An In Silico Investigation of the Molecular Interactions between Volatile Anesthetics and Actin
Abstract
:1. Introduction
2. Results
2.1. Actin
- The first subdomain (SD1) is the N-terminal domain of β-actin. It may be involved in protein interactions and actin filament assembly regulation. This region contains residues 4–39.
- The second subdomain (SD2), the central one, is involved in interactions with actin-binding proteins (ABPs) and other actin monomers to produce filaments. This core region includes residues 40–164.
- The third subdomain (SD3), the C-terminal domain, contains the amino acids found at the C-terminus of β-actin. This domain may interact with other proteins or molecules, regulate actin activities, and take part in many cell signaling cascades. This region comprises residues 165–374.
- Actin polymerization aids in the internalization of membrane vesicles, which helps regulate the composition of the cell membrane and the cell’s interaction with the environment. According to some research, volatile anesthetics can alter cytoplasmic actin polymerization through a variety of methods, including actin filament stability, the suppression of actin polymerization, and the regulation of actin filament activity. A broad variety of actin-binding proteins tightly regulate the polymerization and depolymerization of actin filaments, allowing for the dynamic remodeling of the cytoskeleton in response to cellular demands. Actin has been linked to a variety of pathological illnesses, making it an appealing therapeutic target.
2.2. Anesthetics
2.3. Data Analysis
- SITE 1: 53%;
- SITE 3: 100%;
- SITE 5: 77%;
- SITE 14: 75%;
- SITE 15: 100%.
3. Discussion
4. Material and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anesthesia. Available online: https://www.nigms.nih.gov/education/fact-sheets/Pages/anesthesia.aspx (accessed on 20 October 2023).
- Franks, N.P. Molecular Targets Underlying General Anaesthesia. Br. J. Pharmacol. 2006, 147, S72–S81. [Google Scholar] [CrossRef] [PubMed]
- Borghese, C.M. The Molecular Pharmacology of Volatile Anesthetics. Int. Anesthesiol. Clin. 2015, 53, 28–39. [Google Scholar] [CrossRef] [PubMed]
- General Anaesthesia. Available online: https://www.nhs.uk/conditions/general-anaesthesia/ (accessed on 20 October 2023).
- Craddock, T.J.A.; Kurian, P.; Preto, J.; Sahu, K.; Hameroff, S.R.; Klobukowski, M.; Tuszynski, J.A. Anesthetic Alterations of Collective Terahertz Oscillations in Tubulin Correlate with Clinical Potency: Implications for Anesthetic Action and Post-Operative Cognitive Dysfunction. Sci. Rep. 2017, 7, 9877. [Google Scholar] [CrossRef] [PubMed]
- Craddock, T.J.A.; George, M.S.; Freedman, H.; Barakat, K.H.; Damaraju, S.; Hameroff, S.; Tuszynski, J.A. Computational Predictions of Volatile Anesthetic Interactions with the Microtubule Cytoskeleton: Implications for Side Effects of General Anesthesia. PLoS ONE 2012, 7, e37251. [Google Scholar] [CrossRef]
- Zizzi, E.A.; Cavaglià, M.; Tuszynski, J.A.; Deriu, M.A. Insights into the Interaction Dynamics between Volatile Anesthetics and Tubulin through Computational Molecular Modelling. J. Biomol. Struct. Dyn. 2022, 40, 7324–7338. [Google Scholar] [CrossRef] [PubMed]
