Multifaceted Evaluation of Inhibitors of Anti-Apoptotic Proteins in Head and Neck Cancer: Insights from In Vitro, In Vivo, and Clinical Studies (Review)
Abstract
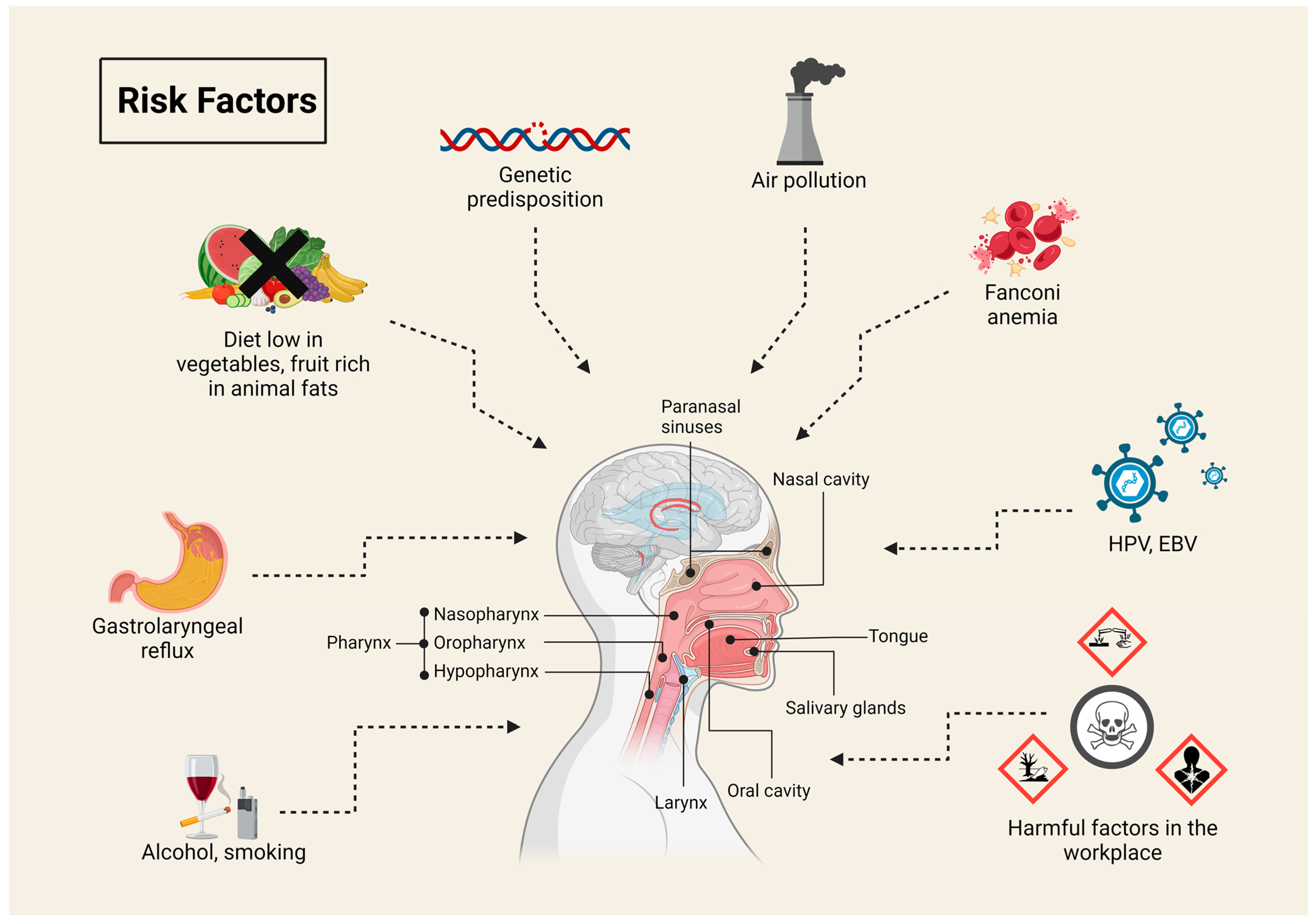
1. Head and Neck Cancer
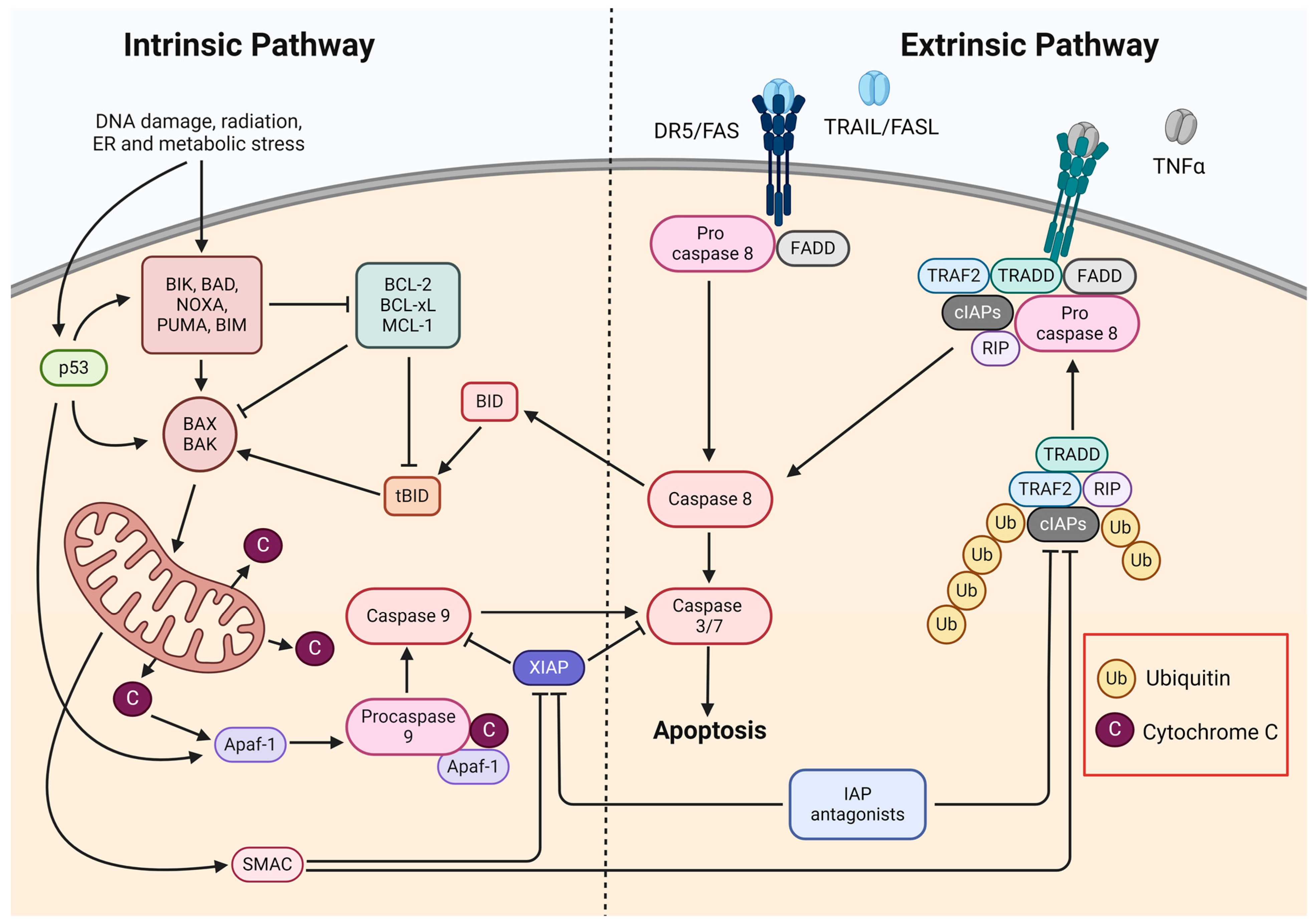
2. Apoptosis
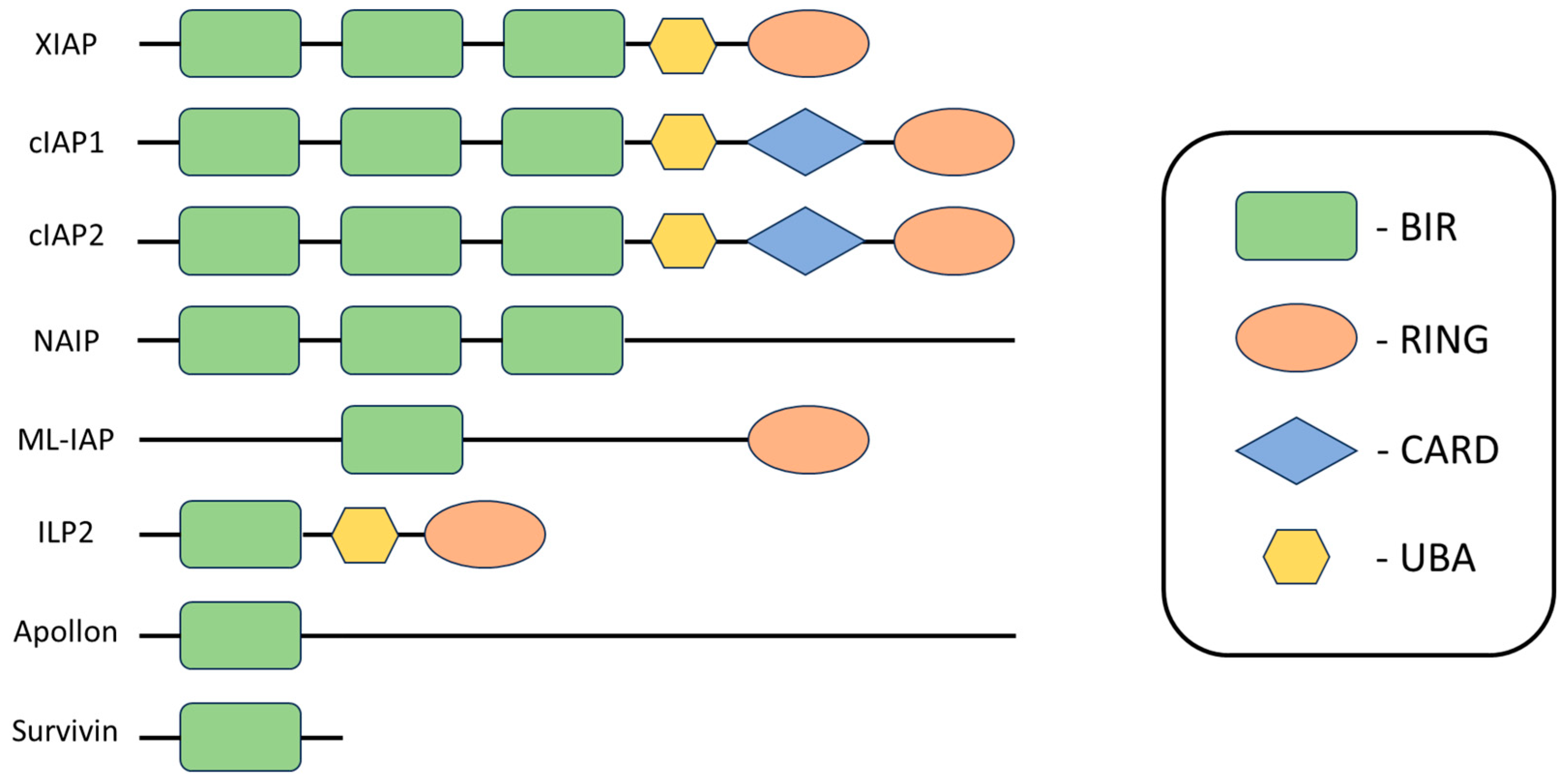
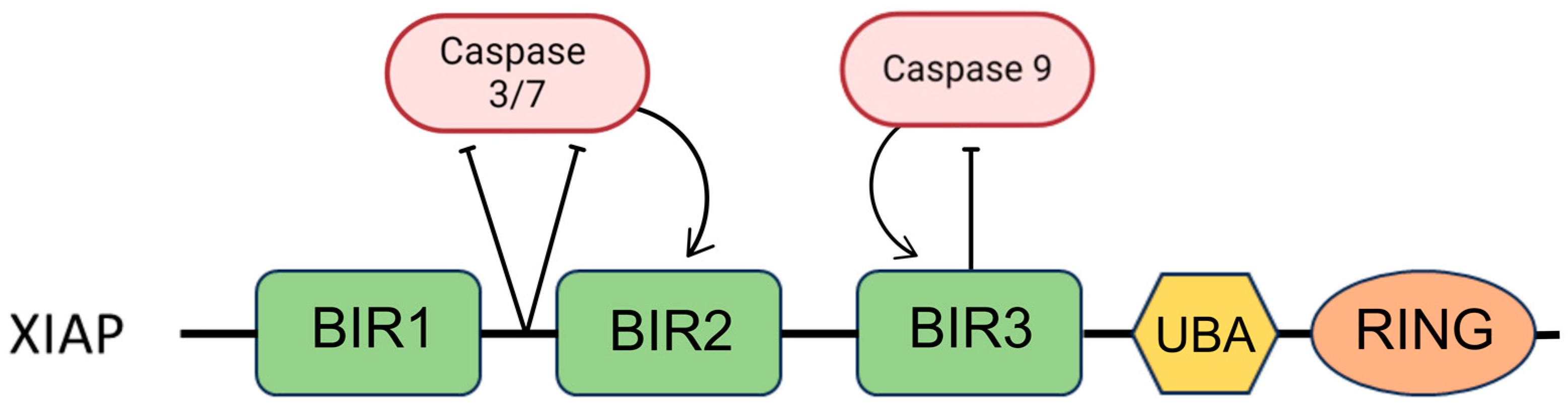
3. Inhibitors of Apoptosis Proteins
4. IAP Antagonists
5. In Vitro Studies
5.1. AZD5582
5.2. LCL161
5.3. Birinapant
5.4. SM-164
5.5. BV6
5.6. ASTX660
5.7. Xevinapant
5.8. APG-1387
5.9. Embelin
| Compound | Form of Treatment | Cell Line | IC50 | Time | Source |
|---|---|---|---|---|---|
| Birinapant | Monotherapy | UM-SCC-1 | >1000 nM | 72 h | [71,75] |
| UM-SCC-6 | >1000 nM | ||||
| UM-SCC-9 | >1000 nM | ||||
| UM-SCC-11A | >1000 nM | ||||
| UM-SCC-11B | >1000 nM | ||||
| UM-SCC-22A | >1000 nM | ||||
| UM-SCC-22B | >1000 nM | ||||
| UM-SCC-38 | >1000 nM | ||||
| UM-SCC-46 | 10.7 nM | ||||
| UM-SCC-74A | >1000 nM | ||||
| UM-SCC-74B | >1000 nM | ||||
| UM-SCC-47 | >1000 nM | ||||
| 93VU147T | >5000 nM | 72 h | [74] | ||
| UM-SCC-47 | >5000 nM | ||||
| UM-SCC-104 | >5000 nM | ||||
| UM-SCC-105 | >5000 nM | ||||
| UPCI-SCC-90 | >5000 nM | ||||
| UPCI-SCC-152 | >5000 nM | ||||
| UPCI-SCC-154 | >5000 nM | ||||
| UD-SCC-2 | >5000 nM | ||||
| 93VU147T | >5000 nM | 120 h | |||
| UM-SCC-47 | 500 nM | ||||
| UM-SCC-104 | >5000 nM | ||||
| UM-SCC-105 | 631 nM | ||||
| UPCI-SCC-90 | >5000 nM | ||||
| UPCI-SCC-152 | >5000 nM | ||||
| UPCI-SCC-154 | >5000 nM | ||||
| UD-SCC-2 | >5000 nM | ||||
| Detroit 562 | >79.5 µM | 72 h | [76] | ||
| FaDu | >58.3 µM | ||||
| PCI-1 | 41.0 µM | ||||
| PCI-9 | 21.4 µM | ||||
| PCI-13 | 31.1 µM | ||||
| PCI-52 | >67 µM | ||||
| PCI-68 | >56.1 µM | ||||
| SCC-9 | >19.0 µM | ||||
| SCC-25 | >4.9 µM | ||||
| Birinapant + 20 ng/mL TNFα | UM-SCC-1 | 45.3 nM | 72 h | [71,75] | |
| UM-SCC-6 | 2.98 nM | ||||
| UM-SCC-9 | >1000 nM | ||||
| UM-SCC-11A | 47.1 nM | ||||
| UM-SCC-11B | 41.7 nM | ||||
| UM-SCC-22A | 1.88 nM | ||||
| UM-SCC-22b | >1000 nM | ||||
| UM-SCC-38 | >1000 nM | ||||
| UM-SCC-46 | 0.72 nM | ||||
| UM-SCC-74A | 8.78 nM | ||||
| UM-SCC-74B | 0.1 nM | ||||
| UM-SCC-47 | >1000 nM | ||||
| 93VU147T | 200 nM | 72 h | [74] | ||
| UM-SCC-47 | 20 nM | ||||
| UM-SCC-104 | 2.5 nM | ||||
| UM-SCC-105 | >5000 nM | ||||
| UPCI-SCC-90 | 5.6 nM | ||||
| UPCI-SCC-152 | 1.1 nM | ||||
| UPCI-SCC-154 | >5000 nM | ||||
| UD-SCC-2 | 794 nM | ||||
| 93VU147T | 177 nM | 120 h | |||
| UM-SCC-47 | 1.6 nM | ||||
| UM-SCC-104 | 0.3 nM | ||||
| UM-SCC-105 | 661 nM | ||||
| UPCI-SCC-90 | 1 nM | ||||
| UPCI-SCC-152 | 0.4 nM | ||||
| UPCI-SCC-154 | >5000 nM | ||||
| UD-SCC-2 | 1995 nM | ||||
| Birinapant + 50 ng/mL TRAIL | UM-SCC-1 | 17.72 nM | 72 h | [71,75] | |
| UM-SCC-6 | 0.29 nM | ||||
| UM-SCC-9 | > 1000 nM | ||||
| UM-SCC-11A | 1.59 nM | ||||
| UM-SCC-11B | 39.9 nM | ||||
| UM-SCC-22A | >1000 nM | ||||
| UM-SCC-22B | >1000 nM | ||||
| UM-SCC-38 | >1000 nM | ||||
| UM-SCC-46 | 0.57 nM | ||||
| UM-SCC-74A | 65.4 nM | ||||
| UM-SCC-74B | 0.27 nM | ||||
| UM-SCC-47 | 160 nM | ||||
| 93VU147T | 100 nM | 72 h | [74] | ||
| UM-SCC-47 | 32 nM | ||||
| UM-SCC-104 | >5000 nM | ||||
| UM-SCC-105 | 2 nM | ||||
| UPCI-SCC-90 | >5000 nM | ||||
| UPCI-SCC-152 | 8.9 nM | ||||
| UPCI-SCC-154 | 0.4 nM | ||||
| UD-SCC-2 | >5000 nM | ||||
| 93VU147T | 126 nM | 120 h | |||
| UM-SCC-47 | 28 nM | ||||
| UM-SCC-104 | >5000 nM | ||||
| UM-SCC-105 | 3.2 nM | ||||
| UPCI-SCC-90 | 25 nM | ||||
| UPCI-SCC-152 | 10 nM | ||||
| UPCI-SCC-154 | 0.6 nM | ||||
| UD-SCC-2 | >5000 nM | ||||
| FasL + IC10 Birinapant | Detroit 562 | 1.3 ng/mL | 72 h | [76] | |
| FaDu | 7.8 ng/mL | ||||
| PCI-1 | 1.5 ng/mL | ||||
| PCI-9 | 14.0 ng/mL | ||||
