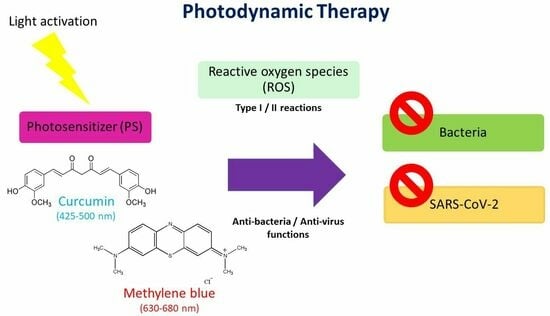
Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review
Abstract
1. Introduction
2. Principles of PDT
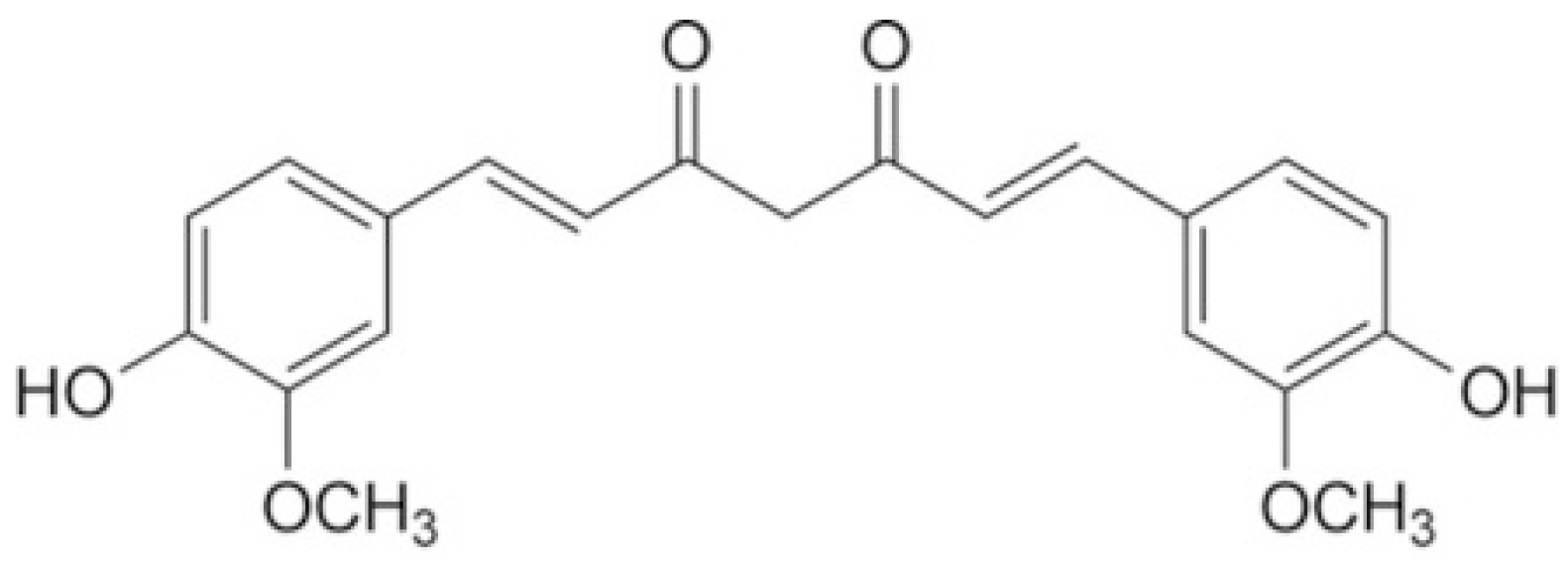
3. Curcumin
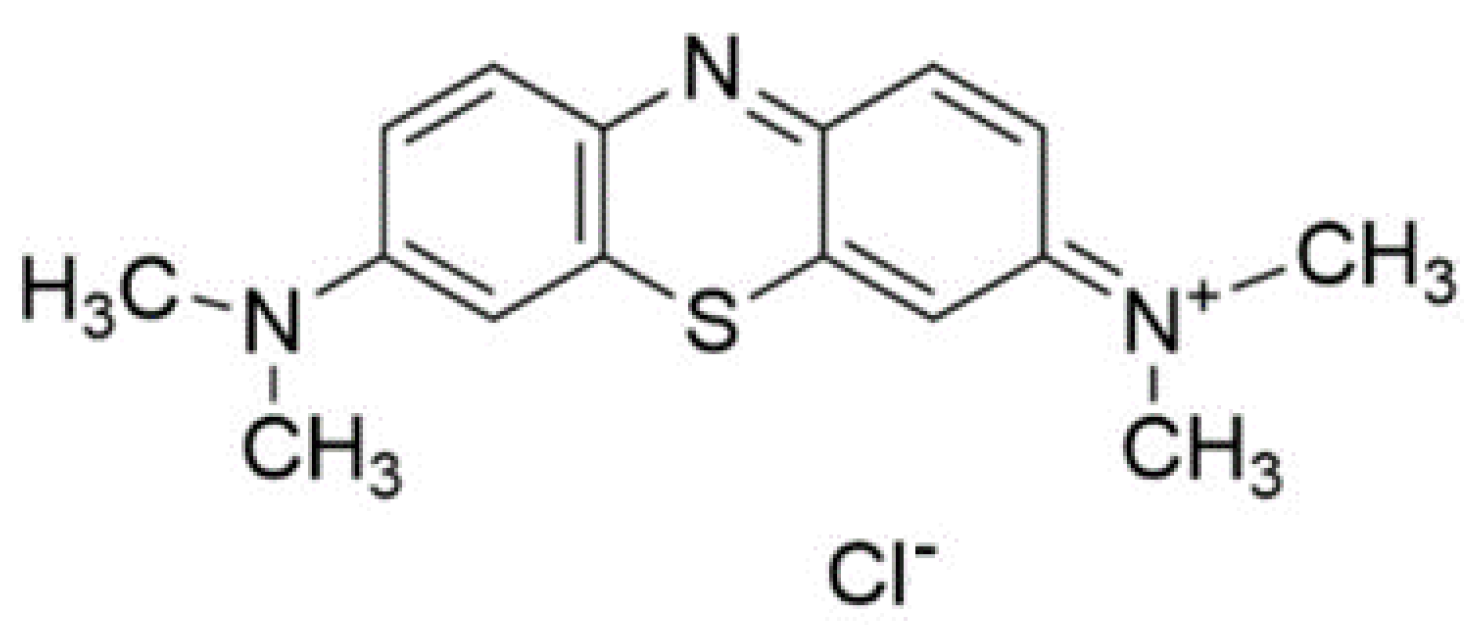
4. Methylene Blue
5. Comparing the Photodynamic Action of Methylene Blue and Curcumin
6. Photodynamic Action against Bacteria
7. Photodynamic Action against SARS-CoV-2
8. Mechanism of Photodynamic Action against SARS-CoV-2
9. Clinical Study for COVID-19
10. Discussion
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lin, Q.; Ran, J.; Musa, S.S.; Yang, G.; Wang, W.; Lou, Y.; Gao, D.; Yang, L.; He, D.; et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020, 92, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Law, S.; Leung, A.W.; Xu, C. Severe acute respiratory syndrome (SARS) and coronavirus disease-2019 (COVID-19): From causes to preventions in Hong Kong. Int. J. Infect. Dis. 2020, 94, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Chirico, F.; Teixeira da Silva, J.A.; Tsigaris, P.; Sharun, K. Safety & effectiveness of COVID-19 vaccines: A narrative review. Indian. J. Med. Res. 2022, 155, 91–104. [Google Scholar]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. GS-US-540-5774 Investigators. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.F.; Neves, M.G.P.M.S. Antimicrobial Photodynamic Therapy in the Control of COVID-19. Antibiotics 2020, 9, 320. [Google Scholar] [CrossRef]
- Courrol, L.C.; de Oliveira Silva, F.R.; Masilamani, V. SARS-CoV-2, hemoglobin and protoporphyrin IX: Interactions and perspectives. Photodiagnosis Photodyn. Ther. 2021, 34, 102324. [Google Scholar] [CrossRef]
- Batishchev, O.V.; Kalutskii, M.A.; Varlamova, E.A.; Konstantinova, A.N.; Makrinsky, K.I.; Ermakov, Y.A.; Meshkov, I.N.; Sokolov, V.S.; Gorbunova, Y.G. Antimicrobial activity of photosensitizers: Arrangement in bacterial membrane matters. Front. Mol. Biosci. 2023, 15, 1192794. [Google Scholar] [CrossRef] [PubMed]
- Ziganshyna, S.; Szczepankiewicz, G.; Kuehnert, M.; Schulze, A.; Liebert, U.G.; Pietsch, C.; Eulenburg, V.; Werdehausen, R. Photodynamic Inactivation of SARS-CoV-2 Infectivity and Antiviral Treatment Effects In Vitro. Viruses 2022, 14, 1301. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.M. Revisiting Tetra-p-Sulphonated Porphyrin as Antimicrobial Photodynamic Therapy Agent. Coatings 2021, 11, 393. [Google Scholar] [CrossRef]
- Benov, L. Photodynamic therapy: Current status and future directions. Med. Princ. Pract. 2015, 24 (Suppl. S1), 14–28. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.S.; da Silva, D.d.F.T.; Cristina Nunez, S.; Zezell, M.D. Laser em baixa intensidade. In Técnicas e Procedimentos Terapêuticos; Quintessense Editora: São Paulo, SP, Brazil, 2004; pp. 945–953. [Google Scholar]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.E.d.H. Terapia fotodinâmica: Princípios, potencial de aplicação e perspectivas. Quim. Nova 2000, 23, 237–243. [Google Scholar] [CrossRef]
- Calixto, G.M.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Boltes Cecatto, R.; Siqueira de Magalhães, L.; Fernanda Setúbal Destro Rodrigues, M.; Pavani, C.; Lino-Dos-Santos-Franco, A.; Teixeira Gomes, M.; Fátima Teixeira Silva, D. Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: The state of the art. Photodiagnosis Photodyn. Ther. 2020, 31, 101828. [Google Scholar] [CrossRef]
- Law, S.; Lo, C.; Han, J.; Yang, F.; Leung, A.W.; Xu, C. Design, Synthesis and Characterization of Novel Curcumin Derivatives. Nat. Prod. Chem. Res. 2020, 8, 367. [Google Scholar]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin-A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [PubMed]
- Jennings, M.R.; Parks, R.J. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S.; Mohammadi, K.; Deilami, I.; Keivan, Z.; Fouladvand, M.; Ramedani, E.; Asayesh, G. Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr. J. Biotechnol. 2008, 7, 3832–3835. [Google Scholar]
- Martins, C.V.; da Silva, D.L.; Neres, A.T.; Magalhães, T.F.; Watanabe, G.A.; Modolo, L.V.; Sabino, A.A.; de Fátima, A.; de Resende, M.A. Curcumin as a promising antifungal of clinical interest. J. Antimicrob. Chemother. 2009, 63, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer. Res. 2003, 23, 363–398. [Google Scholar]
- Leite, D.P.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: A randomized controlled trial. Photomed. Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef]
- Sreedhar, A.; Sarkar, I.; Rajan, P.; Pai, J.; Malagi, S.; Kamath, V.; Barmappa, R. Comparative evaluation of the efficacy of curcumin gel with and without photo activation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A split mouth clinical and microbiological study. J. Nat. Sci. Biol. Med. 2015, 6 (Suppl. S1), S102–S109. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Dahl, T.A.; McGowan, W.M.; Shand, M.A.; Srinivasan, V.S. Photokilling of bacteria by the natural dye curcumin. Arch. Microbiol. 1989, 151, 183–185. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics. 2023, 15, 639. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.H.; Childs, C.J.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Friedlaender, P. Fortschritte der Theerfarbenfabrikation und Verwandter Industriezweige; Springer: Berlin, Germany, 1877. [Google Scholar]