- Filippakopoulos, P.; Picaud, S.; Fedorov, O.; Keller, M.; Wrobel, M.; Morgenstern, O.; Bracher, F.; Knapp, S. Benzodiazepines and Benzotriazepines as Protein Interaction Inhibitors Targeting Bromodomains of the BET Family. Bioorganic Med. Chem. 2012, 20, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tsien, J.Z. Memory and the NMDA Receptors. N. Engl. J. Med. 2009, 361, 302–303. [Google Scholar] [CrossRef]
- Eckenhoff, R.G.; Maze, M.; Xie, Z.; Culley, D.J.; Goodlin, S.J.; Zuo, Z.; Wei, H.; Whittington, R.A.; Terrando, N.; Orser, B.A.; et al. Perioperative Neurocognitive Disorder: State of the Preclinical Science. Anesthesiology 2020, 132, 55–68. [Google Scholar] [CrossRef]
- Eckenhoff, R.G.; Johansson, J.S.; Wei, H.; Carnini, A.; Kang, B.; Wei, W.; Pidikiti, R.; Keller, J.M.; Eckenhoff, M.F. Inhaled Anesthetic Enhancement of Amyloid-β Oligomerization and Cytotoxicity. Anesthesiology 2004, 101, 703–709. [Google Scholar] [CrossRef]
- Colon, E.; Bittner, E.A.; Kussman, B.; McCann, M.E.; Soriano, S.; Borsook, D. Anesthesia, Brain Changes, and Behavior: Insights from Neural Systems Biology. Prog. Neurobiol. 2017, 153, 121–160. [Google Scholar] [CrossRef]
- Yan, Z.; Kim, E.; Datta, D.; Lewis, D.A.; Soderling, S.H. Synaptic Actin Dysregulation, a Convergent Mechanism of Mental Disorders? In Proceedings of the Journal of Neuroscience, San Diego, CA, USA, 9 November 2016; Society for Neuroscience: Washington, DC, USA, 2016; Volume 36, pp. 11411–11417. [Google Scholar] [CrossRef]
- Rae, I.D. Theory versus Practice in the Twentieth-Century Search for the Ideal Anaesthetic Gas. Ambix 2016, 63, 46–65. [Google Scholar] [CrossRef]
- Granak, S.; Hoschl, C.; Ovsepian, S.V. Dendritic Spine Remodeling and Plasticity under General Anesthesia. Brain Struct. Funct. 2021, 226, 2001–2017. [Google Scholar] [CrossRef]
- Matus, A.; Brinkhaus, H.; Wagner, U. Actin Dynamics in Dendritic Spines: A Form of Regulated Plasticity at Excitatory Synapses. Hippocampus 2000, 10, 555–560. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of Cholesterol and Sphingolipids in Brain Development and Neurological Diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef]
- Egawa, J.; Pearn, M.L.; Lemkuil, B.P.; Patel, P.M.; Head, B.P. Membrane Lipid Rafts and Neurobiology: Age-Related Changes in Membrane Lipids and Loss of Neuronal Function. J. Physiol. 2016, 594, 4565–4579. [Google Scholar] [CrossRef]
- Franks, N.P.; Lieb, W.R. The Structure of Lipid Bilayers and the Effects of General Anaesthetics an X-ray and Neutron Diffraction Study. J. Mol. Biol. 1979, 133, 469–500. [Google Scholar] [CrossRef]
- Kita, Y.; Bennett, L.J.; Miller, K.W. The Partial Molar Volumes of Anesthetics in Lipid Bilayers. Biochim. Biophys. Acta 1981, 647, 130–139. [Google Scholar] [CrossRef]
- Cantor, R.S. Breaking the Meyer-Overton Rule: Predicted Effects of Varying Stiffness and Interfacial Activity on the Intrinsic Potency of Anesthetics. Biophys. J. 2001, 80, 2284–2297. [Google Scholar] [CrossRef]