| PCI-13 | 3.3 ng/mL | ||||
| PCI-52 | - | ||||
| PCI-68 | 27.4 ng/mL | ||||
| SCC-9 | - | ||||
| SCC-25 | 12.9 ng/mL | ||||
| AZD5582 | Monotherapy | Cal27 | 4.21 µM | 72 h | [64] |
| SCC25 | 0.54 µM | ||||
| FaDu | 3.02 µM | ||||
| VU974-T | 3.9 nM | 96 h | [16] | ||
| VU1604 | 3.9 nM | ||||
| VU1365-T | 250 nM | ||||
| CCH-FAHNSCC-1 | >1000 nM | ||||
| CCH-FAHNSCC-2 | >1000 nM | ||||
| VU1131-T | >1000 nM | ||||
| AZD5582 + irradiation 2 Gy | Cal27 | 3.03 µM | 72 h | [64] | |
| SCC25 | 0.43 µM | ||||
| FaDu | 2.79 µM | ||||
| AZD5582 + irradiation 4 Gy | Cal27 | 2.70 µM | 72 h | [64] | |
| SCC25 | 0.27 µM | ||||
| FaDu | 3.43 µM | ||||
| AZD5582 + irradiation 8 Gy | Cal27 | 1.57 µM | 72 h | [64] | |
| SCC25 | 0.17 µM | ||||
| FaDu | 2.67 µM | ||||
| LCL161 | Monotherapy | Cal27, UM-SCC-1, UM-SCC-74A, UM-SCC-11B, UM-SCC-47, UD-SCC-2, UPC1-SCC-090, 93VU147T | 32–95 µM | 72 h | [66] |
| PCI-1 | 12/30.6 µM | 72 h | [67,68] | ||
| PCI-9 | 26/36.1 µM | ||||
| PCI-13 | 11/41.1 µM | ||||
| PCI-52 | 13/40.2 µM | ||||
| PCI-68 | 17/49.3 µM | ||||
| Detroit 562 | 48.7 µM | [67] | |||
| FaDu | 61.2 µM | ||||
| SCC-9 | 21.6 µM | ||||
| SCC-25 | 19.8 µM | ||||
| A-253 | 64.3 µM | 72 h | [69] | ||
| FasL + IC10 LCL161 | Detroit 562 | 1.7 ng/mL | 72 h | [68] | |
| FaDu | 5.2 ng/mL | ||||
| PCI-1 | 1.8 ng/mL | ||||
| PCI-9 | 4.9 ng/mL | ||||
| PCI-13 | 1.7 ng/mL | ||||
| PCI-52 | - | ||||
| PCI-68 | 24.8 ng/mL | ||||
| SCC-9 | - | ||||
| SCC-25 | 17 ng/mL | ||||
| A-253 | - | [69] | |||
| SM-164 | Monotherapy | UMSCC-1 | 32.1 µM | 24 h | [79] |
| UMSCC-12 | 55.2 µM | ||||
| UMSCC-17B | 22.3 µM | ||||
| UMSCC-74B | 56.7 µM | ||||
| HSC3 | 0.03 nM | 72 h | [80] | ||
| HSC3M3 | 0.47 nM | ||||
| HN5 | 0.25 nM | ||||
| S18 | 5.07 µM | 48 h | [81] | ||
| S26 | 1.37 µM | ||||
| BV6 | Monotherapy | Detroit 562 | 8.7 µM | 72 h | [68] |
| FaDu | 3.6 µM | ||||
| PCI-1 | 4.5 µM | ||||
| PCI-9 | 3.7 µM | ||||
| PCI-13 | 5.6 µM | ||||
| PCI-52 | 6.6 µM | ||||
| PCI-68 | 3.5 µM | ||||
| SCC-9 | 1.6 µM | ||||
| SCC-25 | 1.2 µM | ||||
| SCC-4 | 2.0 ± 0.5 μM | [82] | |||
| SCC-4cisR | 2.0 ± 0.2 μM | ||||
| FasL + IC10 BV6 | Detroit 562 | 1.3 ng/mL | 72 h | [68] | |
| FaDu | 2.4 ng/mL | ||||
| PCI-1 | 1.9 ng/mL | ||||
| PCI-9 | 13.9 ng/mL | ||||
| PCI-13 | 2.3 ng/mL | ||||
| PCI-52 | - | ||||
| PCI-68 | 26.3 ng/mL | ||||
| SCC-9 | - | ||||
| SCC-25 | 10.3 ng/ml | ||||
| ASTX660 | Monotherapy | UM-SCC-11B | >1000 nM | 72 h | [84] |
| UM-SCC-22B | >1000 nM | ||||
| UM-SCC-38 | >1000 nM | ||||
| UM-SCC-46 | 4.3 nM | ||||
| UM-SCC-74A | >1000 nM | ||||
| UM-SCC-47 | >1000 nM | ||||
| UM-SCC-104 | >1000 nM | ||||
| UPCI-SCC-90 | >1000 nM | ||||
| UPCI-SCC-152 | >1000 nM | ||||
| UD-SCC-2 | >1000 nM | ||||
| 93-VU-147T | >1000 nM | ||||
| ASTX660 + 20 ng/mL TNFα | UM-SCC-11B | 68.2 nM | 72 h | [84] | |
| UM-SCC-22B | 0.1 nM | ||||
| UM-SCC-38 | >1000 nM | ||||
| UM-SCC-46 | 0.9 nM | ||||
| UM-SCC-74A | 4.1 nM | ||||
| UM-SCC-47 | 285 nM | ||||
| UM-SCC-104 | >1000 nM | ||||
| UPCI-SCC-90 | 14.3 nM | ||||
| UPCI-SCC-152 | 43.9 nM | ||||
| UD-SCC-2 | >1000 nM | ||||
| 93-VU-147T | >1000 nM | ||||
| ASTX660 + 50 ng/mL TRAIL | UM-SCC-11B | 27.7 nM | 72 h | [84] | |
| UM-SCC-22B | >1000 nM | ||||
| UM-SCC-38 | >1000 nM | ||||
| UM-SCC-46 | 0.5 nM | ||||
| UM-SCC-74A | 0.3 nM | ||||
| UM-SCC-47 | 18.9 nM | ||||
| UM-SCC-104 | >1000 nM | ||||
| UPCI-SCC-90 | 21.2 nM | ||||
| UPCI-SCC-152 | 24.3 nM | ||||
| UD-SCC-2 | >1000 nM | ||||
| 93-VU-147T | >1000 nM | ||||
| Xevinapant | Monotherapy | S18 | 233.9 µM | 48 h | [81] |
| S26 | 20.92 µM | ||||
| SQ20B | 16.71 µM | Nd. | [87] | ||
| FaDu | 2.45 µM | ||||
| Cal27 | 1.16 µM | ||||
| RPMI-2650 | 1.69 µM | ||||
| SCC-4 | 0.41 µM | ||||
| SCC-15 | 1.21 µM | ||||
