Advances in Neuroprotection in Glaucoma: Pharmacological Strategies and Emerging Technologies
Abstract
:1. Introduction
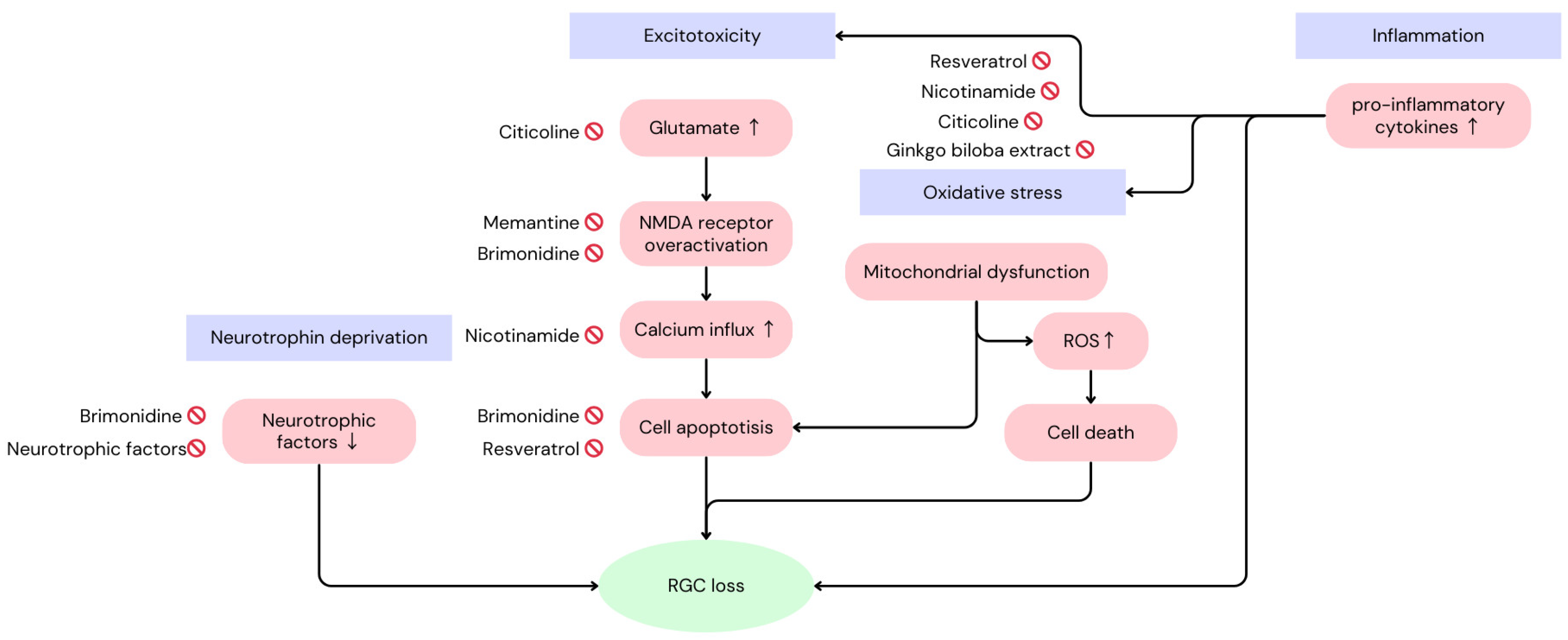
2. Mechanisms of Loss of Retinal Functions in Glaucoma
3. Pharmacological Interventions (Summary in Table 1)
3.1. Brimonidine
| Active Compound | Mechanism of Action | Route of Administration | Status in Clinical Studies | Clinical Trials |
|---|---|---|---|---|
| Brimonidine | Binds alpha-2 adrenergic receptors, regulates apoptotic proteins, upregulates neurotrophic factors, reduces NMDA 1 receptor excitotoxicity | Topical | FDA-approved for IOP 2 reduction, neuroprotective effects under investigation | Krupin et al., 2011 [8]; De Moraes et al., 2012 [9] |
| Neurotrophic Factors (BDNF 3, CNTF 4, NGF 5) | Binds to specific receptors (TrkB and TrkA), promotes survival of neurons, enhances axon regeneration, modulates apoptosis | Implants (CTNF), topical (rhNGF 6) | Ongoing Phase II trials (CTNF), Phase Ib trial showed safety (rhNGF) | Goldberg et al., 2023 [10]; Beykin et al., 2022 [11] |
| Memantine | Non-competitive NMDA receptor antagonist, reduces calcium influx, protects neurons from glutamate-induced excitotoxicity | Oral | Failed large-scale clinical trials | Weinreb et al., 2018 [12] |
| GBE 7 | Scavenges ROS, reduces oxidative stress, stabilizes mitochondria, antagonizes PAF | Oral | Mixed results in clinical trials | Quaranta et al., 2003 [13]; Lee et al., 2013 [14]; Guo et al., 2014 [15] |
| Citicoline | Serves as precursor for phospholipids, enhances neurotransmitter synthesis | Oral, topical | Ongoing large-scale clinical trials | NCT05315206 NCT05710198 |
| Nicotinamide | Replenishes NAD levels, supports mitochondrial function, regulates calcium homeostasis, reduces oxidative stress | Oral | Ongoing large-scale long-term trials | NCT05275738 NCT05405868 |
3.2. Neurotrophic Factors
3.3. Memantine
3.4. Ginkgo Biloba Extract
3.5. Citicoline
3.6. Nicotinamide
3.7. Insulin
3.8. Resveratrol
3.9. Other Novel Drugs
4. Emerging Technologies
4.1. Stem Cell Therapy
4.2. Gene Therapy
4.3. Mitochondrial-Targeted Therapies and Transplantation
4.4. Nanotechnologies
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| RGCs | Retinal Ganglion Cells |
| IOP | Intraocular Pressure |
| BDNF | Brain-Derived Neurotrophic Factor |
| CNTF | Ciliary Neurotrophic Factor |
| NMDA | N-Methyl-D-Aspartate |
| ROS | Reactive Oxygen Species |
| TNF-α | Tumor Necrosis Factor-Alpha |
| FGF | Fibroblast Growth Factor |
| RNFL | Retinal Nerve Fiber Layer |
| NTG | Normal Tension Glaucoma |
| POAG | Primary Open-Angle Glaucoma |
| AH | Aqueous Humor |
| MAPK | Mitogen-Activated Protein Kinase |
| NGF | Nerve Growth Factor |
| p75NTR | p75 Neurotrophin Receptor |
| rhNGF | Recombinant Human Nerve Growth Factor |
| VEGF | Vascular Endothelial Growth Factor |
| NAD | Nicotinamide Adenine Dinucleotide |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate Hydrogen |
| DA | Dopamine |
| DACs | Dopaminergic Amacrine Cells |
| DRD1 | Dopamine Receptor D1 |
| ROCK | Rho-Associated Protein Kinase |
| EP2 | E Prostanoid Receptor 2 |
| CAMKII | Calcium/Calmodulin-Dependent Protein Kinase II |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| MYOC | Myocilin |
| TEK | Tunica Interna Endothelial Cell Kinase |
| AAV | Adeno-Associated Virus |
| XIAP | X-Linked Inhibitor of Apoptosis |
| SOD2 | Superoxide Dismutase 2 |
| LHON | Leber Hereditary Optic Neuropathy |
| MSCs | Mesenchymal Stem Cells |
| PDGF | Platelet-Derived Growth Factor |
| hESCs | Human Embryonic Stem Cells |
| OPCs | Oligodendrocyte Precursor Cells |
| miPSC | Mouse-Induced Pluripotent Stem Cell |
| mESC | Mouse Embryonic Stem Cell |
| BMSC | Bone Marrow Mesenchymal Stem Cells |
| sEVs | Small Extracellular Vesicles |
| LNP | Lipid Nanoparticle |
| TPGS | Tocopheryl Polyethylene Glycol Succinate |
| EVs | Extracellular Vesicles |
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Casson, R.J. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin. Exp. Ophthalmol. 2006, 34, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Fan Gaskin, J.C.; Shah, M.H.; Chan, E.C. Oxidative Stress and the Role of NADPH Oxidase in Glaucoma. Antioxidants 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Vohra, R.; Tsai, J.C.; Kolko, M. The role of inflammation in the pathogenesis of glaucoma. Surv. Ophthalmol. 2013, 58, 311–320. [Google Scholar] [CrossRef]
- Chen, S.D.; Wang, L.; Zhang, X.L. Neuroprotection in glaucoma: Present and future. Chin. Med. J. 2013, 126, 1567–1577. [Google Scholar] [CrossRef]
- Pasutto, F.; Matsumoto, T.; Mardin, C.Y.; Sticht, H.; Brandstatter, J.H.; Michels-Rautenstrauss, K.; Weisschuh, N.; Gramer, E.; Ramdas, W.D.; van Koolwijk, L.M.; et al. Heterozygous NTF4 mutations impairing neurotrophin-4 signaling in patients with primary open-angle glaucoma. Am. J. Hum. Genet. 2009, 85, 447–456. [Google Scholar] [CrossRef]
- Malik, J.M.; Shevtsova, Z.; Bahr, M.; Kugler, S. Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL gene transfer. Mol. Ther. 2005, 11, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Krupin, T.; Liebmann, J.M.; Greenfield, D.S.; Ritch, R.; Gardiner, S.; Low-Pressure Glaucoma Study Group. A randomized trial of brimonidine versus timolol in preserving visual function: Results from the Low-Pressure Glaucoma Treatment Study. Am. J. Ophthalmol. 2011, 151, 671–681. [Google Scholar] [CrossRef]
- De Moraes, C.G.; Liebmann, J.M.; Greenfield, D.S.; Gardiner, S.K.; Ritch, R.; Krupin, T.; Low-pressure Glaucoma Treatment Study Group. Risk factors for visual field progression in the low-pressure glaucoma treatment study. Am. J. Ophthalmol. 2012, 154, 702–711. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Beykin, G.; Satterfield, K.R.; Nunez, M.; Lam, B.L.; Albini, T.A. Phase I NT-501 Ciliary Neurotrophic Factor Implant Trial for Primary Open-Angle Glaucoma: Safety, Neuroprotection, and Neuroenhancement. Ophthalmol. Sci. 2023, 3, 100298. [Google Scholar] [CrossRef]
- Beykin, G.; Stell, L.; Halim, M.S.; Nunez, M.; Popova, L.; Nguyen, B.T.; Groth, S.L.; Dennis, A.; Li, Z.; Atkins, M.; et al. Phase 1b Randomized Controlled Study of Short Course Topical Recombinant Human Nerve Growth Factor (rhNGF) for Neuroenhancement in Glaucoma: Safety, Tolerability, and Efficacy Measure Outcomes. Am. J. Ophthalmol. 2022, 234, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Liebmann, J.M.; Cioffi, G.A.; Goldberg, I.; Brandt, J.D.; Johnson, C.A.; Zangwill, L.M.; Schneider, S.; Badger, H.; Bejanian, M. Oral Memantine for the Treatment of Glaucoma: Design and Results of 2 Randomized, Placebo-Controlled, Phase 3 Studies. Ophthalmology 2018, 125, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, L.; Bettelli, S.; Uva, M.G.; Semeraro, F.; Turano, R.; Gandolfo, E. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology 2003, 110, 359–362; discussion 362–354. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, S.W.; Kee, C. Effect of Ginkgo biloba extract on visual field progression in normal tension glaucoma. J. Glaucoma 2013, 22, 780–784. [Google Scholar] [CrossRef]
- Guo, X.; Kong, X.; Huang, R.; Jin, L.; Ding, X.; He, M.; Liu, X.; Patel, M.C.; Congdon, N.G. Effect of Ginkgo biloba on visual field and contrast sensitivity in Chinese patients with normal tension glaucoma: A randomized, crossover clinical trial. Investig. Ophthalmol. Vis. Sci. 2014, 55, 110–116. [Google Scholar] [CrossRef]
