Anti-Alzheimer’s Potency of Rich Phenylethanoid Glycosides Extract from Marrubium vulgare L.: In Vitro and In Silico Studies
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Composition of the M. vulgare Extract
2.2. Biological Activities of the M. vulgare Extract
2.2.1. Antioxidant Potencies of the M. Vulgare Extract
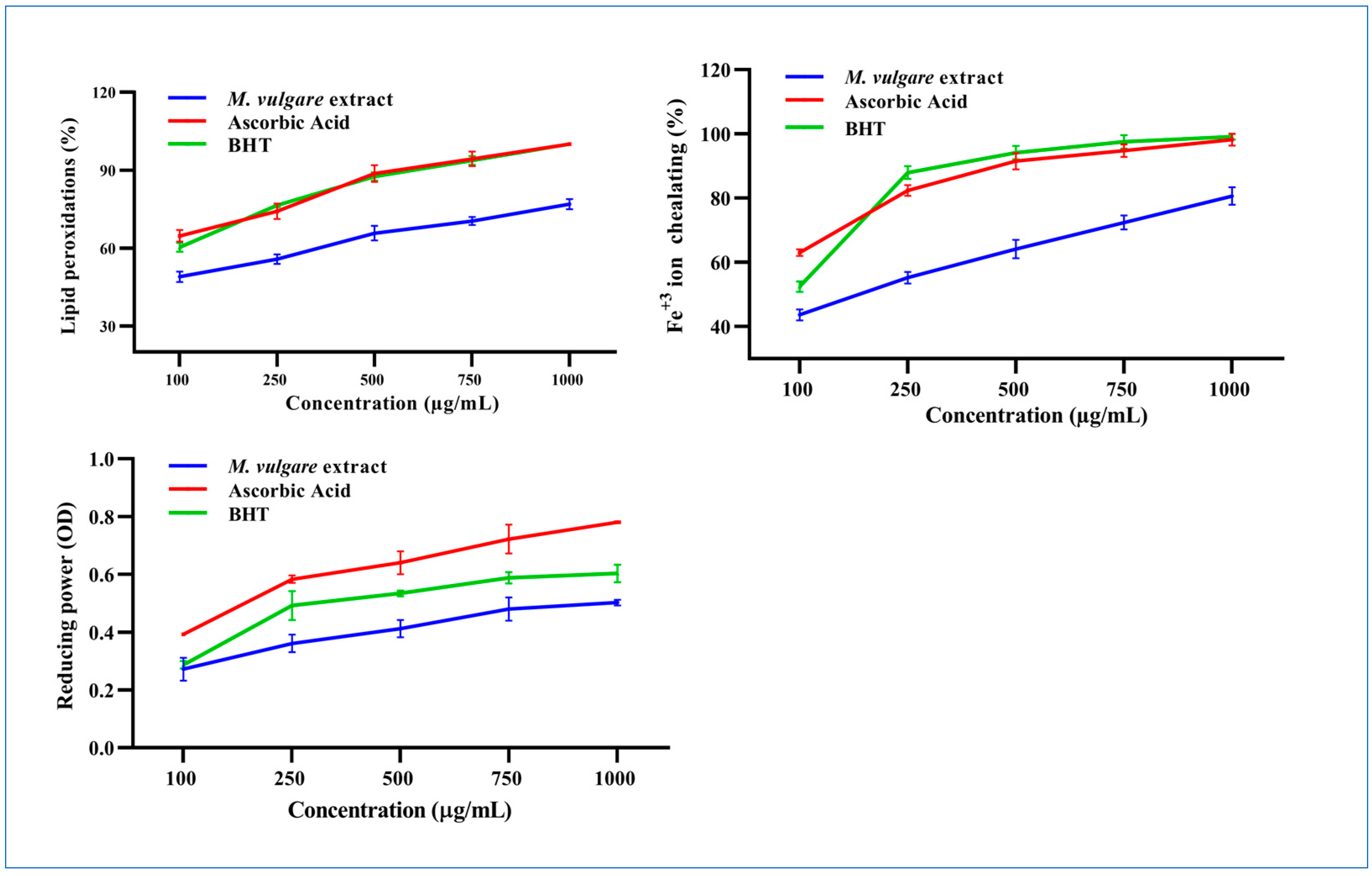
Inhibition of Lipid Peroxidation
Fe2+ Chelation Ability
Reducing Power of M. vulgare
2.2.2. Scavenging Activity
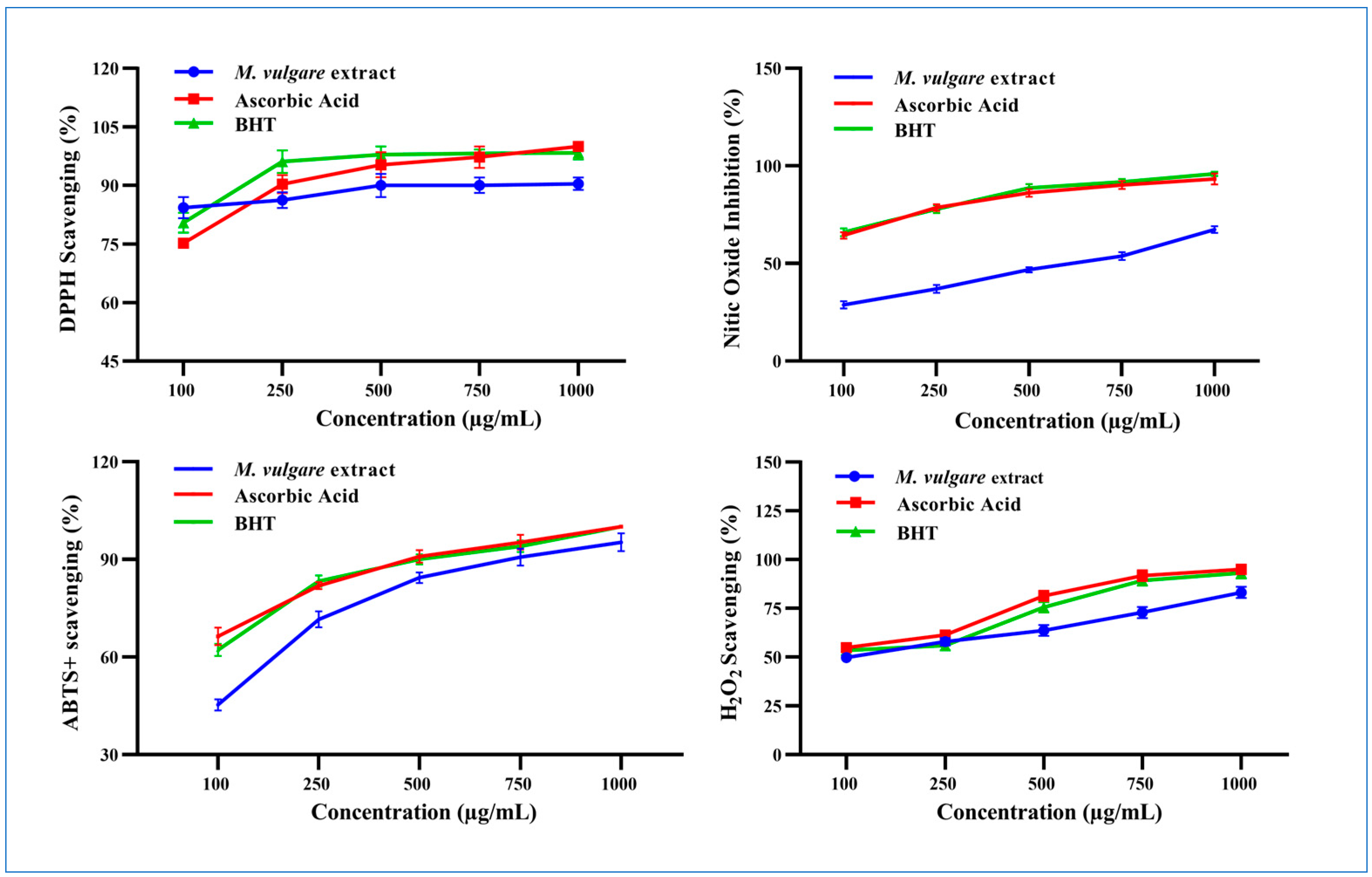
DPPH• Scavenging Activity
NO• Scavenging Activity
ABTS•+ Scavenging Power
H2O2 Scavenging Activity
2.2.3. Anti-Inflammatory Effects of the Crude M. vulgare Extract
COX-1 Inhibition
COX-2 Inhibition
2.2.4. Effect of M. vulgare on the Alzheimer’s Syndrome Biological Markers
Inhibition of Neurotransmitter Degradation
- Acetylcholinesterase Inhibition
- 2.
- Butyrylcholinesterase Inhibition
Inhibition of Tyrosine (Neurofibrillary Tangle Formation)
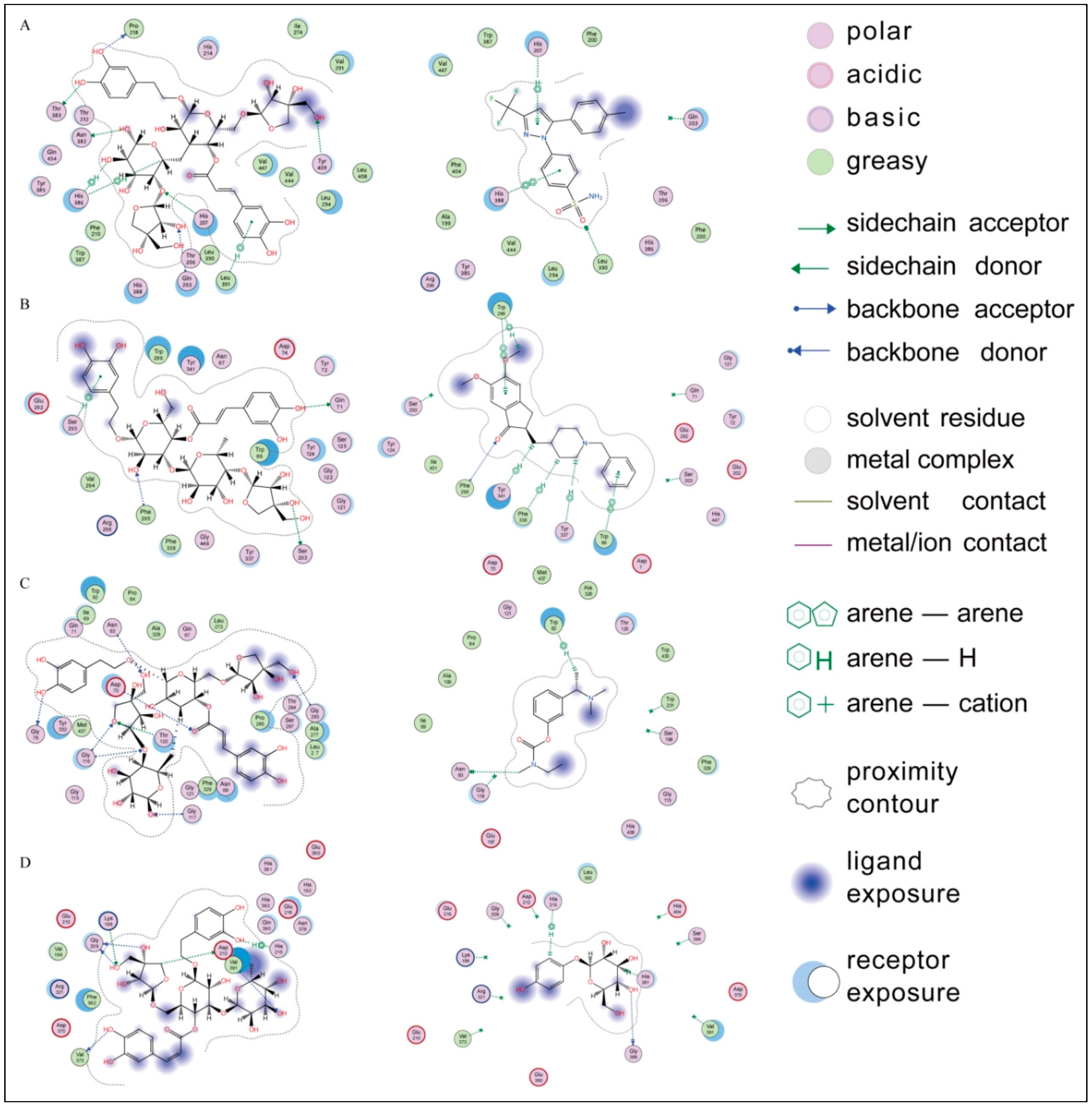
2.3. Molecular Docking Simulation
3. Discussion
4. Materials and Methods
4.1. M. vulgare Assemblage and Extract Preparations
4.2. Phytochemical Analysis
4.2.1. Preliminary Chemical Composition of the M. vulgare Extract
4.2.2. GCMS Analysis
Unsaponifiable Matter (USM)
Saponifiable Matter (SM) Analysis and the Preparation of the Fatty Acid Methyl Esters (FAMEs)
4.2.3. LC-ESI-MS/MS Analysis
4.3. Biological Potencies of the M. vulgare Extract
4.3.1. Antioxidant Potencies of the M. vulgare
Lipid Peroxidation-[NH4] SCN (Ammonium Thiocyanate)
Reduction of Ferric Ions (Fe3+)
Ferrous Ions (Fe2+)-Chelating Capacity
4.3.2. Scavenging Properties
NO• Radical-Scavenging Power
DPPH• Radical-Scavenging Power
ABTS Radical Cation Capture Power
H2O2 Scavenging Activity
4.3.3. Anti-Inflammatory Activity Assay
Cyclooxygenase Inhibition Protocol
4.3.4. In Vitro Evaluation of the Anti-Alzheimer Effectiveness of the M. vulgare Extract
Inhibition of Acetylcholinesterase
Inhibition of Tyrosinase
4.4. Molecular Docking Study
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aćimović, M.; Jeremić, K.; Salaj, N.; Gavarić, N.; Kiprovski, B.; Sikora, V.; Zeremski, T. Marrubium vulgare L.: A phytochemical and pharmacological overview. Molecules 2020, 25, 2898. [Google Scholar] [CrossRef] [PubMed]
- Sewidan, N.; Khalaf, R.A.; Mohammad, H. In-vitro studies on selected Jordanian plants as dipeptidyl peptidase-IV inhibitors for management of diabetes mellitus. Iran. J. Pharm. Res. 2020, 19, 95. [Google Scholar] [PubMed]
- Lodhi, S.; Vadnere, G.P.; Sharma, V.K.; Usman, M.R. Marrubium vulgare L.: A review on phytochemical and pharmacological aspects. J. Intercult. Ethnopharmacol. 2017, 6, 429–452. [Google Scholar] [CrossRef]
- Paula de Oliveira, A.; Santin, J.R.; Lemos, M.; Klein Júnior, L.C.; Couto, A.G.; Meyre da Silva Bittencourt, C.; Filho, V.C.; Faloni de Andrade, S. Gastroprotective activity of methanol extract and marrubiin obtained from leaves of Marrubium vulgare L. (Lamiaceae). J. Pharm. Pharmacol. 2011, 63, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Akther, N.; Shawl, A.; Sultana, S.; Chandan, B.; Akhter, M. Hepatoprotective activity of Marrubium vulgare against paracetamol induced toxicity. J. Pharm. Res. 2013, 7, 565–570. [Google Scholar] [CrossRef]