| SCC-4 | >100 μM | 72 h | [82] | ||
| SCC-4cisR | >100 μM | ||||
| Embelin | Monotherapy | SCC-4 | >100 μM | 72 h | [82] |
| SCC-4cisR | >100 μM |
6. Animal Studies
6.1. Birinapant
6.2. LCL161
6.3. APG-1387
6.4. SM-164
6.5. ASTX660
6.6. Xevinapant
7. Clinical Studies
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Kumar, S.; Noronha, V.; Patil, V.; Joshi, A.; Menon, N.; Prabhash, K. Advances in Pharmacotherapy for Head and Neck Cancer. Expert. Opin. Pharmacother. 2021, 22, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Cancer Today. Available online: https://gco.iarc.who.int/today/ (accessed on 2 May 2024).
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and Neck Cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical Update on Head and Neck Cancer: Molecular Biology and Ongoing Challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Debbaneh, P.; Dhir, S.; Anderson, M.; Rivero, A. Electronic Cigarettes: A Narrative Review and Cohort Study of Electronic Cigarette Users in the Otolaryngology Clinic. Perm. J. 2022, 26, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Lopez, M.; Perry, M.D.; Garbinski, L.D.; Manevski, M.; Andre, M.; Ceyhan, Y.; Caobi, A.; Paul, P.; Lau, L.S.; Ramelow, J.; et al. Health Effects and Known Pathology Associated with the Use of E-Cigarettes. Toxicol. Rep. 2022, 9, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Meng Yip, H.; Garg, M. E-Cigarettes and Oral Cancer: What Do We Know so Far? Br. J. Oral Maxillofac. Surg. 2023, 61, 380–382. [Google Scholar] [CrossRef]
- de Lima, J.M.; Macedo, C.C.S.; Barbosa, G.V.; Castellano, L.R.C.; Hier, M.P.; Alaoui-Jamali, M.A.; da Silva, S.D. E-Liquid Alters Oral Epithelial Cell Function to Promote Epithelial to Mesenchymal Transition and Invasiveness in Preclinical Oral Squamous Cell Carcinoma. Sci. Rep. 2023, 13, 3330. [Google Scholar] [CrossRef] [PubMed]
- Löhler, J.; Wollenberg, B. Are Electronic Cigarettes a Healthier Alternative to Conventional Tobacco Smoking? Eur. Arch. Otorhinolaryngol. 2019, 276, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer—Nature Reviews Clinical Oncology. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-Associated Oropharyngeal Cancer: Epidemiology, Molecular Biology and Clinical Management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Samara, P.; Athanasopoulos, M.; Mastronikolis, S.; Kyrodimos, E.; Athanasopoulos, I.; Mastronikolis, N.S. The Role of Oncogenic Viruses in Head and Neck Cancers: Epidemiology, Pathogenesis, and Advancements in Detection Methods. Microorganisms 2024, 12, 1482. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.S. Epstein–Barr Virus: A Master Epigenetic Manipulator. Curr. Opin. Virol. 2017, 26, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Roohollahi, K.; de Jong, Y.; Pai, G.; Zaini, M.A.; de Lint, K.; Sie, D.; Rooimans, M.A.; Rockx, D.; Hoskins, E.E.; Ameziane, N.; et al. BIRC2–BIRC3 Amplification: A Potentially Druggable Feature of a Subset of Head and Neck Cancers in Patients with Fanconi Anemia. Sci. Rep. 2022, 12, 45. [Google Scholar] [CrossRef]
- Lim, Y.X.; Mierzwa, M.L.; Sartor, M.A.; D’Silva, N.J. Clinical, Morphologic and Molecular Heterogeneity of HPV-Associated Oropharyngeal Cancer. Oncogene 2023, 42, 2939–2955. [Google Scholar] [CrossRef]