- Guo, X.; Namekata, K.; Kimura, A.; Noro, T.; Azuchi, Y.; Semba, K.; Harada, C.; Yoshida, H.; Mitamura, Y.; Harada, T. Brimonidine suppresses loss of retinal neurons and visual function in a murine model of optic neuritis. Neurosci. Lett. 2015, 592, 27–31. [Google Scholar] [CrossRef]
- Lambert, W.S.; Ruiz, L.; Crish, S.D.; Wheeler, L.A.; Calkins, D.J. Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons. Mol. Neurodegener. 2011, 6, 4. [Google Scholar] [CrossRef]
- Lindsey, J.D.; Duong-Polk, K.X.; Hammond, D.; Chindasub, P.; Leung, C.K.; Weinreb, R.N. Differential protection of injured retinal ganglion cell dendrites by brimonidine. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1789–1804. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.P.; Villegas-Perez, M.P.; Mayor, S.; Aguilera, M.E.; Miralles de Imperial, J.; Vidal-Sanz, M. Neuroprotective effects of brimonidine against transient ischemia-induced retinal ganglion cell death: A dose response in vivo study. Exp. Eye Res. 2002, 74, 181–189. [Google Scholar] [CrossRef]
- Vidal-Sanz, M.; Lafuente, M.P.; Mayor-Torroglosa, S.; Aguilera, M.E.; Miralles de Imperial, J.; Villegas-Perez, M.P. Brimonidine’s neuroprotective effects against transient ischaemia-induced retinal ganglion cell death. Eur. J. Ophthalmol. 2001, 11 (Suppl. S2), S36–S40. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Rezania, F.; Trounce, I.A.; Crowston, J.G. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg-Cohen, N.; Dadon-Bar-El, S.; Hasanreisoglu, M.; Avraham-Lubin, B.C.; Dratviman-Storobinsky, O.; Cohen, Y.; Weinberger, D. Possible neuroprotective effect of brimonidine in a mouse model of ischaemic optic neuropathy. Clin. Exp. Ophthalmol. 2009, 37, 718–729. [Google Scholar] [CrossRef]
- Aktas, Z.; Gurelik, G.; Akyurek, N.; Onol, M.; Hasanreisoglu, B. Neuroprotective effect of topically applied brimonidine tartrate 0.2% in endothelin-1-induced optic nerve ischaemia model. Clin. Exp. Ophthalmol. 2007, 35, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Qiao, X.; Cantor, L.B.; WuDunn, D. Up-regulation of brain-derived neurotrophic factor expression by brimonidine in rat retinal ganglion cells. Arch. Ophthalmol. 2002, 120, 797–803. [Google Scholar] [CrossRef]
- Jung, K.I.; Kim, J.H.; Han, J.S.; Park, C.K. Exploring Neuroprotective Effects of Topical Brimonidine in Experimental Diabetic Retinopathy. Vivo 2024, 38, 1609–1620. [Google Scholar] [CrossRef]
- Semba, K.; Namekata, K.; Kimura, A.; Harada, C.; Mitamura, Y.; Harada, T. Brimonidine prevents neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 2014, 5, e1341. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.I.; Kim, J.H.; Park, C.K. alpha2-Adrenergic modulation of the glutamate receptor and transporter function in a chronic ocular hypertension model. Eur. J. Pharmacol. 2015, 765, 274–283. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.Y.; Noh, Y.H.; Chai, S.; Lindsey, J.D.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.K. Brimonidine blocks glutamate excitotoxicity-induced oxidative stress and preserves mitochondrial transcription factor a in ischemic retinal injury. PLoS ONE 2012, 7, e47098. [Google Scholar] [CrossRef]
- Lee, K.Y.; Nakayama, M.; Aihara, M.; Chen, Y.N.; Araie, M. Brimonidine is neuroprotective against glutamate-induced neurotoxicity, oxidative stress, and hypoxia in purified rat retinal ganglion cells. Mol. Vis. 2010, 16, 246–251. [Google Scholar]
- Goldblum, D.; Kipfer-Kauer, A.; Sarra, G.M.; Wolf, S.; Frueh, B.E. Distribution of amyloid precursor protein and amyloid-beta immunoreactivity in DBA/2J glaucomatous mouse retinas. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5085–5090. [Google Scholar] [CrossRef]
- Nizari, S.; Guo, L.; Davis, B.M.; Normando, E.M.; Galvao, J.; Turner, L.A.; Bizrah, M.; Dehabadi, M.; Tian, K.; Cordeiro, M.F. Non-amyloidogenic effects of alpha2 adrenergic agonists: Implications for brimonidine-mediated neuroprotection. Cell. Death Dis. 2016, 7, e2514. [Google Scholar] [CrossRef] [PubMed]
- Feke, G.T.; Bex, P.J.; Taylor, C.P.; Rhee, D.J.; Turalba, A.V.; Chen, T.C.; Wand, M.; Pasquale, L.R. Effect of brimonidine on retinal vascular autoregulation and short-term visual function in normal tension glaucoma. Am. J. Ophthalmol. 2014, 158, 105–112.e101. [Google Scholar] [CrossRef]
- Lonngren, U.; Napankangas, U.; Lafuente, M.; Mayor, S.; Lindqvist, N.; Vidal-Sanz, M.; Hallbook, F. The growth factor response in ischemic rat retina and superior colliculus after brimonidine pre-treatment. Brain Res. Bull. 2006, 71, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Ben Simon, G.J.; Bakalash, S.; Aloni, E.; Rosner, M. A rat model for acute rise in intraocular pressure: Immune modulation as a therapeutic strategy. Am. J. Ophthalmol. 2006, 141, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Namekata, K.; Noro, T.; Nishijima, E.; Sotozono, A.; Guo, X.; Harada, C.; Shinozaki, Y.; Mitamura, Y.; Nakano, T.; Harada, T. Drug combination of topical ripasudil and brimonidine enhances neuroprotection in a mouse model of optic nerve injury. J. Pharmacol. Sci. 2024, 154, 326–333. [Google Scholar] [CrossRef]
- Otsubo, M.; Sase, K.; Tsukahara, C.; Fujita, N.; Arizono, I.; Tokuda, N.; Kitaoka, Y. Axonal protection by combination of ripasudil and brimonidine with upregulation of p-AMPK in TNF-induced optic nerve degeneration. Int. Ophthalmol. 2024, 44, 173. [Google Scholar] [CrossRef]
- Evans, D.W.; Hosking, S.L.; Gherghel, D.; Bartlett, J.D. Contrast sensitivity improves after brimonidine therapy in primary open angle glaucoma: A case for neuroprotection. Br. J. Ophthalmol. 2003, 87, 1463–1465. [Google Scholar] [CrossRef]
- Tsai, J.C.; Chang, H.W. Comparison of the effects of brimonidine 0.2% and timolol 0.5% on retinal nerve fiber layer thickness in ocular hypertensive patients: A prospective, unmasked study. J. Ocul. Pharmacol. Ther. 2005, 21, 475–482. [Google Scholar] [CrossRef]
- Scuteri, D.; Bagetta, G.; Nucci, C.; Aiello, F.; Cesareo, M.; Tonin, P.; Corasaniti, M.T. Evidence on the neuroprotective properties of brimonidine in glaucoma. Prog. Brain Res. 2020, 257, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration. Int. J. Mol. Sci. 2016, 17, 1584. [Google Scholar] [CrossRef]
- Chang, E.E.; Goldberg, J.L. Glaucoma 2.0: Neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 2012, 119, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Saragovi, H.U.; Hamel, E.; Di Polo, A. A neurotrophic rationale for the therapy of neurodegenerative disorders. Curr. Alzheimer Res. 2009, 6, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Aloe, L.; Centofanti, M.; Parisi, V.; Bao, S.N.; Mantelli, F.; Colafrancesco, V.; Manni, G.L.; Bucci, M.G.; Bonini, S.; et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc. Natl. Acad. Sci. USA 2009, 106, 13469–13474. [Google Scholar] [CrossRef] [PubMed]
- Oddone, F.; Roberti, G.; Micera, A.; Busanello, A.; Bonini, S.; Quaranta, L.; Agnifili, L.; Manni, G. Exploring Serum Levels of Brain Derived Neurotrophic Factor and Nerve Growth Factor Across Glaucoma Stages. PLoS ONE 2017, 12, e0168565. [Google Scholar] [CrossRef]
- Pease, M.E.; Zack, D.J.; Berlinicke, C.; Bloom, K.; Cone, F.; Wang, Y.; Klein, R.L.; Hauswirth, W.W.; Quigley, H.A. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2194–2200. [Google Scholar] [CrossRef]
