Thermal Compatibility of New ACEI Derivatives with Popular Excipients Used to Produce Solid Pharmaceutical Formulations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Confirmation of the Chemical Structure of the New ACEI Derivatives Tested
2.2. Assessment of the Compatibility of IND-1 and IND-2 Derivatives with Selected Excipients Using the Thermal Methods
2.3. Colorimetric Analysis
2.4. UV–Vis Spectrophotometry
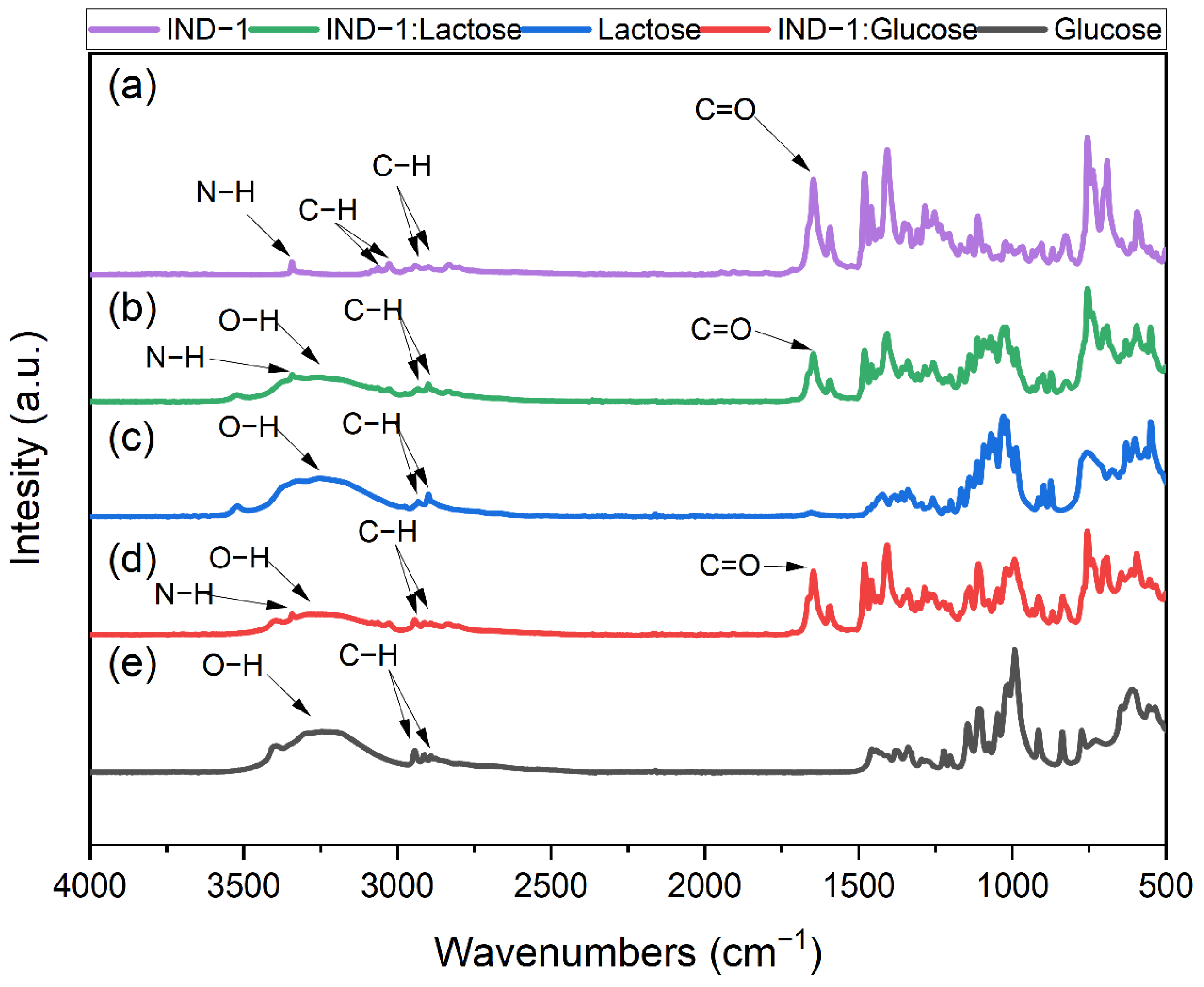
2.5. FTIR Measurements
3. Materials and Methods
3.1. ACEI Indole Derivatives Used in the Research
- 2-chloro-1-(indolin-1-yl)propoan-1-one (1, IND-2)
- 2-chloro-1-(indolin-1-yl)ethan-1-one (2)
- 2-(benzylamino)-1-(2,3-dihydroindol-1-yl)ethanone (3, IND-1)
3.2. Excipients Used in the Research
3.3. Performing Thermogravimetric Analyses of TG, DTG and c-DTA
3.4. Analysis of Mixtures under Room and Stress Conditions
3.5. Performing Colorimetric Analysis
3.6. UV–Vis Analysis
3.7. FTIR Meansuremenets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sznitowska, M. (Ed.) Farmacja Stosowana; PZWL: Warsaw, Poland, 2017. [Google Scholar]
- Kostowski, W.; Herman, Z.S. (Eds.) Farmakologia; PZWL: Wasaw, Poland, 2016. [Google Scholar]
- Tzourio, C.; Anderson, C.; Chapman, N.; Woodward, M.; Neal, B.; MacMahon, S.; Chalmers, J. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. 2003, 163, 1069–1075. [Google Scholar] [PubMed]
- Sink, K.M.; Leng, X.; Williamson, J.; Kritchevsky, S.B.; Yaffe, K.; Kuller, L.; Yasar, S.; Atkinson, H.; Robbins, M.; Psaty, B.; et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2009, 169, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, A.; Ramos, P.; Szczołko, W.; Pilawa, B.; Stanisz, B. Can angiotensin-converting enzyme inhibitors interfere with the free radicals? Measurement of antioxidant capacity using DPPH radical reduction examined by UV-VIS method. Acta Pol. Pharm. 2019, 76, 233–239. [Google Scholar]