- Del Mistro, A.; Frayle, H.; Menegaldo, A.; Favaretto, N.; Gori, S.; Nicolai, P.; Spinato, G.; Romeo, S.; Tirelli, G.; da Mosto, M.C.; et al. Age-independent increasing prevalence of Human Papillomavirus-driven oropharyngeal carcinomas in North-East Italy—Scientific Reports. Sci. Rep. 2020, 10, 9320. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of Head-and-Neck Cancer from Exhaled Breath. Br. J. Cancer 2011, 104, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Bassani, S.; Santonicco, N.; Eccher, A.; Scarpa, A.; Vianini, M.; Brunelli, M.; Bisi, N.; Nocini, R.; Sacchetto, L.; Munari, E.; et al. Artificial Intelligence in Head and Neck Cancer Diagnosis. J. Pathol. Inform. 2022, 13, 100153. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pulido, G.; Medina, D.I.; Barani, M.; Rahdar, A.; Sargazi, G.; Baino, F.; Pandey, S. Nanomaterials for the Diagnosis and Treatment of Head and Neck Cancers: A Review. Materials 2021, 14, 3706. [Google Scholar] [CrossRef] [PubMed]
- Koster, H.J.; Guillen-Perez, A.; Gomez-Diaz, J.S.; Navas-Moreno, M.; Birkeland, A.C.; Carney, R.P. Fused Raman Spectroscopic Analysis of Blood and Saliva Delivers High Accuracy for Head and Neck Cancer Diagnostics. Sci. Rep. 2022, 12, 18464. [Google Scholar] [CrossRef] [PubMed]
- Arantes, L.M.R.B.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, Plasma and Saliva Biomarkers for Head and Neck Cancer. Expert. Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hoseini, S.J.; Milan, P.B.; Hooshmand, S.; Kim, H.-W.; Mozafari, M. Quantum Dots: A Review from Concept to Clinic. Biotechnol. J. 2020, 15, 2000117. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Chen, X.; Luo, H.; Meng, C.; Zhu, D. Cancer Stem Cells of Head and Neck Squamous Cell Carcinoma; Distance towards Clinical Application; a Systematic Review of Literature. Am. J. Cancer Res. 2023, 13, 4315–4345. [Google Scholar] [PubMed]
- McHugh, K.J.; Jing, L.; Behrens, A.M.; Jayawardena, S.; Tang, W.; Gao, M.; Langer, R.; Jaklenec, A. Biocompatible Semiconductor Quantum Dots as Cancer Imaging Agents. Adv. Mater. 2018, 30, 1706356. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.; Shaban, M.; Rajpoot, N.; Khurram, S.A. Artificial Intelligence-Based Methods in Head and Neck Cancer Diagnosis: An Overview. Br. J. Cancer 2021, 124, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Burtness, B. Treating Head and Neck Cancer in the Age of Immunotherapy: A 2023 Update. Drugs 2023, 83, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Moy, J.; Ferris, R.L. Immunotherapy for Head and Neck Squamous Cell Carcinoma. Curr. Oncol. Rep. 2018, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.-T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer Consensus Statement on Immunotherapy for the Treatment of Squamous Cell Carcinoma of the Head and Neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef]
- Ringash, J.; Bernstein, L.J.; Devins, G.; Dunphy, C.; Giuliani, M.; Martino, R.; McEwen, S. Head and Neck Cancer Survivorship: Learning the Needs, Meeting the Needs. Semin. Radiat. Oncol. 2018, 28, 64–74. [Google Scholar] [CrossRef]
- Mazahir, F.; Sharma, R.; Yadav, A.K. Bioinspired Theranostic Quantum Dots: Paving the Road to a New Paradigm for Cancer Diagnosis and Therapeutics. Drug Discov. Today 2023, 28, 103822. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Kumar, M.; Tiwari, A.; Tiwari, V.; Kaushik, D.; Verma, R.; Bhatt, S.; Sahoo, B.M.; Bhattacharya, T.; Alshehri, S.; et al. Quantum Dots: An Emerging Approach for Cancer Therapy. Front. Mater. 2022, 8, 798440. [Google Scholar] [CrossRef]
- Raudenská, M.; Balvan, J.; Masařík, M. Cell Death in Head and Neck Cancer Pathogenesis and Treatment. Cell Death Dis. 2021, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.; Liu, Y.; Han, B. Bivalent SMAC Mimetics for Treating Cancer by Antagonizing Inhibitor of Apoptosis Proteins. ChemMedChem 2019, 14, 1951–1962. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Smith, D.C.; Wang, S. Small-Molecule SMAC Mimetics as New Cancer Therapeutics. Pharmacol. Ther. 2014, 144, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, Pyroptosis and Apoptosis: An Intricate Game of Cell Death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, X.; Yuan, Y.; Li, R.; Zhao, Y.; Fu, J.; Wang, J.; Su, J. HTRA2/OMI-Mediated Mitochondrial Quality Control Alters Macrophage Polarization Affecting Systemic Chronic Inflammation. Int. J. Mol. Sci. 2024, 25, 1577. [Google Scholar] [CrossRef] [PubMed]
