 114, 152–160. [Google Scholar] [CrossRef]
- Lee, K.C.; Ladizinski, B.; Federman, D.G. Complications associated with use of levamisole-contaminated cocaine: An emerging public health challenge. Mayo Clin. Proc. 2012, 87, 581–586. [Google Scholar] [CrossRef]
- Graebner, R.W. Levamisole toxicity. Mayo Clin. Proc. 2012, 87, 1033–1034, author reply 1034. [Google Scholar] [CrossRef]
- Solomon, N.; Hayes, J. Levamisole: A High Performance Cutting Agent. Acad. Forensic Pathol. 2017, 7, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Brunt, T.M.; van den Berg, J.; Pennings, E.; Venhuis, B. Adverse effects of levamisole in cocaine users: A review and risk assessment. Arch. Toxicol. 2017, 91, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Casale, J.F.; Colley, V.L.; Legatt, D.F. Determination of phenyltetrahydroimidazothiazole enantiomers (Levamisole/Dexamisole) in illicit cocaine seizures and in the urine of cocaine abusers via chiral capillary gas chromatography-flame-ionization detection: Clinical and forensic perspectives. J. Anal. Toxicol. 2012, 36, 130–135. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yang, J.H.; Kung, S.L.; Hsiao, Y.P. Levamisole-induced myopathy and leukocytoclastic vasculitis: A case report and literature review. Dermatol. Ther. 2013, 26, 476–480. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, C.; Rozas-Muñoz, E.; Mir-Bonafé, J.F. Levamisole-Induced Vasculopathy. JAMA Dermatol. 2021, 157, 338. [Google Scholar] [CrossRef]
- Bernardi, S.; Innocenti, S.; Charbit, M.; Boyer, O. Late Onset of ANCA Vasculitis as a Side Effect of Levamisole Treatment in Nephrotic Syndrome. Medicina 2022, 58, 650. [Google Scholar] [CrossRef]
- Gampfer, T.M.; Wagmann, L.; Park, Y.M.; Cannaert, A.; Herrmann, J.; Fischmann, S.; Westphal, F.; Müller, R.; Stove, C.P.; Meyer, M.R. Toxicokinetics and toxicodynamics of the fentanyl homologs cyclopropanoyl-1-benzyl-4′-fluoro-4-anilinopiperidine and furanoyl-1-benzyl-4-anilinopiperidine. Arch. Toxicol. 2020, 94, 2009–2025. [Google Scholar] [CrossRef] [PubMed]
- Braeuning, A.; Marx-Stoelting, P. Mixture prioritization and testing: The importance of toxicokinetics. Arch. Toxicol. 2021, 95, 1863–1864. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, X.; Nepovimova, E.; Miron, A.; Liu, Q.; Wang, Y.; Su, D.; Yang, H.; Li, L.; Kuca, K. Trichothecenes: Immunomodulatory effects, mechanisms, and anti-cancer potential. Arch. Toxicol. 2017, 91, 3737–3785. [Google Scholar] [CrossRef]
