Improvement of the Thermal Stability and Aqueous Solubility of Three Matrine Salts Assembled by the Similar Structure Salt Formers
Abstract
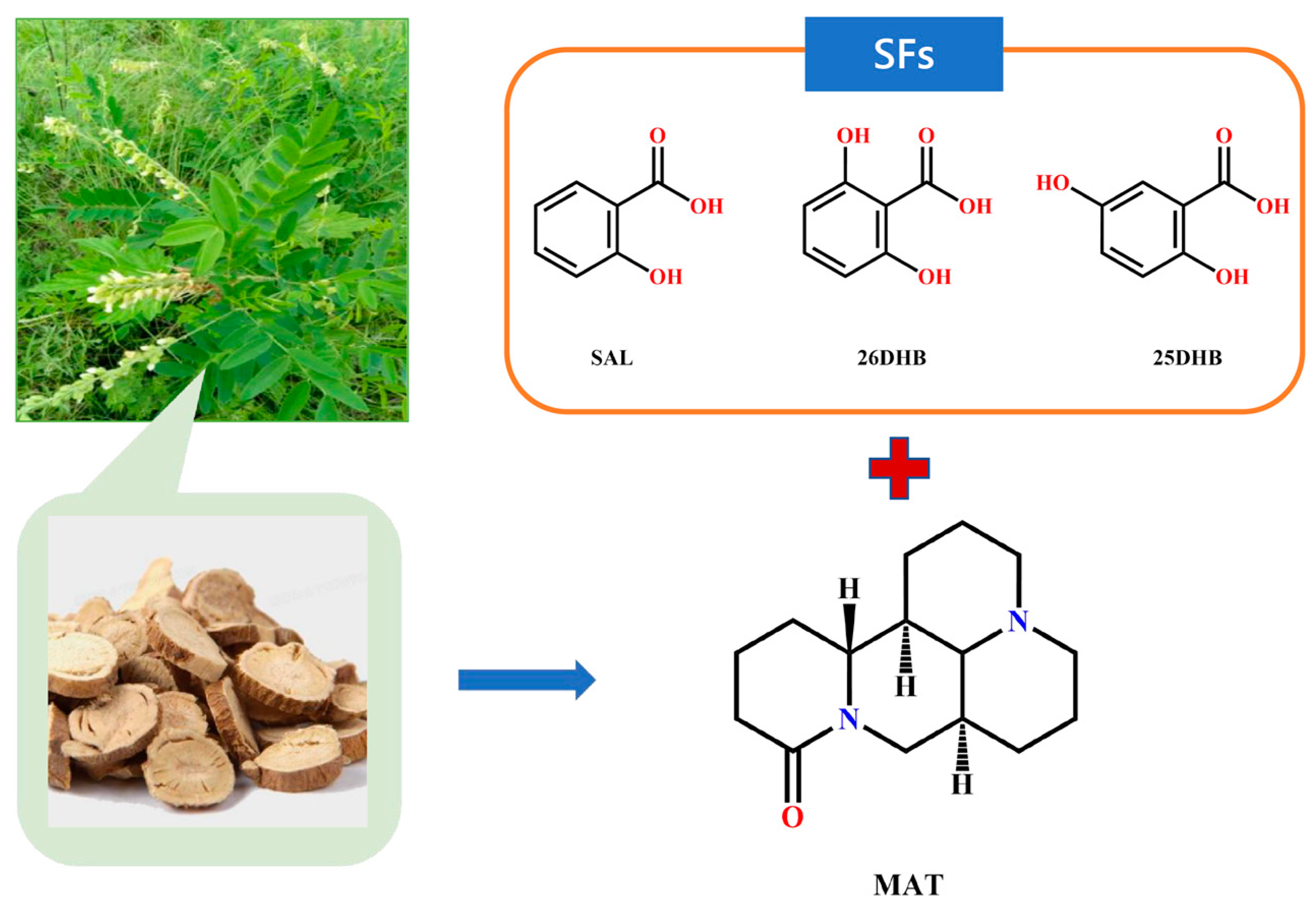
:1. Introduction
2. Results and Discussion
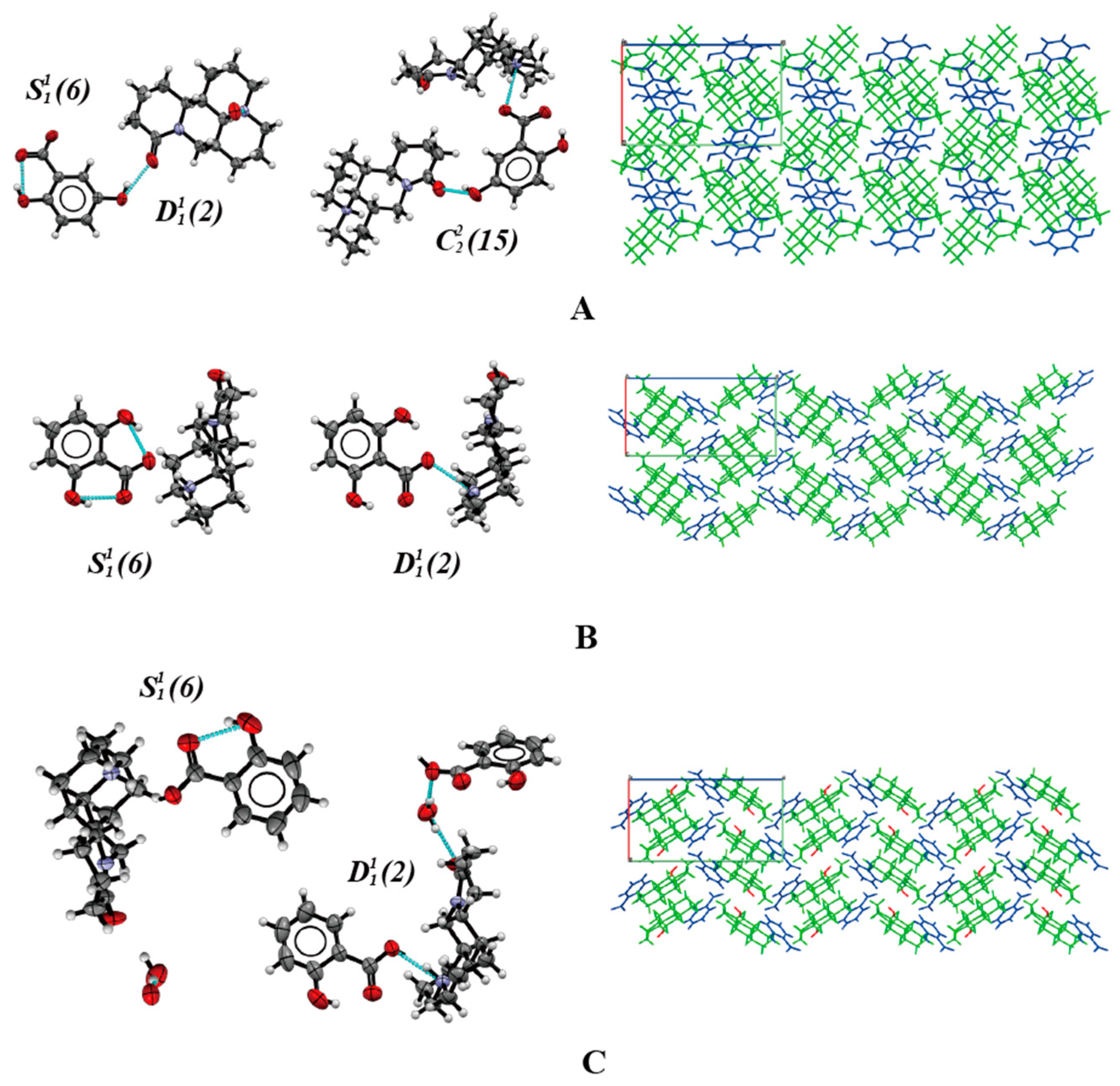
2.1. Crystal Structure Analysis
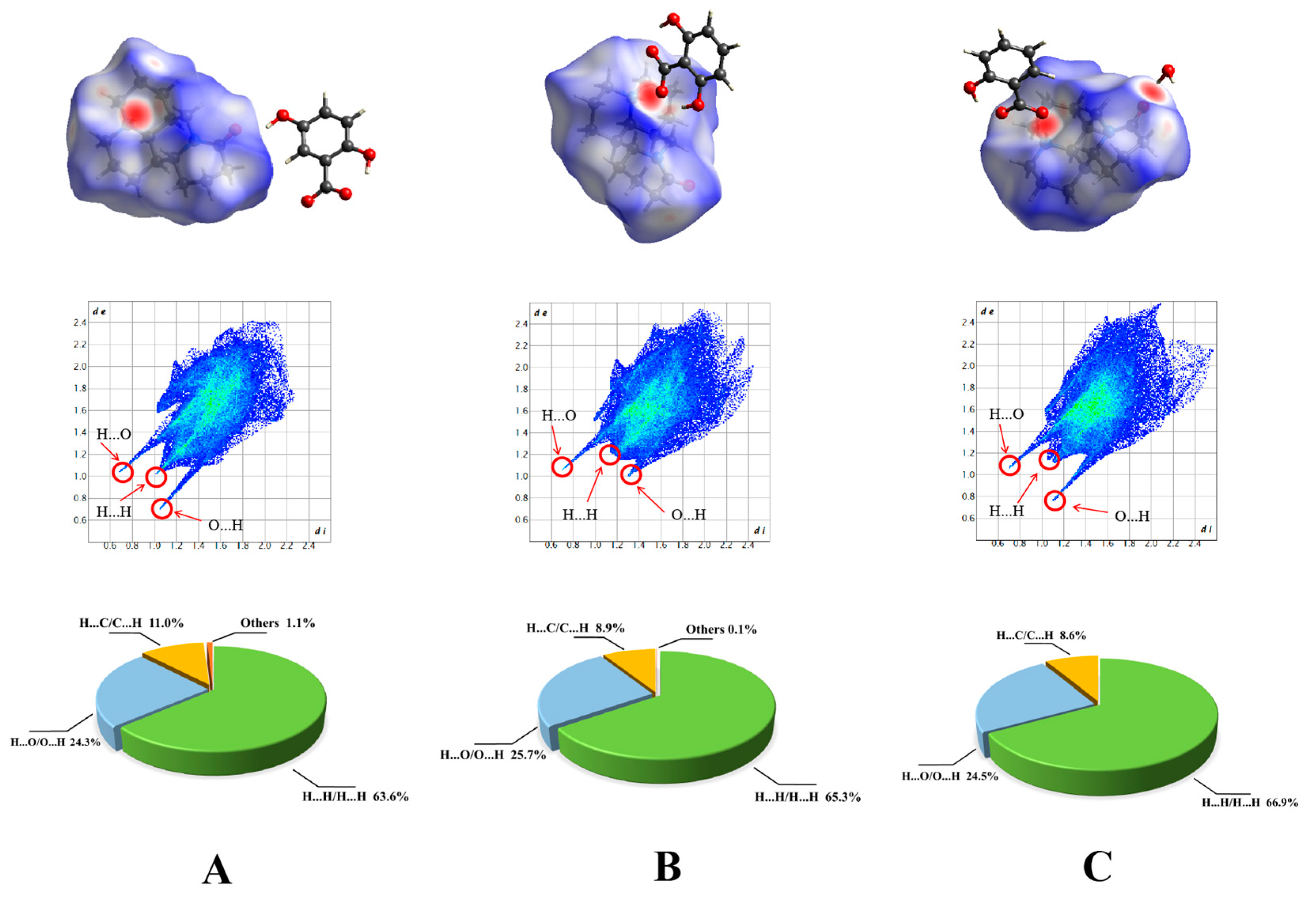
2.2. Hirshfield Surface Analysis
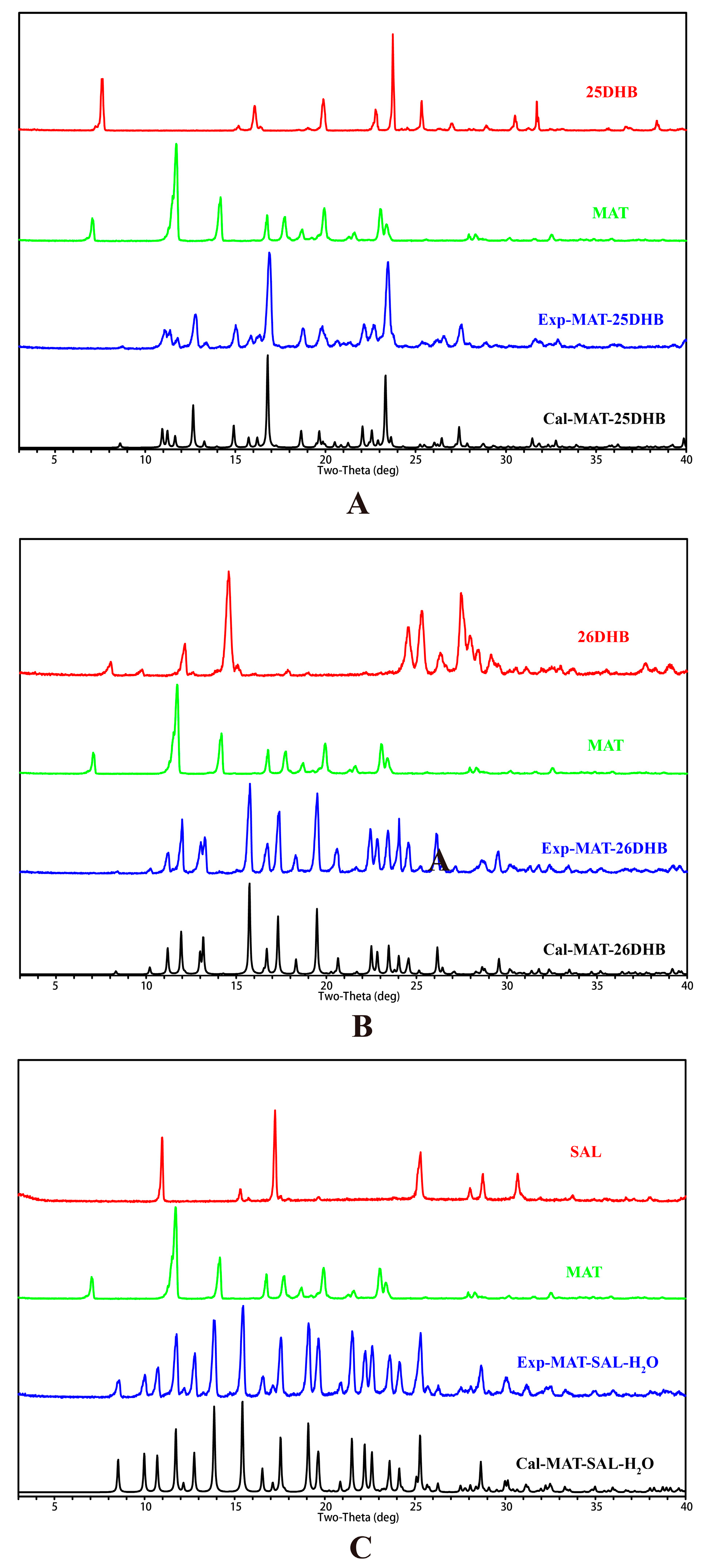
2.3. PXRD Analysis
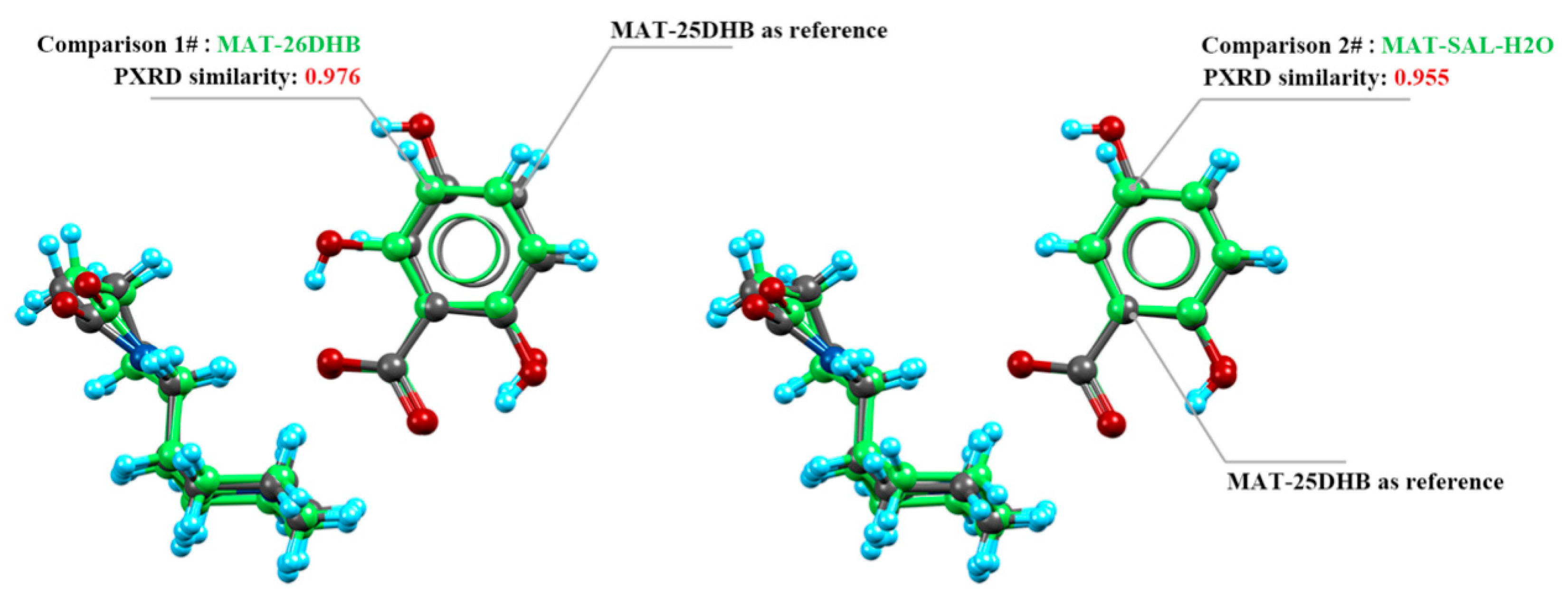
2.4. Crystal Packing Similarity Analysis
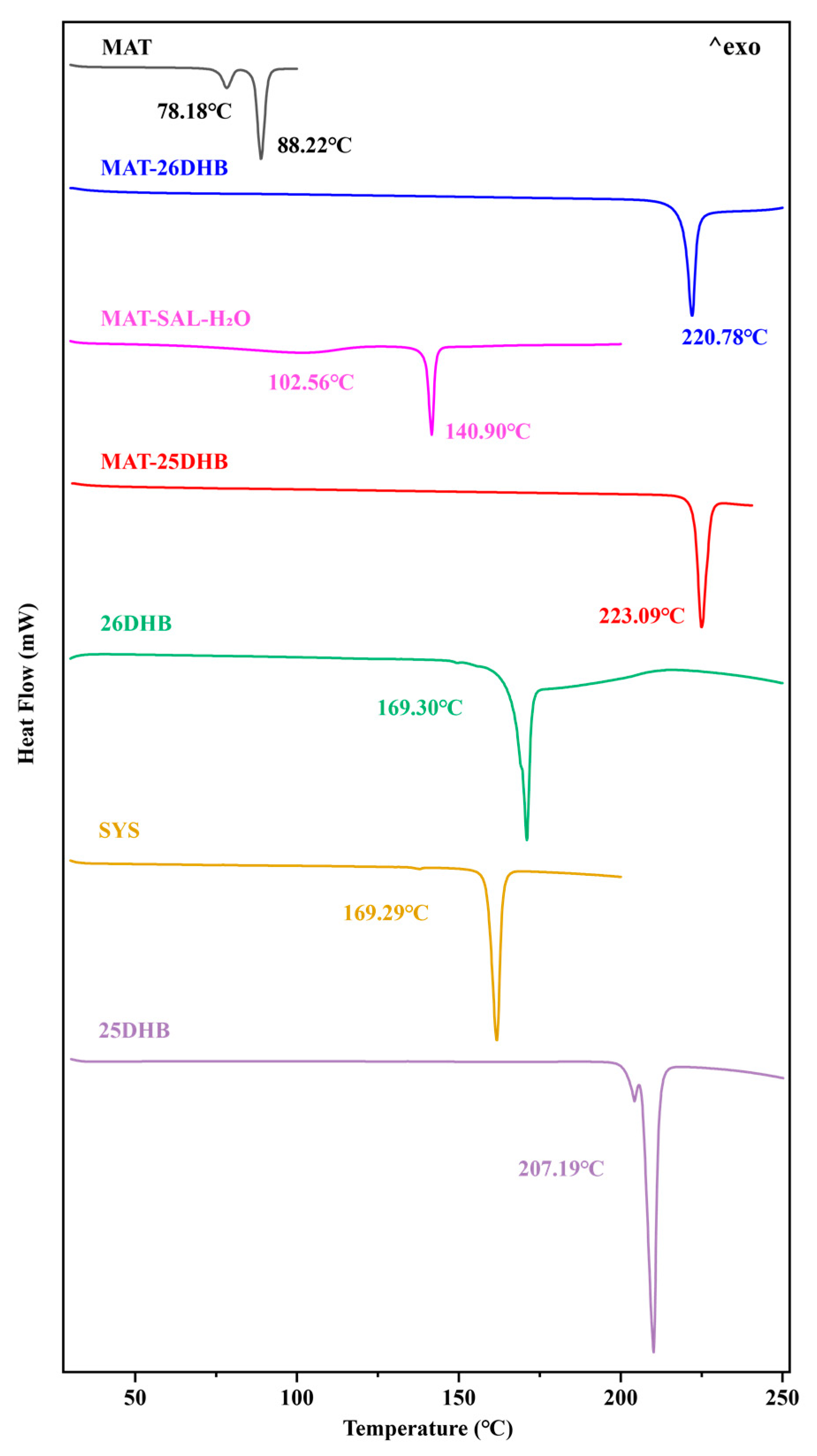
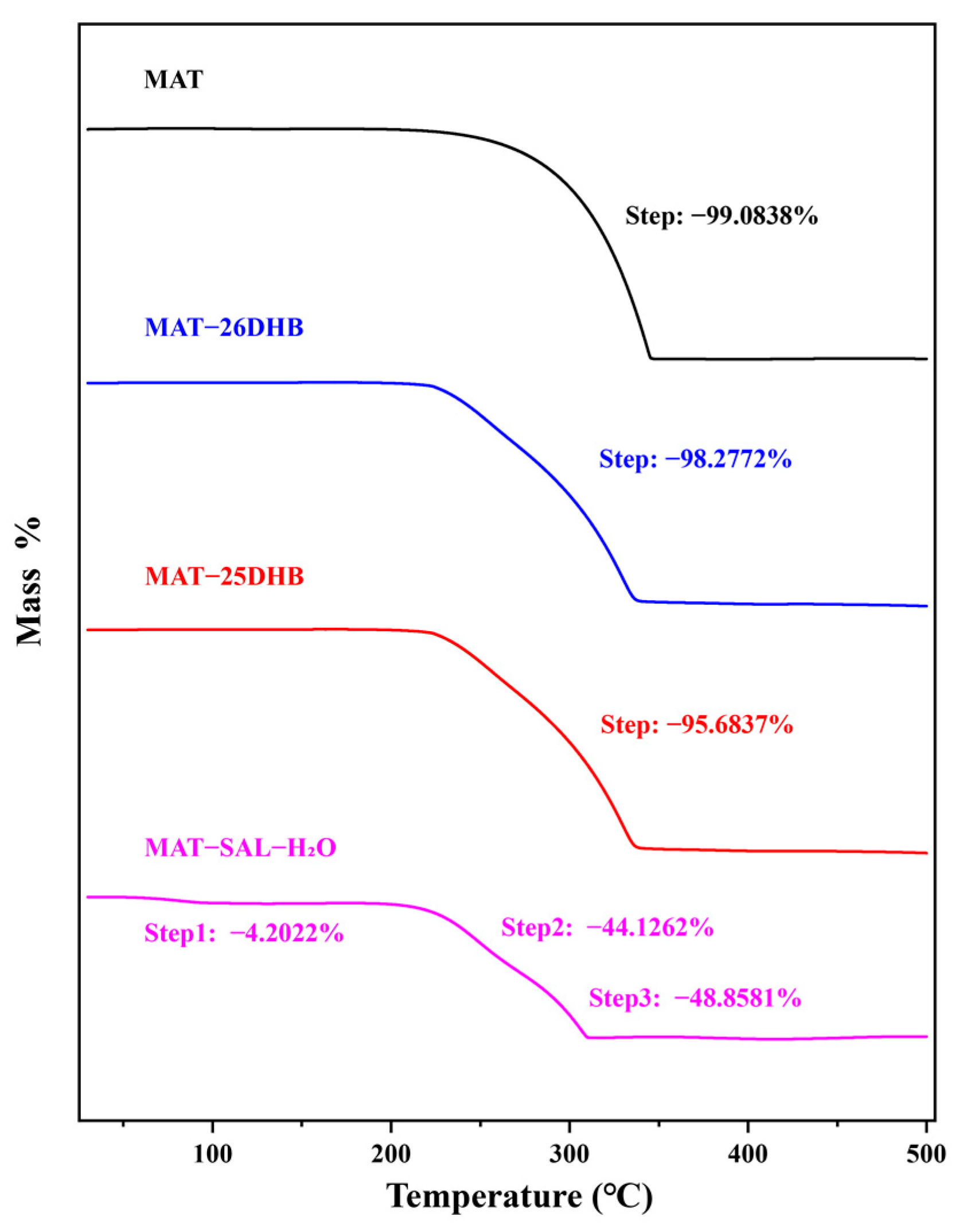
2.5. Thermal Analysis
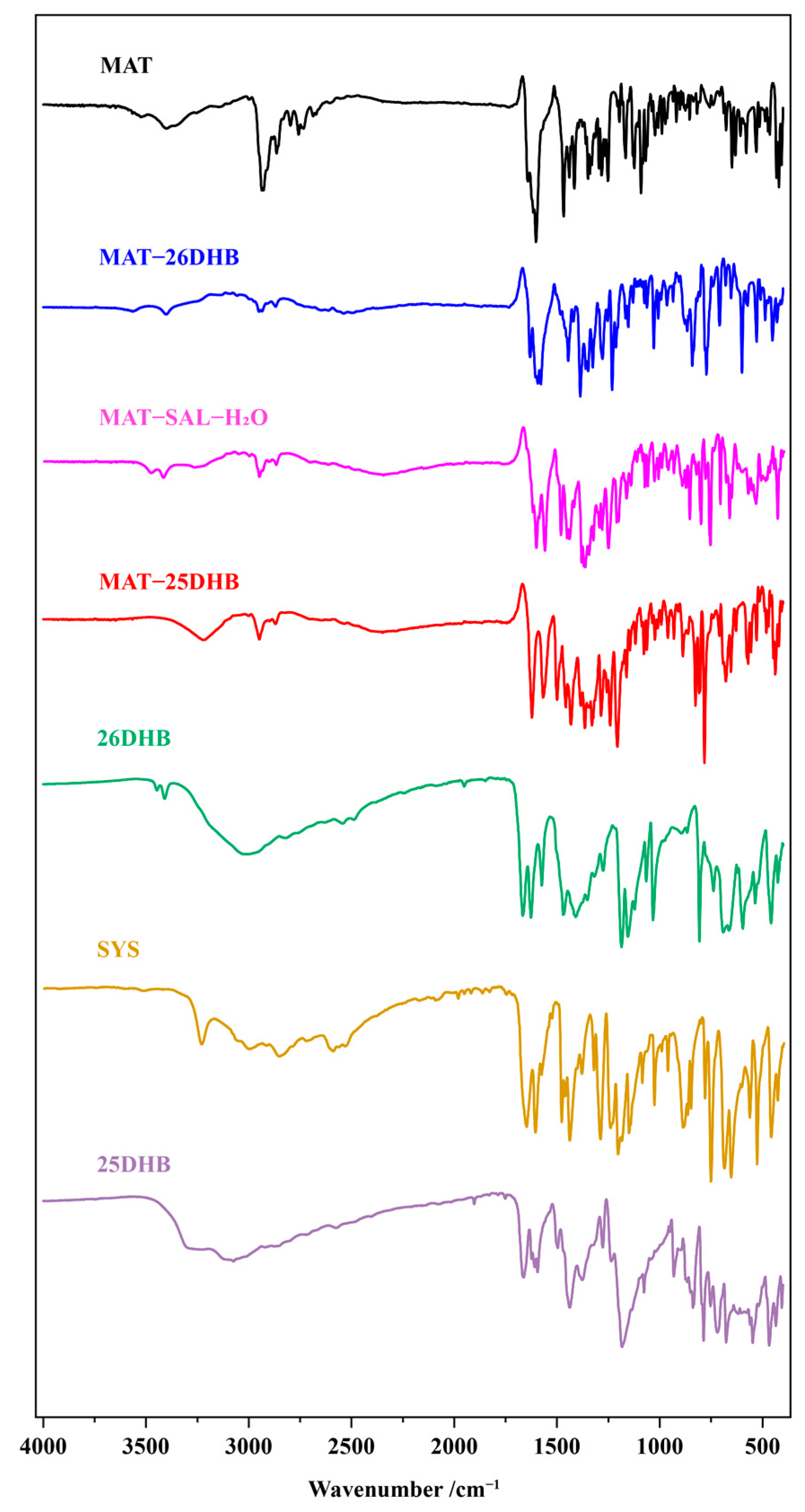
2.6. IR Analysis
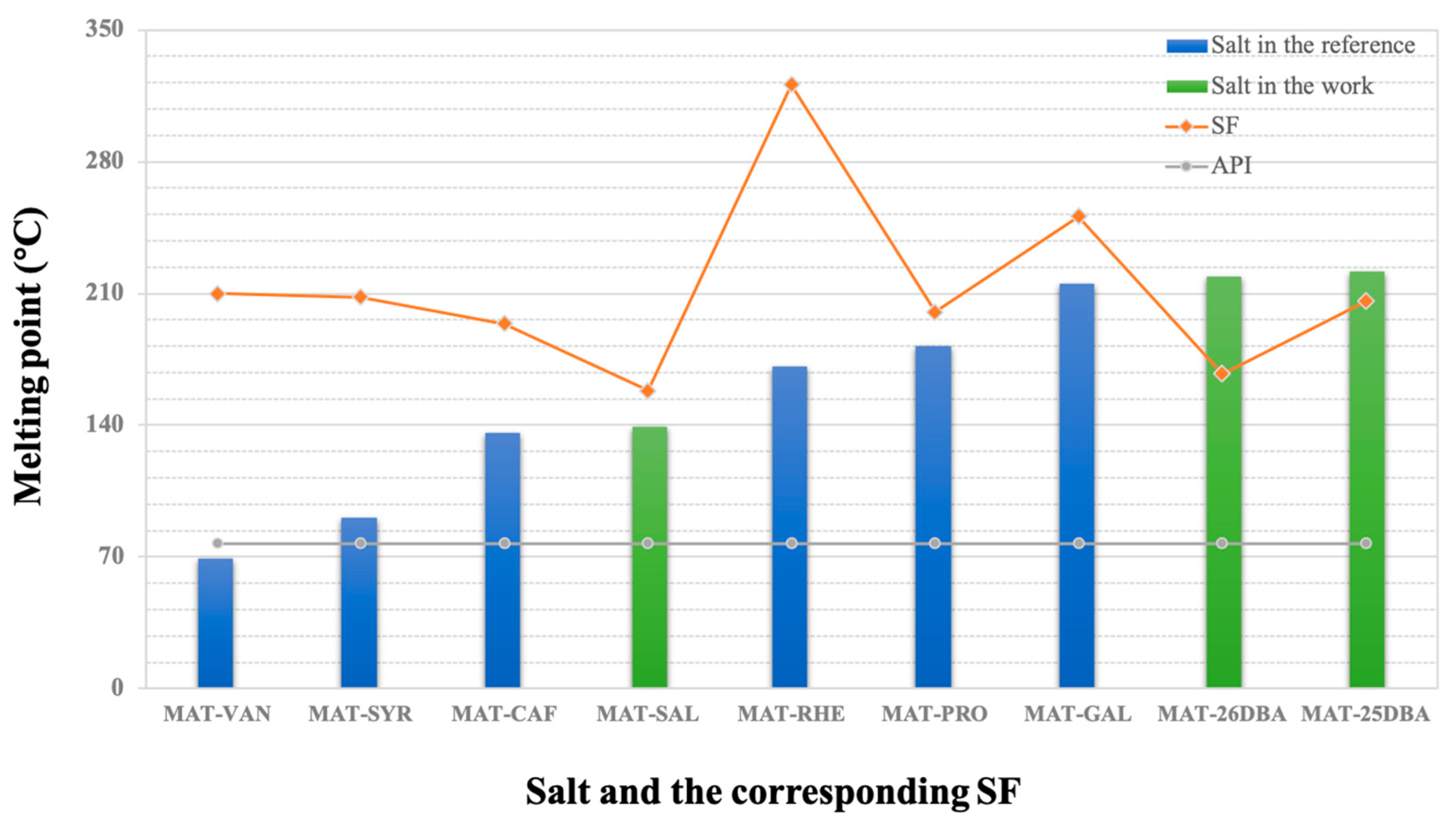
2.7. Equilibrium Solubility
2.8. Theoretical Calculations
2.8.1. Lattice Energy
2.8.2. Solvation Free Energy
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation
3.2.2. SXRD Analysis
3.2.3. PXRD Analysis
3.2.4. Thermal Analysis
3.2.5. IR Analysis
3.2.6. Solubility Tests
3.2.7. Theoretical Computation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Wei, S.; Marabada, D.; Wang, Z.; Huang, Q. Research Progress of Natural Matrine Compounds and Synthetic Matrine Derivatives. Molecules 2023, 28, 5780. [Google Scholar] [CrossRef] [PubMed]
- Chi, G.; Xu, D.; Zhang, B.; Yang, F. Matrine induces apoptosis and autophagy of glioma cell line U251 by regulation of circRNA-104075/BCL-9. Chem.-Biol. Interact. 2019, 308, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, X.; Wei, W.; Zhang, N.; Wu, H.; Ma, Z.; Li, L.; Deng, W.; Tang, Q. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharm. Sin. B 2019, 9, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, L.; Sun, X.; Yang, Q.; Wan, L.; Guo, C. Matrine: A Promising Natural Product With Various Pharmacological Activities. Front. Pharmacol. 2020, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Gu, Y.-Y.; Zhang, A.L.; Sze, D.M.-Y.; Mo, S.