- Ehrlich, P. Ueber die Methylenblaureaction der lebenden Nervensubstanz. Dtsch. Med. Wochenschr. 1886, 12, 49–52. [Google Scholar] [CrossRef][Green Version]
- Ehrlich, P.; Leppmann, A. Ueber schmerzstillende wirkung des methylenblau. Dtsch. Med. Wochenschr. 1890, 16, 493–494. [Google Scholar] [CrossRef][Green Version]
- Kaufmann, P. Über die Wirkung von Methylenblau bei Malaria. Dtsch. Med. Wochenschr. 1919, 45, 1365. [Google Scholar] [CrossRef]
- Orth, K.; Beck, G.; Genze, F.; Rück, A. Methylene blue mediated photodynamic therapy in experimental colorectal tumors in mice. J. Photochem. Photobiol. B 2000, 57, 186–192. [Google Scholar] [CrossRef]
- Kupfer, A.; Aeschilmann, C.; Wermuth, B.; Cerny, T. Prophylaxis and reversal of ifosfamide encephalopathy with methylene-blue. Lancet 1994, 343, 763–764. [Google Scholar] [CrossRef]
- McEvoy, G.K. AHFS Drug Information. Oncology Issues 2017, 9, 12–13. [Google Scholar] [CrossRef]
- Bisland, S.K.; Chien, C.; Wilson, B.C.; Burch, S. Pre-clinical in vitro and in vivo studies to examine the potential use of photodynamic therapy in the treatment of osteomyelitis. Photochem. Photobiol. Sci. 2006, 5, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, J.; Williams-Lockhart, S.; Bensadoun, R.J.; Hanna, R. A Novel Approach of Combining Methylene Blue Photodynamic Inactivation, Photobiomodulation and Oral Ingested Methylene Blue in COVID-19 Management: A Pilot Clinical Study with 12-Month Follow-Up. Antioxidants 2022, 11, 2211. [Google Scholar] [CrossRef] [PubMed]
- PDQ Integrative, Alternative, and Complementary Therapies Editorial Board. Curcumin (Curcuma, Turmeric) and Cancer (PDQ®): Health Professional Version. 1 September 2023. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Ucuncu, M.; Mills, B.; Duncan, S.; Staderini, M.; Dhaliwal, K.; Bradley, M. Polymyxin-based photosensitizer for the potent and selective killing of Gram-negative bacteria. Chem. Commun. 2020, 56, 3757–3760. [Google Scholar] [CrossRef] [PubMed]
- Sivieri-Araujo, G.; Strazzi-Sahyon, H.B.; Jacomassi, D.P.; Dos Santos, P.H.; Cintra, L.T.A.; Kurachi, C.; Bagnato, V.S. Effects of methylene blue and curcumin photosensitizers on the color stability of endodontically treated intraradicular dentin. Photodiagnosis Photodyn. Ther. 2022, 37, 102650. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, A.; Karami, N.; Zabihi, R.; Fazeliyan, E.; Abbasi, A.; Karimi, S.; Barbosa de Farias, M.; Adeodato Vieira, M.G.; Shahsavani, E.; Mousavi Khaneghah, A. Green synthesis, characterization, and application of Fe3O4 nanoparticles for methylene blue removal: RSM optimization, kinetic, isothermal studies, and molecular simulation. Environ. Res. 2023, 225, 115507. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.P.; Vieira, T.; Silva, J.C.; Ribeiro, P.A.; Raposo, M. Phototoxic Potential of Different DNA Intercalators for Skin Cancer Therapy: In Vitro Screening. Int. J. Mol. Sci. 2023, 24, 5602. [Google Scholar] [CrossRef] [PubMed]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updates 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Hu, F.; Xu, S.; Liu, B. Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications. Adv. Mater. 2018, 30, e1801350. [Google Scholar] [CrossRef]
- Harris, F.; Chatfield, L.; Phoenix, D. Phenothiazinium Based Photosensitisers—Photodynamic Agents with a Multiplicity of Cellular Targets and Clinical Applications. Curr. Drug Targets, 2005; Epub ahead of print. [Google Scholar]
- Bartolomeu, M.; Rocha, S.; Cunha, Â.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Effect of Photodynamic Therapy on the Virulence Factors of Staphylococcus aureus. Front. Microbiol. 2016, 7, 267. [Google Scholar] [CrossRef]
- Wang, C.; Chen, P.; Qiao, Y.; Kang, Y.; Yan, C.; Yu, Z.; Wang, J.; He, X.; Wu, H. pH responsive superporogen combined with PDT based on poly Ce6 ionic liquid grafted on SiO2 for combating MRSA biofilm infection. Theranostics 2020, 10, 4795–4808. [Google Scholar] [CrossRef]
- Ribeiro, I.P.; Pinto, J.G.; Souza, B.M.N.; Miñán, A.G.; Ferreira-Strixino, J. Antimicrobial photodynamic therapy with curcumin on methicillin-resistant Staphyloccocus aureus biofilm. Photodiagnosis Photodyn. Ther. 2022, 37, 102729. [Google Scholar] [CrossRef]
- Muniz, I.P.R.; Galantini, M.P.L.; Ribeiro, I.S.; Gonçalves, C.V.; Dos Santos, D.P.; Moura, T.C.; Silva, E.S.; Silva, N.R.; Cipriano, B.P.; Correia, T.M.L.; et al. Antimicrobial photodynamic therapy (aPDT) with curcumin controls intradermal infection by Staphyloccocus aureus in mice with type 1 diabetes mellitus: A pilot study. J. Photochem. Photobiol. B 2021, 224, 112325. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, A.W.; Hua, H.; Rao, X.; Xu, C. Photodynamic Action of LED-Activated Curcumin against Staphyloccocus aureus Involving Intracellular ROS Increase and Membrane Damage. Int. J. Photoenergy. 2014, 2014, 637601. [Google Scholar] [CrossRef]