- Cossu, F.; Milani, M.; Mastrangelo, E.; Lecis, D. Targeting the BIR Domains of Inhibitor of Apoptosis (IAP) Proteins in Cancer Treatment. Comput. Struct. Biotechnol. J. 2019, 17, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Targeting IAP Proteins in Combination with Radiotherapy. Radiat. Oncol. 2015, 10, 105. [Google Scholar] [CrossRef]
- Vucic, D.; Fairbrother, W.J. The Inhibitor of Apoptosis Proteins as Therapeutic Targets in Cancer. Clin. Cancer Res. 2007, 13, 5995–6000. [Google Scholar] [CrossRef]
- Dumétier, B.; Zadoroznyj, A.; Dubrez, L. IAP-Mediated Protein Ubiquitination in Regulating Cell Signaling. Cells 2020, 9, 1118. [Google Scholar] [CrossRef] [PubMed]
- Budhidarmo, R.; Day, C.L. IAPs: Modular Regulators of Cell Signalling. Semin. Cell Dev. Biol. 2015, 39, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Silke, J.; Vucic, D. Chapter Two—IAP Family of Cell Death and Signaling Regulators. In Methods in Enzymology; Ashkenazi, A., Wells, J.A., Yuan, J., Eds.; Regulated Cell Death Part B; Academic Press: Cambridge, MA, USA, 2014; Volume 545, pp. 35–65. [Google Scholar]
- Tu, H.; Costa, M. XIAP’s Profile in Human Cancer. Biomolecules 2020, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Obexer, P.; Ausserlechner, M.J. X-Linked Inhibitor of Apoptosis Protein—A Critical Death Resistance Regulator and Therapeutic Target for Personalized Cancer Therapy. Front. Oncol. 2014, 4, 197. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Butterworth, M.; Malladi, S.; Duckett, C.S.; Cohen, G.M.; Bratton, S.B. The E3 Ubiquitin Ligase cIAP1 Binds and Ubiquitinates Caspase-3 and -7 via Unique Mechanisms at Distinct Steps in Their Processing. J. Biol. Chem. 2009, 284, 12772–12782. [Google Scholar] [CrossRef] [PubMed]
- Gyrd-Hansen, M.; Meier, P. IAPs: From Caspase Inhibitors to Modulators of NF-κB, Inflammation and Cancer. Nat. Rev. Cancer 2010, 10, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Eckelman, B.P.; Salvesen, G.S.; Scott, F.L. Human Inhibitor of Apoptosis Proteins: Why XIAP Is the Black Sheep of the Family. EMBO Rep. 2006, 7, 988–994. [Google Scholar] [CrossRef]
- Fulda, S. Molecular Pathways: Targeting Inhibitor of Apoptosis Proteins in Cancer—From Molecular Mechanism to Therapeutic Application. Clin. Cancer Res. 2014, 20, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Gyrd-Hansen, M.; Darding, M.; Miasari, M.; Santoro, M.M.; Zender, L.; Xue, W.; Tenev, T.; da Fonseca, P.C.A.; Zvelebil, M.; Bujnicki, J.M.; et al. IAPs Contain an Evolutionarily Conserved Ubiquitin-Binding Domain That Regulates NF-κB as Well as Cell Survival and Oncogenesis. Nat. Cell Biol. 2008, 10, 1309. [Google Scholar] [CrossRef]
- Silke, J.; Meier, P. Inhibitor of Apoptosis (IAP) Proteins–Modulators of Cell Death and Inflammation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008730. [Google Scholar] [CrossRef] [PubMed]
- Dougan, S.K.; Dougan, M. Regulation of Innate and Adaptive Antitumor Immunity by IAP Antagonists. Immunotherapy 2018, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Pflug, K.M.; Sitcheran, R. Targeting NF-κB-Inducing Kinase (NIK) in Immunity, Inflammation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8470. [Google Scholar] [CrossRef]
- Targeting NF-κB Pathway for the Therapy of Diseases: Mechanism and Clinical Study—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7506548/ (accessed on 18 February 2024).