- Demartini, C.; Greco, R.; Zanaboni, A.M.; Francavilla, M.; Facchetti, S.; Tassorelli, C. URB937 Prevents the Development of Mechanical Allodynia in Male Rats with Trigeminal Neuralgia. Pharmaceuticals 2023, 16, 1626. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Hwang, J.S.; Kim, I.H.; Hwang, D.Y.; Kang, H.G. Basic data on the hematology, serum biochemistry, urology, and organ weights of beagle dogs. Lab. Anim. Res. 2011, 27, 283–291. [Google Scholar] [CrossRef]
- Han, S.H.; Zheng, G.B.; Lee, J.H. The toxicological effect of 4-week repeated intravenous injection of activin a/BMP-2 chimera and 2-week recovery study in Beagle dog. Drug Chem. Toxicol. 2021, 44, 250–258. [Google Scholar] [CrossRef]
- Dolan, L.C.; Karikachery, A.R.; Thipe, V.C.; Arceneaux, B.G.; Katti, K.K.; Katti, K.V.; Chesne, A.M. Toxicity Investigations of (R)-3-Hydroxybutyrate Glycerides In Vitro and in Male and Female Rats. Nutrients 2022, 14, 4426. [Google Scholar] [CrossRef]
- Amacher, D.E.; Schomaker, S.J.; Burkhardt, J.E. The relationship among microsomal enzyme induction, liver weight and histological change in beagle dog toxicology studies. Food Chem. Toxicol. 2001, 39, 817–825. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, C.; He, C.; Mei, C.; Liao, A.; Huang, D. The Immune Characteristics of the Epididymis and the Immune Pathway of the Epididymitis Caused by Different Pathogens. Front. Immunol. 2020, 11, 2115. [Google Scholar] [CrossRef]
- Han, J.; Shin, H.C.; Kim, J.C.; Kim, B. Subacute toxicity and toxicokinetics of CJ-10882, a type IV phosphodiesterase inhibitor, after 4-week repeated oral administration in dogs. Food Chem. Toxicol. 2004, 42, 373–380. [Google Scholar] [CrossRef]
- Cui, R.T.; He, H.H.; Yu, D.A.; Li, Z.; Jiang, C.H.; Liu, D.F.; Ou-Yang, T.; Xie, N.; Yan, S.S. Single- and repeated-dose toxicity studies on the novel HIV maturation inhibitor QF-036 in Sprague-Dawley rats. Toxicol. Lett. 2020, 329, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.C.; She, J.H.; Jiang, H.; Li, Z.Y.; Yuan, B.J. Sixty-day repeated dose toxicity of sinafloxacin in rats and dogs. Food Chem. Toxicol. 2008, 46, 575–580. [Google Scholar] [CrossRef]
- Eapen, A.K.; de Cock, P.; Crincoli, C.M.; Means, C.; Wismer, T.; Pappas, C. Acute and sub-chronic oral toxicity studies of erythritol in Beagle dogs. Food Chem. Toxicol. 2017, 105, 448–455. [Google Scholar] [CrossRef]
- Nyska, A.; Hayashi, S.M.; Koyanagi, M.; Davis, J.P.; Jokinen, M.P.; Ramot, Y.; Maronpot, R.R. Ninety-day toxicity and single-dose toxicokinetics study of alpha-glycosyl isoquercitrin in Sprague-Dawley rats. Food Chem. Toxicol. 2016, 97, 354–366. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, Y.; Du, G.; Liu, B.; Yu, X.; Ye, L.; Mao, Y.; Wang, H.; Tian, J. Non-clinical pharmacology and toxicology studies of LPM6690061, a novel 5-hydroxytryptamine (5-HT)(2A) receptor inverse agonist. Food Chem. Toxicol. 2023, 176, 113800. [Google Scholar] [CrossRef] [PubMed]