- Juszczak, A.; Ramos, P.; Szczołko, W.; Pilawa, B.; Stanisz, B. Evaluation of antioxidant properties of angiotensin-converting enzyme inhibitors-interactions with free radicals model examined by EPR spectroscopy. Pharm. Pharmacol. Int. J. 2020, 8, 25–32. [Google Scholar] [CrossRef]
- Janiec, W. (Ed.) Farmakodynamika; PZWL: Warsaw, Poland, 2022. [Google Scholar]
- Kollamaram, G.; Faucher, A.; Croker, D.M.; Walker, G.M. Valvejet Technology for the Production of a Personalised Fixed Dose Combination of Ramipril and Glimepiride: An Investigative Study on the Stability of Ramipril. Pharm. Res. 2018, 35, 181. [Google Scholar] [CrossRef]
- Paszun, S.; Stanisz, B. Cilazapril decomposition kinetics and mechanism in the solid state versus stability of the other ester pro-drug angiotensin converting enzyme inhibitors. React. Kinet. Mech. Catal. 2013, 109, 285–300. [Google Scholar] [CrossRef]
- Stanisz, B. Evaluation of stability of enalapril maleate in solid phase. J. Pharm. Biomed. Anal. 2003, 31, 375–380. [Google Scholar] [CrossRef]
- Stanisz, B. The influence of relative humidity and temperature on stability of moexipril hydrochloride in solid phase. Acta Polon. Pharm. 2004, 61, 91–97. [Google Scholar]
- Polski, A.; Iwaniak, K.; Naleśniak, M.; Poleszak, E. The excipients used in the non-coated tablests—A review. Med. Int. Revuo. 2014, 102, 10–18. [Google Scholar]