- Chen, D.J.; Huerta, S. Smac mimetics as new cancer therapeutics. Anti-Cancer Drugs 2009, 20, 646. [Google Scholar] [CrossRef] [PubMed]
- Probst, B.L.; Liu, L.; Ramesh, V.; Li, L.; Sun, H.; Minna, J.D.; Wang, L. Smac Mimetics Increase Cancer Cell Response to Chemotherapeutics in a TNF-α-Dependent Manner. Cell Death Differ. 2010, 17, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Smac Mimetics as IAP Antagonists. Semin. Cell Dev. Biol. 2015, 39, 132–138. [Google Scholar] [CrossRef]
- Fulda, S. Promises and Challenges of Smac Mimetics as Cancer Therapeutics. Clin. Cancer Res. 2015, 21, 5030–5036. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.A.; Caruso, S.; Baxter, A.A.; Poon, I.K.H.; Hawkins, C.J. Smac Mimetics Can Provoke Lytic Cell Death That Is Neither Apoptotic nor Necroptotic. Apoptosis 2020, 25, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Y.; Wang, X.-Y.; Wei, Q.-Y.; Xu, Y.-M.; Lau, A.T.Y. Potency and Selectivity of SMAC/DIABLO Mimetics in Solid Tumor Therapy. Cells 2020, 9, 1012. [Google Scholar] [CrossRef]
- Kikuchi, S.; Sugama, Y.; Takada, K.; Kamihara, Y.; Wada, A.; Arihara, Y.; Nakamura, H.; Sato, T. Simultaneous XIAP and cIAP1/2 Inhibition by a Dimeric SMAC Mimetic AZD5582 Induces Apoptosis in Multiple Myeloma. J. Pharmacol. Sci. 2024, 154, 30–36. [Google Scholar] [CrossRef]
- Kadletz, L.; Enzenhofer, E.; Kotowski, U.; Altorjai, G.; Heiduschka, G. AZD5582, an IAP Antagonist That Leads to Apoptosis in Head and Neck Squamous Cell Carcinoma Cell Lines and Is Eligible for Combination with Irradiation. Acta Oto-Laryngol. 2017, 137, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Carter, B.Z.; Bose, P.; Jain, N.; Kadia, T.M.; Garcia-Manero, G.; Bueso-Ramos, C.E.; DiNardo, C.D.; Bledsoe, S.; Daver, N.G.; et al. Final Results of a Phase 2 Clinical Trial of LCL161, an Oral SMAC Mimetic for Patients with Myelofibrosis. Blood Adv. 2021, 5, 3163. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kumar, B.; Shen, C.; Zhao, S.; Blakaj, D.; Li, T.; Romito, M.; Teknos, T.N.; Williams, T.M. LCL161, a SMAC Mimetic, Preferentially Radiosensitizes Human Papillomavirus Negative Head and Neck Squamous Cell Carcinoma. Mol. Cancer Ther. 2019, 18, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Cytotoxic Effects of SMAC-Mimetic Compound LCL161 in Head and Neck Cancer Cell Lines|Clinical Oral Investigations. Available online: https://link.springer.com/article/10.1007/s00784-016-1741-3 (accessed on 13 June 2024).
- Brands, R.C.; Scheurer, M.J.J.; Hartmann, S.; Seher, A.; Freudlsperger, C.; Moratin, J.; Linz, C.; Kübler, A.C.; Müller-Richter, U.D.A. Sensitization of Head and Neck Squamous Cell Carcinoma to Apoptosis by Combinational SMAC Mimetic and Fas Ligand-Fc Treatment in Vitro. J. Cranio-Maxillofac. Surg. 2020, 48, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.J.J.; Seher, A.; Steinacker, V.; Linz, C.; Hartmann, S.; Kübler, A.C.; Müller-Richter, U.D.A.; Brands, R.C. Targeting Inhibitors of Apoptosis in Oral Squamous Cell Carcinoma in Vitro. J. Cranio-Maxillofac. Surg. 2019, 47, 1589–1599. [Google Scholar] [CrossRef]
- Xie, X.; Lee, J.; Liu, H.; Pearson, T.; Lu, A.Y.; Tripathy, D.; Devi, G.R.; Bartholomeusz, C.; Ueno, N.T. Birinapant Enhances Gemcitabine’s Anti-Tumor Efficacy in Triple-Negative Breast Cancer by Inducing Intrinsic Pathway–Dependent Apoptosis. Mol. Cancer Ther. 2021, 20, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Eytan, D.F.; Snow, G.E.; Carlson, S.; Derakhshan, A.; Saleh, A.; Schiltz, S.; Cheng, H.; Mohan, S.; Cornelius, S.; Coupar, J.; et al. SMAC Mimetic Birinapant plus Radiation Eradicates Human Head and Neck Cancers with Genomic Amplifications of Cell Death Genes FADD and BIRC2. Cancer Res. 2016, 76, 5442–5454. [Google Scholar] [CrossRef] [PubMed]
- Benetatos, C.A.; Mitsuuchi, Y.; Burns, J.M.; Neiman, E.M.; Condon, S.M.; Yu, G.; Seipel, M.E.; Kapoor, G.S.; LaPorte, M.G.; Rippin, S.R.; et al. Birinapant (TL32711), a Bivalent SMAC Mimetic, Targets TRAF2-Associated cIAPs, Abrogates TNF-Induced NF-κB Activation, and Is Active in Patient-Derived Xenograft Models. Mol. Cancer Ther. 2014, 13, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Toni, T.; Viswanathan, R.; Robbins, Y.; Gunti, S.; Yang, X.; Huynh, A.; Cheng, H.; Sowers, A.L.; Mitchell, J.B.; Allen, C.T.; et al. Combined Inhibition of IAPs and WEE1 Enhances TNFα- and Radiation-Induced Cell Death in Head and Neck Squamous Carcinoma. Cancers 2023, 15, 1029. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Jeon, J.; Sun, L.; Derakhshan, A.; Chen, J.; Carlson, S.; Cheng, H.; Silvin, C.; Yang, X.; Van Waes, C.; et al. Death Agonist Antibody against TRAILR2/DR5/TNFRSF10B Enhances Birinapant Anti-Tumor Activity in HPV-Positive Head and Neck Squamous Cell Carcinomas. Sci. Rep. 2021, 11, 6392. [Google Scholar] [CrossRef]
- Eytan, D.F.; Snow, G.E.; Carlson, S.G.; Schiltz, S.; Chen, Z.; Van Waes, C. Combination Effects of SMAC Mimetic Birinapant with TNFα, TRAIL, and Docetaxel in Preclinical Models of HNSCC. Laryngoscope 2015, 125, E118–E124. [Google Scholar] [CrossRef] [PubMed]
- Brands, R.C.; Scheurer, M.J.J.; Hartmann, S.; Seher, A.; Kübler, A.C.; Müller-Richter, U.D.A. Apoptosis-Sensitizing Activity of Birinapant in Head and Neck Squamous Cell Carcinoma Cell Lines. Oncol. Lett. 2018, 15, 4010–4016. [Google Scholar] [CrossRef]
- Uzunparmak, B.; Gao, M.; Lindemann, A.; Erikson, K.; Wang, L.; Lin, E.; Frank, S.J.; Gleber-Netto, F.O.; Zhao, M.; Skinner, H.D.; et al. Caspase-8 Loss Radiosensitizes Head and Neck Squamous Cell Carcinoma to SMAC Mimetic–Induced Necroptosis. JCI Insight 2020, 5, e139837. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Nikolovska-Coleska, Z.; Lu, J.; Meagher, J.L.; Yang, C.-Y.; Qiu, S.; Tomita, Y.; Ueda, Y.; Jiang, S.; Krajewski, K.; et al. Design, Synthesis and Characterization of A Potent, Non-Peptide, Cell-Permeable, Bivalent Smac Mimetic That Concurrently Targets Both the BIR2 and BIR3 Domains in XIAP. J. Am. Chem. Soc. 2007, 129, 15279–15294. [Google Scholar] [CrossRef]