- Robles-Zepeda, R.E.; Velázquez-Contreras, C.A.; Garibay-Escobar, A.; Gálvez-Ruiz, J.C.; Ruiz-Bustos, E. Antimicrobial activity of Northwestern Mexican plants against Helicobacter pylori. J. Med. Food 2011, 14, 1280–1283. [Google Scholar] [CrossRef]
- Dallali, S.; Rouz, S.; Aichi, H.; Hassine, H.B. Phenolic contentand allelopathic potential of leavesand rhizosphere soilaqueous extracts of white horehound (Maribum vulgare L.). J. New Sci. 2017, 39, 3. [Google Scholar]
- Yabrir, B. Essential oil of Marrubium vulgare: Chemical composition and biological activities. A review. Nat. Prod. Sci. 2019, 25, 81–91. [Google Scholar] [CrossRef]
- Evans, W.C. Trease and Evans’ Pharmacognosy; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Izzo, A.A.; Hoon-Kim, S.; Radhakrishnan, R.; Williamson, E.M. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 2016, 30, 691–700. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W.; Doerr, C. Alzheimer Disease (Nursing); StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Health, U.D.o.; Services, H. Alzheimer’s Disease Fact Sheet. National Institute on Aging; US Department of Health and Human Services: Washington, DC, USA, 2019. [Google Scholar]
- Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- Ibrahim, A.; El-Newary, S.; Hendawy, S.F.; Ibrahim, A.E. Balanites aegyptiaca extract to treat risk factors of Alzheimer’s disease: An in vitro study. Egypt. J. Chem. 2021, 64, 781–792. [Google Scholar] [CrossRef]
- Samai, Z.; Toudert, N.; Djilani, S.E.; Dadda, N.; Zakkad, F.; Hamel, T. Chemical composition and in vitro antioxidant, anti-alzheimer, anti-diabetic, anti-tyrosinase, and antimicrobial properties of essential oils and extracts derived from various parts of the algerian Calendula suffruticosa vahlsubsp. boissieri Lanza. Chem. Biodivers. 2023, 20, e202200620. [Google Scholar] [CrossRef] [PubMed]
- Kenchappa, P.G.; Karthik, Y.; Vijendra, P.D.; Hallur, R.L.; Khandagale, A.S.; Pandurangan, A.K.; Jayanna, S.G.; Alshehri, M.A.; Alasmari, A.; Sayed, S. In vitro evaluation of the neuroprotective potential of Olea dioica against Aβ peptide-induced toxicity in human neuroblastoma SH-SY5Y cells. Front. Pharmacol. 2023, 14, 1139606. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The neuromodulator of long-term synaptic plasticity, reward and movement control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Greggio, E.; Bergantino, E.; Carter, D.; Ahmad, R.; Costin, G.E.; Hearing, V.J.; Clarimon, J.; Singleton, A.; Eerola, J.; Hellström, O. Tyrosinase exacerbates dopamine toxicity but is not genetically associated with Parkinson’s disease. J. Neurochem. 2005, 93, 246–256. [Google Scholar] [CrossRef]
- Rodboon, T.; Palipoch, S.; Okada, S.; Charoenchon, N.; Nakornpakdee, Y.; Suwannalert, P. Oxyresveratrol inhibits cellular tyrosinase-related oxidative stress-induced melanogenesis in B16 melanoma cells. J. Appl. Pharm. Sci. 2020, 10, 008–013. [Google Scholar]