-L.; May, B.H. Biological effects and mechanisms of matrine and other constituents of Sophora flavescens in colorectal cancer. Pharmacol. Res. 2021, 171, 105778. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Mehan, S. Matrine exerts its neuroprotective effects by modulating multiple neuronal pathways. Metab. Brain Dis. 2023, 38, 1471–1499. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yin, T. Novel paeonol-matrine deep eutectic solvent: Physicochemical properties and cytotoxicity. J. Mol. Liq. 2022, 348, 118068. [Google Scholar] [CrossRef]
- Zhang, M.; Li, A.; Yang, Q.; Li, J.; Zheng, L.; Wang, G.; Sun, Y.; Huang, Y.; Zhang, M.; Song, Z. Matrine alleviates depres-sive-like behaviors via modulating microbiota–gut–brain axis in CUMS-induced mice. J. Transl. Med. 2023, 21, 145. [Google Scholar] [CrossRef]
- Li, H.; Bao, J.; Tian, L.; ALisa; Dong, Z. Borjigidai Almaz. Biopharmaceutical classification and transport mechanism of 4 alka-loids in Mongolian herbal medicine Sophorae Flavescentis Radix. China J. Chin. Mater. Med. 2021, 46, 4721–4729. [Google Scholar]
- ur Rashid, H.; Xu, Y.; Muhammad, Y.; Wang, L.; Jiang, J. Research advances on anticancer activities of matrine and its de-rivatives: An updated overview. Eur. J. Med. Chem. 2019, 161, 205–238. [Google Scholar] [CrossRef]
- Xu, Y.; Jing, D.; Zhao, D.; Wu, Y.; Xing, L.; Rashid, H.U.; Wang, H.; Wang, L.; Cao, H. New modification strategy of matrine as Hsp90 inhibitors based on its specific L conformation for cancer treatment. Bioorganic Med. Chem. 2020, 28, 115305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, Z.; Wen, L.; Jiang, C.; Feng, Q. Matrine: A review of its pharmacology, pharmacokinetics, toxicity, clinical ap-plication and preparation researches. J. Ethnopharmacol. 2021, 269, 113682. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Yang, C.; Du, Y.; Wang, W.; Sun, M.; Liu, J.; Ma, B.; Pang, L.; Zeng, Y.; Zhang, Z.; et al. A Systematic Review of the Pharmacology, Toxicology and Pharmacokinetics of Matrine. Front. Pharmacol. 