- Elder, D.P.; Kuentz, M.; Holm, R. Pharmaceutical excipients—Quality, regulatory and biopharmaceutical considerations. Eur. J. Pharm. Scien. 2016, 87, 88–99. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B.; Bajaj, A.N. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: A comprehensive review. J. Excip. Food Chem. 2010, 1, 3–26. [Google Scholar]
- Lundin, P.M.; Fu, G.C. Asymmetric Suzuki cross-couplings of activated secondary alkyl electrophiles: Arylations of racemic alpha-chloroamides. J. Am. Chem. Soc. 2010, 132, 11027–11029. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Murakami, M.; Miura, T. Photoassisted cross-coupling reaction of α-chlorocarbonyl compounds with arylboronic acids. Org. Lett. 2022, 24, 1616–1619. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Habdbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA, 2009. [Google Scholar]
- Ramos, P. Compatibility studies of selected mucolytic drugs with excipients used in solid dosage forms: Thermogravimetry analysis. Farmacia 2021, 69, 585–594. [Google Scholar] [CrossRef]
- Ramos, P. Thermal compatibility assessment of selected excipients used in the oral anti-cancer formulation containing busulfan. Farmacia 2022, 70, 417–424. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Ramos, P.; Kłósek, M.; Bobela, E.; Czuba, Z.P.; Balwierz, R.; Olczyk, P. Propolis as a cariostatic agent in lozenges and impact of storage conditions on the stability of propolis. Pharmaceutics 2023, 15, 1768. [Google Scholar] [CrossRef]
- Pitucha, M.; Ramos, P.; Wojtunik-Kulesza, K.; Głogowska, A.; Stefańska, J.; Kowalczuk, D.; Drózd, M.; Augustynowicz-Kopeć, E. Thermal analysis, antimicrobial and antioxidant studies of thiosemicarbazone derivatives. J. Therm. Anal. Calorim. 2023, 2023, 4223–4234. [Google Scholar] [CrossRef]
