Assessment of Some Unsymmetrical Porphyrins as Promising Molecules for Photodynamic Therapy of Cutaneous Disorders
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photophysical Characterization of the Investigated Porphyrins
2.1.1. Absorption Spectra
2.1.2. Emission Spectra
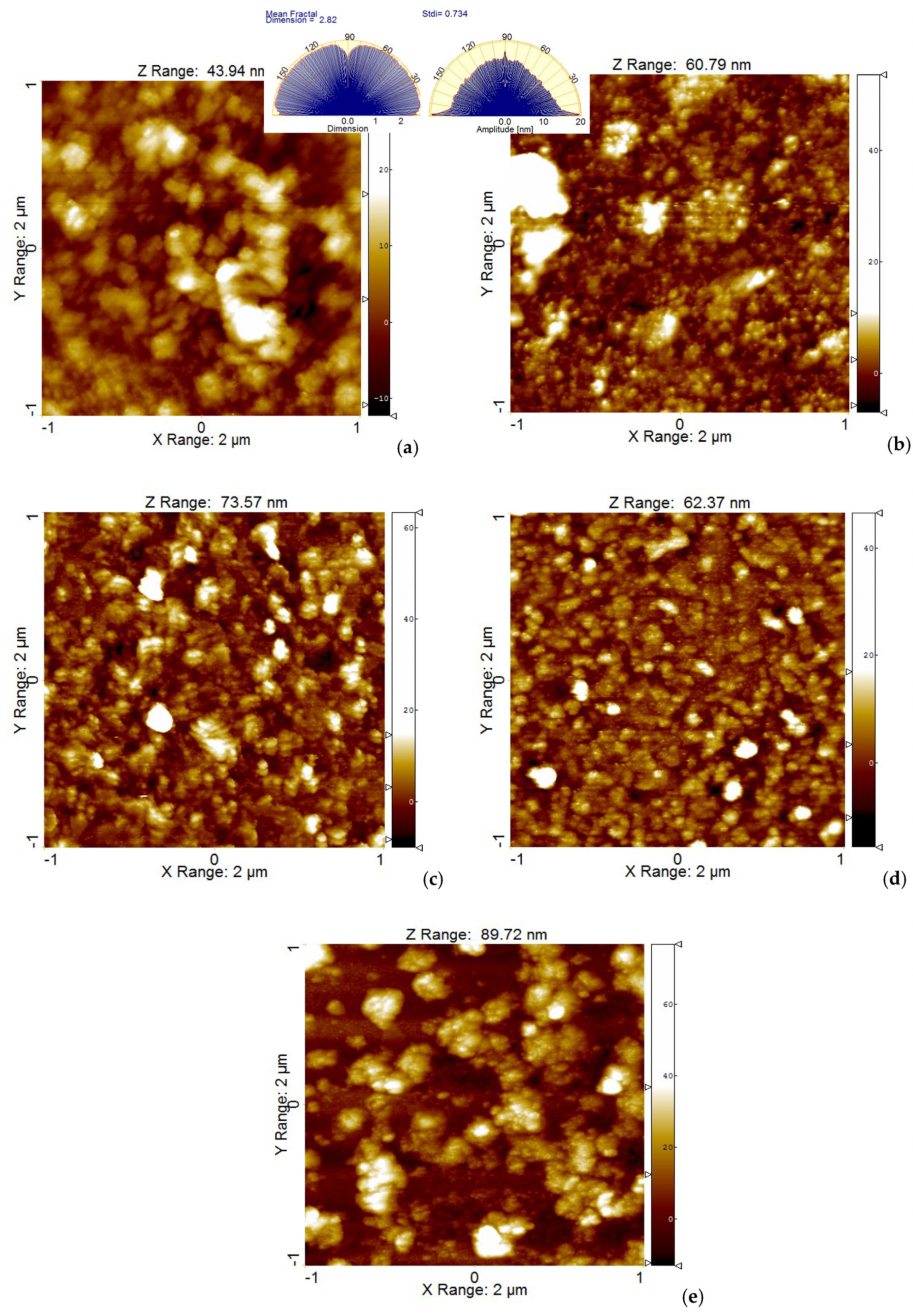
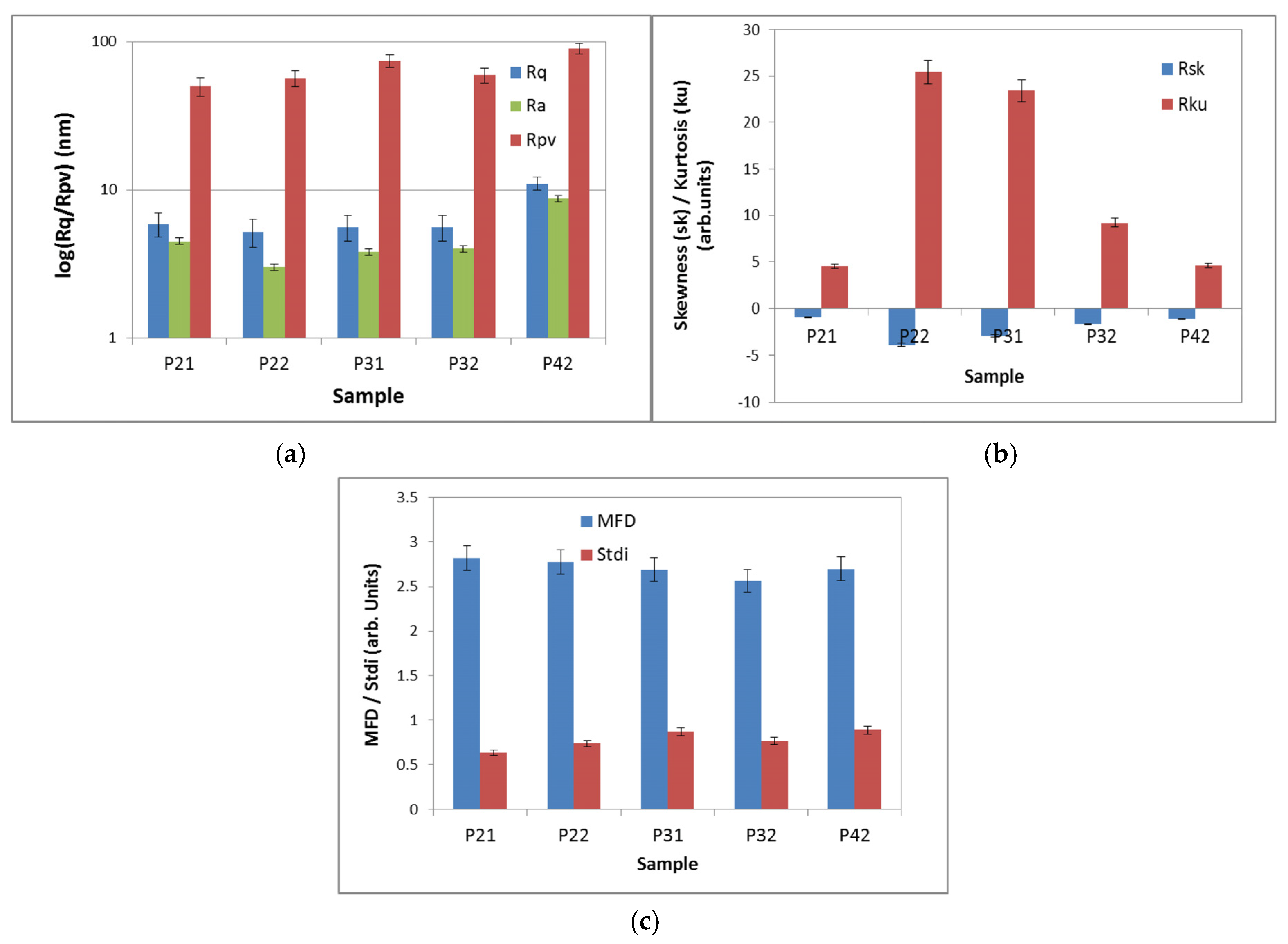
2.2. Aggregation Study on Porphyrinic Structures
2.3. In Silico Approaches
2.3.1. Prediction of the Diffusion of Porphyrin Structures through the Cell Membrane
2.3.2. Prediction of ADMET Properties
2.4. Cellular Uptake of the Investigated Porphyrins
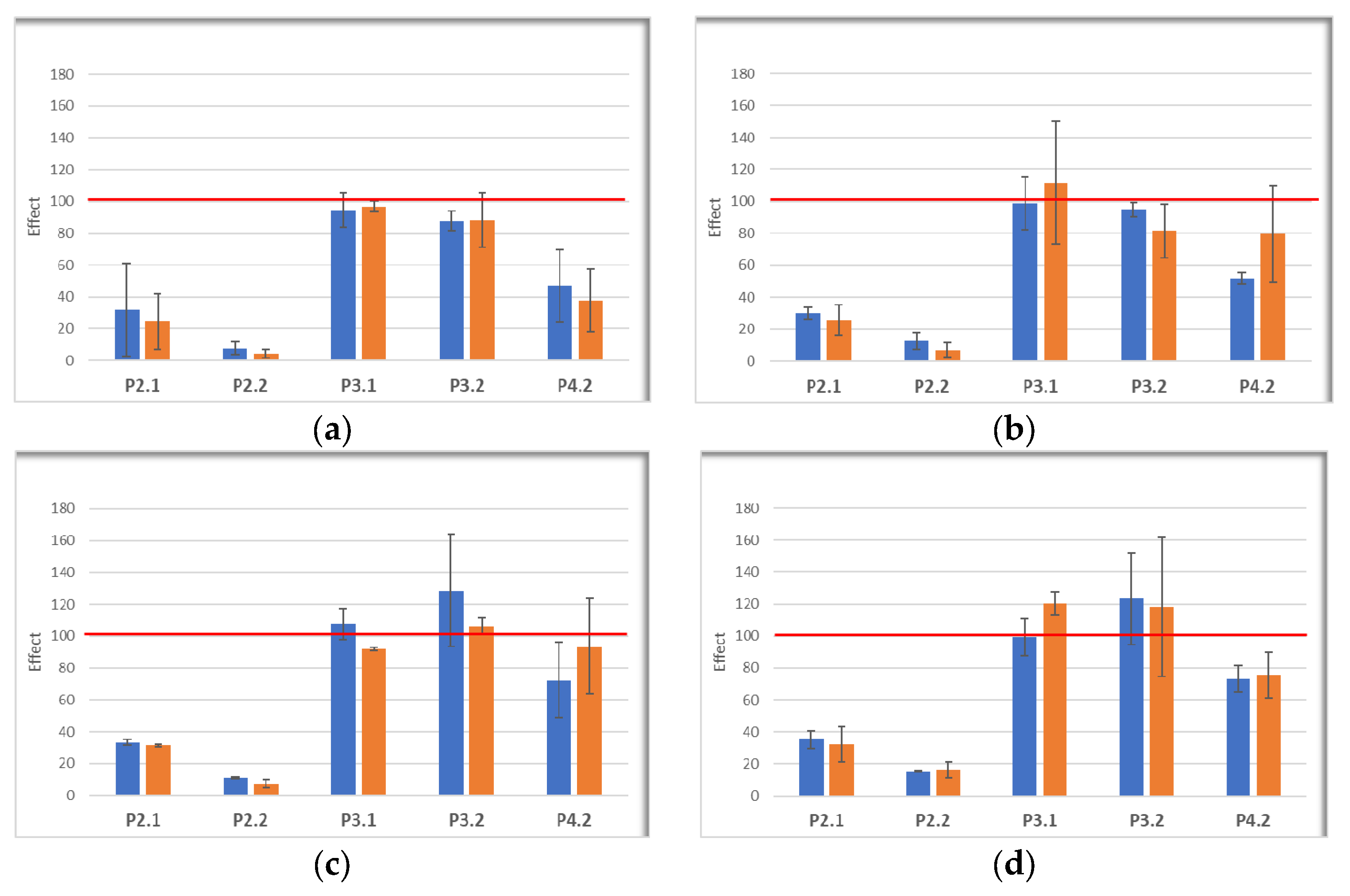
2.5. In Vitro Effects of Photodynamic Therapy on Investigated Porphyrins
3. Materials and Methods
3.1. General Information Materials
3.2. Photophysical Characterization of Porphyrins
3.3. Evaluation of the Aggregation Statuss of Porphyrins
3.4. Computational Studies
3.4.1. Permeability across Cell Membrane Prediction
3.4.2. ADMET Profile Prediction
3.5. Cellular Studies
3.5.1. Cells
3.5.2. Loading of Cells with Porphyrins
3.5.3. Evaluation of Porphyrin Uptake by Cells
3.5.4. In Vitro PDT
3.5.5. Post-PDT Cellular Investigations
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Recent advances in photosensitizers as multifunctional theranostic agents for imaging-guided photodynamic therapy of cancer. Theranostics 2021, 11, 9054–9088. [Google Scholar] [CrossRef]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef]
- Plekhova, N.; Shevchenko, O.; Korshunova, O.; Stepanyugina, A.; Tananaev, I.; Apanasevich, V. Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering 2022, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R.; Radulea, N.; Manda, G.; Machado, I.F.; Socoteanu, R.P.; Lupuliasa, D.; Burloiu, A.M.; Mihai, D.P.; Ferreira, L.F.V. Porphyrin Macrocycles: General Properties and Theranostic Potential. Molecules 2023, 28, 1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, C.; Longo, J.P.F.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef]