2020, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Duan, Y.; Zhao, D.; Liu, C.; Xu, S..; Liang, D.; Zhang, F.; Shi, W. Integrative Analysis of Transcriptomic and Metab-olomic Data for Identification of Pathways Related to Matrine-Induced Hepatotoxicity. Chem. Res. Toxicol. 2022, 35, 2271–2284. [Google Scholar] [CrossRef]
- Wang, W.; You, R.-L.; Qin, W.-J.; Hai, L.-N.; Fang, M.-J.; Huang, G.-H.; Kang, R.-X.; Li, M.-H.; Qiao, Y.-F.; Li, J.-W.; et al. Anti-tumor activities of active ingredients in Compound Kushen Injection. Acta Pharmacol. Sin. 2015, 36, 676–679. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Jia, L.-Y.; Rong, Z.; Zhou, X.; Cao, L.-Q.; Li, A.-H.; Guo, M.; Jin, J.; Wang, Y.-D.; Huang, L.; et al. Research Advances on Matrine. Front. Chem. 2022, 10, 867318. [Google Scholar] [CrossRef]
- Luo, D.; Dai, X.; Tian, H.; Fan, C.; Xie, H.; Chen, N.; Wang, J.; Huang, L.; Wang, H.; Wang, G.; et al. Sophflarine A, a novel matrine-derived alkaloid from Sophora flavescens with therapeutic potential for non-small cell lung cancer through ROS-mediated pyroptosis and autophagy. Phytomedicine 2023, 116, 154909. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Zhang, C.; Yang, D.; Lu, Y.; Zhou, Z. The enhanced pH-dependent solubility behavior of three novel lamotrigine-acid salts. J. Mol. Liq. 2023, 382, 121929. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Yuan, P.; An, Q.; Su, B.; Yu, M.; Chen, T.; Hu, K.; Zhang, L.; Lu, Y.; et al. Cocrystal virtual screening based on the XGBoost machine learning model. Chin. Chem. Lett. 2023, 34, 107964. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Liu, M.; Xing, W.; Hu, K.; Yang, S.; He, G.; Gong, N.; Du, G.; Lu, Y. Design, Preparation, Characterization and Evaluation of Five Cocrystal Hydrates of Fluconazole with Hydroxybenzoic Acids. Pharmaceutics 2022, 14, 2486. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liang, M.; An, Q.; Wang, W.; Zhang, B.; Yang, S.; Zhou, J.; Yang, X.; Yang, D.; Zhang, L.; et al. Versatile Solid Modifications of Multicomponent Pharmaceutical Salts: Novel Metformin–Rhein Salts Based on Advantage Complementary Strategy Design. Pharmaceutics 2023, 15, 1196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Y.; An, Q.; Li, W.; Li, J.; Liu, H.; Yang, D.; Lu, Y.; Zhou, Z. Discovered two polymorphs and two solvates of lamotrigine-tolfenamic acid salt: Thermal behavior and crystal morphological differences. Int. J. Pharm. 2022, 628, 122310. [Google Scholar] [CrossRef]
- Xing, W.; Xing, C.; Liu, M.; Yu, H.; Zhang, B.; Yang, S.; Gong, N.; Lu, Y.; Du, G. Preparation, characterization, and stability enhancement of metoprolol pharmaceutical multicomponent crystals. J. Mol. Struct. 2023, 1291, 136029. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, P.; Heng, T.; Du, L.; An, Q.; Zhang, B.; Zhang, L.; Yang, D.; Du, G.; Lu, Y. Insight into the Formation of Cocrystal and Salt of Tenoxicam from the Isomer and Conformation. Pharmaceutics 2022, 14, 1968. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Z.; Zhang, J.; Zhan, J.; Wu, C.; Yu, W.; Fan, W.; Tang, J.; Zhang, Q.; Zhang, J. Sustainable Bioactive Salts Fully Composed of Natural Products for Enhanced Pharmaceutical Applicability. ACS Sustain. Chem. Eng. 2022, 10, 10369–10382. [Google Scholar] [CrossRef]
- Wang, H.; Yang, D.; Zhang, W.; Song, J.; Gong, N.; Yu, M.; Yang, S.; Zhang, B.; Liu, Q.; Du, G.; et al. An innovative rhein-matrine cocrystal: Synthesis, characterization, formation mechanism and pharmacokinetic study. Chin. Chem. Lett. 2023, 34, 107258. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Zhang, J.; Zhang, L.; Wang, W.; Zhan, J.; Liao, Y.; Wu, C.; Yu, W.; Zhang, J. A matrine-based supra-molecular ionic salt that enhances the water solubility, transdermal delivery, and bioactivity of salicylic acid. Chem. Eng. J. 2023, 468, 143480. [Google Scholar] [CrossRef]