- Zizzi, E.A.; Cavaglià, M.; Tuszynski, J.A.; Deriu, M.A. Alteration of Lipid Bilayer Mechanics by Volatile Anesthetics: Insights from Μs-Long Molecular Dynamics Simulations. iScience 2022, 25, 103946. [Google Scholar] [CrossRef]
- Rao, B.D.; Sarkar, P.; Chattopadhyay, A. Effect of Tertiary Amine Local Anesthetics on G Protein-Coupled Receptor Lateral Diffusion and Actin Cytoskeletal Reorganization. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183547. [Google Scholar] [CrossRef]
- Weizenmann, N.; Huster, D.; Scheidt, H.A. Interaction of Local Anesthetics with Lipid Bilayers Investigated by 1H MAS NMR Spectroscopy. Biochim. Biophys. Acta BBA-Biomembr. 2012, 1818, 3010–3018. [Google Scholar] [CrossRef]
- Butterworth, J.F.; Strichartz, G.R. Molecular Mechanisms of Local Anesthesia. Anesthesiology 1990, 72, 711–734. [Google Scholar] [CrossRef]
- Johansson, J.S.; Eckenhoff, R.G. Minimum Structural Requirement for an Inhalational Anesthetic Binding Site on a Protein Target. Biochim. Biophys. Acta 1996, 1290, 63–68. [Google Scholar] [CrossRef]
- Eckenhoff, R.G.; Johansson, J.S. Molecular Interactions between Inhaled Anesthetics and Proteins. Pharmacol. Rev. 1997, 49, 343–367. [Google Scholar]
- Eckenhoff, R.G.; Shuman, H. Halothane Binding to Soluble Proteins Determined by Photoaffinity Labeling. Anesthesiology 1993, 79, 96–106. [Google Scholar] [CrossRef]
- Dubois, B.W.; Cherian, S.F.; Evers, A.S. Volatile Anesthetics Compete for Common Binding Sites on Bovine Serum Albumin: A 19F-NMR Study. Proc. Natl. Acad. Sci. USA 1993, 90, 6478–6482. [Google Scholar] [CrossRef]
- Johansson, J.S.; Eckenhoff, R.G.; Dutton, P.L. Binding of Halothane to Serum Albumin Demonstrated Using Tryptophan Fluorescence. Anesthesiology 1995, 83, 316–324. [Google Scholar] [CrossRef]
- Johansson, J.S.; Manderson, G.A.; Ramoni, R.; Grolli, S.; Eckenhoff, R.G. Binding of the Volatile General Anesthetics Halothane and Isoflurane to a Mammalian β-Barrel Protein. FEBS J. 2005, 272, 573–581. [Google Scholar] [CrossRef]
- Wague, A.; Joseph, T.T.; Woll, K.A.; Bu, W.; Vaidya, K.A.; Bhanu, N.V.; Garcia, B.A.; Nimigean, C.M.; Eckenhoff, R.G.; Riegelhaupt, P.M. Mechanistic Insights into Volatile Anesthetic Modulation of K2p Channels. Elife 2020, 9, e59839. [Google Scholar] [CrossRef]
- Vemparala, S.; Domen, C.; Klein, M.L. Computational Studies on the Interactions of Inhalational Anesthetics with Proteins. Acc. Chem. Res. 2010, 43, 103–110. [Google Scholar] [CrossRef]
- Woll, K.A.; Peng, W.; Liang, Q.; Zhi, L.; Jacobs, J.A.; Maciunas, L.; Bhanu, N.; Garcia, B.A.; Covarrubias, M.; Loll, P.J.; et al. Photoaffinity Ligand for the Inhalational Anesthetic Sevoflurane Allows Mechanistic Insight into Potassium Channel Modulation. ACS Chem. Biol. 2017, 12, 1353–1362. [Google Scholar] [CrossRef]
- Kinde, M.N.; Bondarenko, V.; Granata, D.; Bu, W.; Grasty, K.C.; Loll, P.J.; Carnevale, V.; Klein, M.L.; Eckenhoff, R.G.; Tang, P.; et al. Fluorine-19 NMR and Computational Quantification of Isoflurane Binding to the Voltage-Gated Sodium Channel NaChBac. Proc. Natl. Acad. Sci. USA 2016, 113, 13762–13767. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, E.; Wells, M.M.; Bondarenko, V.; Woll, K.; Carnevale, V.; Granata, D.; Klein, M.L.; Eckenhoff, R.G.; Dailey, W.P.; et al. Propofol Inhibits the Voltage-Gated Sodium Channel NaChBac at Multiple Sites. J. Gen. Physiol. 2018, 150, 1317–1332. [Google Scholar] [CrossRef]