- Grozdanic, S.D.; Lazic, T.; Kuehn, M.H.; Harper, M.M.; Kardon, R.H.; Kwon, Y.H.; Lavik, E.B.; Sakaguchi, D.S. Exogenous modulation of intrinsic optic nerve neuroprotective activity. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1105–1116. [Google Scholar] [CrossRef]
- Parrilla-Reverter, G.; Agudo, M.; Sobrado-Calvo, P.; Salinas-Navarro, M.; Villegas-Perez, M.P.; Vidal-Sanz, M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: A quantitative in vivo study. Exp. Eye Res. 2009, 89, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.J.; Viswanathan, S.; Ramanathan, C.; Harman, C.D. Combined application of BDNF to the eye and brain enhances ganglion cell survival and function in the cat after optic nerve injury. Investig. Ophthalmol. Vis. Sci. 2010, 51, 327–334. [Google Scholar] [CrossRef]
- Flachsbarth, K.; Jankowiak, W.; Kruszewski, K.; Helbing, S.; Bartsch, S.; Bartsch, U. Pronounced synergistic neuroprotective effect of GDNF and CNTF on axotomized retinal ganglion cells in the adult mouse. Exp. Eye Res. 2018, 176, 258–265. [Google Scholar] [CrossRef]
- Hameed, S.S.; Bodi, N.E.; Miller, R.C.; Sharma, T.P. Neuritin 1 Drives Therapeutic Preservation of Retinal Ganglion Cells in an Ex Vivo Human Glaucoma Model. J. Ocul. Pharmacol. Ther. 2024. ahead of print. [Google Scholar] [CrossRef]
- Johnson, T.V.; Bull, N.D.; Martin, K.R. Neurotrophic factor delivery as a protective treatment for glaucoma. Exp. Eye Res. 2011, 93, 196–203. [Google Scholar] [CrossRef]
- Vecino, E.; Garcia-Crespo, D.; Garcia, M.; Martinez-Millan, L.; Sharma, S.C.; Carrascal, E. Rat retinal ganglion cells co-express brain derived neurotrophic factor (BDNF) and its receptor TrkB. Vis. Res. 2002, 42, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.W.; Kim, S.T. Serum and aqueous humor levels of brain-derived neurotrophic factor in patients with primary open-angle glaucoma and normal-tension glaucoma. Int. Ophthalmol. 2021, 41, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Uzel, M.M.; Elgin, U.; Boral, B.; Cicek, M.; Sen, E.; Sener, B.; Yilmazbas, P. The effect of trabeculectomy on serum brain-derived neurotrophic factor levels in primary open-angle glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Lazaldin, M.A.M.; Iezhitsa, I.; Agarwal, R.; Agarwal, P.; Ismail, N.M. Neuroprotective effects of exogenous brain-derived neurotrophic factor on amyloid-beta 1-40-induced retinal degeneration. Neural Regen. Res. 2023, 18, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Binley, K.E.; Ng, W.S.; Barde, Y.A.; Song, B.; Morgan, J.E. Brain-derived neurotrophic factor prevents dendritic retraction of adult mouse retinal ganglion cells. Eur. J. Neurosci. 2016, 44, 2028–2039. [Google Scholar] [CrossRef]
- Shpak, A.A.; Guekht, A.B.; Druzhkova, T.A.; Kozlova, K.I.; Gulyaeva, N.V. Ciliary neurotrophic factor in patients with primary open-angle glaucoma and age-related cataract. Mol. Vis. 2017, 23, 799–809. [Google Scholar]
- Boulton, T.G.; Stahl, N.; Yancopoulos, G.D. Ciliary neurotrophic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J. Biol. Chem. 1994, 269, 11648–11655. [Google Scholar] [CrossRef]
- Escartin, C.; Pierre, K.; Colin, A.; Brouillet, E.; Delzescaux, T.; Guillermier, M.; Dhenain, M.; Deglon, N.; Hantraye, P.; Pellerin, L.; et al. Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J. Neurosci. 2007, 27, 7094–7104. [Google Scholar] [CrossRef]
- Lee, K.; Choi, J.O.; Hwang, A.; Bae, H.W.; Kim, C.Y. Ciliary Neurotrophic Factor Derived From Astrocytes Protects Retinal Ganglion Cells Through PI3K/AKT, JAK/STAT, and MAPK/ERK Pathways. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4. [Google Scholar] [CrossRef]
- Leibinger, M.; Andreadaki, A.; Diekmann, H.; Fischer, D. Neuronal STAT3 activation is essential for CNTF- and inflammatory stimulation-induced CNS axon regeneration. Cell Death Dis. 2013, 4, e805. [Google Scholar] [CrossRef]
- Muller, A.; Hauk, T.G.; Leibinger, M.; Marienfeld, R.; Fischer, D. Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol. Cell Neurosci. 2009, 41, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Mey, J.; Thanos, S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993, 602, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Yip, H.K.; Zhao, R.C.; So, K.F.; Harvey, A.R. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol. Cell Neurosci. 2003, 22, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Winton, M.J.; Rodriguez-Hernandez, N.; Campenot, R.B.; McKerracher, L. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J. Neurosci. 2005, 25, 1113–1121. [Google Scholar] [CrossRef]
- Lingor, P.; Tonges, L.; Pieper, N.; Bermel, C.; Barski, E.; Planchamp, V.; Bahr, M. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain 2008, 131, 250–263. [Google Scholar] [CrossRef]
- Kauper, K.; McGovern, C.; Sherman, S.; Heatherton, P.; Rapoza, R.; Stabila, P.; Dean, B.; Lee, A.; Borges, S.; Bouchard, B.; et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7484–7491. [Google Scholar] [CrossRef]
- Talcott, K.E.; Ratnam, K.; Sundquist, S.M.; Lucero, A.S.; Lujan, B.J.; Tao, W.; Porco, T.C.; Roorda, A.; Duncan, J.L. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2219–2226. [Google Scholar] [CrossRef]
- Zhang, K.; Hopkins, J.J.; Heier, J.S.; Birch, D.G.; Halperin, L.S.; Albini, T.A.; Brown, D.M.; Jaffe, G.J.; Tao, W.; Williams, G.A. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6241–6245. [Google Scholar] [CrossRef]
- Tirassa, P.; Rosso, P.; Iannitelli, A. Ocular Nerve Growth Factor (NGF) and NGF Eye Drop Application as Paradigms to Investigate NGF Neuroprotective and Reparative Actions. Methods Mol. Biol. 2018, 1727, 19–38. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.; Liao, S.; Gao, Y.; Liao, R.; Little, P.J.; Xu, J.; Feng, Z.P.; Zheng, Y.; Zheng, W. Nerve growth factor protects retinal ganglion cells against injury induced by retinal ischemia-reperfusion in rats. Growth. Factors 2015, 33, 149–159. [Google Scholar] [CrossRef]
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Colafrancesco, V.; Parisi, V.; Sposato, V.; Rossi, S.; Russo, M.A.; Coassin, M.; Lambiase, A.; Aloe, L. Ocular application of nerve growth factor protects degenerating retinal ganglion cells in a rat model of glaucoma. J. Glaucoma 2011, 20, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Davis, B.M.; Ravindran, N.; Galvao, J.; Kapoor, N.; Haamedi, N.; Shamsher, E.; Luong, V.; Fico, E.; Cordeiro, M.F. Topical recombinant human Nerve growth factor (rh-NGF) is neuroprotective to retinal ganglion cells by targeting secondary degeneration. Sci. Rep. 2020, 10, 3375. [Google Scholar] [CrossRef] [PubMed]
- Lebrun-Julien, F.; Bertrand, M.J.; De Backer, O.; Stellwagen, D.; Morales, C.R.; Di Polo, A.; Barker, P.A. ProNGF induces TNFalpha-dependent death of retinal ganglion cells through a p75NTR non-cell-autonomous signaling pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 3817–3822. [Google Scholar] [CrossRef]
- Mesentier-Louro, L.A.; Rosso, P.; Carito, V.; Mendez-Otero, R.; Santiago, M.F.; Rama, P.; Lambiase, A.; Tirassa, P. Nerve Growth Factor Role on Retinal Ganglion Cell Survival and Axon Regrowth: Effects of Ocular Administration in Experimental Model of Optic Nerve Injury. Mol. Neurobiol. 2019, 56, 1056–1069. [Google Scholar] [CrossRef]