- del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. 2017, 57, 3084–3103. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Z.; Li, D.; Ye, K.; Xie, C.; Zhang, S.; Jiang, L.; Zheng, K.; Pang, Q. Determination of protonation state in molecular salt of minoxidil and 2,4-dihydroxybenzoic acid through a combined experimental and theoretical study: Influence of proton transfer on biological activities. J. Mol. Struct. 2022, 1249, 131560. [Google Scholar] [CrossRef]
- Tang, L.; Zhu, W. Theoretical study on the structure, electronic properties, intermolecular interactions, and detonation per-formance of DAF: ADNP co-crystal. J. Mol. Model. 2023, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jeevananthan, V.; Varathan, E.; Shanmugan, S. Supramolecular cocrystals of dispiro-P3N3-dicarboxylic acid: Synthesis, structural characterization and hirshfeld surface analysis. J. Mol. Struct. 2023, 1289, 135888. [Google Scholar] [CrossRef]
- Pindelska, E.; Sokal, A.; Kolodziejski, W. Pharmaceutical cocrystals, salts and polymorphs: Advanced characterization techniques. Adv. Drug Deliv. Rev. 2017, 117, 111–146. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liang, W.; Chen, X.; Wang, J.; Deng, Z.; Zhang, H. Pharmaceutical cocrystals of naringenin with improved dissolution performance. CrystEngComm 2018, 20, 3025–3033. [Google Scholar] [CrossRef]
- Rahman, Z.; Agarabi, C.; Zidan, A.S.; Khan, S.R.; Khan, M.A. Physico-mechanical and Stability Evaluation of Carbamazepine Cocrystal with Nicotinamide. Aaps Pharmscitech 2011, 12, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Sathisaran, I.; Dalvi, S.V. Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef]
- Cheng, X.; Ye, J.; He, H.; Liu, Z.; Xu, C.; Wu, B.; Xiong, X.; Shu, X.; Jiang, X.; Qin, X. Synthesis, characterization and in vitro biological evaluation of two matrine derivatives. Sci. Rep. 2018, 8, 15686. [Google Scholar] [CrossRef] [PubMed]