- Oyim, J.; Omolo, C.A.; Amuhaya, E.K. Photodynamic Antimicrobial Chemotherapy: Advancements in Porphyrin-Based Photosensitize Development. Front. Chem. 2021, 9, 635344. [Google Scholar] [CrossRef]
- De Annunzio, S.R.; Costa, N.C.S.; Mezzina, R.D.; Graminha, M.A.S.; Fontana, C.R. Chlorin, Phthalocyanine, and Porphyrin Types Derivatives in Phototreatment of Cutaneous Manifestations: A Review. Int. J. Mol. Sci. 2019, 20, 3861. [Google Scholar] [CrossRef]
- Manda, G.; Hinescu, M.E.; Neagoe, I.V.; Ferreira, L.F.V.; Boscencu, R.; Vasos, P.; Basaga, S.H.; Cuadrado, A. Emerging Therapeutic Targets in Oncologic Photodynamic Therapy. Curr. Pharm. Des. 2019, 24, 5268–5295. [Google Scholar] [CrossRef]
- Farberg, A.S.; Marson, J.W.; Soleymani, T. Advances in Photodynamic Therapy for the Treatment of Actinic Keratosis and Nonmelanoma Skin Cancer: A Narrative Review. Dermatol. Ther. 2023, 13, 689–716. [Google Scholar] [CrossRef] [PubMed]
- Del Regno, L.; Catapano, S.; Di Stefani, A.; Cappilli, S.; Peris, K. A Review of Existing Therapies for Actinic Keratosis: Current Status and Future Directions. Am. J. Clin. Dermatol. 2022, 23, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Nesi-Reis, V.; Lera-Nonose, D.S.S.L.; Oyama, J.; Silva-Lalucci, M.P.P.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Contribution of photodynamic therapy in wound healing: A systematic review. Photodiagnosis Photodyn. Ther. 2018, 21, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Slomp, A.M.; Barreira, S.M.W.; Carrenho, L.Z.B.; Vandresen, C.C.; Zattoni, I.F.; Ló, S.S.; Dallagnol, J.C.C.; Ducatti, D.R.B.; Orsato, A.; Duarte, M.E.R.; et al. Photodynamic effect of meso-(aryl) porphyrins and meso-(1-methyl-4-pyridinium) porphyrins on HaCaT keratinocytes. Bioorganic Med. Chem. Lett. 2017, 27, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Carrenho, L.Z.; Moreira, C.G.; Vandresen, C.C.; Gomes