- Freitas, M.A.A.; Pereira, A.H.C.; Pinto, J.G.; Casas, A.; Ferreira-Strixino, J. Bacterial viability after antimicrobial photodynamic therapy with curcumin on multiresistant Staphylococcus aureus. Future Microbiol. 2019, 14, 42. [Google Scholar] [CrossRef]
- Astuti, S.D.; Mahmud, A.F.; Pudjiyanto; Mukhammad, Y.; Fitriyah, N. Antimicrobial photodynamic of blue LED for activation of curcumin extract (curcuma longa) on Staphyloccocus aureus bacteria, an in vitro study. J. Phys. Conf. Ser. 2018, 1120, 012073. [Google Scholar] [CrossRef]
- Almeida, P.P.; Pereira, Í.S.; Rodrigues, K.B.; Leal, L.S.; Marques, A.S.; Rosa, L.P.; da Silva, F.C.; da Silva, R.A.A. Photodynamic therapy controls of Staphyloccocus aureus intradermal infection in mice. Lasers Med. Sci. 2017, 32, 1337–1342. [Google Scholar] [CrossRef]
- Haukvik, T.; Bruzell, E.; Kristensen, S.; Tønnesen, H.H. Photokilling of bacteria by curcumin in different aqueous preparations. Studies on curcumin and curcuminoids XXXVII. Pharmazie 2009, 64, 666–673. [Google Scholar]
- Mushtaq, S.; Yasin, T.; Saleem, M.; Dai, T.; Yameen, M.A. Potentiation of Antimicrobial Photodynamic Therapy by Curcumin-loaded Graphene Quantum Dots. Photochem. Photobiol. 2022, 98, 202–210. [Google Scholar] [CrossRef]
- Wikene, K.O.; Hegge, A.B.; Bruzell, E.; Tønnesen, H.H. Formulation and characterization of lyophilized curcumin solid dispersions for antimicrobial photodynamic therapy (aPDT): Studies on curcumin and curcuminoids LII. Drug Dev. Ind. Pharm. 2015, 41, 969–977. [Google Scholar] [CrossRef]
- Moradi, M.; Fazlyab, M.; Pourhajibagher, M.; Chiniforush, N. Antimicrobial action of photodynamic therapy on Enterococcus faecalis biofilm using curing light, curcumin and riboflavin. Aust. Endod. J. 2022, 48, 274–282. [Google Scholar] [CrossRef]
- Cusicanqui Méndez, D.A.; Cardenas Cuéllar, M.R.; Feliz Pedrinha, V.; Velásquez Espedilla, E.G.; Bombarda de Andrade, F.; Rodrigues, P.A.; Cruvinel, T. Effects of curcumin-mediated antimicrobial photodynamic therapy associated to different chelators against Enterococcus faecalis biofilms. Photodiagnosis Photodyn. Ther. 2021, 35, 102464. [Google Scholar] [CrossRef]
- Abdulrahman, H.; Misba, L.; Ahmad, S.; Khan, A.U. Curcumin induced photodynamic therapy mediated suppression of quorum sensing pathway of Pseudomonas aeruginosa: An approach to inhibit biofilm in vitro. Photodiagnosis Photodyn. Ther. 2020, 30, 101645. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lin, S.; Zhang, J.; Kong, Z.; Tan, B.K.; Hamzah, S.S.; Hu, J. Enhancement of photodynamic bactericidal activity of curcumin against Pseudomonas aeruginosa using polymyxin B. Photodiagnosis Photodyn. Ther. 2022, 37, 102677. [Google Scholar] [CrossRef] [PubMed]
- Geralde, M.C.; Corrêa, T.Q.; Vollet-Filho, J.D.; Kurachi, C.; Bagnato, V.S.; Pratavieira, S.; de Souza, C.W.O. Evaluation of curcumin incubation time in Staphyloccocus aureus and Pseudomonas aeruginosa Photodynamic Inactivation. In 2021 SBFoton International Optics and Photonics Conference (SBFoton IOPC); IEEE: Toulouse, France, 2021; pp. 1–4. [Google Scholar]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy: What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Rezusta, A.; Gilaberte, Y. Photodynamic therapy using methylene blue, combined or not with gentamicin, against Staphyloccocus aureus and Pseudomonas aeruginosa. Photodiagnosis Photodyn. Ther. 2020, 31, 101810. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, P.S.; Packer, S.; Singer, M.; Nair, S.P.; Bennett, J.; Street, C.; Wilson, M. In vivo killing of Staphyloccocus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009, 9, 27. [Google Scholar] [CrossRef]
- Virych, P.; Nadtoka, O.; Virych, P.; Kutsevol, N. Antimicrobial photoinactivation with methylene blue of Staphylococcus aureus. Mol. Cryst. Liq. 2021, 716, 123–128. [Google Scholar] [CrossRef]
- MiyabeI, M.; JunqueiraI, J.C.; Pereira da CostaI, A.C.B.; JorgeI, A.O.C.; RibeiroII, M.S.; FeistII, I.S. Effect of photodynamic therapy on clinical isolates of Staphylococcus spp. Braz. Oral Res. 2011, 25, 230–234. [Google Scholar] [CrossRef]
- de Oliveira, B.P.; dos Santos Accioly Lins, C.C.; Diniz, F.A.; Melo, L.L.; de Castro, C.M.M.B. In Vitro antimicrobial photoinactivation with methylene blue in different microorganisms. Braz. J. Oral. Sci. 2014, 13, 53–57. [Google Scholar] [CrossRef][Green Version]
- Rosa, L.P.; Silva, F.C.; Nader, S.A.; Meira, G.A.; Viana, M.S. Effectiveness of antimicrobial photodynamic therapy using a 660 nm laser and methyline blue dye for inactivating Staphyloccocus aureus biofilms in compact and cancellous bones: An in vitro study. Photodiagnosis Photodyn. Ther. 2015, 12, 276–281. [Google Scholar] [CrossRef]
- Hampden-Martin, A.; Fothergill, J.; El Mohtadi, M.; Chambers, L.; Slate, A.J.; Whitehead, K.A.; Shokrollahi, K. Photodynamic antimicrobial chemotherapy coupled with the use of the photosensitizers methylene blue and temoporfin as a potential novel treatment for Staphylococcus aureus in burn infections. Access Microbiol. 2021, 3, 000273. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.H.C.; Pinto, J.G.; Freitas, M.A.A.; Fontana, L.C.; Pacheco Soares, C.; Ferreira-Strixino, J. Methylene blue internalization and photodynamic action against clinical and ATCC Pseudomonas aeruginosa and Staphyloccocus aureus strains. Photodiagnosis Photodyn. Ther. 2018, 22, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.; Coutinho-Filho, W.P.; Andrade, A.O.; Herrera, D.R.; Coutinho-Filho, T.S.; Krebs, R.L. Evaluation of photodynamic therapy using a diode laser and different photosensitizers against Enterococcus faecalis. Acta Odontol. Latinoam. 2014, 27, 63–65. [Google Scholar] [PubMed]