- Bassi, P.; Kaur, G. pH modulation: A mechanism to obtain pH-independent drug release. Expert Opin. Drug Deliv. 2010, 7, 845–857. [Google Scholar] [CrossRef]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-Hornedo, N. Pharmaceutical cocrystals and poorly soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef]
- Guo, W.; Du, S.; Lin, Y.; Lu, B.; Yang, C.; Wang, J.; Zeng, Y. Structural and computational insights into the enhanced solubility of dipfluzine by complexation: Salt and salt-cocrystal. New J. Chem. 2018, 42, 15068–15078. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, Q. HPLC determination of matrine fuyanping capsules. Pharm J. Chin. People’s Lib. Army 2008, 24, 542–543. [Google Scholar]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ho, J.; Klamt, H.; Coote, M.L. Comment on the Correct Use of Continuum Solvent Models. Phys. Chem. A 2010, 114, 13442–13444. [Google Scholar] [CrossRef]
- Ribeiro, R.F.; Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Use of solution-phase vibrational frequencies in continuum models for the free energy of solvation. J. Phys. Chem. B. 2011, 115, 14556. [Google Scholar] [CrossRef]
- Palatinus, L.; Brázda, P.; Boullay, P.; Perez, O.; Klementová, M.; Petit, S.; Eigner, V.; Zaarour, M.; Mintova, S. Hydrogen positions in single nanocrystals revealed by electron diffraction. Science 2017, 355, 166–169. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
| Parameters | MAT-25DHB | MAT-26DHB | MAT-SAL-H2O |
|---|---|---|---|
| Empirical formula | (C15H25N2O)+·(C7H5O4)− | (C15H25N2O)+·(C7H5O4)− | (C15H25N2O)+·(C7H5O3)−·H2O |
| Formula weight (Da) | 402.49 | 402.49 | 386.49 |
| Crystal size(mm) | 0.30 × 0.40 × 0.40 | 0.20 × 0.20 × 0.20 | 0.20 × 0.20 × 0.20 |
| Description | block | block | block |
| Crystal system | orthorhombic | orthorhombic | orthorhombic |
| Space group | P 212121 | P 212121 | P 212121 |
| a (Å) | 9.5462(2) | 8.8803(2) | 9.3422(19) |