Junior, R.; Gonçalves, A.G.; Barreira, S.M.; Noseda, M.D.; Duarte, M.E.; Ducatti, D.R.; Dietrich, M.; et al. Investigation of anti-inflammatory and anti-proliferative activities promoted by photoactivated cationic porphyrin. Photodiagnosis Photodyn. Ther. 2015, 12, 444–458. [Google Scholar] [CrossRef]
- Sandland, J.; Malatesti, N.; Boyle, R. Porphyrins and related macrocycles: Combining photosensitization with radio- or optical-imaging for next generation theranostic agents. Photodiagnosis Photodyn. Ther. 2018, 23, 281–294. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Pandey, R.K.; Zheng, G. Porphyrins as photosensitizers in photodynamic therapy. In The Porphyrin Handbook; Kadish, K.M., Guilard, R., Smith, K.M., Eds.; Academic Press: New York, NY, USA, 2000; Volume 6, pp. 157–230. [Google Scholar]
- Simpson, M.C.; Novikova, I.N. Porphyrins: Electronic structure and ultraviolet/visible absorption spectroscopy. In Fundamentals of Porphyrin Chemistry: A 21st Century Approach; Brothers, P.J., Senge, O.M., Eds.; John Wiley & Sons Ltd.: New Jersey, NJ, USA, 2022; Volume 1, pp. 505–586. [Google Scholar]
- Boyle, R.B.; Dolphin, D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 1996, 64, 469–485. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Monro, S.; Colón, L.K.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.; Lilge, L.; Cameron, G.C.; McFarland, A.S. Ttransition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J.; Fukami, S.; Ichikawa, M.; Mohamed, A.; Kohno, M. Intraoperative photodiagnosis for malignant glioma using photosensitizer talaporfin sodium. Front. Surg. 2019, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R.; Manda, G.; Socoteanu, R.P.; Hinescu, M.E.; Radulea, N.; Neagoe, I.V.; Vieira Ferreira, L.F. Tetrapyrrolic Compound with Theranostic Applications and Obtaining Process. Patent No. 131946, 29 March 2019. [Google Scholar]