- Ferrari, M.P.; Mantelli, F.; Sacchetti, M.; Antonangeli, M.I.; Cattani, F.; D’Anniballe, G.; Sinigaglia, F.; Ruffini, P.A.; Lambiase, A. Safety and pharmacokinetics of escalating doses of human recombinant nerve growth factor eye drops in a double-masked, randomized clinical trial. BioDrugs 2014, 28, 275–283. [Google Scholar] [CrossRef]
- Parsons, C.G.; Stoffler, A.; Danysz, W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system–too little activation is bad, too much is even worse. Neuropharmacology 2007, 53, 699–723. [Google Scholar] [CrossRef]
- Osborne, N.N. Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol. 2009, 87, 450–454. [Google Scholar] [CrossRef]
- Hare, W.A.; WoldeMussie, E.; Lai, R.K.; Ton, H.; Ruiz, G.; Chun, T.; Wheeler, L. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, I: Functional measures. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2625–2639. [Google Scholar] [CrossRef]
- Seki, M.; Lipton, S.A. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog. Brain Res. 2008, 173, 495–510. [Google Scholar] [CrossRef]
- Yucel, Y.H.; Gupta, N.; Zhang, Q.; Mizisin, A.P.; Kalichman, M.W.; Weinreb, R.N. Memantine protects neurons from shrinkage in the lateral geniculate nucleus in experimental glaucoma. Arch. Ophthalmol. 2006, 124, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Kim, D.M.; Park, K.H.; Kim, H. Neuroprotective effect of memantine in a rabbit model of optic nerve ischemia. Korean J. Ophthalmol. 2002, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, C.F. The non-human primate experimental glaucoma model. Exp. Eye Res. 2015, 141, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Wamsley, S.; Gabelt, B.T.; Dahl, D.B.; Case, G.L.; Sherwood, R.W.; May, C.A.; Hernandez, M.R.; Kaufman, P.L. Vitreous glutamate concentration and axon loss in monkeys with experimental glaucoma. Arch. Ophthalmol. 2005, 123, 64–70. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Quigley, H.A.; Kerrigan-Baumrind, L.A.; D’Anna, S.A.; Kerrigan, D.; Pease, M.E. Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 975–982. [Google Scholar]
- Vianna, J.R.; Chauhan, B.C. How to detect progression in glaucoma. Prog. Brain Res. 2015, 221, 135–158. [Google Scholar] [CrossRef]
- Eckert, A.; Keil, U.; Scherping, I.; Hauptmann, S.; Muller, W.E. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann. N. Y. Acad. Sci. 2005, 1056, 474–485. [Google Scholar] [CrossRef]
- Ritch, R. Potential role for Ginkgo biloba extract in the treatment of glaucoma. Med. Hypotheses 2000, 54, 221–235. [Google Scholar] [CrossRef]
- Kang, J.M.; Lin, S. Ginkgo biloba and its potential role in glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 116–120. [Google Scholar] [CrossRef]
- Hirooka, K.; Tokuda, M.; Miyamoto, O.; Itano, T.; Baba, T.; Shiraga, F. The Ginkgo biloba extract (EGb 761) provides a neuroprotective effect on retinal ganglion cells in a rat model of chronic glaucoma. Curr. Eye Res. 2004, 28, 153–157. [Google Scholar] [CrossRef]
- Ma, K.; Xu, L.; Zhan, H.; Zhang, S.; Pu, M.; Jonas, J.B. Dosage dependence of the effect of Ginkgo biloba on the rat retinal ganglion cell survival after optic nerve crush. Eye 2009, 23, 1598–1604. [Google Scholar] [CrossRef]
- Ma, K.; Xu, L.; Zhang, H.; Zhang, S.; Pu, M.; Jonas, J.B. The effect of ginkgo biloba on the rat retinal ganglion cell survival in the optic nerve crush model. Acta Ophthalmol. 2010, 88, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Gross, J.; Moore, N.; Do, T.; Huang, A.; Gama, W.; Siesky, B. The effects of antioxidants on ocular blood flow in patients with glaucoma. Acta Ophthalmol. 2018, 96, e237–e241. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kwon, H.J.; Chung, W.S.; Kim, C.Y.; Seong, G.J. Short-term effects of Ginkgo biloba extract on peripapillary retinal blood flow in normal tension glaucoma. Korean J. Ophthalmol. 2011, 25, 323–328. [Google Scholar] [CrossRef]
- Faiq, M.A.; Wollstein, G.; Schuman, J.S.; Chan, K.C. Cholinergic nervous system and glaucoma: From basic science to clinical applications. Prog. Retin. Eye Res. 2019, 72, 100767. [Google Scholar] [CrossRef]
- Roberti, G.; Tanga, L.; Michelessi, M.; Quaranta, L.; Parisi, V.; Manni, G.; Oddone, F. Cytidine 5’-Diphosphocholine (Citicoline) in Glaucoma: Rationale of Its Use, Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2015, 16, 28401–28417. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F.; Dempsey, R.J. Effects of citicoline on phospholipid and glutathione levels in transient cerebral ischemia. Stroke 2001, 32, 2376–2381. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, Y.; Yang, X.; Ho, L.C.; Yu, Y.; Chau, Y.; Leung, C.K.-S.; Conner, I.P.; Steketee, M.B.; Wollstein, G.; Schuman, J.S.; et al. Citicoline preserves optic nerve integrity and visuomotor function following chronic intraocular pressure elevation. Investig. Ophthalmol. Vis. Sci. 2016, 57. [Google Scholar]
- Virno, M.; Pecori-Giraldi, J.; Liguori, A.; De Gregorio, F. The protective effect of citicoline on the progression of the perimetric defects in glaucomatous patients (perimetric study with a 10-year follow-up). Acta Ophthalmol. Scand. Suppl. 2000, 78, 56–57. [Google Scholar] [CrossRef]
- Parisi, V. Electrophysiological assessment of glaucomatous visual dysfunction during treatment with cytidine-5’-diphosphocholine (citicoline): A study of 8 years of follow-up. Doc. Ophthalmol. 2005, 110, 91–102. [Google Scholar] [CrossRef]
- Rejdak, R.; Toczolowski, J.; Kurkowski, J.; Kaminski, M.L.; Rejdak, K.; Stelmasiak, Z.; Grieb, P. Oral citicoline treatment improves visual pathway function in glaucoma. Med. Sci. Monit. 2003, 9, PI24-8. [Google Scholar] [PubMed]
- Parisi, V.; Coppola, G.; Centofanti, M.; Oddone, F.; Angrisani, A.M.; Ziccardi, L.; Ricci, B.; Quaranta, L.; Manni, G. Evidence of the neuroprotective role of citicoline in glaucoma patients. Prog. Brain Res. 2008, 173, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Ottobelli, L.; Manni, G.L.; Centofanti, M.; Iester, M.; Allevena, F.; Rossetti, L. Citicoline oral solution in glaucoma: Is there a role in slowing disease progression? Ophthalmologica 2013, 229, 219–226. [Google Scholar] [CrossRef]
- Rossetti, L.; Iester, M.; Tranchina, L.; Ottobelli, L.; Coco, G.; Calcatelli, E.; Ancona, C.; Cirafici, P.; Manni, G. Can Treatment With Citicoline Eyedrops Reduce Progression in Glaucoma? The Results of a Randomized Placebo-controlled Clinical Trial. J. Glaucoma 2020, 29, 513–520. [Google Scholar] [CrossRef]
- Parisi, V.; Centofanti, M.; Ziccardi, L.; Tanga, L.; Michelessi, M.; Roberti, G.; Manni, G. Treatment with citicoline eye drops enhances retinal function and neural conduction along the visual pathways in open angle glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1327–1340. [Google Scholar] [CrossRef]
- Parisi, V.; Oddone, F.; Roberti, G.; Tanga, L.; Carnevale, C.; Ziccardi, L.; Manni, G. Enhancement of Retinal Function and of Neural Conduction Along the Visual Pathway Induced by Treatment with Citicoline Eye Drops in Liposomal Formulation in Open Angle Glaucoma: A Pilot Electrofunctional Study. Adv. Ther. 2019, 36, 987–996. [Google Scholar] [CrossRef]
- Roberti, G.; Tanga, L.; Parisi, V.; Sampalmieri, M.; Centofanti, M.; Manni, G. A preliminary study of the neuroprotective role of citicoline eye drops in glaucomatous optic neuropathy. Indian J. Ophthalmol. 2014, 62, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Grieb, P.; Junemann, A.; Rekas, M.; Rejdak, R. Citicoline: A Food Beneficial for Patients Suffering from or Threated with Glaucoma. Front. Aging Neurosci. 2016, 8, 73. [Google Scholar] [CrossRef]