- Guillen Quispe, Y.N.; Hwang, S.H.; Wang, Z.; Lim, S.S. Screening of peruvian medicinal plants for tyrosinase inhibitory properties: Identification of tyrosinase inhibitors in Hypericum laricifolium Juss. Molecules 2017, 22, 402. [Google Scholar] [CrossRef]
- Qu, Y.; Zhan, Q.; Du, S.; Ding, Y.; Fang, B.; Du, W.; Wu, Q.; Yu, H.; Li, L.; Huang, W. Catalysis-based specific detection and inhibition of tyrosinase and their application. J. Pharm. Anal. 2020, 10, 414–425. [Google Scholar] [CrossRef]
- Tiwari, P.; Dwivedi, S.; Singh, M.P.; Mishra, R.; Chandy, A. Basic and modern concepts on cholinergic receptor: A review. Asian Pac. J. Trop. Dis. 2013, 3, 413–420. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Zeng, F.; Shen, Y.; Wang, Y.-Y.; Zhang, N.; Geng, F. Bioguided isolation and structure identification of acetylcholinesterase enzyme inhibitors from Drynariae rhizome. J. Anal. Methods Chem. 2020, 2020, 2971841. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Alzheimer’s pathogenesis: Is there neuron-to-neuron propagation? Acta Neuropathol. 2011, 121, 589–595. [Google Scholar] [CrossRef]
- Usman, K. Medicinal plants anticholinesterase activity and the potential for Alzheimer’s disease treatment. J. Dis. Med. Plants 2017, 3, 68–82. [Google Scholar]
- Bursal, E.; Aras, A.; Kılıç, Ö. Evaluation of antioxidant capacity of endemic plant Marrubium astracanicum subsp. macrodon: Identification of its phenolic contents by using HPLC-MS/MS. Nat. Prod. Res. 2019, 33, 1975–1979. [Google Scholar] [CrossRef]
- Nawwar, M.A.; El-Mousallamy, A.M.; Barakat, H.H.; Buddrus, J.; Linscheid, M. Flavonoid lactates from leaves of Marrubium vulgare. Phytochemistry 1989, 28, 3201–3206. [Google Scholar] [CrossRef]
- Amri, B.; Martino, E.; Vitulo, F.; Corana, F.; Ben-Kaâb, L.B.; Rui, M.; Rossi, D.; Mori, M.; Rossi, S.; Collina, S. Marrubium vulgare L. leave extract: Phytochemical composition, antioxidant and wound healing properties. Molecules 2017, 22, 1851. [Google Scholar] [CrossRef]
- Hashemi, M.; Kharazian, N. Identification of flavonoids from Marrubium and Ballota species (Lamiaceae) and determination of chemotaxonomic markers using high performance liquid chromatography mass spectrometer. J. Sci. Islam. Repub. Iran 2021, 32, 305–320. [Google Scholar]
- Abdelhameed, R.F.; Ali, A.I.; Elhady, S.S.; Abo Mansour, H.E.; Mehanna, E.T.; Mosaad, S.M.; Ibrahim, S.A.; Hareeri, R.H.; Badr, J.M.; Eltahawy, N.A. Marrubium alysson L. ameliorated methotrexate-induced testicular damage in mice through regulation of apoptosis and miRNA-29a expression: LC-MS/MS metabolic profiling. Plants 2022, 11, 2309. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Q.; Zhang, Z.; Zhang, X. Elsholtzia rugulosa: Phytochemical profile and antioxidant, anti-Alzheimer’s disease, antidiabetic, antibacterial, cytotoxic and hepatoprotective activities. Plant Foods Hum. Nutr. 2022, 77, 62–67. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sayeed, M.S.B.; Haque, M.A.; Hassan, M.M.; Islam, S.A. Phytochemical screening, antioxidant, anti-Alzheimer and anti-diabetic activities of Centella asiatica. J. Nat. Prod. Plant Resour. 2012, 2, 504–511. [Google Scholar]