- Bartyzel, A.; Kaczor, A.A.; Głuchowska, H.; Pitucha, M.; Wróbel, T.M.; Matosiuk, D. Thermal and spectroscopic studies of 2,3,5-trisubstituted and 1,2,3,5-tetrassubstituted indoles as non-competitive antagonists of GluK1/GluK2 receptors. J. Therm. Anal. Calorim. 2018, 133, 935–944. [Google Scholar] [CrossRef]
- Saavedra-Leosa, M.Z.; Alvarez-Salasb, C.; EsneiderAlcala, M.A.; Toxqui-Terán, A.; Pérez-García, S.A.; RuizCabrera, M.A. Towards an improved calorimetric methodology for glass transition temperature determination in amorphous sugars. CyTA—J. Food 2012, 10, 258–267. [Google Scholar] [CrossRef]
- Wei-Hsien, H.; Wen-Ting, C.; Ling-Chun, C.; HongLiang, L.; Shan-Yang, L. Non-isothermal dehydration kinetics of glucose monohydrate, maltose monohydrate and trehalose dihydrate by thermal analysis and DSC-FTIR study. J. Biomed. Pharm. Sci. 2018, 1, 1–6. [Google Scholar]
- Zhao, Z.; Hayashi, S.; Xu, W.; Wu, Z.; Tanaka, S.; Sun, S.; Zhang, M.; Kanayama, K.; Umemura, K. A Novel EcoFriendly Wood Adhesive Composed by Sucrose and Ammonium Dihydrogen Phosphate. Polymers 2018, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dou, B.; Song, Y.; Chen, H.; Yang, M.; Xu, Y. Kinetic study on non-isothermal pyrolysis of sucrose biomass. Energy Fuels 2014, 28, 3793–3801. [Google Scholar] [CrossRef]
- Ilyes, K.; Casian, T.; Hales, D.; Borodi, G.; Rus, L.; Stiufius, R.; Tomuta, I. Applying the principles of quality by design (QbD) coupled with multivariate data analysis (MVDA) in establishing the impact of raw material variability for extended release tablets. Farmacia 2021, 69, 481–497. [Google Scholar] [CrossRef]
- Lavor, E.P.; Navarro, M.V.M.; Freire, F.D.; Aragão, C.F.S.; Raffin, F.N.; Barbosa, E.G.; de Lima e Moura, T.F.A. Application of thermal analysis to the study of antituberculosis drugs–excipient compatibility. J. Therm. Anal. Calorim. 2014, 115, 2303–2309. [Google Scholar] [CrossRef]