- Boscencu, R.; Socoteanu, R.P.; Manda, G.; Radulea, N.; Anastasescu, M.; Gama, A.; Ferreira Machado, I.; Vieira Ferreira, L.F. New A3B porphyrins as potential candidates for theranostic. Synthesis and photochemical behaviour. Dyes Pigments 2019, 160, 410–417. [Google Scholar] [CrossRef]
- Boscencu, R.; Manda, G.; Radulea, N.; Socoteanu, R.P.; Ceafalan, L.C.; Neagoe, I.V.; Ferreira Machado, I.; Basaga, S.H.; Vieira Ferreira, L.F. Studies on the synthesis, photophysical and biological evaluation of some unsymmetrical meso-tetrasubstituted phenyl porphyrins. Molecules 2017, 22, 1815. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R.; Manda, G.; Olariu, L.; Neagoe, I.V.; Socoteanu, R.P.; Hinescu, M.E.; Vieira Ferreira, L.F.; Cuadrado, A.; Basaga, H. Tetrapyrrolic Derivative for Antitumor Photodynamic Therapy and Obtaining Process. Patent Application 201900799, 30 September 2020. [Google Scholar]
- Boscencu, R.; Manda, G.; Socoteanu, R.P.; Hinescu, M.E.; Neagoe, I.V.; Olariu, L.; Dumitriu, B. Porphyrin Derivative for Theranostic Use. Patent No. 132752 B1, 29 November 2023. [Google Scholar]
- Socoteanu, R.; Manda, G.; Boscencu, R.; Vasiliu, G.; Oliveira, A.S. Synthesis, Spectral Analysis and Preliminary In Vitro Evaluation of Some Tetrapyrrolic Complexes with 3d Metal Ions. Molecules 2015, 20, 15488–15499. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.V.; Ferreira, D.P.; Oliveira, A.S.; Boscencu, R.; Socoteanu, R.; Ilie, M.; Constantin, C.; Neagu, M. Synthesis, photophysical and cytotoxicity evaluation of A3B type mesoporphyrinic compounds. Dyes Pigments 2012, 95, 296–303. [Google Scholar] [CrossRef]
- Boscencu, R. Microwave Synthesis under Solvent-Free Conditions and Spectral Studies of Some Mesoporphyrinic Complexes. Molecules 2012, 17, 5592–5603. [Google Scholar] [CrossRef]