- Paul, A.; Zothantluanga, J.H.; Rakshit, G.; Celik, I.; Rudrapal, M.; Zaman, M.K. Computational simulations reveal the synergistic action of phytochemicals of Morus alba to exert anti-Alzheimer activity via inhibition of acetylcholinesterase and glycogen synthase kinase-3β. Polycycl. Aromat. Compd. 2023, 44, 3476–3500. [Google Scholar] [CrossRef]
- Lopa, S.S.; Al-Amin, M.Y.; Hasan, M.K.; Ahammed, M.S.; Islam, K.M.; Alam, A.; Tanaka, T.; Sadik, M.G. Phytochemical analysis and cholinesterase inhibitory and antioxidant activities of Enhydra fluctuans relevant in the management of Alzheimer’s disease. Int. J. Food Sci. 2021, 2021, 8862025. [Google Scholar] [CrossRef]
- Neganova, M.; Afanas’eva, S.; Klochkov, S.; Shevtsova, E. Mechanisms of antioxidant effect of natural sesquiterpene lactone and alkaloid derivatives. Bull. Exp. Biol. Med. 2012, 152, 720. [Google Scholar] [CrossRef]
- Malinowska, P. Effect of flavonoids content on antioxidant activity of commercial cosmetic plant extracts. Herba Pol. 2013, 59, 63–75. [Google Scholar] [CrossRef]
- Kondratyuk, T.P.; Pezzuto, J.M. Natural product polyphenols of relevance to human health. Pharm. Biol. 2004, 42, 46–63. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Bendlin, B.; Carlsson, C.; Gleason, C.; Johnson, S.; Sodhi, A.; Gallagher, C.; Puglielli, L.; Engelman, C.; Ries, M.; Xu, G. Midlife predictors of Alzheimer’s disease. Maturitas 2010, 65, 131–137. [Google Scholar] [CrossRef]
- Quinn, D.M. Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Schlemper, V.; Ribas, A.; Nicolau, M.; Cechinel Filho, V. Antispasmodic effects of hydroalcoholic extract of Marrubium vulgare on isolated tissues. Phytomedicine 1996, 3, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.H.; Orhan, I.; Şenol, F.; Kartal, M.; Şener, B.; Dvorská, M.; Šmejkal, K.; Šlapetová, T. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chem. Biol. Interact. 2009, 181, 383–389. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase activity of phenolic acids and their derivatives. Z. Naturforschung C 2013, 68, 125–132. [Google Scholar] [CrossRef]
- Neagu, E.; Radu, G.L.; Albu, C.; Paun, G. Antioxidant activity, acetylcholinesterase and tyrosinase inhibitory potential of Pulmonaria officinalis and Centarium umbellatum extracts. Saudi J. Biol. Sci. 2018, 25, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kartal, M.; Tosun, F.; Şener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Naturforschung C 2007, 62, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Shahwar, D.; Rehman, S.U.; Raza, M.A. Acetyl cholinesterase inhibition potential and antioxidant activities of ferulic acid isolated from Impatiens bicolor Linn. J. Med. Plants Res 2010, 4, 260–266. [Google Scholar]