- Listiohadi, Y.; Hourigan, J.A.; Sleigh, R.W.; Steele, R.J. Thermal analysis of amorphous lactose and α-lactose monohydrate. Dairy. Sci. Technol. 2009, 89, 43–67. [Google Scholar] [CrossRef]
- Suxia, R.; Xiuxuan, S.; Tingzhou, L.; Qinglin, W. The effect of chemical and high-pressure homogenization treatment conditions on the morphology of cellulose nanoparticles. J. Nanomat. 2014, 2014, 582913. [Google Scholar]
- Kuthi, F.A.A.; Norzali, N.R.A.; Badri, K.H. Thermal characteristics of microcrystalline cellulose from oil palm biomass. Malaysian J. Analyt. Sci. 2016, 20, 1112–1122. [Google Scholar]
- Katugampola, P.; Winstead, C.; Adeleke, A. Thermal stability of carboxymethyl chitosan varying the degree of substitution. Int. J. Pharm. Sci. Invent. 2014, 3, 42–48. [Google Scholar]
- Liao, S.K.; Hung, C.C.; Lin, M.F. A kinetic study of thermal degradations of chitosan/polycaprolactam blends. Macromolec. Res. 2004, 12, 466–473. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, S.; Zhang, B.; Qiao, D. Structural features and thermal property of propionylated starches with different amylose/amylopectin ratio. Int. J. Biol. Macromol. 2017, 97, 123–130. [Google Scholar] [CrossRef]
- Kaczmarska, K.; Żymankowska-Kumon, S.; Grabowska, B.; Bobrowski, A.; Cukrowicz, S. Study of thermal degradation of starch-based binder by TG-DTG-DSC Py-GC/MS and DRIFTS. Arch. Found. Engine. 2019, 19, 21–26. [Google Scholar] [CrossRef]
- Tița, B.; Fuliaș, A.; Bandur, G.; Tiťa, D.; Babe, V. Application of thermal analysis to study the compatibility of sodium diclofenac with different pharmaceutical excipients. Rev. Chim. 2011, 62, 443–454. [Google Scholar]