- Woll, K.A.; Zhou, X.; Bhanu, N.V.; Garcia, B.A.; Covarrubias, M.; Miller, K.W.; Eckenhoff, R.G. Identification of Binding Sites Contributing to Volatile Anesthetic Effects on GABA Type A Receptors. FASEB J. 2018, 32, 4172–4189. [Google Scholar] [CrossRef]
- Oscarsson, A.; Juhas, M.; Sjölander, A.; Eintrei, C. The Effect of Propofol on Actin, ERK-1/2 and GABAA Receptor Content in Neurones. Acta Anaesthesiol. Scand. 2007, 51, 1184–1189. [Google Scholar] [CrossRef]
- Coyle, J.E.; Nikolov, D.B. GABARAP: Lessons for Synaptogenesis. Neuroscientist 2003, 9, 205–216. [Google Scholar] [CrossRef]
- Pimm, M.L.; Henty-Ridilla, J.L. New Twists in Actin-Microtubule Interactions. Mol. Biol. Cell 2021, 32, 211–217. [Google Scholar] [CrossRef]
- Platholi, J.; Herold, K.F.; Hemmings, H.C.; Halpain, S. Isoflurane Reversibly Destabilizes Hippocampal Dendritic Spines by an Actin-Dependent Mechanism. PLoS ONE 2014, 9, e102978. [Google Scholar] [CrossRef]
- Kaech, S.; Brinkhaus, H.; Matus, A. Volatile Anesthetics Block Actin-Based Motility in Dendritic Spines. Proc. Natl. Acad. Sci. USA 1999, 96, 10433–10437. [Google Scholar] [CrossRef]
- Hinkley, R.E.; Telser, A.G. The Effects of Halothane on Cultured Mouse Neuroblastoma Cells. I. Inhibition of Morphological Differentiation. J. Cell Biol. 1974, 63, 531–540. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin Structure and Function. Annu Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef]
- Dugina, V.B.; Shagieva, G.S.; Kopnin, P.B. Biological Role of Actin Isoforms in Mammalian Cells. Biochemistry 2019, 84, 583–592. [Google Scholar] [CrossRef]
- Dugina, V.; Zwaenepoel, I.; Gabbiani, G.; Clément, S.; Chaponnier, C. β- and γ-Cytoplasmic Actins Display Distinct Distribution and Functional Diversity. J. Cell Sci. 2009, 122, 2980–2988. [Google Scholar] [CrossRef]
- Gupton, S.L.; Gertler, F.B. Filopodia: The Fingers That Do the Walking. Sci. STKE 2007, 2007, re5. [Google Scholar] [CrossRef]
- Gehler, S.; Shaw, A.E.; Sarmiere, P.D.; Bamburg, J.R.; Letourneau, P.C. Brain-Derived Neurotrophic Factor Regulation of Retinal Growth Cone Filopodial Dynamics Is Mediated through Actin Depolymerizing Factor/Cofilin. J. Neurosci. 2004, 24, 10741–10749. [Google Scholar] [CrossRef]
- Paunola, E.; Mattila, P.K.; Lappalainen, P. WH2 Domain: A Small, Versatile Adapter for Actin Monomers. FEBS Lett. 2002, 513, 92–97. [Google Scholar] [CrossRef]
- Slupe, A.M.; Kirsch, J.R. Effects of Anesthesia on Cerebral Blood Flow, Metabolism, and Neuroprotection. J. Cereb. Blood Flow Metab. 2018, 38, 2192–2208. [Google Scholar] [CrossRef]
- Pinho, J.; Marcut, C.; Fonseca, R. Actin Remodeling, the Synaptic Tag and the Maintenance of Synaptic Plasticity. IUBMB Life 2020, 72, 577–589. [Google Scholar] [CrossRef]