- Safhi, A.Y.; Siddique, W.; Zaman, M.; Sarfraz, R.M.; Shafeeq Ur Rahman, M.; Mahmood, A.; Salawi, A.; Sabei, F.Y.; Alsalhi, A.; Zoghebi, K. Statistically Optimized Polymeric Buccal Films of Eletriptan Hydrobromide and Itopride Hydrochloride: An In Vivo Pharmacokinetic Study. Pharmaceuticals 2023, 16, 1551. [Google Scholar] [CrossRef] [PubMed]
- Golasik, M.; Herman, M.; Olbert, M.; Librowski, T.; Szklarzewicz, J.; Piekoszewski, W. Toxicokinetics and tissue distribution of titanium in ionic form after intravenous and oral administration. Toxicol. Lett. 2016, 247, 56–61. [Google Scholar] [CrossRef]
| Parameters | 0 mg/kg | 5 mg/kg | 15 mg/kg | 30 mg/kg |
|---|---|---|---|---|
| RBC (×1012/L) | 6.64 ± 0.72 | 6.40 ± 0.63 | 6.53 ± 0.47 | 6.10 ± 0.14 |
| WBC (109/L) | 15.09 ± 2.34 | 12.00 ± 2.10 * | 13.04 ± 1.12 | 10.31 ± 0.99 ** |
| HGB (g/L) | 144.40 ± 13.26 | 144.40 ± 19.72 | 141.80 ± 10.94 | 130.60 ± 5.27 |
| HCT (%) | 42.08 ± 3.42 | 42.58 ± 5.94 | 40.72 ± 2.94 | 37.30 ± 1.85 |
| MCV (fL) | 63.52 ± 2.28 | 66.30 ± 3.35 | 62.40 ± 1.43 | 61.10 ± 1.76 |
| MCH (pg) | 21.76 ± 0.61 | 22.50 ± 1.10 | 21.72 ± 0.48 | 21.40 ± 0.50 |
| NEUT (%) | 55.34 ± 4.41 | 55.06 ± 4.81 | 56.08 ± 4.83 | 55.66 ± 6.49 |
| LYMPH (%) | 35.84 ± 4.95 | 34.36 ± 5.74 | 32.74 ± 5.53 | 32.86 ± 8.35 |
| MONO (%) | 5.44 ± 1.60 | 7.42 ± 1.17 | 7.78 ± 2.31 | 9.18 ± 1.97 * |
| EO (%) | 3.24 ± 0.72 | 2.94 ± 1.18 | 3.16 ± 0.85 | 2.04 ± 1.07 |
| BASO (%) | 0.14 ± 0.05 | 0.22 ± 0.08 | 0.24 ± 0.15 | 0.26 ± 0.15 |
| NEUT (109/L) | 8.36 ± 1.48 | 6.55 ± 0.839 * | 7.30 ± 0.85 | 5.74 ± 0.92 ** |
| LYMPH (×109/L) | 5.40 ± 0.98 | 4.19 ± 1.27 | 4.27 ± 0.77 | 3.39 ± 0.92 * |
| MONO (×109/L) | 0.82 ± 0.24 | 0.88 ± 0.13 | 1.02 ± 0.34 | 0.94 ± 0.18 |
| EO (×109/L) | 0.50 ± 0.19 | 0.36 ± 0.16 | 0.42 ± 0.13 | 0.21 ± 0.11 * |
| BASO (×109/L) | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.03 ± 0.02 |
| PLT (×109/L) | 307.68 ± 26.30 | 306.16 ± 81.07 | 273.12 ± 119.42 | 331.56 ± 72.21 |
| PT (sec) | 8.46 ± 2.80 | 7.08 ± 0.38 | 7.18 ± 0.20 | 7.24 ± 0.76 |
| APTT (sec) | 24.62 ± 1.35 | 24.62 ± 1.39 | 23.08 ± 1.56 | 24.04 ± 1.34 |
| Parameters | 0 mg/kg | 5 mg/kg | 15 mg/kg | 30 mg/kg |
|---|---|---|---|---|
| CREA (μM) | 54.22 ± 7.23 | 58.26 ± 10.31 | 57.38 ± 5.90 | 57.14 ± 3.73 |
| BUN (mM) | 3.34 ± 0.60 | 4.04 ± 1.51 | 4.14 ± 0.88 | 4.32 ± 0.81 |