- Fuliaş, A.; Ledeţi, I.; Vlase, G.; Popoiu, C.; Hegheş, A.; Bilanin, M.; Vlase, T.; Gheorgheosu, D.; Craina, M.; Ardelean, S.; et al. Thermal behaviour of procaine and benzocaine Part II: Compatibility study with some pharmaceutical excipients used in solid dosage forms. Chem. Cent. J. 2013, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Wesołowski, M.; Rojek, B. Thermogravimetric detection of incompatibilities between atenolol and excipients using multivariate techniques. J. Therm. Anal. Calorim. 2013, 113, 169–177. [Google Scholar] [CrossRef]
- Rojek, B.; Wesołowski, M. Compatibility studies of hydrocortisone with excipients using thermogravimetric analysis supported by multivariate statistical analysis. J. Therm. Anal. Calorim. 2017, 127, 543–553. [Google Scholar] [CrossRef]
- Sip, S.; Paczkowska-Walendowska, M.; Rosiak, N.; Miklaszewski, A.; Grabańska-Martyńska, K.; Samarzewska, K.; Cielecka-Piontek, J. Chitosan as Valuable Excipient for Oral and Topical Carvedilol Delivery Systems. Pharmaceuticals 2021, 14, 712. [Google Scholar] [CrossRef]
- Kepsutlu, A.R.; Savaser, A.; Ozkan, Y.; Dikmen, N.; Isimer, A. Evaluation of chitosan used as an excipient in tablet formulations. Acta Pol. Pharm. 1999, 56, 227–235. [Google Scholar]
- Ray, S.D. Potential aspects of chitosan as pharmaceutical excipient. Acta Pol. Pharm. 2011, 68, 619–622. [Google Scholar]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef]
- Echavarría, A.; Pagán, J.; Ibarz, A. Kinetics of color development in glucose/amino acid model systems at different temperatures. Sci. Agropecu. 2016, 7, 15–21. [Google Scholar] [CrossRef]