- Mozayeni, M.A.; Vatandoost, F.; Asnaashari, M.; Shokri, M.; Azari-Marhabi, S.; Asnaashari, N. Comparing the Efficacy of Toluidine Blue, Methylene Blue and Curcumin in Photodynamic Therapy Against Enterococcus faecalis. J. Lasers Med. Sci. 2020, 11 (Suppl. S1), S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, W.; Zhang, Q.; Xu, K.; Ye, G.; Wu, W.; Sun, Z.; Liu, F.; Wu, K.; Zhong, B.; et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg. Microbes. Infect. 2020, 9, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Lenard, J.; Rabson, A.; Vanderoef, R. Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: Inhibition of fusion and syncytia formation. Proc. Natl. Acad. Sci. USA 1993, 90, 158–162. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Sharshov, K.; Solomatina, M.; Kurskaya, O.; Kovalenko, I.; Kholina, E.; Fedorov, V.; Meerovich, G.; Rubin, A.; Strakhovskaya, M. The Photosensitizer Octakis(Cholinyl)Zinc Phthalocyanine with Ability to Bind to a Model Spike Protein Leads to a Loss of SARS-CoV-2 Infectivity In Vitro When Exposed to Far-Red LED. Viruses 2021, 13, 643. [Google Scholar] [CrossRef]
- Zupin, L.; Fontana, F.; Clemente, L.; Borelli, V.; Ricci, G.; Ruscio, M.; Crovella, S. Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer. Viruses 2022, 14, 2132. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Azimi, M.; Haddadi-Asl, V.; Ahmadi, H.; Gholamzad, M.; Ghorbanpour, S.; Bahador, A. Robust antimicrobial photodynamic therapy with curcumin-poly (lactic-co-glycolic acid) nanoparticles against COVID-19: A preliminary in vitro study in Vero cell line as a model. Photodiagnosis Photodyn. Ther. 2021, 34, 102286. [Google Scholar] [CrossRef] [PubMed]
- Preis, E.; Baghdan, E.; Agel, M.R.; Anders, T.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Spray dried curcumin loaded nanoparticles for antimicrobial photodynamic therapy. Eur. J. Pharm. Biopharm. 2019, 142, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K.; Lo, C.M.; Han, J.; Leung, A.W.; Xu, C.S. Could Curcumin hydrogel for photodynamic therapy fight against SARS-CoV-2? Biointerface Res. Appl. Chem. 2023, 13, 145. [Google Scholar]
- Lobo, C.S.; Rodrigues-Santos, P.; Pereira, D. Photodynamic disinfection of SARS-CoV-2 clinical samples using a methylene blue formulation. Photochem. Photobiol. Sci. 2022, 21, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Arentz, J.; von der Heide, H.J. Evaluation of methylene blue based photodynamic inactivation (PDI) against intracellular B-CoV and SARS-CoV2 viruses under different light sources in vitro as a basis for new local treatment strategies in the early phase of a Covid19 infection. Photodiagnosis Photodyn. Ther. 2022, 37, 102642. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. Lest we forget you--methylene blue. Neurobiol. Aging. 2011, 32, 2325–2416. [Google Scholar] [CrossRef]
- Bistas, E.; Sanghavi, D. Methylene Blue; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tobin, M.J.; Laghi, F.; Jubran, A. Why COVID-19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med. 2020, 202, 356–360. [Google Scholar] [CrossRef]
- Vahedian-Azimi, A.; Abbasifard, M.; Rahimi-Bashar, F.; Guest, P.C.; Majeed, M.; Mohammadi, A.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients 2022, 14, 256. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, Y.; Cao, X.; Liu, Y.; Ying, R.; Huang, Q.; Gao, P.; Zhang, C. Curcumin as an antiviral agent and immune-inflammatory modulator in COVID-19: A scientometric analysis. Heliyon 2023, 9, e21648. [Google Scholar] [CrossRef]
- Golpour-Hamedani, S.; Pourmasoumi, M.; Askari, G.; Bagherniya, M.; Majeed, M.; Guest, P.C.; Sahebkar, A. Antiviral Mechanisms of Curcumin and Its Derivatives in Prevention and Treatment of COVID-19: A Review. Adv. Exp. Med. Biol. 2023, 1412, 397–411. [Google Scholar] [PubMed]
- Imran, M.; Thabet, H.K.; Alaqel, S.I.; Alzahrani, A.R.; Abida, A.; Alshammari, M.K. The therapeutic and prophylactic potential of quercetin against COVID-19: An outlook on the clinical studies, inventive compositions, and patent literature. Antioxidants 2022, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, H.; Abdolmohammadi-Vahid, S.; Danshina, S.; Ziya Gencer, M.; Ammari, A.; Sadeghi, A. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharm. 2020, 89 Pt B, 107088. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega. 2023, 8, 10713–10746. [Google Scholar] [CrossRef] [PubMed]
- Ghahestani, S.M.; Shahab, E.; Karimi, S.; Madani, M.H. Methylene blue may have a role in the treatment of COVID-19. Med. Hypotheses. 2020, 144, 110163. [Google Scholar] [CrossRef] [PubMed]
- Top, W.M.; Gillman, P.K.; de Langen, C.J.; Kooy, A. Fatal methylene blue associated serotonin toxicity. Neth. J. Med. 2014, 72, 179–181. [Google Scholar] [PubMed]