| GLU (mM) | 7.08 ± 0.28 | 6.52 ± 0.62 | 6.54 ± 0.34 | 6.68 ± 0.49 |
| TP (g/L) | 60.12 ± 6.04 | 60.10 ± 4.96 | 60.20 ± 3.80 | 60.14 ± 2.62 |
| ALB (g/L) | 28.32 ± 1.61 | 28.88 ± 1.54 | 29.36 ± 2.82 | 28.96 ± 2.49 |
| TBIL (μM) | 2.78 ± 0.22 | 2.98 ± 0.19 | 2.78 ± 0.37 | 2.40 ± 1.56 |
| AST (U/L) | 32.22 ± 3.60 | 29.02 ± 3.94 | 43.04 ± 22.65 | 50.58 ± 50.16 |
| ALT (U/L) | 35.68 ± 7.47 | 27.64 ± 5.01 | 31.02 ± 2.87 | 43.14 ± 19.47 |
| CK (U/L) | 238.38 ± 79.69 | 197.64 ± 69.00 | 258.64 ± 84.01 | 598.26 ± 828.12 |
| ALP (U/L) | 156.72 ± 99.59 | 234.92 ± 76.42 | 138.86 ± 54.65 | 160.86 ± 29.12 |
| GGT (U/L) | 2.44 ± 0.52 | 2.16 ± 0.72 | 2.64 ± 0.95 | 2.36 ± 0.44 |
| TG (mM) | 0.58 ± 0.07 | 0.76 ± 0.06 ** | 0.72 ± 0.11 * | 0.79 ± 0.08 ** |
| CHOL (mM) | 4.06 ± 0.55 | 6.27 ± 1.14 | 6.59 ± 1.24 | 8.92 ± 2.54 *** |
| Na+ (mM) | 139.62 ± 0.50 | 141.80 ± 1.08 ** | 139.14 ± 1.13 | 139.70 ± 1.08 |
| K+ (mM) | 4.69 ± 0.15 | 4.97 ± 0.31 | 4.47 ± 0.36 | 4.71 ± 0.43 |
| Cl- (mM) | 114.24 ± 1.25 | 115.12 ± 0.51 | 115.68 ± 0.54 | 113.88 ± 1.01 |
| Parameters | 0 mg/kg | 5 mg/kg | 15 mg/kg | 30 mg/kg |
|---|---|---|---|---|
| Myeloblast (%) | 1.33 ± 0.94 | 1.67 ± 0.47 | 1.33 ± 1.25 | 0.33 ± 0.47 |
| Progranulocyte (%) | 7.00 ± 2.65 | 5.00 ± 4.36 | 5.33 ± 2.51 | 10.3 ± 3.51 |
| Progranulocyte (%) | 3.83 ± 1.61 | 3.33 ± 1.53 | 2.83 ± 0.76 | 12.8 ± 3.88 |
| Metamylocyte (%) | 17.5 ± 5.50 | 18.3 ± 2.57 | 13.3 ± 1.04 | 13.3 ± 1.15 |
| Eosinophils (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.17 ± 0.29 | 0.00 ± 0.00 |
| Eosinophils (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Pronormoblasts (%) | 0.67 ± 0.76 | 0.50 ± 0.00 | 0.67 ± 0.76 | 1.80 ± 0.76 |
| Prorubricyte (%) | 2.67 ± 1.61 | 4.50 ± 2.00 | 2.17 ± 1.76 | 2.67 ± 1.04 |
| Polychromatic (%) | 8.83 ± 3.51 | 12.7 ± 4.19 | 9.67 ± 1.26 | 12.2 ± 2.25 |
| Metarubricyte (%) | 36.3 ± 7.64 | 28.2 ± 3.79 | 36.5 ± 3.50 | 33.7 ± 11.7 |
| Myeloid erythroid ratio | 0.46 ± 0.14 | 0.53 ± 0.04 | 0.45 ± 0.03 | 0.50 ± 0.25 |
| Metarubricyte (%) | 5.17 ± 2.75 | 4.67 ± 1.61 | 4.17 ± 3.25 | 1.83 ± 0.58 |
| Monocyte (%) | 3.67 ± 2.02 | 1.00 ± 0.87 | 3.00 ± 2.18 | 1.33 ± 0.58 |
| Plasmocyte (%) | 0.17 ± 0.29 | 0.50 ± 0.00 | 0.33 ± 0.29 | 0.33 ± 0.29 |
| Organ | 0 mg/kg | 5 mg/kg | 15 mg/kg | 30 mg/kg |
|---|---|---|---|---|
| Brain | 0.961 ± 0.102 | 0.906 ± 0.045 | 0.893 ± 0.043 | 0.897 ± 0.075 |
| Thyroid | 0.013 ± 0.002 | 0.012 ± 0.004 | 0.009 ± 0.001 | 0.010 ± 0.002 |
| Heart | 0.689 ± 0.086 | 0.718 ± 0.019 | 0.754 ± 0.009 | 0.765 ± 0.155 |
| Liver | 3.013 ± 0.077 | 2.893 ± 0.275 | 3.082 ± 0.223 | 3.198 ± 0.274 |