- Janoff, A.S.; Pringle, M.J.; Miller, K.W. Correlation of General Anesthetic Potency with Solubility in Membranes. Biochim. Biophys. Acta BBA-Biomembr. 1981, 649, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Joksovic, P.M.; Todorovic, S.M. Isoflurane Modulates Neuronal Excitability of the Nucleus Reticularis Thalami in Vitro. In Proceedings of the Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2010; Volume 1199, pp. 36–42. [Google Scholar]
- Mapelli, J.; Gandolfi, D.; Giuliani, E.; Casali, S.; Congi, L.; Barbieri, A.; D’Angelo, E.; Bigiani, A. The Effects of the General Anesthetic Sevoflurane on Neurotransmission: An Experimental and Computational Study. Sci. Rep. 2021, 11, 4335. [Google Scholar] [CrossRef]
- Lee, S. Can Desflurane Be an Alternative to Sevoflurane in Neuroanesthesia? Korean J. Anesthesiol. 2019, 72, 207–208. [Google Scholar] [CrossRef]
- Kapoor, M.C.; Vakamudi, M. Desflurane-Revisited. J. Anaesthesiol. Clin. Pharmacol. 2012, 28, 92–100. [Google Scholar] [CrossRef]
- Azad, S.S.; Bartkowski, R.R.; Witkowski, T.A.; Marr, A.T.; Lessin, J.B.; Seltzer, J.L. A Comparison of Desflurane and Isoflurane in Prolonged Surgery. J. Clin. Anesth. 1992, 5, 122–128. [Google Scholar] [CrossRef]
- Gupta, A.; Stierer, T.; Zuckerman, R.; Sakima, N.; Parker, S.D.; Fleisher, L.A. Comparison of Recovery Profile after Ambulatory Anesthesia with Propofol, Isoflurane, Sevoflurane and Desflurane: A Systematic Review. Anesth. Analg. 2004, 98, 632–641. [Google Scholar] [CrossRef]
- Kalenderova, S.; Ohridski, K. Changes in A 549 Cells Morphology in Response to the Toxic Effect of Halothane. Acta Morphol. Et Anthropol. 2005, 10, 126–130. [Google Scholar]
- Liu, R.; Loll, P.J.; Eckenhoff, R.G. Structural Basis for High-affinity Volatile Anesthetic Binding in a Natural 4-helix Bundle Protein. FASEB J. 2005, 19, 567–576. [Google Scholar] [CrossRef]
- Janus, C.; Pearson, J.; McLaurin, J.; Mathews, P.M.; Jiang, Y.; Schmidt, S.D.; Chishti, M.A.; Horne, P.; Heslin, D.; French, J.; et al. Aβ Peptide Immunization Reduces Behavioural Impairment and Plaques in a Model of Alzheimer’s Disease. Nature 2000, 408, 979–982. [Google Scholar] [CrossRef]
- Franks, N.P.; Lieb, W.R. Molecular and Cellular Mechanisms of General Anaesthesia. Nature 1994, 367, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Rebecchi, M.J.; Pentyala, S.N. Anaesthetic Actions on Other Targetes: Protein Kinase C and Guanine Nucleotide-Binding Proteins. Br. J. Anaesth. 2002, 89, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal Chemistry and the Molecular Operating Environment (MOE): Application of QSAR and Molecular Docking to Drug Discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- Arora, A.S.; Huang, H.L.; Singh, R.; Narui, Y.; Suchenko, A.; Hatano, T.; Heissler, S.M.; Balasubramanian, M.K.; Chinthalapudi, K. Structural Insights into Actin Isoforms. Elife 2023, 12, e82015. [Google Scholar] [CrossRef]
- Pliska, V.; Fauchère, J.-L. Hydrophobic Parameters II of Amino Acid Side-Chains from the Partitioning of N-Acetyl-Amino Acid Amides. Eur. J. Med. Chem. 1983, 18, 369–375. [Google Scholar]