- Anton, A.; Garcia, V.; Munoz, M.; Gonzales, K.; Ayala, E.; Del Mar Sanchez, E.; Morilla-Grasa, A. The Effect of Oral Citicoline and Docosahexaenoic Acid on the Visual Field of Patients with Glaucoma: A Randomized Trial. Life 2022, 12, 1481. [Google Scholar] [CrossRef]
- Marino, P.F.; Rossi, G.C.M.; Campagna, G.; Capobianco, D.; Costagliola, C.; on behalf of QUALICOS Study Group. Effects of Citicoline, Homotaurine, and Vitamin E on Contrast Sensitivity and Visual-Related Quality of Life in Patients with Primary Open-Angle Glaucoma: A Preliminary Study. Molecules 2020, 25, 5614. [Google Scholar] [CrossRef]
- Bonechi, C.; Mahdizadeh, F.F.; Talarico, L.; Pepi, S.; Tamasi, G.; Leone, G.; Consumi, M.; Donati, A.; Magnani, A. Liposomal Encapsulation of Citicoline for Ocular Drug Delivery. Int. J. Mol. Sci. 2023, 24, 16864. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. NAD(+) in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Cimaglia, G.; Votruba, M.; Morgan, J.E.; Andre, H.; Williams, P.A. Potential Therapeutic Benefit of NAD(+) Supplementation for Glaucoma and Age-Related Macular Degeneration. Nutrients 2020, 12, 2871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ying, W. NAD(+) Deficiency Is a Common Central Pathological Factor of a Number of Diseases and Aging: Mechanisms and Therapeutic Implications. Antioxid Redox Signal 2019, 30, 890–905. [Google Scholar] [CrossRef]
- Araie, M.; Mayama, C. Use of calcium channel blockers for glaucoma. Prog. Retin. Eye Res. 2011, 30, 54–71. [Google Scholar] [CrossRef]
- Pasquale, L.R. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr. Opin. Ophthalmol. 2016, 27, 94–101. [Google Scholar] [CrossRef]
- Resch, H.; Garhofer, G.; Fuchsjager-Mayrl, G.; Hommer, A.; Schmetterer, L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009, 87, 4–12. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cardozo, B.H.; Cochran, K.E.; John, S.W.M. Nicotinamide and WLD(S) Act Together to Prevent Neurodegeneration in Glaucoma. Front. Neurosci. 2017, 11, 232. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cochran, K.E.; Philip, V.M.; Porciatti, V.; Smithies, O.; John, S.W. Vitamin B(3) modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017, 355, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Tribble, J.R.; Otmani, A.; Sun, S.; Ellis, S.A.; Cimaglia, G.; Vohra, R.; Joe, M.; Lardner, E.; Venkataraman, A.P.; Dominguez-Vicent, A.; et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol 2021, 43, 101988. [Google Scholar] [CrossRef]
- Yu, N.; Wu, X.; Zhang, C.; Qin, Q.; Gu, Y.; Ke, W.; Liu, X.; Zhang, Q.; Liu, Z.; Chen, M.; et al. NADPH and NAC synergistically inhibits chronic ocular hypertension-induced neurodegeneration and neuroinflammation through regulating p38/MAPK pathway and peroxidation. Biomed. Pharmacother. 2024, 175, 116711. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.Y.; Rhim, W.K.; Cimaglia, G.; Want, A.; Morgan, J.E.; Williams, P.A.; Park, C.G.; Han, D.K.; Rho, S. Extracellular vesicle encapsulated nicotinamide delivered via a trans-scleral route provides retinal ganglion cell neuroprotection. Acta Neuropathol. Commun. 2024, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Felici, R.; Lapucci, A.; Ramazzotti, M.; Chiarugi, A. Insight into molecular and functional properties of NMNAT3 reveals new hints of NAD homeostasis within human mitochondria. PLoS ONE 2013, 8, e76938. [Google Scholar] [CrossRef]
- Tribble, J.R.; Joe, M.; Varricchio, C.; Otmani, A.; Canovai, A.; Habchi, B.; Daskalakis, E.; Chaleckis, R.; Loreto, A.; Gilley, J.; et al. NMNAT2 is a druggable target to drive neuronal NAD production. Nat. Commun. 2024, 15, 6256. [Google Scholar] [CrossRef]
- Kouassi Nzoughet, J.; Chao de la Barca, J.M.; Guehlouz, K.; Leruez, S.; Coulbault, L.; Allouche, S.; Bocca, C.; Muller, J.; Amati-Bonneau, P.; Gohier, P.; et al. Nicotinamide Deficiency in Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2509–2514. [Google Scholar] [CrossRef]
- Hui, F.; Tang, J.; Williams, P.A.; McGuinness, M.B.; Hadoux, X.; Casson, R.J.; Coote, M.; Trounce, I.A.; Martin, K.R.; van Wijngaarden, P.; et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin. Exp. Ophthalmol. 2020, 48, 903–914. [Google Scholar] [CrossRef]
- De Moraes, C.G.; John, S.W.M.; Williams, P.A.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M. Nicotinamide and Pyruvate for Neuroenhancement in Open-Angle Glaucoma: A Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.K.S.; Ren, S.T.; Chan, P.P.M.; Wan, K.H.N.; Kam, A.K.W.; Lai, G.W.K.; Chiu, V.S.M.; Ko, M.W.L.; Yiu, C.K.F.; Yu, M.C.Y. Nicotinamide riboside as a neuroprotective therapy for glaucoma: Study protocol for a randomized, double-blind, placebo-control trial. Trials 2022, 23, 45. [Google Scholar] [CrossRef]
- Mehmel, M.; Jovanovic, N.; Spitz, U. Nicotinamide Riboside-The Current State of Research and Therapeutic Uses. Nutrients 2020, 12, 1616. [Google Scholar] [CrossRef]
- Di Polo, A. Dendrite pathology and neurodegeneration: Focus on mTOR. Neural Regen. Res. 2015, 10, 559–561. [Google Scholar] [CrossRef]
- Agostinone, J.; Alarcon-Martinez, L.; Gamlin, C.; Yu, W.Q.; Wong, R.O.L.; Di Polo, A. Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 2018, 141, 1963–1980. [Google Scholar] [CrossRef] [PubMed]
- Jhanwar-Uniyal, M.; Wainwright, J.V.; Mohan, A.L.; Tobias, M.E.; Murali, R.; Gandhi, C.D.; Schmidt, M.H. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 2019, 72, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Kalaitzidis, G.; Malli, A.; Kalaitzoglou, D.; Myserlis, P.G.; Lioutas, V.A. Intranasal insulin in Alzheimer’s dementia or mild cognitive impairment: A systematic review. J. Neurol. 2018, 265, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef]
- Wennberg Smith, Z.; Beykin, G.; Saludares, M.; Nunez, M.; Wang, Q.; Di Polo, A.; Goldberg, J.L. A Phase 1 Trial of Topical Insulin for Patients with Glaucoma. Investig. Ophthalmol. Vis. Sci. 2024, 65, 668. [Google Scholar]
- Pezzuto, J.M. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef]
- Pang, Y.; Qin, M.; Hu, P.; Ji, K.; Xiao, R.; Sun, N.; Pan, X.; Zhang, X. Resveratrol protects retinal ganglion cells against ischemia induced damage by increasing Opa1 expression. Int. J. Mol. Med. 2020, 46, 1707–1720. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, Y.; Wei, W.; Shao, A.; Deng, C.; Li, X.; Chang, H.; Hu, P.; Liu, X.; Zhang, X. Resveratrol protects retinal ganglion cell axons through regulation of the SIRT1-JNK pathway. Exp. Eye Res. 2020, 200, 108249. [Google Scholar] [CrossRef]
- Ji, K.; Li, Z.; Lei, Y.; Xu, W.; Ouyang, L.; He, T.; Xing, Y. Resveratrol attenuates retinal ganglion cell loss in a mouse model of retinal ischemia reperfusion injury via multiple pathways. Exp. Eye Res. 2021, 209, 108683. [Google Scholar] [CrossRef]
- Luo, H.; Zhuang, J.; Hu, P.; Ye, W.; Chen, S.; Pang, Y.; Li, N.; Deng, C.; Zhang, X. Resveratrol Delays Retinal Ganglion Cell Loss and Attenuates Gliosis-Related Inflammation From Ischemia-Reperfusion Injury. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3879–3888. [Google Scholar] [CrossRef]