| Spleen | 0.325 ± 0.038 | 0.293 ± 0.059 | 0.317 ± 0.081 | 0.281 ± 0.039 |
| Kidneys | 0.514 ± 0.024 | 0.518 ± 0.050 | 0.494 ± 0.042 | 0.481 ± 0.050 |
| Adrenal glands | 0.015 ± 0.004 | 0.012 ± 0.002 | 0.018 ± 0.007 | 0.019 ± 0.011 |
| Thymus | 0.305 ± 0.036 | 0.279 ± 0.024 | 0.184 ± 0.065 * | 0.155 ± 0.062 * |
| Testis | 0.043 ± 0.031 | 0.054 ± 0.049 | 0.101 ± 0.031 | 0.081 ± 0.033 |
| Epididymis | 0.010 ± 0.002 | 0.013 ± 0.007 | 0.024 ± 0.006 * | 0.022 ± 0.007 * |
| Concentration of LVM in Plasma (ng/mL) | ||||
|---|---|---|---|---|
| LLOQ (0.5 ng/mL) | LQC (1.5 ng/mL) | MQC (10 ng/mL) | HQC (150 ng/mL) | |
| Mean value of intra-day | 0.48 | 1.61 | 10.15 | 145.83 |
| SD | 0.01 | 0.08 | 0.52 | 5.23 |
| RSD (%) | 2.61 | 5.04 | 5.14 | 3.59 |
| Accuracy (%) | 95.4 | 107.4 | 101.5 | 97.2 |
| Mean value of inter-day | 0.49 | 1.58 | 10.45 | 146.72 |
| SD | 0.06 | 0.08 | 0.41 | 4.18 |
| RSD (%) | 12.00 | 5.16 | 3.91 | 2.85 |
| Accuracy (%) | 98.3 | 105.3 | 104.5 | 97.8 |
| Dosage | Day | Tmax (h) | Cmax (ng/mL) | AUC0-t (h*ng/mL) |
|---|---|---|---|---|
| 5 mg/kg | 1 | 0.90 ± 0.65 | 994.68 ± 435.86 | 2999.50 ± 778.80 |
| 28 | 0.50 ± 0.00 | 1304.28 ± 367.21 | 4851.26 ± 1299.42 | |
| 15 mg/kg | 1 | 1.00 ± 0.55 | 3568.07 ± 1337.19 | 12,326.52 ± 3144.35 |
| 28 | 0.50 ± 0.00 | 5027.63 ± 739.03 | 15,990.54 ± 6690.98 | |
| 30 mg/kg | 1 | 1.60 ± 1.47 | 8102.45 ± 4913.85 | 30,387.45 ± 9603.64 |
| 28 | 1.90 ± 1.34 | 6275.85 ± 5456.97 | 32,740.81 ± 23,859.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, J.; Chen, L.; Yu, X.; Zhang, S.; Yu, Y. Toxicity and Toxicokinetics of a Four-Week Repeated Gavage of Levamisole in Male Beagle Dogs: A Good Laboratory Practice Study. Pharmaceuticals 2024, 17, 141. https://doi.org/10.3390/ph17010141
Zhang J, Wang J, Chen L, Yu X, Zhang S, Yu Y. Toxicity and Toxicokinetics of a Four-Week Repeated Gavage of Levamisole in Male Beagle Dogs: A Good Laboratory Practice Study. Pharmaceuticals. 2024; 17(1):141. https://doi.org/10.3390/ph17010141
Chicago/Turabian StyleZhang, Jiahui, Junxiang Wang, Lingfan Chen, Xiangbin Yu, Shuihua Zhang, and Yue Yu. 2024. "Toxicity and Toxicokinetics of a Four-Week Repeated Gavage of Levamisole in Male Beagle Dogs: A Good Laboratory Practice Study" Pharmaceuticals 17, no. 1: 141. https://doi.org/10.3390/ph17010141
APA StyleZhang, J., Wang, J., Chen, L., Yu, X., Zhang, S., & Yu, Y. (2024). Toxicity and Toxicokinetics of a Four-Week Repeated Gavage of Levamisole in Male Beagle Dogs: A Good Laboratory Practice Study. Pharmaceuticals, 17(1), 141. https://doi.org/10.3390/ph17010141