- Heo, K.S.; Lee, S.J.; Ko, J.H.; Lim, K.; Lim, K.T. Glycoprotein isolated from Solanum nigrum L. inhibits the DNA-binding activities of NF-κB and AP-1, and increases the production of nitric oxide in TPA-stimulated MCF-7 cells. Toxicol. Vitr. 2004, 18, 755–763. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef]
- Konrath, E.L.; Passos, C.d.S.; Klein-Júnior, L.C.; Henriques, A.T. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef]
- Storga, D.; Vrecko, K.; Birkmayer, J.; Reibnegger, G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci. Lett. 1996, 203, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Guler, G.O.; Aktumsek, A.; Ceylan, R.; Picot, C.M.N.; Mahomoodally, M.F. Enzyme inhibitory properties, antioxidant activities, and phytochemical profile of three medicinal plants from Turkey. Adv. Pharmacol. Pharm. Sci. 2015, 2015, 410675. [Google Scholar] [CrossRef]
- Baek, H.S.; Rho, H.S.; Yoo, J.W.; Ahn, S.M.; Lee, J.Y.; Jeonga-Lee, J.-L.; Kim, M.-K.; Kim, D.H.; Chang, I.S. The inhibitory effect of new hydroxamic acid derivatives on melanogenesis. Bull. Korean Chem. Soc. 2008, 29, 43–46. [Google Scholar]
- Salah, N.M.; Souleman, A.M.; Shaker, K.H.; Hawary, S.; El-Hady, F. Acetylcholinesterase, alpha-glucosidase and tyrosinase inhibitors from Egyptian propolis. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 528–536. [Google Scholar] [CrossRef]
- Aparadh, V.; Naik, V.; Karadge, B.A. Antioxidative properties (TPC, DPPH, FRAP, metal chelating ability, reducing power and TAC) within some Cleome species. Ann. Bot. 2012, 2, 49–56. [Google Scholar]
- Prajit, R.; Sritawan, N.; Suwannakot, K.; Naewla, S.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Chrysin protects against memory and hippocampal neurogenesis depletion in D-galactose-induced aging in rats. Nutrients 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, S.C.H.; Silva, J.; Alves, C.; Pinteus, S.; Pedrosa, R.; Laufer, S.; Goettert, M.I. Neuroprotective effect of luteolin-7-O-glucoside against 6-OHDA-induced damage in undifferentiated and RA-differentiated SH-SY5Y Cells. Int. J. Mol. Sci. 2022, 23, 2914. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Tong, Y.; Chuan, J.; Bai, L.; Shi, J.; Zhu, Y. Protective effect of ethyl vanillin against Aβ-induced neurotoxicity in PC12 cells via the reduction of oxidative stress and apoptosis. Exp. Ther. Med. 2019, 17, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Zhao, J.; Xing, X.; Zhang, C.; Meng, H. Neuroprotective effects of D-(-)-quinic acid on aluminum chloride-induced dementia in rats. Evid. Based Complement. Altern. Med. 2020, 2020, 5602597. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.Y.D.; Dobrachinski, F.; Silva, H.B.; Lopes, J.P.; Gonçalves, F.Q.; Soares, F.A.; Porciúncula, L.O.; Andrade, G.M.; Cunha, R.A.; Tomé, A.R. Neuromodulation and neuroprotective effects of chlorogenic acids in excitatory synapses of mouse hippocampal slices. Sci. Rep. 2021, 11, 10488. [Google Scholar] [CrossRef]
- Shao, S.-Y.; Zhang, F.; Yang, Y.-N.; Feng, Z.-M.; Jiang, J.-S.; Zhang, P.-C. Neuroprotective and anti-inflammatory phenylethanoid glycosides from the fruits of Forsythia suspensa. Bioorganic Chem. 2021, 113, 105025. [Google Scholar] [CrossRef]