- Wiehe, A.; Senge, M.O. The Photosensitizer Temoporfin (mTHPC)—Chemical, Pre-clinical and Clinical Developments in the Last Decade. Photochem. Photobiol. 2023, 99, 356–419. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of Porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Gouterman, M. Optical Spectra and Electronic Structure of Porphyrins and Related Rings. In The Porphyrins; Dolphin, D., Ed.; Academic Press: New York, NY, USA, 1978; Volume 3, pp. 11–87. [Google Scholar]
- Gouterman, M.; Wagniere, G.H.; Snyder, L.C. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar] [CrossRef]
- Nifiatis, F.; Su, W.; Haley, J.E.; Slagle, J.E.; Cooper, T.M. Comparison of the photophysical properties of a planar, PtOEP, and a nonplanar, PtOETPP, porphyrin in solution and doped films. J. Phys. Chem. A 2011, 115, 13764–13772. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Nicholls, I.A.; Karlsson, B.C.G.; Rosengren, A.M.; Ballabio, D.; Consonni, V.; Todeschini, R. Towards global QSAR model building for acute toxicity: Munro database case study. Int. J. Mol. Sci. 2014, 15, 18162–18174. [Google Scholar] [CrossRef] [PubMed]
- Dobre, M.; Boscencu, R.; Neagoe, I.V.; Surcel, M.; Milanesi, E.; Manda, G. Insight into the web of stress responses triggered at gene expression level by porphyrin-PDT in HT29 human colon carcinoma cells. Pharmaceutics 2021, 13, 1032. [Google Scholar] [CrossRef]
- Chan, F.K.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [PubMed]
- Mushtaq, A.; Zhang, H.; Cui, M.Y.; Lin, X.; Huang, S.; Tang, Z.; Hou, Y.; Iqbal, M.Z.; Kong, X. ROS-responsive chlorin e6 and silk fibroin loaded ultrathin magnetic hydroxyapatite nanorods for T1-magnetic resonance imaging guided photodynamic therapy in vitro. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130513. [Google Scholar] [CrossRef]
- Mushtaq, A.; Ma, X.; Farheen, J.; Lin, X.; Tayyab, M.; Iqbal, M.Z.; Kong, X. Facile synthesis of metformin loaded Mn3O4-HAp magnetic hydroxyapatite nanocomposites for T1-magnetic resonance imaging guided targeted chemo-phototherapy in vitro. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131911. [Google Scholar] [CrossRef]