- Jayaraj, V.; Suhanya, R.; Vijayasarathy, M.; Anandagopu, P.; Rajasekaran, E. Role of Large Hydrophobic Residues in Proteins. Bioinformation 2009, 3, 409–412. [Google Scholar] [CrossRef]
- Reynolds, M.J.; Hachicho, C.; Carl, A.G.; Gong, R.; Alushin, G.M. Bending Forces and Nucleotide State Jointly Regulate F-Actin Structure. Nature 2022, 611, 380–386. [Google Scholar] [CrossRef]
- Wetlaufer, D.B.; Lovrien, R. Induction of Reversible Structural Changes in Proteins By Nonpolar. J. Biol. Chem. 1964, 239, 596–603. [Google Scholar] [CrossRef]
- Hawkins, M.; Pope, B.; Maciver, S.K.; Weeds, A.G. Human Actin Depolymerizing Factor Mediates a PH-Sensitive Destruction of Actin Filaments. Biochemistry 1993, 32, 9985–9993. [Google Scholar] [CrossRef]
- Kurdi, M.; Patel, T. The Role of Melatonin in Anaesthesia and Critical Care. Indian J. Anaesth. 2013, 57, 137–144. [Google Scholar] [CrossRef]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Aversa, S.; Arrigo, T.; Reiter, R.; Gitto, E. Analgesic, Anxiolytic and Anaesthetic Effects of Melatonin: New Potential Uses in Pediatrics. Int. J. Mol. Sci. 2015, 16, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Mowafi, H.; Ismail, S. The Uses of Melatonin in Anesthesia and Surgery. Saudi J. Med. Med. Sci. 2014, 2, 134. [Google Scholar] [CrossRef]
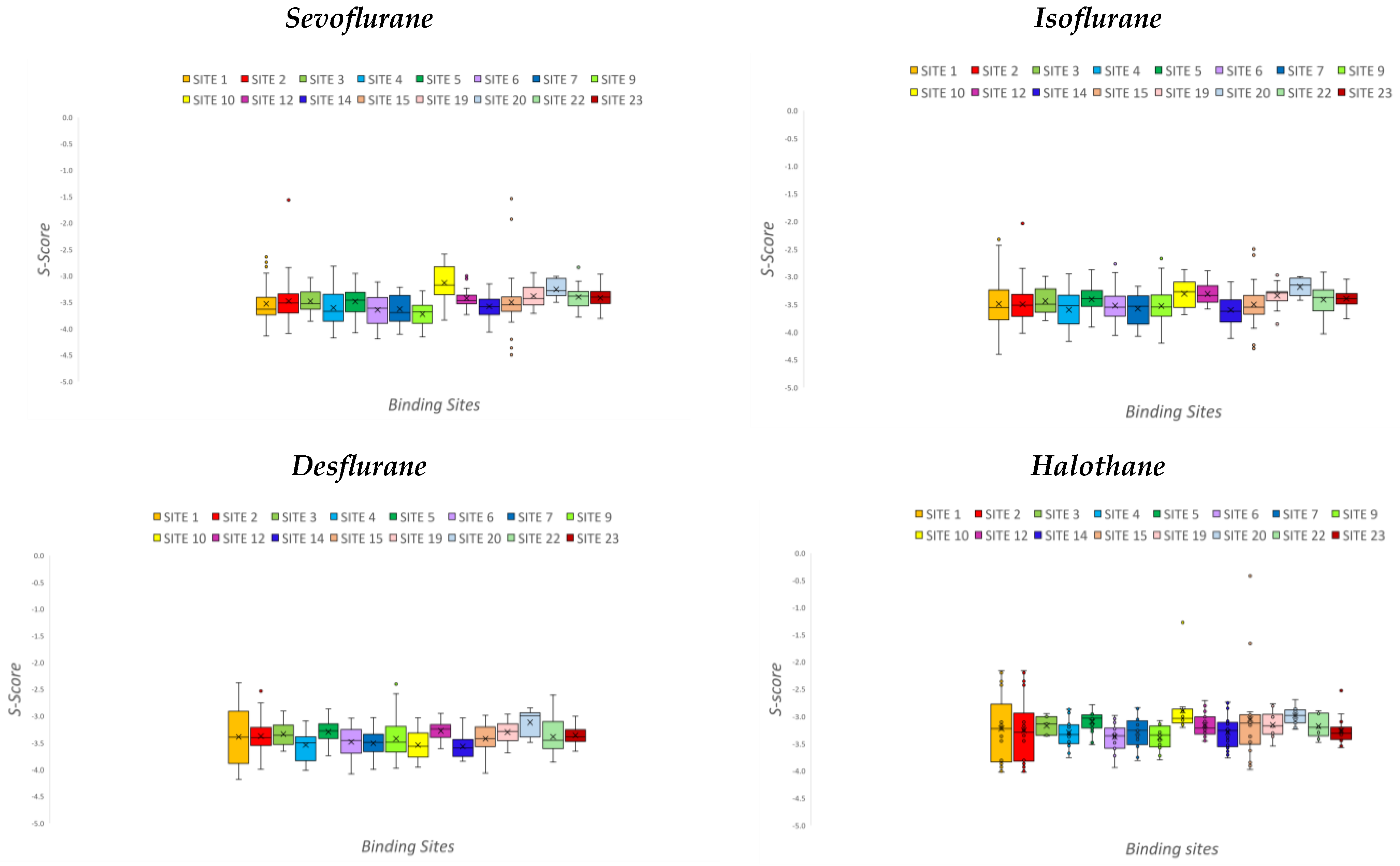





| Anesthetic | Binding Affinity (kcal/mol) |
|---|---|
| Desflurane | −3.39 ± 0.12 |
| Halothane | −3.19 ± 0.14 |
| Isoflurane | −3.44 ± 0.12 |
| Sevoflurane | −3.48 ± 0.15 |
| Anesthetic | RMSD Value |
|---|---|
| Desflurane | 159.53 ± 13.10 |
| Halothane | 160.59 ± 12.68 |
| Isoflurane | 124.30 ± 12.29 |
| Sevoflurane | 160.62 ± 13.03 |
| Site | PLB | Hydrophobic Residues | Number of Hydrophobic Residues |
|---|---|---|---|
| 1 | 4.55 | VAL9 MET15 ILE70 VAL75 MET81 LEU104 MET118 VAL158 VAL338 | 9 |
| 2 | 0.25 | PRO108 LEU109 VAL133 ILE135 PRO171 ILE174 VAL369 PHE374 | 8 |
| 3 | 0.21 | VAL53 | 1 |
| 4 | 0.12 | MET0 ILE4 PHE20 LEU348 | 4 |
| 5 | 0.11 | PRO37 VAL42 MET43 MET46 ILE63 | 5 |
| 6 | 0.05 | VAL133 ILE135 VAL138 LEU139 ILE164 LEU345 PHE351 MET354 PHE374 | 9 |
| 7 | 0.03 | ILE340 ILE344 | 2 |
| 8 | −0.02 | MET189 LEU192 PHE199 | 3 |
| 9 | −0.03 | MET15 LEU215 MET304 PRO306 | 4 |
| 10 | −0.18 | VAL297 PRO331 ILE340 | 3 |
| 11 | −0.19 | ILE70 | 1 |
| 12 | −0.29 | PHE199 ILE207 LEU241 PRO242 | 4 |
| 13 | −0.29 | LEU66 PRO69 | 2 |
| 14 | −0.35 | VAL138 LEU141 ILE164 | 3 |
| 15 | −0.37 | PRO108 LEU109 PRO111 ILE135 | 4 |
| 16 | −0.38 | LEU235 | 1 |
| 17 | −0.39 | - | 0 |
| 18 | −0.39 | LEU220 PHE222 | 2 |
| 19 | −0.41 | LEU175 LEU177 | 2 |
| 20 | −0.41 | PRO26 ILE340 | 2 |
| 21 | −0.42 | ILE63 LEU64 | 2 |
| 22 | −0.56 | VAL286 ILE288 MET324 | 3 |
| 23 | −0.64 | ILE4 PRO101 PRO129 | 3 |
| Code | Method | Resolution | Upload Date | Sequence Length | Missing Residues | Modified Residues | Related Study |
|---|---|---|---|---|---|---|---|
| 6ICT | X-RAY DIFFRACTION | 1.95 Å | FEB 2019 | 23—Chain E, G, H, I (66–88) | 66, 86–88 | 73—HIC | Structure of SETD3 bound to SAH and methylated actin |
| 7W28 | X-RAY DIFFRACTION | 1.79 Å | OCT 2022 | 16—Chain P (66–81) | - | 73—N9P | Crystal Structure of SETD3-SAH in complex with betaA-4PyrAla73 peptide |