- Yang, J.; Ju, B.; Hu, J. Effects of phenylethanoid glycosides extracted from Herba cistanches on the learning and memory of the APP/PSI transgenic mice with Alzheimer’s disease. BioMed Res. Int. 2021, 2021, 1291549. [Google Scholar] [CrossRef]
- Ji, S.; Wu, Y.; Zhu, R.; Guo, D.; Jiang, Y.; Huang, L.; Ma, X.; Yu, L. Novel Phenylethanoid glycosides improve hippocampal synaptic plasticity via the cyclic adenosine monophosphate-CREB-brain-derived neurotrophic growth factor pathway in APP/PS1 transgenic mice. Gerontology 2023, 69, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Onwuka, G. Soaking, boiling and antinutritional factors in pigeon peas (Cajanus cajan) and cowpeas (Vigna unguiculata). J. Food Process. Preserv. 2006, 30, 616–630. [Google Scholar] [CrossRef]
- Ahmed, W.; Kamal, A.; Ibrahim, R. Phytochemical screening and chemical investigation of lipoidal matter of Arenga engleri leaves. J. Adv. Pharm. Res. 2019, 3, 83–89. [Google Scholar] [CrossRef]
- Johnson, A.R.; Davenport, J.B. Biochemistry and Methodology of Lipids; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1971. [Google Scholar]
- Fang, K.; Pan, X.; Huang, B.; Liu, J.; Wang, Y.; Gao, J. Simultaneous derivatization of hydroxyl and ketone groups for the analysis of steroid hormones by GC–MS. Chromatographia 2010, 72, 949–956. [Google Scholar] [CrossRef]
- Seif, M.; Aati, H.; Amer, M.; Ragauskas, A.J.; Seif, A.; El-Sappah, A.H.; Aati, A.; Madboli, A.E.-N.A.; Emam, M. Mitigation of hepatotoxicity via boosting antioxidants and reducing oxidative stress and inflammation in carbendazim-treated rats using Adiantum capillus-veneris L. Extract. Molecules 2023, 28, 4720. [Google Scholar] [CrossRef] [PubMed]
- Vogel, I. Practical Organic Chemistry; CiteSeer: Princeton, NJ, USA, 1974. [Google Scholar]
- Hassan, M.; Mohdaly, A.; Elneairy, N.; Mahmoud, A. Influence of extraction systems on oil yield, wastes and olive oil properties. Fayoum J. Agric. Res. Dev. 2022, 36, 1–11. [Google Scholar] [CrossRef]
- Farid, M.M.; Aboul Naser, A.F.; Salem, M.M.; Ahmed, Y.R.; Emam, M.; Hamed, M.A. Chemical compositions of Commiphora opobalsamum stem bark to alleviate liver complications in streptozotocin-induced diabetes in rats: Role of oxidative stress and DNA damage. Biomarkers 2022, 27, 671–683. [Google Scholar] [CrossRef]
- Duh, P.-D.; Tu, Y.-Y.; Yen, G.-C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT-Food Sci. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, L.; Maguire, J.J.; Droylefaix, M.T.; Packer, L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Terao, J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1, 1-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.A. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and blackcurrant drink. Food Chem. 1997, 60, 331–337. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.-j.