- Cao, K.; Ishida, T.; Fang, Y.; Shinohara, K.; Li, X.; Nagaoka, N.; Ohno-Matsui, K.; Yoshida, T. Protection of the Retinal Ganglion Cells: Intravitreal Injection of Resveratrol in Mouse Model of Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef]
- Chen, S.; Zhi, Z.; Ruan, Q.; Liu, Q.; Li, F.; Wan, F.; Reinach, P.S.; Chen, J.; Qu, J.; Zhou, X. Bright Light Suppresses Form-Deprivation Myopia Development With Activation of Dopamine D1 Receptor Signaling in the ON Pathway in Retina. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2306–2316. [Google Scholar] [CrossRef]
- Lin, J.; Xue, J.; Xu, Q.; Liu, Z.; Zhao, C.; Tang, J.; Han, J.; A, S.; Wang, W.; Zhuo, Y.; et al. In situ-crosslinked hydrogel-induced experimental glaucoma model with persistent ocular hypertension and neurodegeneration. Biomater. Sci. 2022, 10, 5006–5017. [Google Scholar] [CrossRef]
- Zhang, Q.; Xue, J.; Tang, J.; Wu, S.; Liu, Z.; Wu, C.; Liu, C.; Liu, Y.; Lin, J.; Han, J.; et al. Modulating amacrine cell-derived dopamine signaling promotes optic nerve regeneration and preserves visual function. Sci. Adv. 2024, 10, eado0866. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Takaseki, S.; Yoshitomi, T. Effects of ripasudil hydrochloride hydrate (K-115), a Rho-kinase inhibitor, on ocular blood flow and ciliary artery smooth muscle contraction in rabbits. Jpn. J. Ophthalmol. 2017, 61, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Tanna, A.P.; Johnson, M. Rho Kinase Inhibitors as a Novel Treatment for Glaucoma and Ocular Hypertension. Ophthalmology 2018, 125, 1741–1756. [Google Scholar] [CrossRef]
- Nakamura, N.; Honjo, M.; Yamagishi-Kimura, R.; Sakata, R.; Watanabe, S.; Aihara, M. Neuroprotective effect of omidenepag on excitotoxic retinal ganglion cell death regulating COX-2-EP2-cAMP-PKA/Epac pathway via Neuron-Glia interaction. Neuroscience 2024, 553, 145–159. [Google Scholar] [CrossRef]
- Kapic, A.; Zaman, K.; Nguyen, V.; Neagu, G.C.; Sumien, N.; Prokai, L.; Prokai-Tatrai, K. The Prodrug DHED Delivers 17beta-Estradiol into the Retina for Protection of Retinal Ganglion Cells and Preservation of Visual Function in an Animal Model of Glaucoma. Cells 2024, 13, 1126. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, S.Y.; Zhang, L.J.; Lei, B.; Wang, Y.C.; Wang, Z. Neuroprotection of the P2X7 receptor antagonist A740003 on retinal ganglion cells in experimental glaucoma. Neuroreport 2024, 35, 822–831. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, S.; Chen, X.; Rong, Y.; Huang, L.; Chen, Z.; Yao, K.; Chen, W.; Deng, C.; Wang, J. Irisin attenuates acute glaucoma-induced neuroinflammation by activating microglia-integrin alphaVbeta5/AMPK and promoting autophagy. Int. Immunopharmacol. 2024, 138, 112545. [Google Scholar] [CrossRef]
- Wu, L.H.; Cheng, Y.W.; Lin, F.L.; Hsu, K.C.; Wang, M.H.; Yen, J.L.; Wang, T.J.; Lin, T.E.; Liu, Y.C.; Huang, W.J.; et al. A novel HDAC8 inhibitor H7E exerts retinoprotective effects against glaucomatous injury via ameliorating aberrant Muller glia activation and oxidative stress. Biomed. Pharmacother. 2024, 174, 116538. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; Gallego-Ortega, A.; Norte-Munoz, M.; Vidal-Villegas, B.; Bravo, I.; Boluda-Ruiz, M.; Bernal-Garro, J.M.; Fernandez-Bueno, I.; Pastor-Jimeno, J.C.; Villegas-Perez, M.P.; et al. Evaluation of the neuroprotective efficacy of the gramine derivative ITH12657 against NMDA-induced excitotoxicity in the rat retina. Front. Neuroanat. 2024, 18, 1335176. [Google Scholar] [CrossRef]
- Dahlmann-Noor, A.; Vijay, S.; Jayaram, H.; Limb, A.; Khaw, P.T. Current approaches and future prospects for stem cell rescue and regeneration of the retina and optic nerve. Can. J. Ophthalmol. 2010, 45, 333–341. [Google Scholar] [CrossRef]
- Fu, L.; Kwok, S.S.; Chan, Y.K.; Ming Lai, J.S.; Pan, W.; Nie, L.; Shih, K.C. Therapeutic Strategies for Attenuation of Retinal Ganglion Cell Injury in Optic Neuropathies: Concepts in Translational Research and Therapeutic Implications. Biomed. Res. Int. 2019, 2019, 8397521. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, J.; Starr, C.; Mohns, E.J.; Li, Y.; Chen, E.P.; Yoon, Y.; Kellner, C.P.; Tanaka, K.; Wang, H.; et al. Preservation of vision after CaMKII-mediated protection of retinal ganglion cells. Cell 2021, 184, 4299–4314.e4212. [Google Scholar] [CrossRef]
- Bull, N.D.; Johnson, T.V.; Martin, K.R. Stem cells for neuroprotection in glaucoma. Prog. Brain Res. 2008, 173, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.Y.; Xin, M.; Chen, M.; Yu, P.; Zeng, L.Z. Mesenchymal stem cells for repairing glaucomatous optic nerve. Int. J. Ophthalmol. 2024, 17, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Fellabaum, C.; Arsenijevic, A.; Markovic, B.S.; Djonov, V.; Volarevic, V. Therapeutic Potential of Mesenchymal Stem Cells and Their Secretome in the Treatment of Glaucoma. Stem Cells Int. 2019, 2019, 7869130. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.V.; DeKorver, N.W.; Levasseur, V.A.; Osborne, A.; Tassoni, A.; Lorber, B.; Heller, J.P.; Villasmil, R.; Bull, N.D.; Martin, K.R.; et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2014, 137, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Moisseiev, E.; Bauer, G.; Anderson, J.D.; Grant, M.B.; Zam, A.; Zawadzki, R.J.; Werner, J.S.; Nolta, J.A. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog. Retin. Eye Res. 2017, 56, 148–165. [Google Scholar] [CrossRef]
- Crigler, L.; Robey, R.C.; Asawachaicharn, A.; Gaupp, D.; Phinney, D.G. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp. Neurol. 2006, 198, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Emre, E.; Yuksel, N.; Duruksu, G.; Pirhan, D.; Subasi, C.; Erman, G.; Karaoz, E. Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow-derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy 2015, 17, 543–559. [Google Scholar] [CrossRef]
- Harper, M.M.; Grozdanic, S.D.; Blits, B.; Kuehn, M.H.; Zamzow, D.; Buss, J.E.; Kardon, R.H.; Sakaguchi, D.S. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4506–4515. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.V.; Bull, N.D.; Hunt, D.P.; Marina, N.; Tomarev, S.I.; Martin, K.R. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2051–2059. [Google Scholar] [CrossRef]
- Mead, B.; Amaral, J.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in Rodent Models of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Roubeix, C.; Godefroy, D.; Mias, C.; Sapienza, A.; Riancho, L.; Degardin, J.; Fradot, V.; Ivkovic, I.; Picaud, S.; Sennlaub, F.; et al. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res. Ther. 2015, 6, 177. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, J.; Huang, C.; Li, X.; Chen, Y.; Wu, W.; Wu, R. Human Umbilical Cord-Mesenchymal Stem Cells Survive and Migrate within the Vitreous Cavity and Ameliorate Retinal Damage in a Novel Rat Model of Chronic Glaucoma. Stem Cells Int. 2021, 2021, 8852517. [Google Scholar] [CrossRef]
- Osborne, A.; Sanderson, J.; Martin, K.R. Neuroprotective Effects of Human Mesenchymal Stem Cells and Platelet-Derived Growth Factor on Human Retinal Ganglion Cells. Stem Cells 2018, 36, 65–78. [Google Scholar] [CrossRef]
- Vilela, C.A.P.; Messias, A.; Calado, R.T.; Siqueira, R.C.; Silva, M.J.L.; Covas, D.T.; Paula, J.S. Retinal function after intravitreal injection of autologous bone marrow-derived mesenchymal stromal cells in advanced glaucoma. Doc. Ophthalmol. 2021, 143, 33–38. [Google Scholar] [CrossRef]