- Yang, J.; McEachern, D.; Li, W.; Davis, M.A.; Li, H.; Morgan, M.A.; Bai, L.; Sebolt, J.T.; Sun, H.; Lawrence, T.S.; et al. Radiosensitization of Head and Neck Squamous Cell Carcinoma by a SMAC-Mimetic Compound, SM-164, Requires Activation of Caspases. Mol. Cancer Ther. 2011, 10, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Raulf, N.; El-Attar, R.; Kulms, D.; Lecis, D.; Delia, D.; Walczak, H.; Papenfuss, K.; Odell, E.; Tavassoli, M. Differential Response of Head and Neck Cancer Cell Lines to TRAIL or Smac Mimetics Is Associated with the Cellular Levels and Activity of Caspase-8 and Caspase-10. Br. J. Cancer 2014, 111, 1955–1964. [Google Scholar] [CrossRef]
- Wu, M.; Wang, G.; Zhao, Z.; Liang, Y.; Wang, H.; Wu, M.; Min, P.; Chen, L.; Feng, Q.; Bei, J.; et al. Smac Mimetics in Combination with TRAIL Selectively Target Cancer Stem Cells in Nasopharyngeal Carcinoma. Mol. Cancer Ther. 2013, 12, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Magnano, S.; Rynne, A.; O’Kane, L.; Barroeta, P.H.; Zisterer, D.M. Targeting Inhibitor of Apoptosis Proteins (IAPs) Enhances Susceptibility of Oral Squamous Carcinoma Cells to Cisplatin. Exp. Cell Res. 2024, 437, 113995. [Google Scholar] [CrossRef]
- Xiao, R.; Allen, C.T.; Tran, L.; Patel, P.; Park, S.-J.; Chen, Z.; Van Waes, C.; Schmitt, N.C. Antagonist of cIAP1/2 and XIAP Enhances Anti-Tumor Immunity When Combined with Radiation and PD-1 Blockade in a Syngeneic Model of Head and Neck Cancer. Oncoimmunology 2018, 7, e1471440. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; An, Y.; Ye, W.; Derakhshan, A.; Cheng, H.; Yang, X.; Allen, C.T.; Chen, Z.; Schmitt, N.C.; Van Waes, C. Dual Antagonist of cIAP/XIAP ASTX660 Sensitizes HPV(−) and HPV(+) Head and Neck Cancers To TNFα, TRAIL, and Radiation Therapy. Clin. Cancer Res. 2019, 25, 6463–6474. [Google Scholar] [CrossRef]
- Ye, W.; Gunti, S.; Allen, C.T.; Hong, Y.; Clavijo, P.E.; Van Waes, C.; Schmitt, N.C. ASTX660, an Antagonist of cIAP1/2 and XIAP, Increases Antigen Processing Machinery and Can Enhance Radiation-Induced Immunogenic Cell Death in Preclinical Models of Head and Neck Cancer. Oncoimmunology 2020, 9, 1710398. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, J.; Hildebrand, L.S.; Kuhlmann, L.; Fietkau, R.; Distel, L.V. The Effect of Xevinapant Combined with Ionizing Radiation on HNSCC and Normal Tissue Cells and the Impact of Xevinapant on Its Targeted Proteins cIAP1 and XIAP. Cells 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, O.; Viertl, D.; Tsoutsou, P.; Kadi, L.; Rigotti, S.; Zanna, C.; Wiedemann, N.; Vozenin, M.-C.; Vuagniaux, G.; Bourhis, J. The Radiosensitizing Activity of the SMAC-Mimetic, Debio 1143, Is TNFα-Mediated in Head and Neck Squamous Cell Carcinoma. Radiother. Oncol. 2015, 116, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yu, Y.; Li, Z.-L.; Chen, M.-Y.; Deng, R.; Huang, X.; Wang, G.-F.; Zhang, M.-X.; Yang, Q.; Ravichandran, S.; et al. XIAP Limits Autophagic Degradation of Sox2 and Is A Therapeutic Target in Nasopharyngeal Carcinoma Stem Cells. Theranostics 2018, 8, 1494–1510. [Google Scholar] [CrossRef] [PubMed]
- YANG, S.; LI, S.-S.; YANG, X.-M.; YIN, D.-H.; WANG, L. Embelin Prevents LMP1-Induced TRAIL Resistance via Inhibition of XIAP in Nasopharyngeal Carcinoma Cells. Oncol. Lett. 2016, 11, 4167–4176. [Google Scholar] [CrossRef][Green Version]
- Kansal, V.; Kinney, B.L.C.; Uppada, S.; Saba, N.F.; Stokes, W.A.; Buchwald, Z.S.; Schmitt, N.C. The Expanding Role of IAP Antagonists for the Treatment of Head and Neck Cancer. Cancer Med. 2023, 12, 13958–13965. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-S.; Tao, Y.; Tourneau, C.L.; Pointreau, Y.; Sire, C.; Kaminsky, M.-C.; Coutte, A.; Alfonsi, M.; Boisselier, P.; Martin, L.; et al. Debio 1143 and High-Dose Cisplatin Chemoradiotherapy in High-Risk Locoregionally Advanced Squamous Cell Carcinoma of the Head and Neck: A Double-Blind, Multicentre, Randomised, Phase 2 Study. Lancet Oncol. 2020, 21, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Sun, X.-S.; Pointreau, Y.; Tourneau, C.L.; Sire, C.; Kaminsky, M.-C.; Coutte, A.; Alfonsi, M.; Calderon, B.; Boisselier, P.; et al. Extended Follow-up of a Phase 2 Trial of Xevinapant plus Chemoradiotherapy in High-Risk Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Randomised Clinical Trial. Eur. J. Cancer 2023, 183, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Vugmeyster, Y.; Ravula, A.; Rouits, E.; Diderichsen, P.M.; Kleijn, H.J.; Koenig, A.; Wang, X.; Schroeder, A.; Goteti, K.; Venkatakrishnan, K. Model-Informed Selection of the Recommended Phase III Dose of the Inhibitor of Apoptosis Protein Inhibitor, Xevinapant, in Combination with Cisplatin and Concurrent Radiotherapy in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck. Clin. Pharmacol. Ther. 2024, 115, 52–61. [Google Scholar] [CrossRef] [PubMed]
- TAO, Y.; Sun, X.-S.; Pointreau, Y.; Tourneau, C.L.; Sire, C.; Gollmer, K.; Crompton, P.; Bourhis, J. Long-Term Results from a Clinical Study of Xevinapant Plus Chemoradiotherapy in People with High-Risk Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Plain Language Summary. Future Oncol. 2023, 19, 1769–1776. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Schilder, R.J.; Martin, L.P.; Levin, M.; Graham, M.A.; Weng, D.E.; Adjei, A.A. A Phase I Study of the SMAC-Mimetic Birinapant in Adults with Refractory Solid Tumors or Lymphoma. Mol. Cancer Ther. 2015, 14, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Noonan, A.M.; Bunch, K.P.; Chen, J.-Q.; Herrmann, M.A.; Lee, J.-M.; Kohn, E.C.; O’Sullivan, C.C.; Jordan, E.; Houston, N.; Takebe, N.; et al. Pharmacodynamic Markers and Clinical Results from the Phase 2 Study of the SMAC Mimetic Birinapant in Women with Relapsed Platinum-Resistant or -Refractory Epithelial Ovarian Cancer. Cancer 2016, 122, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Dees, E.C.; Olszanski, A.J.; Dhuria, S.V.; Sen, S.; Cameron, S.; Cohen, R.B. Phase I Dose-Escalation Study of LCL161, an Oral Inhibitor of Apoptosis Proteins Inhibitor, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2014, 32, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Chesi, M.; Mirza, N.N.; Garbitt, V.M.; Sharik, M.E.; Dueck, A.C.; Asmann, Y.W.; Akhmetzyanova, I.; Kosiorek, H.E.; Calcinotto, A.; Riggs, D.L.; et al. IAP Antagonists Induce Anti-Tumor Immunity in Multiple Myeloma. Nat. Med. 2016, 22, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-R.; Wei, X.