| b (Å) | 13.9464(3) | 13.4444(2) | 12.7748(2) |
| c (Å) | 15.1799(3) | 17.3365(3) | 17.7168(3) |
| α (°) | 90 | 90 | 90 |
| β (°) | 90 | 90 | 90 |
| γ (°) | 90 | 90 | 90 |
| Z | 4 | 4 | 4 |
| Volume (Å3) | 2020.98(8) | 2069.81(8) | 2114.41(7) |
| Density(g/cm3) | 1.323 | 1.292 | 1.271 |
| R1 | 0.0484 | 0.0361 | 0.0524 |
| wR2 | 0.1259 | 0.0958 | 0.1540 |
| Reflections collected | 4045 | 3894 | 4016 |
| independent reflections | 3835 | 3442 | 3582 |
| Completeness (%) | 99.7 | 100 | 99.9 |
| CCDC number | 2,297,779 | 2,297,780 | 2,297,778 |
| Salt | D–H…A | d(D–H)/Å | d(H…A)/Å | d(D…A)/Å | ∠DHA/° |
|---|---|---|---|---|---|
| MAT-25DHB | O2B–H2B…O3B | 0.820 | 1.793 | 2.517(4) | 146.34 |
| N2A–H2A…O4B | 0.980 | 1.756 | 2.719(3) | 166.51 | |
| O1B–H1B…O1A | 0.820 | 1.899 | 2.653(4) | 152.39 | |
| MAT-26DHB | O1B–H1B…O2B | 0.820 | 1.809 | 2.541(3) | 147.92 |
| O4B–H4B…O3B | 0.820 | 1.801 | 2.536(3) | 148.50 | |
| N2A–H2A…O2B | 0.980 | 1.802 | 2.764(3) | 166.53 | |
| MAT-SAL-H2O | O3B–H3B…O2B | 0.820 | 1.784 | 2.517(4) | 148.00 |
| O1W–H1W…O1A | 0.877 | 1.982 | 2.855(4) | 173.14 | |
| O1W–H1W…O1B | 0.855 | 2.058 | 2.907(4) | 172.50 | |
| N2A–H2A…O1B | 0.980 | 1.799 | 2.761(4) | 166.53 |
| Samples | Concentration (mg/mL) | |||
|---|---|---|---|---|
| H2O | pH = 1.2 | pH = 4.5 | pH = 6.8 | |
| MAT | 65.36 ± 0.53 | 90.89 ± 1.01 | 91.79 ± 2.31 | 64.89 ± 1.13 |
| MAT-SAL-H2O | 29.00 ± 1.28 | 55.89 ± 6.54 | 40.07 ± 1.15 | 25.09 ± 0.82 |
| MAT-26DHB | 7.42 ± 0.45 | 13.08 ± 1.52 | 8.23 ± 0.10 | 5.71 ± 0.75 |
| MAT-25DHB | 613.60 ± 62.57 | 795.11 ± 18.28 | 606.03 ± 36.97 | 482.55 ± 31.93 |
| Salts | E (Bulk) | E (Mol, Bulk) | E (Mol, Ghost) | BSSE | E (Lattice) |
|---|---|---|---|---|---|
| MAT-SAL-H2O | −5368.4772 | −1342.0654 | −1342.0955 | 0.0301 | −14.90 |
| MAT–26–DHB | −5363.7148 | −1340.8063 | −1340.8063 | 0.0000 | −76.80 |
| MAT–25–DHB | −5363.6795 | −1340.7405 | −1340.7794 | 0.0389 | −88.13 |
| Symbol | Physical Meaning | |