- Subert, J.; Cizmárik, J. Application of instrumental colour measurement in development and quality control of drugs and pharmaceutical excipients. Die Pharm. 2008, 63, 331–336. [Google Scholar]
- MacDougall, D.B.; Mirjana Granov, M. Relationship between Ultraviolet and Visible Spectra in Maillard Reactions and CIELAB Colour Space and Visual Appearance. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2005; pp. 160–165. [Google Scholar]
- Sun, Y.; Lin, L.; Zhang, P. Color Development Kinetics of Maillard Reactions. Ind. Eng. Chem. Res. 2021, 60, 3495–3501. [Google Scholar] [CrossRef]
- Echavarría, A.P.; Pagán, J.; Ibarz, A. Kinetics of color development of melanoidins formed from fructose/amino acid model systems. Food Sci. Tech. Intern. 2013, 20, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Arachchi, S.J.; Kim, Y.J.; Kim, D.W.; Oh, S.C.; Lee, Y.B. Optimization of Maillard Reaction in Model System of Glucosamine and Cysteine Using Response Surface Methodology. Prev. Nutr. Food Sci. 2017, 22, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sakiroff, L.M.; Chennell, P.; Yessaad, M.; Pereira, B.; Bouattour, Y.; Sautou, V. Evaluation of color changes during stability studies using spectrophotometric chromaticity measurements versus visual examination. Sci. Rep. 2022, 12, 8959. [Google Scholar] [CrossRef]
- Golonka, I.; Wilk, S.; Musiał, W. The influence of UV radiation on the degradation of pharmaceutical formulations containing quercetin. Molecules 2020, 25, 5454. [Google Scholar] [CrossRef]
- Akhtar, F.; Gul, S.; Ashfaq, S.; Rehman, I.; Mirza, A.Z. UV spectroscopic method for optimization and determination of glibenclamide in bulk, pharmaceutical dosage form and its application for in vitro interaction studies. J. Anal. Test. 2020, 4, 281–290. [Google Scholar] [CrossRef]