-L.; Feng, W.-N.; Zhao, H.-Y.; Zhang, Y.; Wang, Z.-Q.; Zhang, D.-S.; Wang, F.-H.; Yang, S.; Pan, W.; et al. Inhibitor of Apoptosis Proteins (IAP) Inhibitor APG-1387 Monotherapy or in Combination with Programmed Cell Death 1 (PD-1) Inhibitor Toripalimab in Patients with Advanced Solid Tumors: Results from Two Phase I Trials. ESMO Open 2024, 9, 103651. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.M.; LoRusso, P.M.; Papadopoulos, K.P.; Gordon, M.S.; Mita, A.C.; Ferraldeschi, R.; Keer, H.; Oganesian, A.; Su, X.Y.; Jueliger, S.; et al. A Phase I Study of ASTX660, an Antagonist of Inhibitors of Apoptosis Proteins, in Adults with Advanced Cancers or Lymphoma. Clin. Cancer Res. 2020, 26, 2819–2826. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzoli, A.B.A.; Moreno, V.; Gomez-Roca, C.A.; Even, C.; Cassier, P.; Guerrero, T.C.H.; de Miguel, M.; Korakis, I.; Purcea, D.; Roy, E.; et al. 560P Safety and Efficacy of Debio 1143, an Antagonist of Inhibitor of Apoptosis Proteins (IAPs), in Combination with Nivolumab in a Phase Ib/II Trial in Patients (Pts) Failing Prior PD-1/PD-L1 Treatment. Ann. Oncol. 2020, 31, S483. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Smith, D.C.; Pitot, H.C.; Brill, J.M.; Chugh, R.; Rouits, E.; Rubin, J.; Strickler, J.; Vuagniaux, G.; Sorensen, J.M.; et al. Safety, Pharmacokinetics, and Pharmacodynamic Properties of Oral DEBIO1143 (AT-406) in Patients with Advanced Cancer: Results of a First-in-Man Study. Cancer Chemother. Pharmacol. 2015, 75, 851–859. [Google Scholar] [CrossRef]



| IAP | Phase | The Aim of the Clinical Trial | Intervention | Condition | Status | Number |
|---|---|---|---|---|---|---|
| Birinapant | I | The side effects and optimal dose of birinapant administered alongside intensity-modulated re-irradiation therapy in the treatment of patients with HNSCC, who have experienced recurrence at or near the site of the original tumor (locally recurrent), have been under investigation. | +IMRT |
| Terminated | NCT03803774 |
| Xevinapant | I | Determination of the optimal safe dose of xevinapant administered in combination with radiotherapy and chemotherapy. | +RT +carboplatin or packlitaxel |
| Active, not recruiting | NCT06110195 |
| III | Evaluation of the efficacy and safety of Xevinapant combined with radiotherapy compared to placebo and radiotherapy in participants with resected HNSCC at high risk of recurrence who are ineligible for high-dose cisplatin treatment. | +IMRT |
| Active, not recruiting | NCT05386550 | |
| II | Assessment of the efficacy and safety of Xevinapant in combination with radiotherapy in elderly patients with locally advanced squamous cell carcinoma of the head and neck (LA-HNSCC) involving the oral cavity, oropharynx, hypopharynx, or larynx. | +IMRT |
| Recruiting | NCT05724602 | |
| Ib | Evaluation of the tolerability and safety of Xevinapant in combination with weekly cisplatin-based chemoradiotherapy for the treatment of patients with unresectable LA-HNSCC eligible for definitive chemoradiotherapy. | +cisplatin +IMRT |
| Active, not recruiting | NCT06056310 | |
| II | Assessment of the efficacy of adding Xevinapant to standard postoperative chemoradiotherapy in high-risk head and neck cancer patients. | +RT +cisplatin |
| Active, not recruiting | NCT06145412 | |
| III | Demonstration of the efficacy of Xevinapant in combination with radiotherapy and cetuximab compared to radiotherapy, cetuximab, and placebo in previously untreated participants with LA-HNSCC who are ineligible for high-dose cisplatin therapy. | +IMRT +cetuximab |
| Suspended | NCT05930938 | |
| III | Demonstration of the efficacy of Xevinapant (Debio 1143) compared to placebo in combination with chemoradiotherapy in LA-HNSCC | +IMRT +cisplatin |
| Active, not recruiting | NCT04459715 | |
| II | Evaluation of whether adding Xevinapant to postoperative adjuvant treatment with cisplatin and radiotherapy, followed by Xevinapant monotherapy, in patients with surgically resected HNSCC with extranodal extension and/or positive margins, will improve disease-free survival. | +IMRT and IGRT +carboplatin +cisplatin |
| Withdrawn | NCT06084845 | |
| I/II | A phase I/II randomized study to determine the maximum tolerated dose, safety, pharmacokinetics and antitumor activity of Debio 1143 combined with concurrent chemo-radiation therapy in patients with locally advanced squamous cell carcinoma of the head and neck. | +RT +cisplatin |
| Completed | NCT02022098 | |
| Tolinapant | I | Demonstration of the safety and side effects of tolinapant administered in combination with radiotherapy in the treatment of patients with head and neck cancer, who are treatment-naïve, have disease that has spread to nearby tissues or lymph nodes, and are ineligible for cisplatin therapy. | +RT |
| Recruiting | NCT05245682 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzykawski, K.; Kubina, R.; Wendlocha, D.; Sarna, R.; Mielczarek-Palacz, A. Multifaceted Evaluation of Inhibitors of Anti-Apoptotic Proteins in Head and Neck Cancer: Insights from In Vitro, In Vivo, and Clinical Studies (Review). Pharmaceuticals 2024, 17, 1308. https://doi.org/10.3390/ph17101308
Krzykawski K, Kubina R, Wendlocha D, Sarna R, Mielczarek-Palacz A. Multifaceted Evaluation of Inhibitors of Anti-Apoptotic Proteins in Head and Neck Cancer: Insights from In Vitro, In Vivo, and Clinical Studies (Review). Pharmaceuticals. 2024; 17(10):1308. https://doi.org/10.3390/ph17101308
Chicago/Turabian StyleKrzykawski, Kamil, Robert Kubina, Dominika Wendlocha, Robert Sarna, and Aleksandra Mielczarek-Palacz. 2024. "Multifaceted Evaluation of Inhibitors of Anti-Apoptotic Proteins in Head and Neck Cancer: Insights from In Vitro, In Vivo, and Clinical Studies (Review)" Pharmaceuticals 17, no. 10: 1308. https://doi.org/10.3390/ph17101308
APA StyleKrzykawski, K., Kubina, R., Wendlocha, D., Sarna, R., & Mielczarek-Palacz, A. (2024). Multifaceted Evaluation of Inhibitors of Anti-Apoptotic Proteins in Head and Neck Cancer: Insights from In Vitro, In Vivo, and Clinical Studies (Review). Pharmaceuticals, 17(10), 1308. https://doi.org/10.3390/ph17101308