- Clifton, J.; Leikin, J.B. Methylene blue. Am. J. Ther. 2003, 10, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Ginimuge, P.R.; Jyothi, S.D. Methylene blue: Revisited. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 517–520. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Cozzolino, M.; Delcanale, P.; Montali, C.; Tognolini, M.; Giorgio, C.; Corrado, M.; Cavanna, L.; Bianchini, P.; Diaspro, A.; Abbruzzetti, S.; et al. Enhanced photosensitizing properties of protein bound curcumin. Life Sci. 2019, 233, 116710. [Google Scholar] [CrossRef]
- Seong, D.Y.; Kim, Y.J. Enhanced photodynamic therapy efficacy of methylene blue-loaded calcium phosphate nanoparticles. J. Photochem. Photobiol. B. 2015, 146, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lin, M.; Huang, J.; Mo, M.; Liu, H.; Jiang, Y.; Cai, X.; Leung, W.; Xu, C. Smart Responsive Nanoformulation for Targeted Delivery of Active Compounds from Traditional Chinese Medicine. Front. Chem. 2020, 8, 559159. [Google Scholar] [CrossRef] [PubMed]
- Law, S.; Leung, A.W.; Xu, C. Could nanotechnology assist traditional Chinese medicine (TCM) in photodynamic therapy (PDT) against SARS-CoV-2? Photodiagnosis Photodyn. Ther. 2021, 36, 102543. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xu, H.; Kopelman, R.; Philbert, M.A. Photodynamic characterization and in vitro application of methylene blue-containing nanoparticle platforms. Photochem. Photobiol. 2005, 81, 242–249. [Google Scholar]
- Wang, S.; Ha, Y.; Huang, X.; Chin, B.; Sim, W.; Chen, R. A New Strategy for Intestinal Drug Delivery via pH-Responsive and Membrane-Active Nanogels. ACS Appl. Mater. Interfaces. 2018, 10, 36622–36627. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Edit. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
| Study | Usage of Light and Energy (J) | Experiment Parameters | Conclusion | References | |
|---|---|---|---|---|---|
| 1 | Antimicrobial photodynamic therapy with curcumin on methicillin-resistant Staphylococcus aureus biofilm | The strains are induced to form biofilm and incubated with curcumin for 20 min, irradiated with a Light-Emitting Diode at 450 nm with 50 J/cm2 for 455 s. | Colony-forming units, scanning electron microscopy, confocal microscopy images, and resazurin dye test. | PDT reduces the growth of the MRSA strain biofilm, making it a relevant alternative possibility for inactivation. | [55] |
| 2 | Antimicrobial photodynamic therapy (aPDT) with curcumin controls intradermal infection by Staphylococcus aureus in mice with type 1 diabetes mellitus: a pilot study | Curcumin is photoactivated ex vivo with LED light at 450 nm delivering a fluency of 13.5 J/cm3. | MRSA ATCC 43,300 strain infection study, immuno-assay in diabetes mellitus. | The therapeutic potential of PDT using curcumin when administered intradermally in the treatment of infections caused by S. aureus in mice with type 1 diabetes mellitus. | [56] |
| 3 | Photodynamic action of LED-activated curcumin against Staphylococcus aureus involving an intracellular ROS increase and membrane damage | S. aureus is incubated with different concentrations of curcumin for 60 min, then irradiated with blue light at 470 nm with a light dose of 3 J/cm2. | Colony-forming units, confocal microscopy images, flow cytometry, FCM analysis with DCFH-DA staining, transmission electron microscopy, and ROS assay. | Blue light-activated curcumin markedly damaged membrane permeability, resulting in cell death of S. aureus, and highlighted that an intracellular ROS increase might be an important event in the photodynamic killing of Staphylococcus aureus. | [57] |
| 4 | Bacterial viability after antimicrobial photodynamic therapy with curcumin on multi-resistant Staphylococcus aureus | Methicillin-resistant S. aureus strains and American-type culture collection (ATCC) of Staphylococcus aureus are evaluated in vitro, after incubation with curcumin for 20 min, and irradiated with LED light at 450 nm. | Colony-forming units, confocal microscopy images, and flow cytometry. | PDT with curcumin may be an interesting therapeutic alternative, because of its in vitro response, for the control of multi-resistant clinical S. aureus strains. | [58] |
| 5 | Antimicrobial photodynamic therapy using blue LED light for the activation of curcumin extracts (curcuma longa) on Staphylococcus aureus bacteria: an in vitro study | Blue LED light of 400–450 nm with an energy density of 16.19 J/cm2 is used to activate exogenous curcumin extracts in S. aureus in vitro. | Colony-forming units, confocal microscopy images, and flow cytometry. | LED irradiation of PDT can activate curcumin extracts to increase the percentage of S. aureus bacterial death (91.49 ± 0.01%) with curcumin extract and 44.88 ± 0.18% without curcumin extract. | [59] |
| 6 | Photodynamic therapy control of Staphylococcus aureus intradermal infection in mice | Blue LED light is associated with curcumin to treat S. aureus intradermal infection in mice at 450 nm with 75 mW/cm2, and 54 J/cm2 for 10 min. | Colony-forming units, confocal microscopy images, and flow cytometry. | PDT may be effective against MRSA infection in a murine model of intradermal infection. | [60] |