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.; Dahl, E.; Bremer, J. Peroxidative oxidation of leuco-dichlorofluorescein by prostaglandin H synthase in prostaglandin biosynthesis from polyunsaturated fatty acids. Biochim. Biophys. Acta Lipids Lipid Metab. 1996, 1299, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ingkaninan, K.; Temkitthawon, P.; Chuenchom, K.; Yuyaem, T.; Thongnoi, W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003, 89, 261–264. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Yi, W.; Ma, C.; Wan, Y.; Zhou, B.; Ma, L.; Song, H. A class of potent tyrosinase inhibitors: Alkylidenethiosemicarbazide compounds. Eur. J. Med. Chem. 2009, 44, 1773–1778. [Google Scholar] [CrossRef]
- Li, Y.-S.; He, M.; Zhou, T.-S.; Wang, Q.; He, L.; Wang, S.-J.; Hu, B.; Wei, B.; Wang, H.; Cui, Z.-N. 2, 5-Disubstituted furan derivatives containing 1, 3, 4-thiadiazole moiety as potent α-glucosidase and E. coli β-glucuronidase inhibitors. Eur. J. Med. Chem. 2021, 216, 113322. [Google Scholar] [CrossRef] [PubMed]
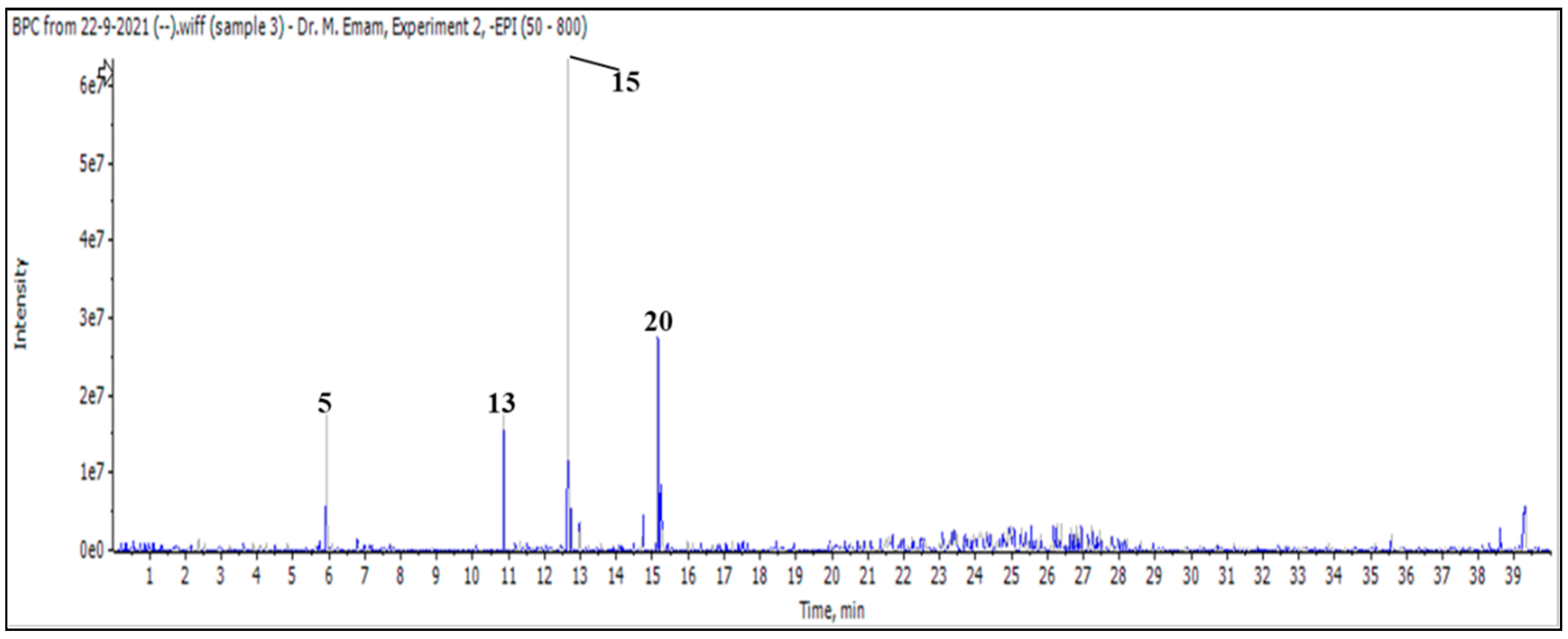





| Peak | tR (min) | Name | Formula | Chemical Class | Conc. |
|---|---|---|---|---|---|
| 1 | 3.12 | Desogestrel | C22H30O | Steroid | 2.92 |
| 2 | 3.16 | 1-Heptatriacotanol | C37H76O | OHC | 2.42 |
| 3 | 3.40 | Cholest-22-ene-21-ol, 3,5-dehydro-6-methoxy-, pivalate | C33H54O3 | Steroid | 1.41 |
| 4 | 3.78 | 3-Ethyl-3-hydroxy-, (5.α.)-androstan-17-one | C21H34O2 | Steroid | 81.39 |
| 5 | 4.07 | Ageratriol, trimethyl ether | C18H30O3 | Sesquiterpene | 2.60 |
| 6 | 4.37 | Cholestan-3-ol, 2-methylene-, (3β, 5.α.)- | C28H48O | Steroid | 3.97 |
| 7 | 6.62 | Stigmasterol | C29H48O | Steroid | 1.83 |
| 8 | 6.97 | (3β, 24S)-Stigmast-5-en-3-ol | C29H50O | Steroid | 2.05 |
| 9 | 7.83 | Doconexent | C22H32O2 | PUSFA | 1.42 |
| Peak | tR (min) | Name | Chemical Formula | Conc. % |
|---|---|---|---|---|
| 1 | 11.269 | Benzoic acid, 2-ethylhexyl ester | C15H22O2 | 0.11 |
| 2 | 11.618 | Hexadecanoic acid, methyl ester | C17H34O2 | 1.94 |
| 3 | 13.473 | Methyl stearate | C19H38O2 | 0.27 |
| 4 | 13.682 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | 0.27 |
| 5 | 14.256 | 9,12-Octadecadienoic acid (Z, Z)-, methyl ester | C19H34O2 | 0.56 |
| 6 | 14.633 | Methyl 5,6-octadecadienoate | C19H34O2 | 0.34 |
| 7 | 15.188 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | C19H32O2 | 0.33 |
| 8 | 16.625 | 17-Octadecynoic acid, methyl ester | C19H34O2 | 0.06 |
| 9 | 16.778 | Methyl 9-cis,11-trans-octadecadienoate | C19H34O2 | 0.06 |
| 10 | 19.038 | Methyl 2-ethylhexyl phthalate | C17H24O4 | 2.95 |
| 11 | 19.392 | Phthalic acid, cyclohexyl methyl ethyl ester | C17H22O4 | 0.08 |
| 12 | 29.567 | Phthalic acid, di(2-propylpentyl) ester | C24H38O4 | 92.25 |
| 13 | 33.184 | Decanedioic acid, bis(2-ethylhexyl) ester | C26H50O4 | 0.24 |
| 14 | 34.048 | Octadecanoic acid, 9,10-dihydroxy-, methyl ester | C19H38O4 | 0.14 |
| 15 | 35.18 | 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | C24H38O4 | 0.4 |
| Sum | 100 | |||
| SAT. FAME | 2.59 | |||
| UNSAT. SFAME | 1.62 | |||
| Benzoic acid derivatives | 95.79 |
| Peak | tR (min) | Identified Metabolites | [M − H]− | Ms/Ms |