- Lopez Sanchez, M.I.; Crowston, J.G.; Mackey, D.A.; Trounce, I.A. Emerging Mitochondrial Therapeutic Targets in Optic Neuropathies. Pharmacol. Ther. 2016, 165, 132–152. [Google Scholar] [CrossRef]
- Chao, J.R.; Lamba, D.A.; Klesert, T.R.; Torre, A.; Hoshino, A.; Taylor, R.J.; Jayabalu, A.; Engel, A.L.; Khuu, T.H.; Wang, R.K.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Cells into the Subretinal Space of a Non-Human Primate. Transl. Vis. Sci. Technol. 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Sluch, V.M.; Davis, C.H.; Ranganathan, V.; Kerr, J.M.; Krick, K.; Martin, R.; Berlinicke, C.A.; Marsh-Armstrong, N.; Diamond, J.S.; Mao, H.Q.; et al. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci. Rep. 2015, 5, 16595. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, P.; Wang, Y.; Nguyen, T.; Huang, A.; Muller, K.J.; Goldberg, J.L. Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 2016, 7, 10472. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Guo, C.; Guo, C.; Sun, Y.; Liao, T.; Beattie, U.; Lopez, F.J.; Chen, D.F.; Lashkari, K. Transplantation of Human Neural Progenitor Cells Expressing IGF-1 Enhances Retinal Ganglion Cell Survival. PLoS ONE 2015, 10, e0125695. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, X.B.; Xiong, S.Q. Neuro-protection of retinal stem cells transplantation combined with copolymer-1 immunization in a rat model of glaucoma. Mol. Cell Neurosci. 2013, 54, 1–8. [Google Scholar] [CrossRef]
- Oswald, J.; Kegeles, E.; Minelli, T.; Volchkov, P.; Baranov, P. Transplantation of miPSC/mESC-derived retinal ganglion cells into healthy and glaucomatous retinas. Mol. Ther. Methods Clin. Dev. 2021, 21, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Suen, H.C.; Qian, Y.; Liao, J.; Luk, C.S.; Lee, W.T.; Ng, J.K.W.; Chan, T.T.H.; Hou, H.W.; Li, I.; Li, K.; et al. Transplantation of Retinal Ganglion Cells Derived from Male Germline Stem Cell as a Potential Treatment to Glaucoma. Stem Cells Dev. 2019, 28, 1365–1375. [Google Scholar] [CrossRef]
- Qin, Y.; Ge, G.; Yang, P.; Wang, L.; Qiao, Y.; Pan, G.; Yang, H.; Bai, J.; Cui, W.; Geng, D. An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity. Adv. Sci. 2023, 10, e2207334. [Google Scholar] [CrossRef]
- Greco, S.J.; Rameshwar, P. Microenvironmental considerations in the application of human mesenchymal stem cells in regenerative therapies. Biologics 2008, 2, 699–705. [Google Scholar] [CrossRef]
- Kasetty, M.A.; Hedges, T.R., 3rd; Witkin, A.J. Bilateral Epiretinal Membrane Formation after Intravitreal Injections of Autologous Mesenchymal Stem Cells. Retin. Cases Brief Rep. 2022, 16, 561–564. [Google Scholar] [CrossRef]
- Jain, A.; Zode, G.; Kasetti, R.B.; Ran, F.A.; Yan, W.; Sharma, T.P.; Bugge, K.; Searby, C.C.; Fingert, J.H.; Zhang, F.; et al. CRISPR-Cas9-based treatment of myocilin-associated glaucoma. Proc. Natl. Acad. Sci. USA 2017, 114, 11199–11204. [Google Scholar] [CrossRef] [PubMed]
- Souma, T.; Tompson, S.W.; Thomson, B.R.; Siggs, O.M.; Kizhatil, K.; Yamaguchi, S.; Feng, L.; Limviphuvadh, V.; Whisenhunt, K.N.; Maurer-Stroh, S.; et al. Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. J. Clin. Investig. 2016, 126, 2575–2587. [Google Scholar] [CrossRef]
- Thomson, B.R.; Heinen, S.; Jeansson, M.; Ghosh, A.K.; Fatima, A.; Sung, H.K.; Onay, T.; Chen, H.; Yamaguchi, S.; Economides, A.N.; et al. A lymphatic defect causes ocular hypertension and glaucoma in mice. J. Clin. Investig. 2014, 124, 4320–4324. [Google Scholar] [CrossRef] [PubMed]
- Osborne, A.; Khatib, T.Z.; Songra, L.; Barber, A.C.; Hall, K.; Kong, G.Y.X.; Widdowson, P.S.; Martin, K.R. Neuroprotection of retinal ganglion cells by a novel gene therapy construct that achieves sustained enhancement of brain-derived neurotrophic factor/tropomyosin-related kinase receptor-B signaling. Cell Death Dis. 2018, 9, 1007. [Google Scholar] [CrossRef]
- Osborne, A.; Wang, A.X.Z.; Tassoni, A.; Widdowson, P.S.; Martin, K.R. Design of a Novel Gene Therapy Construct to Achieve Sustained Brain-Derived Neurotrophic Factor Signaling in Neurons. Hum. Gene Ther. 2018, 29, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Khatib, T.Z.; Osborne, A.; Yang, S.; Ali, Z.; Jia, W.; Manyakin, I.; Hall, K.; Watt, R.; Widdowson, P.S.; Martin, K.R. Receptor-ligand supplementation via a self-cleaving 2A peptide-based gene therapy promotes CNS axonal transport with functional recovery. Sci. Adv. 2021, 7, eabd2590. [Google Scholar] [CrossRef]
- Wojcik-Gryciuk, A.; Gajewska-Wozniak, O.; Kordecka, K.; Boguszewski, P.M.; Waleszczyk, W.; Skup, M. Neuroprotection of Retinal Ganglion Cells with AAV2-BDNF Pretreatment Restoring Normal TrkB Receptor Protein Levels in Glaucoma. Int. J. Mol. Sci. 2020, 21, 6262. [Google Scholar] [CrossRef]
- Alqawlaq, S.; Sivak, J.M.; Huzil, J.T.; Ivanova, M.V.; Flanagan, J.G.; Beazely, M.A.; Foldvari, M. Preclinical development and ocular biodistribution of gemini-DNA nanoparticles after intravitreal and topical administration: Towards non-invasive glaucoma gene therapy. Nanomedicine 2014, 10, 1637–1647. [Google Scholar] [CrossRef]
- Cen, L.P.; Liang, J.J.; Chen, J.H.; Harvey, A.R.; Ng, T.K.; Zhang, M.; Pang, C.P.; Cui, Q.; Fan, Y.M. AAV-mediated transfer of RhoA shRNA and CNTF promotes retinal ganglion cell survival and axon regeneration. Neuroscience 2017, 343, 472–482. [Google Scholar] [CrossRef]
- Hellstrom, M.; Pollett, M.A.; Harvey, A.R. Post-injury delivery of rAAV2-CNTF combined with short-term pharmacotherapy is neuroprotective and promotes extensive axonal regeneration after optic nerve trauma. J. Neurotrauma 2011, 28, 2475–2483. [Google Scholar] [CrossRef]
- Do Rhee, K.; Wang, Y.; Ten Hoeve, J.; Stiles, L.; Nguyen, T.T.T.; Zhang, X.; Vergnes, L.; Reue, K.; Shirihai, O.; Bok, D.; et al. Ciliary neurotrophic factor-mediated neuroprotection involves enhanced glycolysis and anabolism in degenerating mouse retinas. Nat. Commun. 2022, 13, 7037. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.; Guido, B.; Narsineni, L.; Chen, D.W.; Foldvari, M. Gene therapy strategies for glaucoma from IOP reduction to retinal neuroprotection: Progress towards non-viral systems. Adv. Drug. Deliv. Rev. 2023, 196, 114781. [Google Scholar] [CrossRef]
- Bosco, A.; Anderson, S.R.; Breen, K.T.; Romero, C.O.; Steele, M.R.; Chiodo, V.A.; Boye, S.L.; Hauswirth, W.W.; Tomlinson, S.; Vetter, M.L. Complement C3-Targeted Gene Therapy Restricts Onset and Progression of Neurodegeneration in Chronic Mouse Glaucoma. Mol. Ther. 2018, 26, 2379–2396. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xiao, R.; Bair, J.; Wang, F.; Vandenberghe, L.H.; Dartt, D.; Baranov, P.; Ng, Y.S.E. Novel engineered, membrane-localized variants of vascular endothelial growth factor (VEGF) protect retinal ganglion cells: A proof-of-concept study. Cell Death Dis. 2018, 9, 1018. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, P.; Huang, H.; Li, L.; Liu, L.; Webber, H.C.; Dalal, R.; Siew, L.; Fligor, C.M.; Chang, K.C.; et al. Mouse gamma-Synuclein Promoter-Mediated Gene Expression and Editing in Mammalian Retinal Ganglion Cells. J. Neurosci. 2020, 40, 3896–3914. [Google Scholar] [CrossRef] [PubMed]