- Zhou, T.; Xiao, X.; Li, G.; Cai, Z.W. Study of polyethylene glycol as a green solvent in the microwave-assisted extraction of flavone and coumarin compounds from medicinal plants. J. Chromatogr. A 2011, 1218, 3608–3615. [Google Scholar] [CrossRef]
- SPIP™ User’s and Reference Guide; Horsholm, Denmark. 2007. Available online: http://www.imagemet.com/WebHelp6/Default.htm (accessed on 28 September 2023).
- Lomize, A.L.; Hage, J.M.; Schnitzer, K.; Golobokov, K.; LaFaive, M.B.; Forsyth, A.C.; Pogozheva, I.D. PerMM: A Web Tool and Database for Analysis of Passive Membrane Permeability and Translocation Pathways of Bioactive Molecules. J. Chem. Inf. Model. 2019, 59, 3094–3099. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. AdmetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
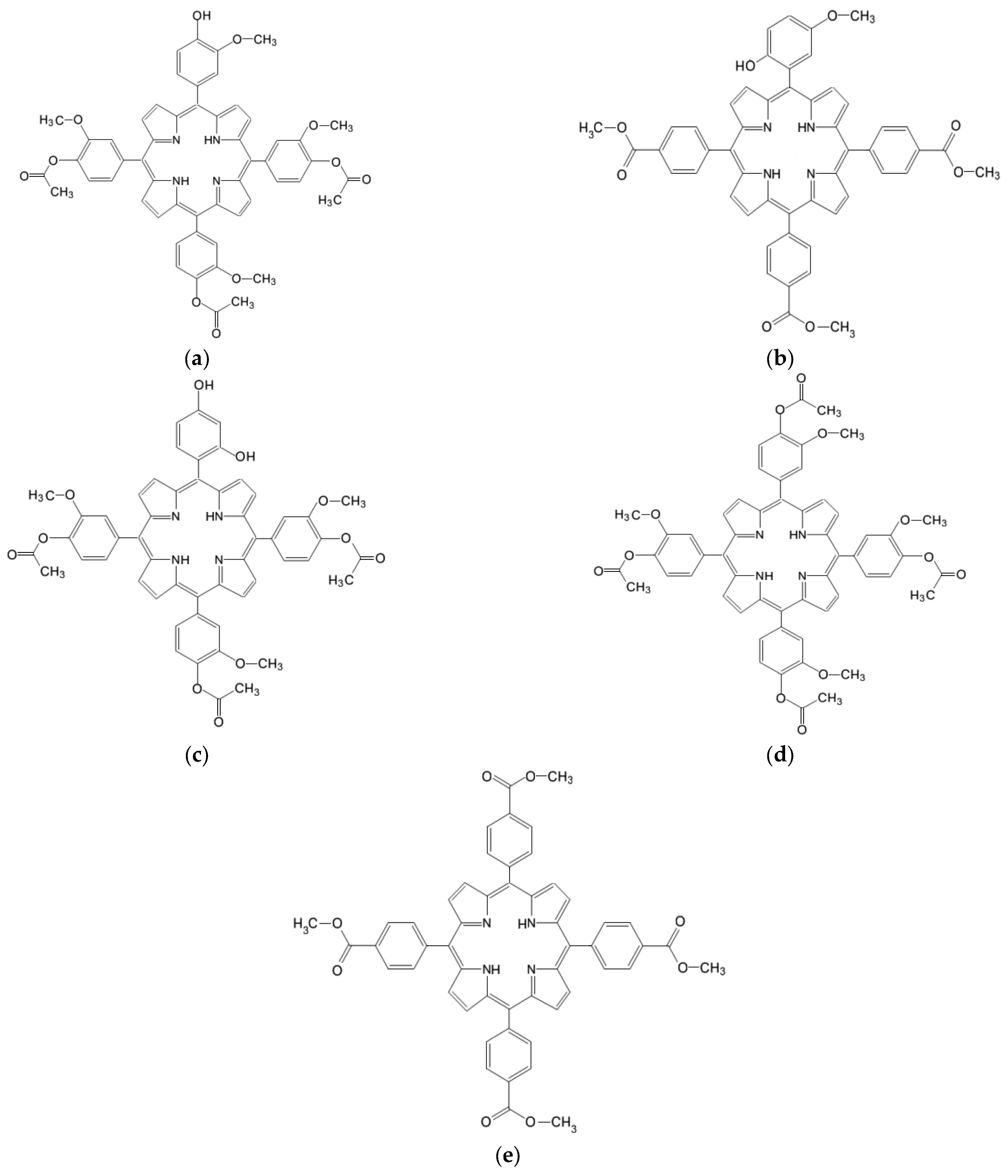








| Solvent | Absorption λmax (nm) [lg ε] (L mol−1 cm−1) | Emission λmax(nm) [F.I.] (a.u.) | ||||
|---|---|---|---|---|---|---|
| Soret Band | Qy(1,0) | Qy(0,0) | Qx(1,0) | Qx(0,0) | ||
| 5,10,15,20-tetrakis-(4-acetoxy-3-methoxyphenyl) porphyrin | ||||||
| PEG 200 | 402 [5.326] | 495 [4.097] | 531 [3.851] | 569 [3.552] | 626 [3.501] | 652 [22.560] |
| PEG 200/PBS | 405 [5.189] | 499 [4.330] | 534 [4.086] | 571 [3.900] | 628 [3.786] | 654 [21.920] |
| 5-(4-hydroxy-3-methoxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl) porphyrin | ||||||
| PEG 200 | 405 [5.383] | 498 [4.136] | 535 [3.947] | 572 [3.556] | 629 [3.585] | 650 [18.395] |
| PEG 200/PBS | 404 [5.332] | 498 [4.247] | 535 [4.094] | 571 [3.902] | 621 [3.890] | 656 [9.523] |
| 5,10,15,20-tetrakis-(4-carboxymethylphenyl) porphyrin | ||||||
| PEG 200 | 400 [5.360] | 494 [4.071] | 527 [3.736] | 568 [3.448] | 625 [3.235] | 650 [21.076] |
| PEG 200/PBS | 406 [5.062] | 498 [4.205] | 531 [3.930] | 571 [3.755] | 628 [3.552] | 656 [19.804] |