| 6OX3 | X-RAY DIFFRACTION | 1.78 Å | AUG 2019 | 19—Chain E, G, H, I (66–84) | - | - | SETD3 in Complex with an Actin Peptide with His73 Replaced with Lysine |
| 6ICV | X-RAY DIFFRACTION | 2.15 Å | FEB 2019 | 23—Chain C, D (66–88) | 66, 84–88 | - | Structure of SETD3 bound to SAH and unmodified actin |
| 6OX0 | X-RAY DIFFRACTION | 1.76 Å | AUG 2019 | 15—Chain Y, Z (66–80) | - | - | SETD3 in Complex with an Actin Peptide with Sinefungin Replacing SAH as Cofactor |
| 6OX2 | X-RAY DIFFRACTION | 2.09 Å | AUG 2019 | 15—Chain Y, Z (66–80) | - | 73—HIC | SETD3 in Complex with an Actin Peptide with the Target Histidine Fully Methylated |
| 6OX1 | X-RAY DIFFRACTION | 1.95 Å | AUG 2019 | 15—Chain Y, Z (66–80) | - | 73—HIC | SETD3 in Complex with an Actin Peptide with Target Histidine Partially Methylated |
| 6OX5 | X-RAY DIFFRACTION | 2.1 Å | AUG 2019 | 18—Chain Y | - | - | SETD3 (N255A) mutant complexed with an actin peptide with His73 replaced by lysine |
| 6MBK | X-RAY DIFFRACTION | 1.69 Å | DEC 2018 | 15—Chains Y, Z | - | - | SETD3, a Histidine Methyltransferase, in Complex with an Actin Peptide and SAH, First P212121 Crystal Form |
| 6MBJ | X-RAY DIFFRACTION | 1.78 Å | DEC 2018 | 15—Chains Y, Z | - | - | SETD3, a Histidine Methyltransferase, in Complex with an Actin Peptide and SAH, P21 Crystal Form |
| 6MBL | X-RAY DIFFRACTION | 2.2 Å | DEC 2018 | 15—Chains Y, Z | - | - | SETD3, a Histidine Methyltransferase, in Complex with an Actin Peptide and SAH, Second P212121 Crystal Form |
| 6OX4 | X-RAY DIFFRACTION | 2.29 Å | AUG 2019 | 15—Chains Y, Z | - | - | SETD3 (N255A) mutant in complex with an actin peptide |
| 7W29 | X-RAY DIFFRACTION | 2.9 Å | OCT 2022 | 16—Chain P | - | 73—ORN | SETD3-SAH crystal structure in complex with the peptide betaA-Orn73 |
| 3D2U | X-RAY DIFFRACTION | 2.21 Å | JUL 2008 | 9—Chains C, G | - | - | Structure of UL18, a Peptide-Binding Viral MHC Mimic, Bound to a Host Inhibitory Receptor |
| 6NBW | X-RAY DIFFRACTION | 2.5 Å | GEN 2020 | 374—Chain A | 41–47 | 73—HIC | Ternary complex of beta/gamma actin with profilin and AnCoA-NAA80 |
| 8DNH | E-MICROSCOPY | 2.99 Å | APRIL 2023 | 375—Chain A, B, C, D | - | 73—HIC | Non muscle beta actin |
| 6LTJ | E-MICROSCOPY | 3.7 Å | FEB 2020 | 375—Chain K | 1, 13–16, 93–96, 373–375 | - | Nucleosome-bound human BAF complex |
| 7VDV | E-MICROSCOPY | 3.4 Å | MAY 2022 | 375—Chain P | 1, 13–16, 33–78, 93–96, 373–375 | - | Human chromatin remodeling PBAF-nucleosome complex |
| 7AS4 | E-MICROSCOPY | 4.13 Å | GEN 2021 | 374—Chain G | 39–48 | - | Recombinant human gTuRC |
| 7P1H | E-MICROSCOPY | 3.9 Å | NOV 2021 | 372—Chain B | 38–44 | 70—HIC | Exo-Y-G-actin -profilin complex |
| 6ANU | E-MICROSCOPY | 7 Å | NOV 2017 | 375—Chain A, B, C, D, E, F | - | - | F-actin complexed with beta-III-spectrin-ABD |
| 7QJ6 | E-MICROSCOPY | 7.8 Å | JAN 2022 | 374- Chain A | 1, 40–49 | - | Structure of recombinant human gamma-Tubulin Ring Complex 10-spoked assembly intermediate |
| 7QJ9 | E-MICROSCOPY | 8.1 Å | JAN 2022 | 372—Chain E | 1, 40–49 | - | Structure of recombinant human gamma-Tubulin Ring Complex 10-spoked assembly intermediate |