| 7 | Photokilling of bacteria via curcumin in different aqueous preparations: Studies on curcumin and curcuminoids XXXVII | The bacteria are exposed to 1 to 25 micron curcumin solubilized in DMSO, cyclodextrins, liposomes, and surfactants for 30 min, then are incubation irradiated with fluorescent tubes emitting blue light at 430 nm with 17 mW/cm2 and a radiant exposure of 0.5–30 J/cm2. | Colony forming units, fluorescence microscopy, and confocal microscopy images. | Aqueous preparations of DMSO, polyethylene glycol, and the copolymer poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) are the most efficient vehicles for curcumin to exert photo killing of Gram-positive and Gram-negative bacteria. | [61] |
| 8 | Potentiation of antimicrobial photodynamic therapy via curcumin-loaded graphene quantum dots | Blue LED light is associated with 100 µm of curcumin at 405 nm in the irradiance of 30 J cm−2 to NIH/3t3 fibroblasts. | Colony-forming units, cytotoxicity assay, and ROS assay. | Curcumin-loaded graphene quantum dots enhance antimicrobial photodynamic effects and can be used as an alternative effective treatment for resistant infections. | [62] |
| 9 | Formulation and characterization of lyophilized curcumin solid dispersions for antimicrobial photodynamic therapy (aPDT): Studies on curcumin and curcuminoids LII | Blue LED light is associated with 0.5 to 10 µM low concentrations of curcumin at 450 nm in the irradiance of 11–16 J/cm2 to E. faecalis and E. coli. | Colony-forming units, cytotoxicity assay, differential scanning calorimetry, photostability, and thermal stability tests. | The optimized curcumin formulations should be explored as an alternative to topical antibiotics in the treatment of wound infections, as it has very high phototoxicity towards both E. faecalis and E. coli, making these lyophilizes suitable for in vivo PDT. | [63] |
| 10 | Antimicrobial action of photodynamic therapy on Enterococcus faecalis biofilm using curing light, curcumin, and riboflavin | Blue LED is associated with 10 µL of curcumin at 460 nm in the irradiance of 60 J cm−2 on E. faecalis biofilm. | Colony-forming units, scanning electron microscopy. | Significantly reducing E. faecalis colony count, aPDT with curcumin and riboflavin can serve as an adjunct to routine root canal disinfection methods. | [64] |
| 11 | Effects of curcumin-mediated antimicrobial photodynamic therapy associated with different chelators against Enterococcus faecalis biofilms | Curcumin with different concentrations of 17 to 18% of EDTA, or HEBP irradiated with LED light in the irradiance of 75 J/cm2. | Colony-forming units, confocal scanning laser microscopy. | The combination of curcumin with EDTA and HEBP similarly improved the effect of aPDT on E. faecalis biofilms. | [65] |
| 12 | Curcumin-induced photodynamic therapy mediated the suppression of quorum-sensing pathways of Pseudomonas aeruginosa: An approach to inhibit biofilm in vitro | A total of 6.75 mM of curcumin at 405 nm is used in the irradiance of 10 J/cm2, which reduces P. aeruginosa biofilm more efficiently than without light, and extracellular polymeric substance production is also reduced by approximately 94% with 10 J/cm2 of light dose. | Crystal violet, XTT, Congo red binding assay, and confocal scanning laser microscopy. | Curcumin-mediated aPDT inhibits biofilm formation of P. aeruginosa through quorum-sensing pathways via the action of singlet oxygen generation, which in turn reduces the extracellular polymeric substances of the biofilm. | [66] |
| 13 | Enhancement of photodynamic bactericidal activity of curcumin against Pseudomonas aeruginosa using polymyxin B | P. aeruginosa is treated with curcumin in the presence of 0.1 to 0.5 mg/L polymyxin B and irradiated with blue LED light in the irradiance of 10 J/cm2. | Colony-forming units, scanning electron microscopy. | Introducing polymyxin B has the potential to enhance the effects of aPDT treatment against Gram-negative skin infections, in particular P. aeruginosa. | [67] |
| 14 | Evaluation of curcumin incubation time in Staphylococcus aureus and Pseudomonas aeruginosa photodynamic inactivation | Different concentrations of curcumin and P. aeruginosa are cultured for 24 h at 37 °C, then irradiated with blue LED light at 460 nm in the irradiance of 30 J/cm2. | Colony-forming units, scanning electron microscopy. | This treatment is reduced to approximately seven logs of 1 µM S. aureus after 30 min of incubation, whereas only two logs are observed for P. aeruginosa at a 50 µM maximum concentration. | [68] |
| 15 | Combination of curcumin photosensitizer with laser diode to reduce antibiotic-resistant bacterial biofilms | Addition of turmeric extract to the MRSA biofilm in 403 nm diode laser irradiation at 30 s, 60 s, 90 s, 120 s, and 150 s with an energy density of 13.56 J/cm2. | Biofilm development assay. | The addition of PS extracts for turmeric increases the effectiveness of photoinactivation of biofilm bacteria that are resistant to antibiotics. | [69] |
| Study | Usage of Light and Energy (J) | Experiment Parameters | Conclusion | References | |