| 5-(2-hydroxy-3-methoxyphenyl)-10,15,20-tris-(4-carboxymethylphenyl) porphyrin | ||||||
| PEG 200 | 400 [5.161] | 495 [3.942] | 526 [3.613] | 568 [3.310] | 626 [3.101] | 656 [13.850] |
| PEG 200/PBS | 405 [4.813] | 499 [3.983] | 530 [3.683] | 571 [3.476] | 629 [3.253] | 657 [5.206] |
| 5-(2,4-dihydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl) porphyrin | ||||||
| PEG 200 | 402 [5.269] | 496 [3.990] | 532 [3.710] | 570 [3.322] | 626 [3.085] | 652 [17.291] |
| PEG 200/PBS | 407 [5.010] | 498 [4.138] | 533 [3.885] | 571 [3.670] | 627 [3.467] | 655 [9.472] |
| Compound | ΔG (kcal/mol) | logPerm |
|---|---|---|
| 2.1 | −6.29 | 0.65 |
| P2.2 | −7.15 | 1.28 |
| P3.1 | −5.86 | −1.50 |
| P3.2 | −5.49 | −3.08 |
| P4.2 | −6.41 | 0.62 |
| Parameter | P2.1 | P2.2 | P3.1 | P3.2 | P4.2 |
|---|---|---|---|---|---|
| Intestinal absorption | yes | yes | yes | yes | yes |
| Oral bioavailability | low | low | low | high | low |
| P-glycoprotein substrate | inc. | inc. | inc. | no | yes |
| P-glycoprotein inhibitor | yes | yes | yes | yes | yes |
| OATP subtypes inhibition | yes | yes | yes | yes | yes |
| Skin permeability (log Kp, cm/h) | −2.735 | −2.735 | −2.735 | −2.735 | −2.735 |
| Subcellular localization | mitochondria | mitochondria | mitochondria | mitochondria | mitochondria |
| CYP isoforms inhibitor | inc. | inc. | inc. | inc. | inc. |
| Plasma protein binding | 0.8118 | 0.9041 | 0.8745 | 0.9889 | 0.8754 |
| VDss (L/kg) | 0.4446 | 0.4217 | 0.5420 | 0.4742 | 0.4305 |
| BBB permeability | yes | no | yes | no | no |
| Total clearance (mL/min/kg) | 4.656 | 4.285 | 8.185 | 7.396 | 4.581 |
| OCT1/2 substrate/inhibitor | no | no | no | no | no |
| Parameter | P2.1 | P2.2 | P3.1 | P3.2 | P4.2 |
|---|---|---|---|---|---|
| Rat LD50 (mg/kg) | 3066 | 3066 | 3066 | 3066 | 3066 |
| Human max. tolerated dose (mg/kg/day) | 2.735 | 2.735 | 2.742 | 2.742 | 2.742 |
| Skin sensitization | no | no | no | no | no |