|---|---|---|---|---|
| 1 | 1.7 | Quinic acid # | 191 | 173, 127, 111 |
| 2 | 3.13 | Aconitic acid # | 173 | 85, 129 |
| 3 | 4.19 | Methyl gallic acid # | 183 | 169, 139, 125 |
| 4 | 4.99 | Malic acid # | 133 | 115, 87, 71 |
| 5 | 5.93 | Apigenin-7-O-xyloside | 401 | 287, 269, 221, 219,113 |
| 6 | 6.6 | Protocatechuic acid # | 153 | 109 |
| 7 | 7.41 | Cinnamic acid | 147 | 103, 77 |
| 8 | 7.73 | Ferulic acid | 193 | 134 |
| 9 | 8.35 | Caffeic acid # | 179 | 135 |
| 10 | 8.90 | 4-hydroxy benzoic acid # | 137 | 93, 65 |
| 11 | 9.55 | Gallic acid # | 169 | 125 |
| 12 | 10.50 | Vanillin # | 151 | 136, 135, 92 |
| 13 | 10.88 | Vitexin “ Apigenin-8-C-glucoside” * | 431 | 341, 311, 283, 269 |
| 14 | 12.631 | Catechin | 289 | 245, 221, 109 |
| 15 | 12.65 | Apigenin-7-O-xyloside | 401 | 287, 269, 221, 219, 113 |
| 16 | 12.97 | p-Coumaric acid # | 163 | 119, 93 |
| 17 | 14.67 | Quercetin * | 301 | 273, 257 |
| 18 | 14.75 | Naringenin # | 271 | 151, 119 |
| 19 | 15.13 | Apigenin-7-O-xyloside | 401 | 287, 269, 221, 219, 113 |
| 20 | 15.15 | Apigenin * | 269 | 151, 117 |
| 21 | 25.24 | luteolin-7-glucoside * | 447 | 285 |
| 22 | 25.67 | Luteolin * | 285 | 151, 133 |
| 23 | 28.17 | luteolin-7-O-lactate * | 357 | 285, 269, 223 |
| 24 | 29.4 | Chrysoeriol * | 299 | 285, 284, 269 |
| 25 | 32.2 | Apigenin * | 269 | 151, 117 |
| 26 | 35.14 | Chrysin # | 253 | 143, 119 |
| Peak | tR (min) | Identified Metabolites | [M − H]− | Ms/Ms |
|---|---|---|---|---|
| 1 | 6.36 | Marruboside  | 887 | 725, 593 |
| 2 | 6.51 | Verbascoside  | 623 | 461, 315 |
| 3 | 6.80 | Forsythoside B  | 755 | 593, 461, 447 |
| 4 | 7.00 | Samioside  | 755 | 593, 461 |
| 5 | 7.45 | Apigenin-7-O-neohesperidoside # | 577 | 431, 269 |
| 6 | 8.00 | Apigenin-7-O-diglucuronide-O-hexoside | 783 | 737, 607, 431, 269 |
| 7 | 8.59 | Apigenin-7-O-diglucuronide-O-hexoside | 783 | 737, 607, 431, 269 |
| 8 | 9.00 | Isoscutellarein-7-O-(6-O-acetylallosyl) glucoside # | 651 | 591, 489, 285, 257, 217, 175 |
| 9 | 9.49 | Apigenin-7-O-neohesperidoside # | 577 | 431, 269 |
| 10 | 10.45 | Vicenin II (apigenin 6,8-di-C-glycoside) # | 593 | 503, 383 |
| 11 | 14.82 | Marrubiin  | 331 | 313, 303, 287, 285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emam, M.; El-Newary, S.A.; Aati, H.Y.; Wei, B.; Seif, M.; Ibrahim, A.Y. Anti-Alzheimer’s Potency of Rich Phenylethanoid Glycosides Extract from Marrubium vulgare L.: In Vitro and In Silico Studies. Pharmaceuticals 2024, 17, 1282. https://doi.org/10.3390/ph17101282
Emam M, El-Newary SA, Aati HY, Wei B, Seif M, Ibrahim AY. Anti-Alzheimer’s Potency of Rich Phenylethanoid Glycosides Extract from Marrubium vulgare L.: In Vitro and In Silico Studies. Pharmaceuticals. 2024; 17(10):1282. https://doi.org/10.3390/ph17101282
Chicago/Turabian StyleEmam, Mahmoud, Samah A. El-Newary, Hanan Y. Aati, Bin Wei, Mohamed Seif, and Abeer Y. Ibrahim. 2024. "Anti-Alzheimer’s Potency of Rich Phenylethanoid Glycosides Extract from Marrubium vulgare L.: In Vitro and In Silico Studies" Pharmaceuticals 17, no. 10: 1282. https://doi.org/10.3390/ph17101282