|---|---|---|---|---|---|
| 1 | Photodynamic therapy using methylene blue, combined or not with gentamicin, against Staphylococcus aureus and Pseudomonas aeruginosa | Different concentrations of methylene blue (0.03–7000 μg/mL), with or without GN (1–20 μg/mL), are added to planktonic cultures or biofilms, and the samples are irradiated with a Light Emitting Diode lamp (625 nm, 7 mW/cm2, 18 J/cm2). | Biofilm development assay. | This combination cannot significantly alter the photo-inactivating effect of methylene blue against Staphylococcus aureus biofilms but exerts a positive bactericidal effect against Pseudomonas aeruginosa biofilms. | [70] |
| 2 | In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent | Irradiation of wounds with 360 J/cm2 of laser light (670 nm) in the presence of 100 µL/mL of methylene blue. | Histological evaluation, and wound temperature studies. | PDT is effective with a 25-fold reduction in the total number of viable epidemic methicillin-resistant Staphylococcus aureus in a wound. | [71] |
| 3 | Antimicrobial photoinactivation with methylene blue of Staphylococcus aureus | Methylene blue is activated with red light (660 nm) of a low energy density. | Colony-forming units. | A reduction in CFU/mL of Staphylococcus aureus to 60% was achieved. | [72] |
| 4 | Effect of photodynamic therapy on clinical isolates of Staphylococcus spp. | The Staphylococcus strain (106 cells/mL) is employed via PDT using 3 mM of methylene blue with a low-power 9.65 J/cm2 laser. | Colony-forming units. | PDT is effective in reducing the number of viable cells, such as Staphylococcus aureus, ranging from 4.89 to 6.83 CFU (log10)/mL. | [73] |
| 5 | In vitro antimicrobial photoinactivation with methylene blue in different microorganisms | A solution of 50 μM methylene blue with a wavelength of 660 nm, 100 mW, at an irradiation time of 3 min in an energy dose of 9 J for each sample. | Colony-forming units. | PDT is effective in reducing the number of viable cells, including 74.90% of Candida albicans, 72.41% of Pseudomonas aeruginosa, 96.44% of Enterococcus faecalis, and 95.42% of Staphylococcus aureus. | [74] |
| 6 | Effectiveness of antimicrobial photodynamic therapy using a 660 nm laser and methylene blue dye for inactivating Staphylococcus aureus biofilms in compact and cancellous bones: An in vitro study | A concentration of 0.1 mg/mL methylene blue with a wavelength of 660 nm, 40 mW, and energy doses of 3.6, 7.2, and 12 J for 90, 180, or 300 s. | Colony-forming units, and cytotoxicity assay. | Antimicrobial photodynamic therapy (aPDT) with methylene blue dye is effective in inactivating Staphylococcus aureus biofilms formed in compact and cancellous bone, which showed a significant reduction (log10 CFU/mL). | [75] |
| 7. | Photodynamic antimicrobial chemotherapy coupled with the use of the photosensitizers methylene blue and temoporfin as a potential novel treatment for Staphyloccocus aureus in burn infections | A concentration of 1 mg/mL methylene blue and 50 μM and 12.5 μM of temoporfin for Staphylococcus aureus with a wavelength of 640 nm for 20 min. | Colony-forming units. | Antimicrobial photodynamic therapy (aPDT) with methylene blue dye resulted in a loss of Staphylococcus aureus viability, with a two-log reduction in bacterial viability for both light and dark conditions after 20 min exposure time. Temoporfin has a greater antimicrobial efficacy against Staphylococcus aureus and Pseudomonas aeruginosa. | [76] |
| 8 | Methylene blue internalization and photodynamic action against clinical and ATCC Pseudomonas aeruginosa and Staphylococcus aureus strains | Methylene blue in concentrations of 100, 300, and 500 μg/mL with test strains is irradiated with an LED lamp (±660 nm) at a fluence of 10 and 25 J/cm2. | Colony-forming units, confocal microscopy, and DNA staining. | PDT with methylene blue can decrease the growth of the tested strains in vitro, which is significantly reduced (p ≤ 0.01) at an average of 5.0 logs. | [77] |
| 9 | Evaluation of photodynamic therapy using a diode laser and different photosensitizers against Enterococcus faecalis | Solutions containing E. faecalis are mixed with methylene blue and malachite green and are irradiated with a diode laser (660 nm) at a distance of 1 mm with 40 mW for 30, 60, or 120 s. | Colony-forming units. | PDT treatments using methylene blue and malachite green have an antibacterial effect against E. faecalis, showing potential to be used as an adjunctive antimicrobial procedure in endodontic therapy. | [78] |
| 10 | Comparing the efficacy of toluidine blue, methylene blue and curcumin in photodynamic therapy against Enterococcus faecalis | Methylene blue is injected into the root canals and remained there for 120 s before irradiation, which was carried out for 60 s with an LED lamp emitting light in the red spectrum with a power peak at 630 nm and output intensity of 2000–4000 mW/cm2. | Colony-forming units, and scanning electron microscopy. | The adjunction of methylene blue-mediated PDT via a light-emitting diode to NaOCl irrigation has antibacterial efficacy against E. faecalis. | [79] |