| Hepatotoxicity | inc. | inc. | inc. | inc. | inc. |
| Nephrotoxicity | yes | yes | yes | yes | yes |
| Reproductive toxicity | yes | yes | yes | yes | yes |
| Immunotoxicity | yes | yes | no | yes | yes |
| Carcinogenicity | inc. | inc. | no | inc. | no |
| Mutagenicity | yes | no | no | no | no |
| Cytotoxicity | no | no | no | no | no |
| Mitochondrial toxicity | yes | yes | no | yes | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burloiu, A.M.; Manda, G.; Lupuliasa, D.; Socoteanu, R.P.; Mihai, D.P.; Neagoe, I.V.; Anghelache, L.-I.; Surcel, M.; Anastasescu, M.; Olariu, L.; et al. Assessment of Some Unsymmetrical Porphyrins as Promising Molecules for Photodynamic Therapy of Cutaneous Disorders. Pharmaceuticals 2024, 17, 62. https://doi.org/10.3390/ph17010062
Burloiu AM, Manda G, Lupuliasa D, Socoteanu RP, Mihai DP, Neagoe IV, Anghelache L-I, Surcel M, Anastasescu M, Olariu L, et al. Assessment of Some Unsymmetrical Porphyrins as Promising Molecules for Photodynamic Therapy of Cutaneous Disorders. Pharmaceuticals. 2024; 17(1):62. https://doi.org/10.3390/ph17010062
Chicago/Turabian StyleBurloiu, Andreea Mihaela, Gina Manda, Dumitru Lupuliasa, Radu Petre Socoteanu, Dragos Paul Mihai, Ionela Victoria Neagoe, Laurentiu-Iliuta Anghelache, Mihaela Surcel, Mihai Anastasescu, Laura Olariu, and et al. 2024. "Assessment of Some Unsymmetrical Porphyrins as Promising Molecules for Photodynamic Therapy of Cutaneous Disorders" Pharmaceuticals 17, no. 1: 62. https://doi.org/10.3390/ph17010062
APA StyleBurloiu, A. M., Manda, G., Lupuliasa, D., Socoteanu, R. P., Mihai, D. P., Neagoe, I. V., Anghelache, L.-I., Surcel, M., Anastasescu, M., Olariu, L., Gîrd, C. E., Barbuceanu, S. F., Ferreira, L. F. V., & Boscencu, R. (2024). Assessment of Some Unsymmetrical Porphyrins as Promising Molecules for Photodynamic Therapy of Cutaneous Disorders. Pharmaceuticals, 17(1), 62. https://doi.org/10.3390/ph17010062