|---|---|---|
| 1 | ΔE (Lattice) | the lattice energy |
| 2 | ∆E (Condensation) | the condensation of molecules that retain the same conformation they had in the crystal |
| 3 | BSSE | the basis set superposition error |
| 4 | E (Bulk) | the total energy per unit cell |
| 5 | Z | the number of molecules in the cell |
| 6 | E (Molecule, Bulk) | the total energy of each molecule in the cell |
| 7 | E (Molecule, Ghost) | the total energy of the molecule obtained by increasing the basis group with the virtual function of the surrounding atoms |
| 8 | ΔG (solv) | Solvation free energy |
| 9 | E (soln) | the single-point energy in the solvent |
| 10 | E (gas) | the single–point energy in the gaseous state |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, B.; Wang, W.; Yuan, P.; Hu, K.; Zhang, L.; Yang, D.; Lu, Y.; Du, G. Improvement of the Thermal Stability and Aqueous Solubility of Three Matrine Salts Assembled by the Similar Structure Salt Formers. Pharmaceuticals 2024, 17, 94. https://doi.org/10.3390/ph17010094
Wang Y, Zhang B, Wang W, Yuan P, Hu K, Zhang L, Yang D, Lu Y, Du G. Improvement of the Thermal Stability and Aqueous Solubility of Three Matrine Salts Assembled by the Similar Structure Salt Formers. Pharmaceuticals. 2024; 17(1):94. https://doi.org/10.3390/ph17010094
Chicago/Turabian StyleWang, Yeyang, Baoxi Zhang, Wenwen Wang, Penghui Yuan, Kun Hu, Li Zhang, Dezhi Yang, Yang Lu, and Guanhua Du. 2024. "Improvement of the Thermal Stability and Aqueous Solubility of Three Matrine Salts Assembled by the Similar Structure Salt Formers" Pharmaceuticals 17, no. 1: 94. https://doi.org/10.3390/ph17010094
APA StyleWang, Y., Zhang, B., Wang, W., Yuan, P., Hu, K., Zhang, L., Yang, D., Lu, Y., & Du, G. (2024). Improvement of the Thermal Stability and Aqueous Solubility of Three Matrine Salts Assembled by the Similar Structure Salt Formers. Pharmaceuticals, 17(1), 94. https://doi.org/10.3390/ph17010094