| Study | Usage of Light and Energy Usage (J) | Experiment Parameters | Conclusion | References | |
|---|---|---|---|---|---|
| 1 | Optimization of anti-SARS-CoV-2 treatments based on curcumin, used alone or employed as a photosensitizer | A total of 10 μM of curcumin is irradiated with laser light at 445 nm with 0.25 W/cm2, fluence 15 J/cm2, in a continuous wave and then transferred to A549 epithelial cells. | Colony-forming units, crystal violet staining. | aPDT presents a higher flexibility and a broad spectrum of activity against different viruses, which can be exploited for different purposes, ranging from blood product decontamination, and surface and liquid disinfection for applications to treat human infections. | [86] |
| 2 | Robust antimicrobial photodynamic therapy with curcumin-poly (lactic-co-glycolic acid) nanoparticles against COVID-19: A preliminary in vitro study with Vero cell lines as a model | Ten wt.% Cur@PLGA-NPs and a blue laser at an energy density of 522.8 J/cm2 to inactivate SARS-CoV-2 in plasma. | Scanning electron microscopy, transmission electron microscopy, Fourier transform infrared, coagulometer, Bradford method, and titration. | aPDT exhibits in vitro anti-COVID-19 activities in the treated plasma containing SARS-CoV-2 without Vero cell apoptosis and any adverse effects on plasma quality in aPDT-exposed plasma. | [87] |
| 3 | Spray-dried curcumin-loaded nanoparticles for antimicrobial photodynamic therapy | LED device for 10 min at 457 nm with a radiant exposure of 13.2 J/cm2. | Colony-forming units, transmission electron microscopy, confocal scanning laser microscopy, dispersibility, aerodynamic assays, and antibacterial photoactivity assay. | A curcumin-loaded nanoformulation was successfully sprayed in the lower respiratory tract, which is used to oppose severe bacterial infections, reduce antimicrobial resistance, and facilitate the treatment of chronic lung disease associated with a high risk of infection. | [88] |
| 4 | Could curcumin hydrogel for photodynamic therapy fight against SARS-CoV-2? | Curcumin hydrogel exposure to blue light with a mean wavelength of 450 nm using a microcatheter into the lung to combat SARS-CoV-2. | Colony-forming units, scanning electron microscopy. | Curcumin incorporated with the hydrogel is a suitable candidate used for the photodynamic treatment of SARS-CoV-2, cancer, wound healing, and bacterial infection. | [89] |
| Study | Usage of Light and Energy (J) | Experiment Parameters | Conclusion | References | |
|---|---|---|---|---|---|
| 1 | A novel approach of combining methylene blue photodynamic inactivation, photobiomodulation, and orally ingested methylene blue in COVID-19 management: A pilot clinical study with 12-month follow-up | The mucosal surfaces were irradiated with methylene blue at 660 nm LED light in a continuous emission mode at an energy density of 49 J/cm2 for photodynamic therapy. | Cycle threshold tests, and polymerase chain reaction (PCR) tests. | Methylene blue has a broad spectrum of activity, which addresses the prevailing and future COVID-19 variants and other infections transmitted via the upper respiratory tract. | [44] |
| 2 | Photodynamic disinfection of SARS-CoV-2 clinical samples using a methylene blue formulation | Concentrations of 15 μM of methylene blue and 4 to 8 min of illumination (~860 mW) at 670 nm to inactivate SARS-CoV-2. | Real-time reverse-transcription quantitative polymerase chain reaction (RT-qPCR). | Pre-treatment of lentiviral vectors with MB-PDT prevented infection to reduce viral loads in the nasal cavity and oropharynx in the early stages of COVID-19, which may be employed to curb the transmission and severity of the disease. | [90] |
| 3 | Evaluation of methylene blue-based photodynamic inactivation (PDI) against intracellular B-CoV and SARS-CoV-2 viruses under different light sources in vitro as a basis for new local treatment strategies in the early phase of a COVID-19 infection | Both 590 nm white broadband LED light and 810 nm coherent laser light at 300 mW/cm2 in conjugation with a low concentration of methylene blue (31 µm and 31, 1 µm) were used to inactivate intracellular B-CoV and SARS-CoV-2. | Cytotoxicity assay, viral titration, and plaque assay. | A minimum concentration of 0.0001% MB and a minimum radiation intensity of 20,000 lx leads to a 99.99% reduction in intracellular and extracellular viruses after one minute of exposure. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Law, S.K.; Leung, A.W.N.; Xu, C. Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review. Pharmaceuticals 2024, 17, 34. https://doi.org/10.3390/ph17010034
Law SK, Leung AWN, Xu C. Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review. Pharmaceuticals. 2024; 17(1):34. https://doi.org/10.3390/ph17010034
Chicago/Turabian StyleLaw, Siu Kan, Albert Wing Nang Leung, and Chuanshan Xu. 2024. "Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review" Pharmaceuticals 17, no. 1: 34. https://doi.org/10.3390/ph17010034
APA StyleLaw, S. K., Leung, A. W. N., & Xu, C. (2024). Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review. Pharmaceuticals, 17(1), 34. https://doi.org/10.3390/ph17010034