- Donahue, R.J.; Fehrman, R.L.; Gustafson, J.R.; Nickells, R.W. BCLX(L) gene therapy moderates neuropathology in the DBA/2J mouse model of inherited glaucoma. Cell Death Dis. 2021, 12, 781. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhuang, P.; Feng, X.; Liu, P.; Liu, D.; Huang, H.; Li, L.; Chen, W.; Liu, L.; Sun, Y.; et al. NMNAT2 is downregulated in glaucomatous RGCs, and RGC-specific gene therapy rescues neurodegeneration and visual function. Mol. Ther. 2022, 30, 1421–1431. [Google Scholar] [CrossRef]
- Lani-Louzada, R.; Marra, C.; Dias, M.S.; de Araujo, V.G.; Abreu, C.A.; Ribas, V.T.; Adesse, D.; Allodi, S.; Chiodo, V.; Hauswirth, W.; et al. Neuroprotective Gene Therapy by Overexpression of the Transcription Factor MAX in Rat Models of Glaucomatous Neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2022, 63, 5. [Google Scholar] [CrossRef]
- Visuvanathan, S.; Baker, A.N.; Lagali, P.S.; Coupland, S.G.; Miller, G.; Hauswirth, W.W.; Tsilfidis, C. XIAP gene therapy effects on retinal ganglion cell structure and function in a mouse model of glaucoma. Gene Ther. 2022, 29, 147–156. [Google Scholar] [CrossRef]
- Jiang, W.; Tang, L.; Zeng, J.; Chen, B. Adeno-associated virus mediated SOD gene therapy protects the retinal ganglion cells from chronic intraocular pressure elevation induced injury via attenuating oxidative stress and improving mitochondrial dysfunction in a rat model. Am. J. Transl. Res. 2016, 8, 799–810. [Google Scholar]
- Petrova, V.; Pearson, C.S.; Ching, J.; Tribble, J.R.; Solano, A.G.; Yang, Y.; Love, F.M.; Watt, R.J.; Osborne, A.; Reid, E.; et al. Protrudin functions from the endoplasmic reticulum to support axon regeneration in the adult CNS. Nat. Commun. 2020, 11, 5614. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, J.; Tomczak, J.; Lisek, M.; Sakowicz, A.; Guo, F.; Boczek, T. NFATc4 Knockout Promotes Neuroprotection and Retinal Ganglion Cell Regeneration After Optic Nerve Injury. Mol. Neurobiol. 2024. ahead of print. [Google Scholar] [CrossRef]
- Thananthirige, K.P.M.; Chitranshi, N.; Basavarajappa, D.; Rajput, R.; Abbasi, M.; Palanivel, V.; Gupta, V.B.; Paulo, J.A.; Koronyo-Hamaoui, M.; Mirzaei, M.; et al. Tau modulation through AAV9 therapy augments Akt/Erk survival signalling in glaucoma mitigating the retinal degenerative phenotype. Acta Neuropathol. Commun. 2024, 12, 89. [Google Scholar] [CrossRef]
- Brown, M.D.; Starikovskaya, E.; Derbeneva, O.; Hosseini, S.; Allen, J.C.; Mikhailovskaya, I.E.; Sukernik, R.I.; Wallace, D.C. The role of mtDNA background in disease expression: A new primary LHON mutation associated with Western Eurasian haplogroup. J. Hum. Genet. 2002, 110, 130–138. [Google Scholar] [CrossRef]
- Alexander, C.; Votruba, M.; Pesch, U.E.; Thiselton, D.L.; Mayer, S.; Moore, A.; Rodriguez, M.; Kellner, U.; Leo-Kottler, B.; Auburger, G.; et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 2000, 26, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Nunez-Alvarez, C.; Joglar, B.; Del Olmo-Aguado, S. Glaucoma: Focus on mitochondria in relation to pathogenesis and neuroprotection. Eur. J. Pharmacol. 2016, 787, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Pappenhagen, N.; Palmer, P.G.; Smith, M.A.; Gevorgyan, V.; Wilson, G.N.; Crish, S.D.; Inman, D.M. Structural and Functional Rescue of Chronic Metabolically Stressed Optic Nerves through Respiration. J. Neurosci. 2018, 38, 5122–5139. [Google Scholar] [CrossRef] [PubMed]
- Oddone, F.; Rossetti, L.; Parravano, M.; Sbardella, D.; Coletta, M.; Ziccardi, L.; Roberti, G.; Carnevale, C.; Romano, D.; Manni, G.; et al. Citicoline in Ophthalmological Neurodegenerative Disease: A Comprehensive Review. Pharmaceuticals 2021, 14, 281. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; Cardozo, B.H.; Foxworth, N.E.; John, S.W.M. Nicotinamide treatment robustly protects from inherited mouse glaucoma. Commun. Integr. Biol. 2018, 11, e1356956. [Google Scholar] [CrossRef]
- Lanza, M.; Gironi Carnevale, U.A.; Mele, L.; Bifani Sconocchia, M.; Bartollino, S.; Costagliola, C. Morphological and Functional Evaluation of Oral Citicoline Therapy in Chronic Open-Angle Glaucoma Patients: A Pilot Study With a 2-Year Follow-Up. Front. Pharmacol. 2019, 10, 1117. [Google Scholar] [CrossRef]
- Gabelein, C.G.; Feng, Q.; Sarajlic, E.; Zambelli, T.; Guillaume-Gentil, O.; Kornmann, B.; Vorholt, J.A. Mitochondria transplantation between living cells. PLoS Biol. 2022, 20, e3001576. [Google Scholar] [CrossRef]
- Nascimento-Dos-Santos, G.; de-Souza-Ferreira, E.; Lani, R.; Faria, C.C.; Araujo, V.G.; Teixeira-Pinheiro, L.C.; Vasconcelos, T.; Goncalo, T.; Santiago, M.F.; Linden, R.; et al. Neuroprotection from optic nerve injury and modulation of oxidative metabolism by transplantation of active mitochondria to the retina. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165686. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Han, Y.; Wang, F.; Su, Y. Progress in exosomes and their potential use in ocular diseases. Int. J. Ophthalmol. 2020, 13, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef]
- Pan, D.; Chang, X.; Xu, M.; Zhang, M.; Zhang, S.; Wang, Y.; Luo, X.; Xu, J.; Yang, X.; Sun, X. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J. Chem. Neuroanat. 2019, 96, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, K.; Hao, H.; Liu, Y.; Yue, Y.; Li, X.; Xing, X.; Zhang, X. Small extracellular vesicles derived from microRNA-22-3p-overexpressing mesenchymal stem cells protect retinal ganglion cells by regulating MAPK pathway. Commun. Biol. 2024, 7, 807. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef]
- Giannaccini, M.; Usai, A.; Chiellini, F.; Guadagni, V.; Andreazzoli, M.; Ori, M.; Pasqualetti, M.; Dente, L.; Raffa, V. Neurotrophin-conjugated nanoparticles prevent retina damage induced by oxidative stress. Cell Mol Life Sci. 2018, 75, 1255–1267. [Google Scholar] [CrossRef]
- Jiang, C.; Moore, M.J.; Zhang, X.; Klassen, H.; Langer, R.; Young, M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol Vis 2007, 13, 1783–1792. [Google Scholar]
- Checa-Casalengua, P.; Jiang, C.; Bravo-Osuna, I.; Tucker, B.A.; Molina-Martinez, I.T.; Young, M.J.; Herrero-Vanrell, R. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J. Control Release 2011, 156, 92–100. [Google Scholar] [CrossRef]
- Jiang, W.; Xiao, D.; Wu, C.; Yang, J.; Peng, X.; Chen, L.; Zhang, J.; Zha, G.; Li, W.; Ju, R.; et al. Circular RNA-based therapy provides sustained and robust neuroprotection for retinal ganglion cells. Mol. Ther. Nucleic Acids 2024, 35, 102258. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H.; et al. Topical Curcumin Nanocarriers are Neuroprotective in Eye Disease. Sci. Rep. 2018, 8, 11066. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Mhatre, S.; Chandler, C.; Opere, C.; Singh, S. Application of Quality by Design in the Development of Hydrogen Sulfide Donor Loaded Polymeric Microparticles. AAPS PharmSciTech 2024, 25, 132. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-H.; Huang, C.-H.; Lin, I.-C. Advances in Neuroprotection in Glaucoma: Pharmacological Strategies and Emerging Technologies. Pharmaceuticals 2024, 17, 1261. https://doi.org/10.3390/ph17101261
Wang L-H, Huang C-H, Lin I-C. Advances in Neuroprotection in Glaucoma: Pharmacological Strategies and Emerging Technologies. Pharmaceuticals. 2024; 17(10):1261. https://doi.org/10.3390/ph17101261
Chicago/Turabian StyleWang, Li-Hsin, Chun-Hao Huang, and I-Chan Lin. 2024. "Advances in Neuroprotection in Glaucoma: Pharmacological Strategies and Emerging Technologies" Pharmaceuticals 17, no. 10: 1261. https://doi.org/10.3390/ph17101261