| 3J82 | E-MICROSCOPY | 7.7 Å | MAY 2015 | 374—Chain B, C, D | - | 72- HIC | C-type lectin domain family 9 member A complexed with F-actin |
| 3BYH | E-MICROSCOPY | 12 Å | FEB 2008 | 374—Chain A | - | - | Actin-fimbrin ABD2 complex |
| 3LUE | E-MICROSCOPY | 15 Å | APR 2010 | 374- Chains A, B, C, D, E, F, G, H, I, J | - | - | Alpha-actinin CH1 model bound to F-actin |
| Code | Method | Resolution | Upload Date | Sequence Length | Missing Residues | Modified Residues | Related Study |
|---|---|---|---|---|---|---|---|
| 6V63 | X-RAY DIFFRACTION | 2.02 Å | JAN 2020 | 23—Chain Y, Z (66–88) | 85–88 | - | SETD3 WT in Complex with an Actin Peptide with His73 Replaced with Glutamine |
| 6WK1 | X-RAY DIFFRACTION | 1.89 Å | JUN 2020 | 23—Chain Y, Z (66–88) | 85–88 | - | SETD3 in Complex with an Actin Peptide with His73 Replaced with Methionine |
| 6WK2 | X-RAY DIFFRACTION | 1.76 Å | JUN 2020 | 23—Chain C, Y (66–88) | 85–88 | - | SETD3 mutant (N255V) in Complex with an Actin Peptide with His73 Replaced with Methionine |
| 6V62 | X-RAY DIFFRACTION | 2.36 Å | JAN 2020 | 23—Chain Y (66–88) | 84–88 | - | SETD3 double mutant (N255F/W273A) in Complex with an Actin Peptide with His73 Replaced with Lysine |
| 7NVM | E-MICROSCOPY | 3.1 Å | MAR 2022 | 375—Chain K | 1–5, 35–49, 193–200, 231–260 | - | Human TRiC complex in closed state with nanobody Nb18, actin and PhLP2A bound |
| 8DNF | E-MICROSCOPY | 3.38 Å | APR 2023 | 375—Chain A, B, C, D | - | 73—HIC | Cryo-EM structure of nonmuscle gamma-actin |
| 5JLH | E-MICROSCOPY | 3.9 Å | JUN 2016 | 374—Chain A, B, C, D, E | - | - | Cryo-EM structure of a human cytoplasmic actomyosin complex at near-atomic resolution |
| 6G2T | E-MICROSCOPY | 9 Å | OTT 2018 | 375—Chain A, B, C, D, E, F | 1–5 | - | Human cardiac myosin binding protein C C1 Ig-domain bound to native cardiac thin filament |
| 6CXJ | E-MICROSCOPY | 11 Å | OTT 2018 | 375—Chain A, B, C, D, E | 1–5 | - | Cardiac thin filament decorated with C0C1 fragment of cardiac myosin binding protein C mode 2 |
| 6CXI | E- MICROSCOPY | 11 Å | OTT 2018 | 375—Chain A, B, C, D, E | 1–5 | - | Cardiac thin filament decorated with C0C1 fragment of cardiac myosin binding protein C mode 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truglia, B.; Carbone, N.; Ghadre, I.; Vallero, S.; Zito, M.; Zizzi, E.A.; Deriu, M.A.; Tuszynski, J.A. An In Silico Investigation of the Molecular Interactions between Volatile Anesthetics and Actin. Pharmaceuticals 2024, 17, 37. https://doi.org/10.3390/ph17010037
Truglia B, Carbone N, Ghadre I, Vallero S, Zito M, Zizzi EA, Deriu MA, Tuszynski JA. An In Silico Investigation of the Molecular Interactions between Volatile Anesthetics and Actin. Pharmaceuticals. 2024; 17(1):37. https://doi.org/10.3390/ph17010037
Chicago/Turabian StyleTruglia, Barbara, Nicola Carbone, Ibrahim Ghadre, Sara Vallero, Marinella Zito, Eric Adriano Zizzi, Marco Agostino Deriu, and J. A. Tuszynski. 2024. "An In Silico Investigation of the Molecular Interactions between Volatile Anesthetics and Actin" Pharmaceuticals 17, no. 1: 37. https://doi.org/10.3390/ph17010037