- Kukulski, T.; Wacławek, S.; Silvestri, D.; Krawczyk, K.; Padil, V.V.T.; Fryczkowski, R.; Janicki, J.; Černík, M. A Polymeric Composite Material (rGO/PANI) for Acid Blue 129 Adsorption. Polymers 2020, 12, 1051. [Google Scholar] [CrossRef]
- Zhang, H.; Ou, J.; Yao, Y.; Wang, H.; Liu, Z.; Wei, Y.; Ye, M. Facile Preparation of Titanium(IV)-Immobilized Hierarchically Porous Hybrid Monoliths. Anal. Chem. 2017, 89, 4655–4662. [Google Scholar] [CrossRef]
- Silvestri, D.; Wacławek, S.; Venkateshaiah, A.; Krawczyk, K.; Sobel, B.; Padil, V.V.T.; Černík, M.; Varma, R.S. Synthesis of Ag Nanoparticles by a Chitosan-Poly(3-Hydroxybutyrate) Polymer Conjugate and Their Superb Catalytic Activity. Carbohydr. Polym. 2020, 232, 115806. [Google Scholar] [CrossRef]
- Silvestri, D.; Wacławek, S.K.; Ramakrishnan, R.; Venkateshaiah, A.; Krawczyk, K.; Padil, V.V.T.; Sobel, B.; Černík, M. The Use of a Biopolymer Conjugate for an Eco-Friendly One-Pot Synthesis of Palladium-Platinum Alloys. Polymers 2019, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- ICH. ICH Harmonised Tripartite Guideline: Stability Testing of New Drug Substances and Products, Q1A (R2); ICH: Geneva, Switzerland, 2003. [Google Scholar]
- Ding, P.; Ling, Y.S. Brownikg assessment methods and polyphenol oxidase in UV-C irradiated Berangan banana fruit. Intern. Food Res. J. 2014, 21, 1667–1674. [Google Scholar]
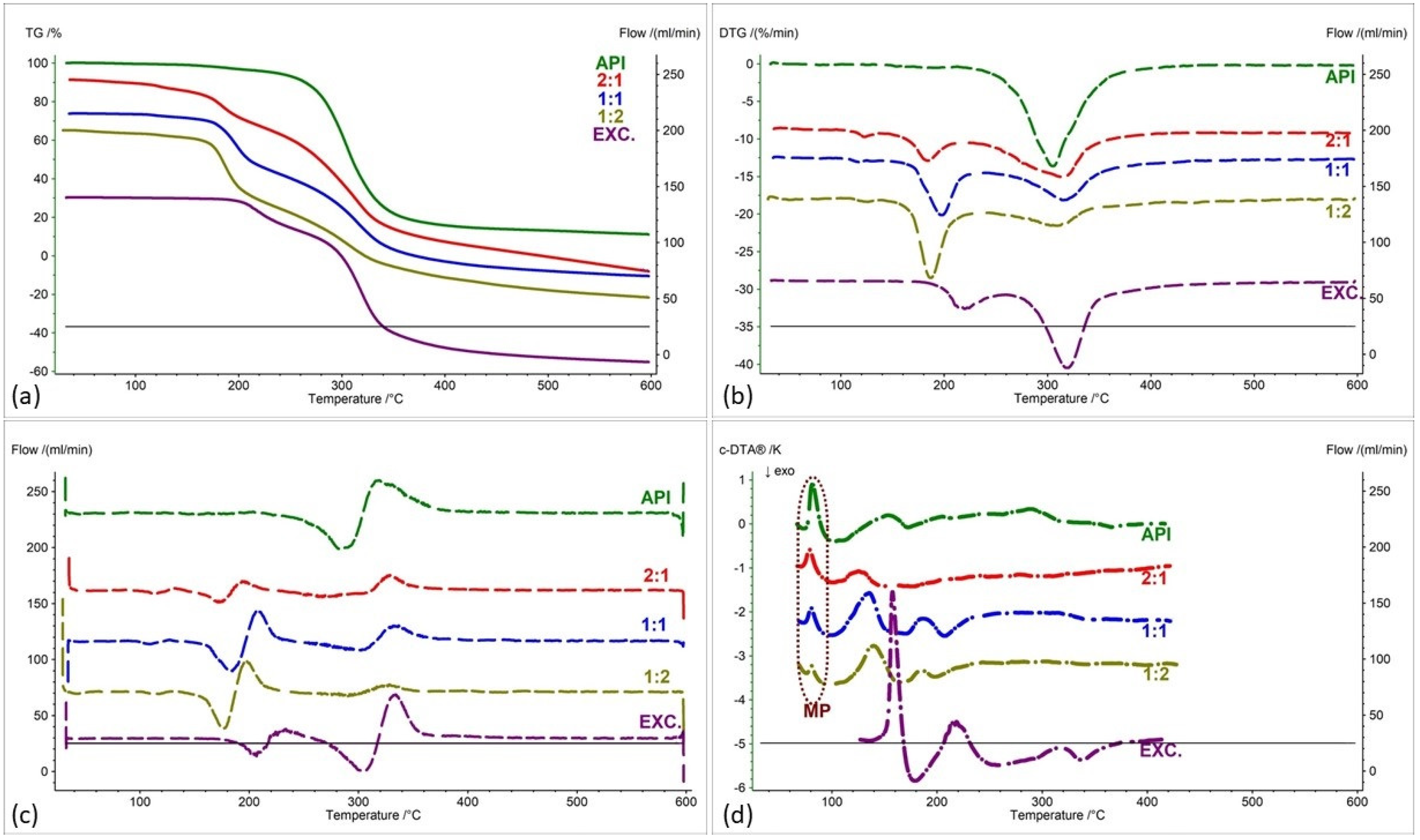
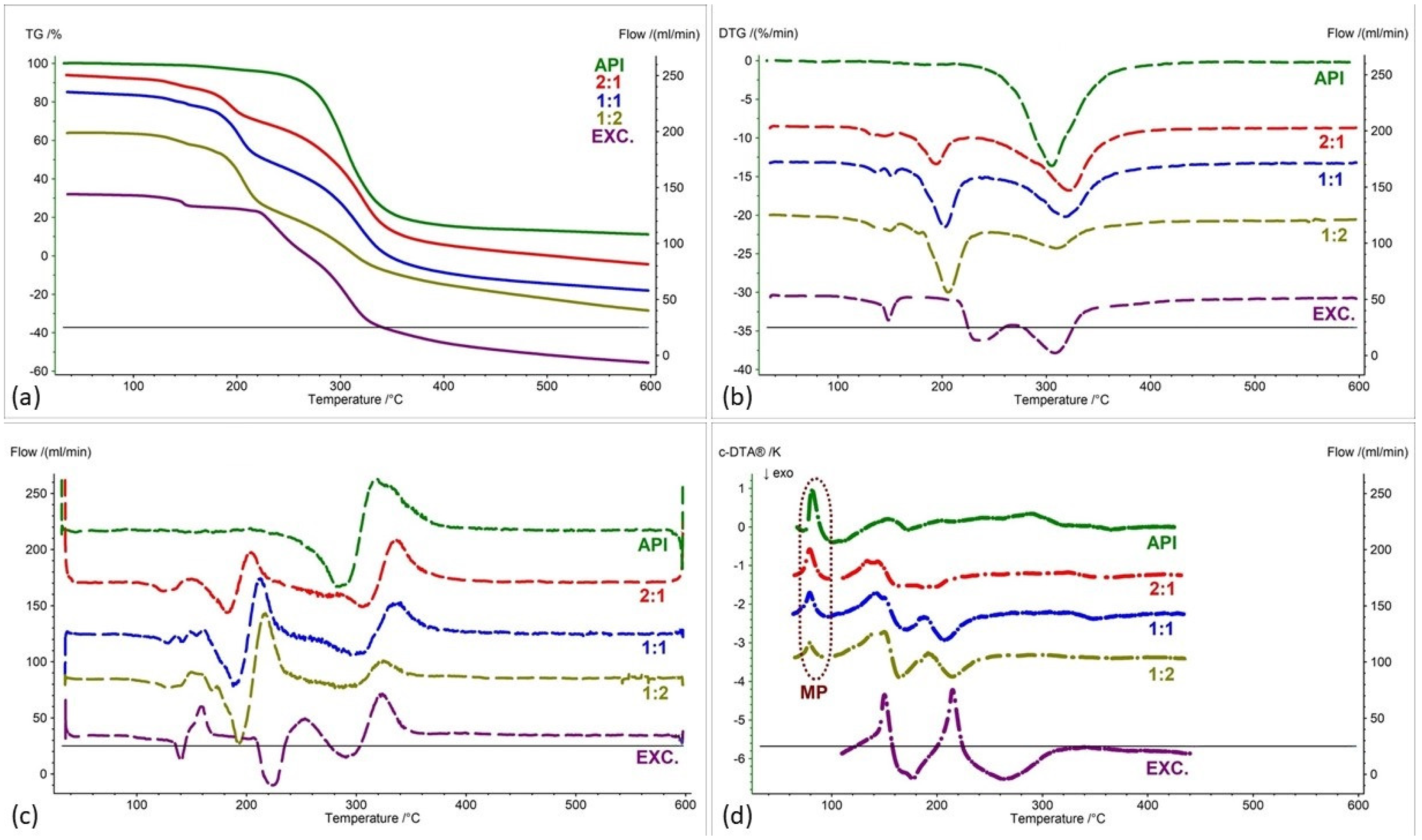
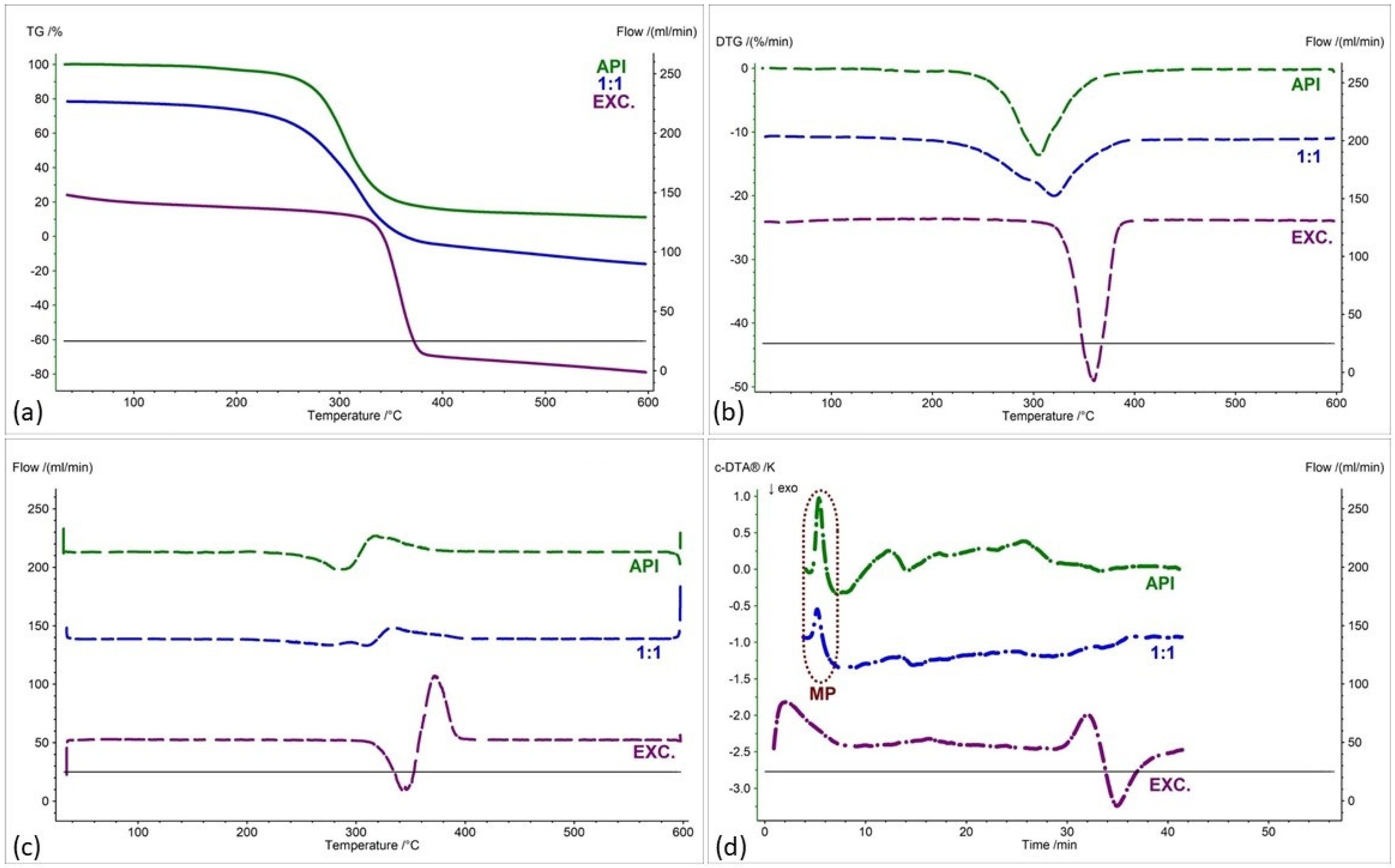
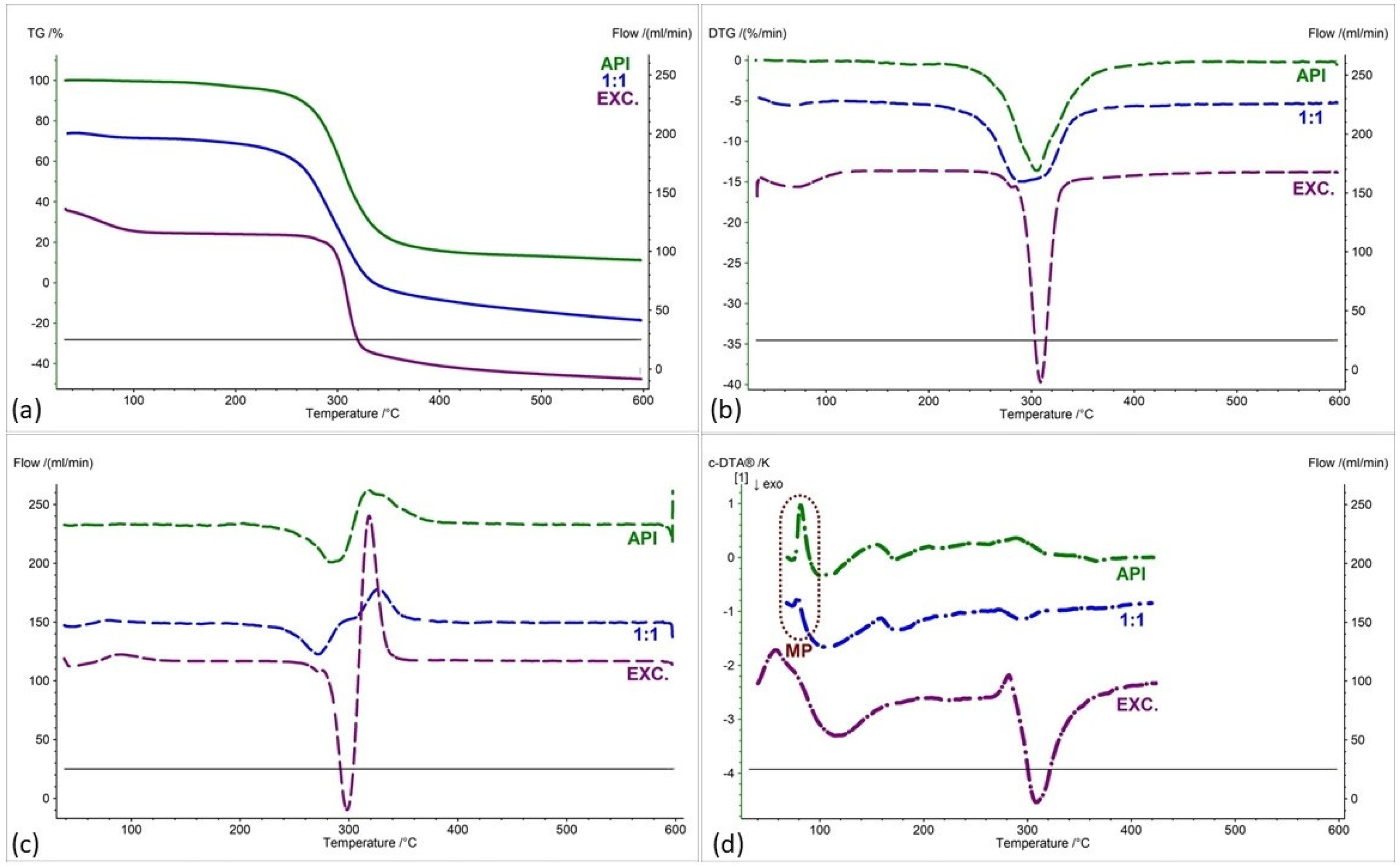
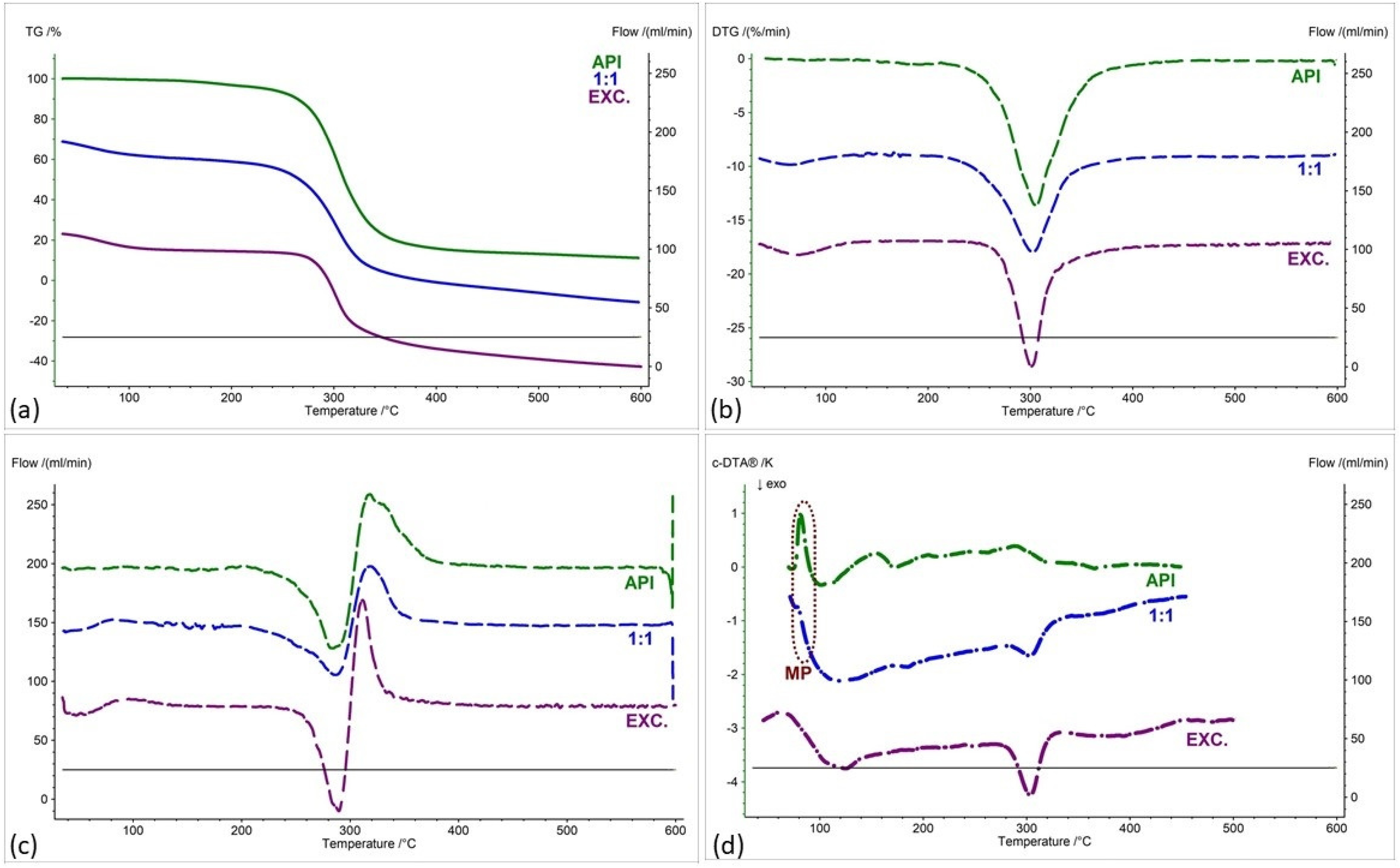
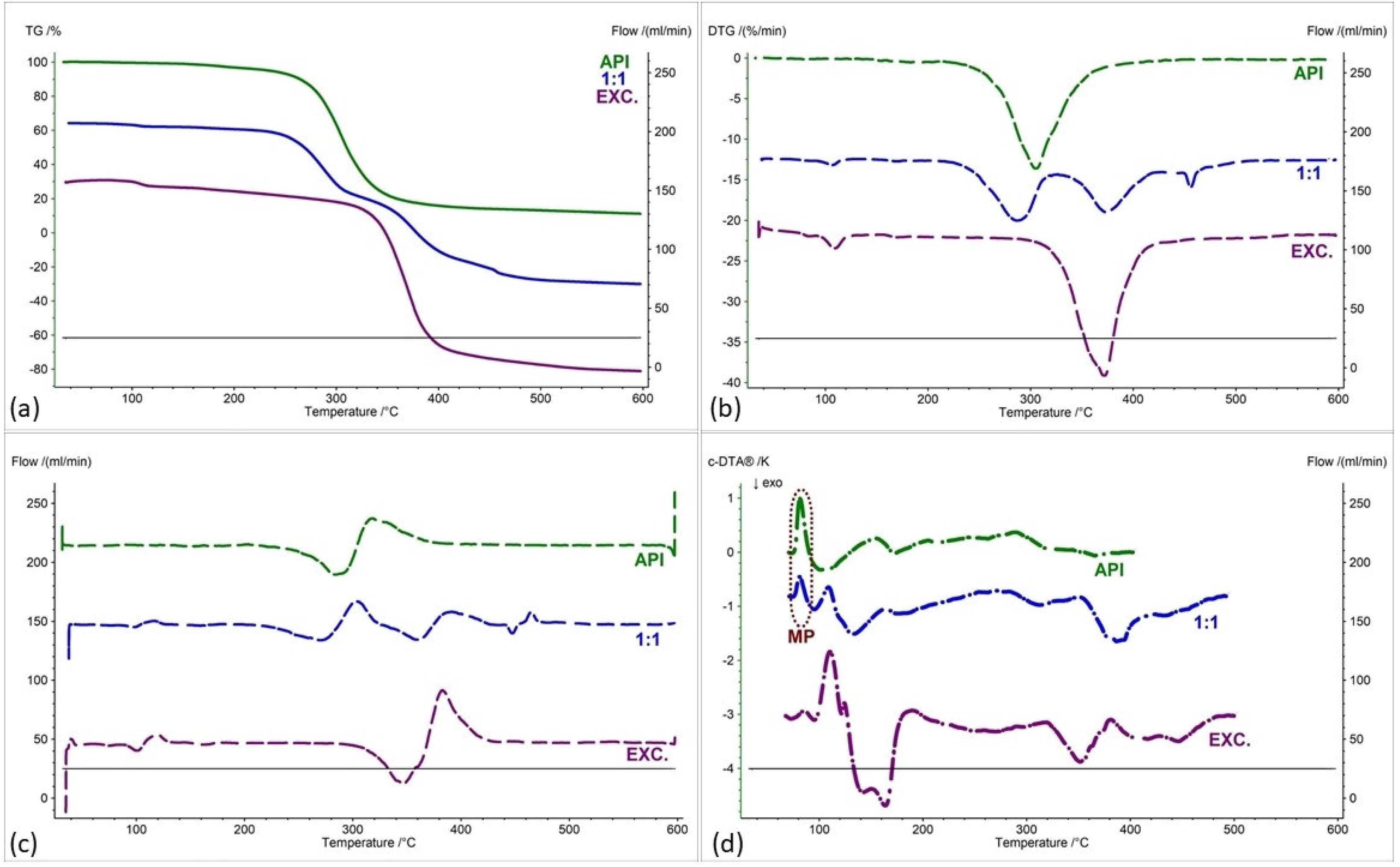
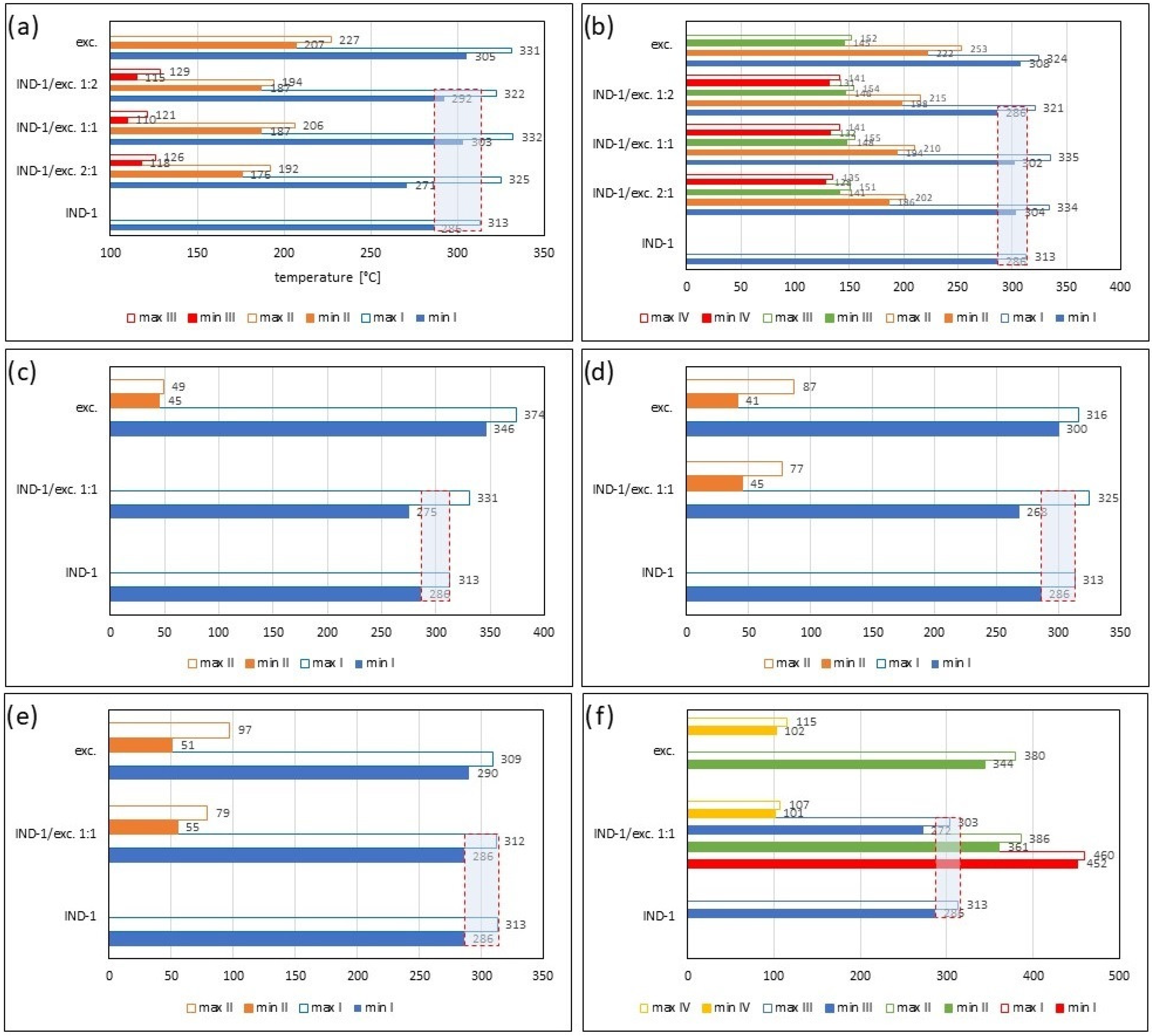
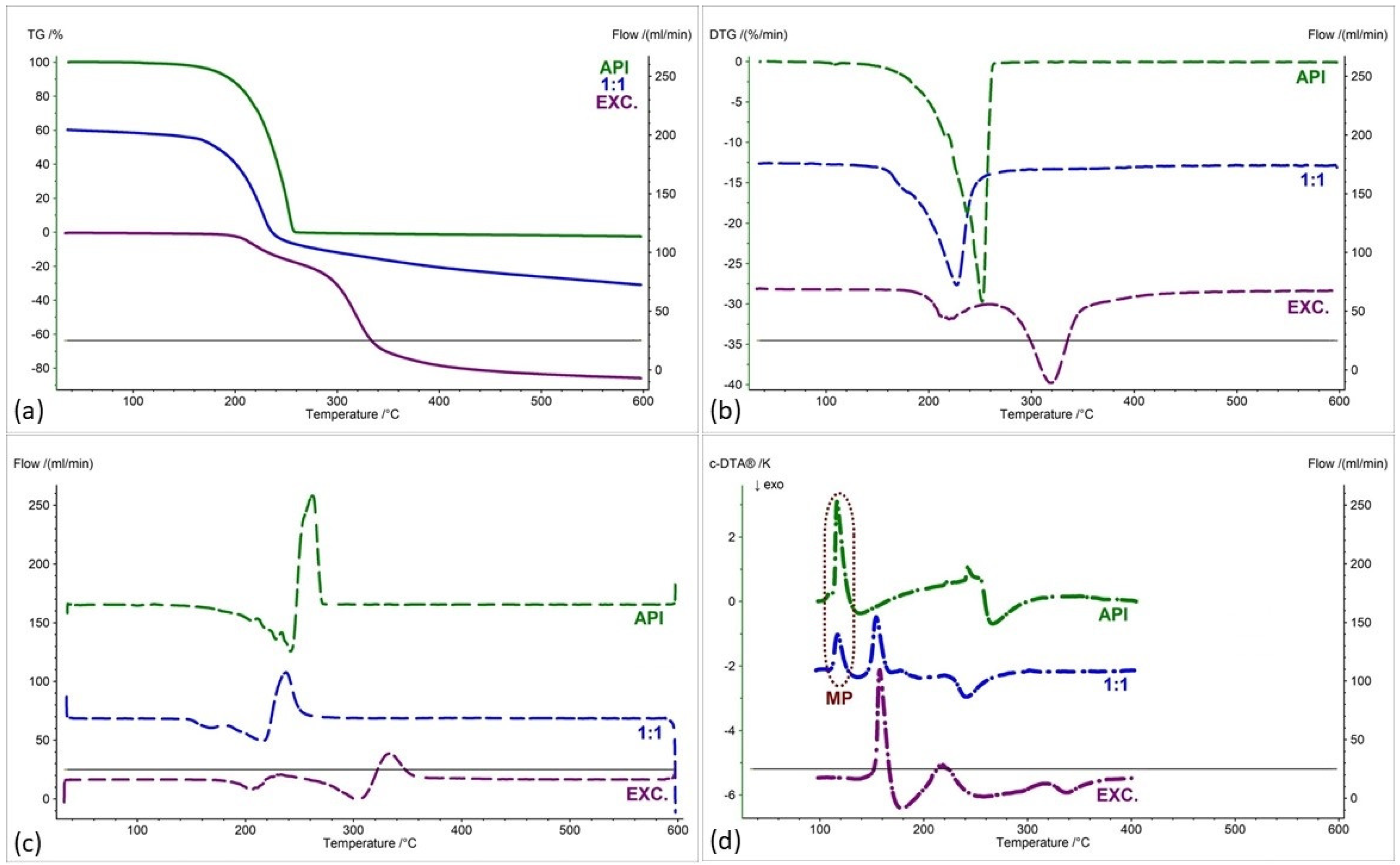

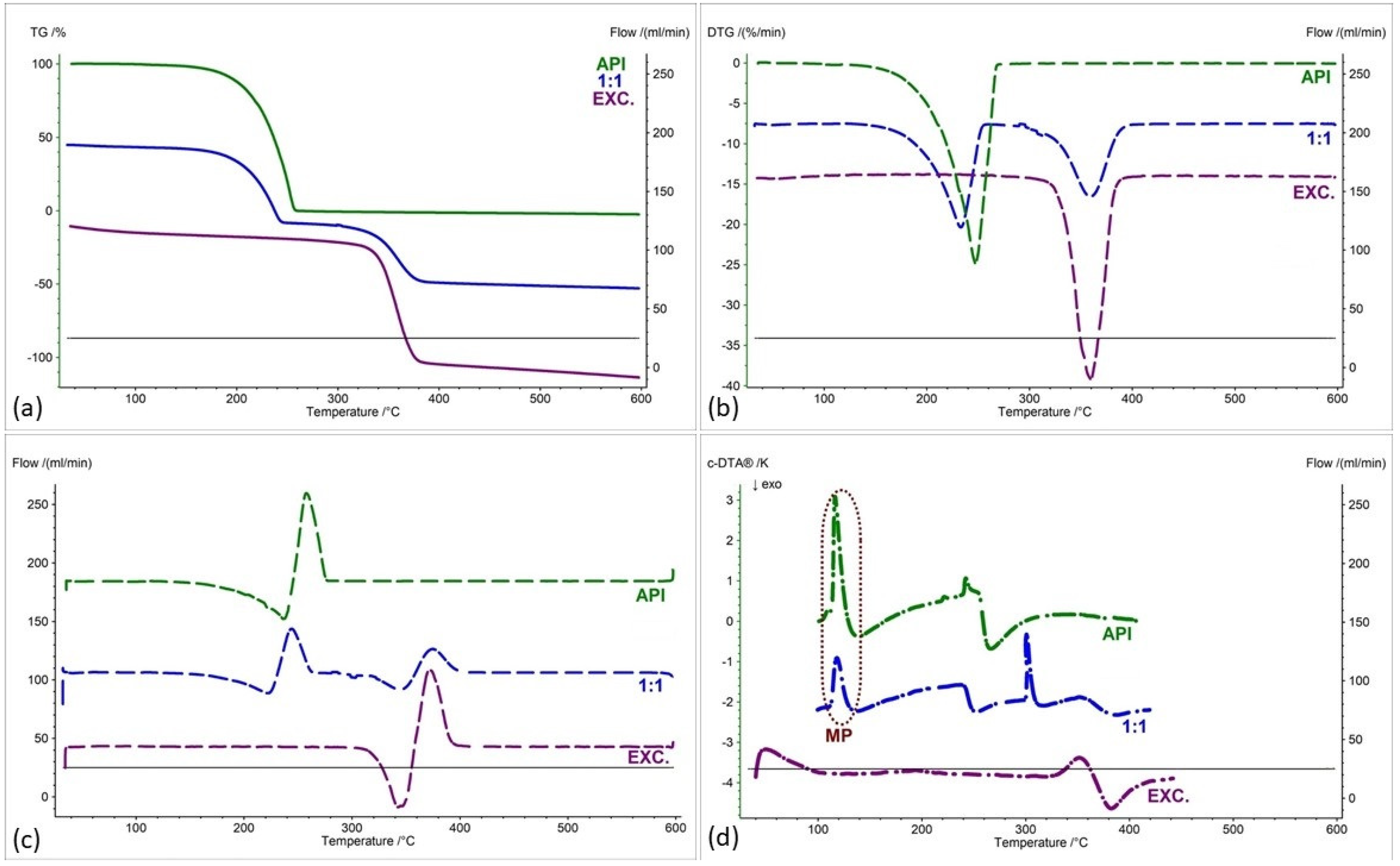

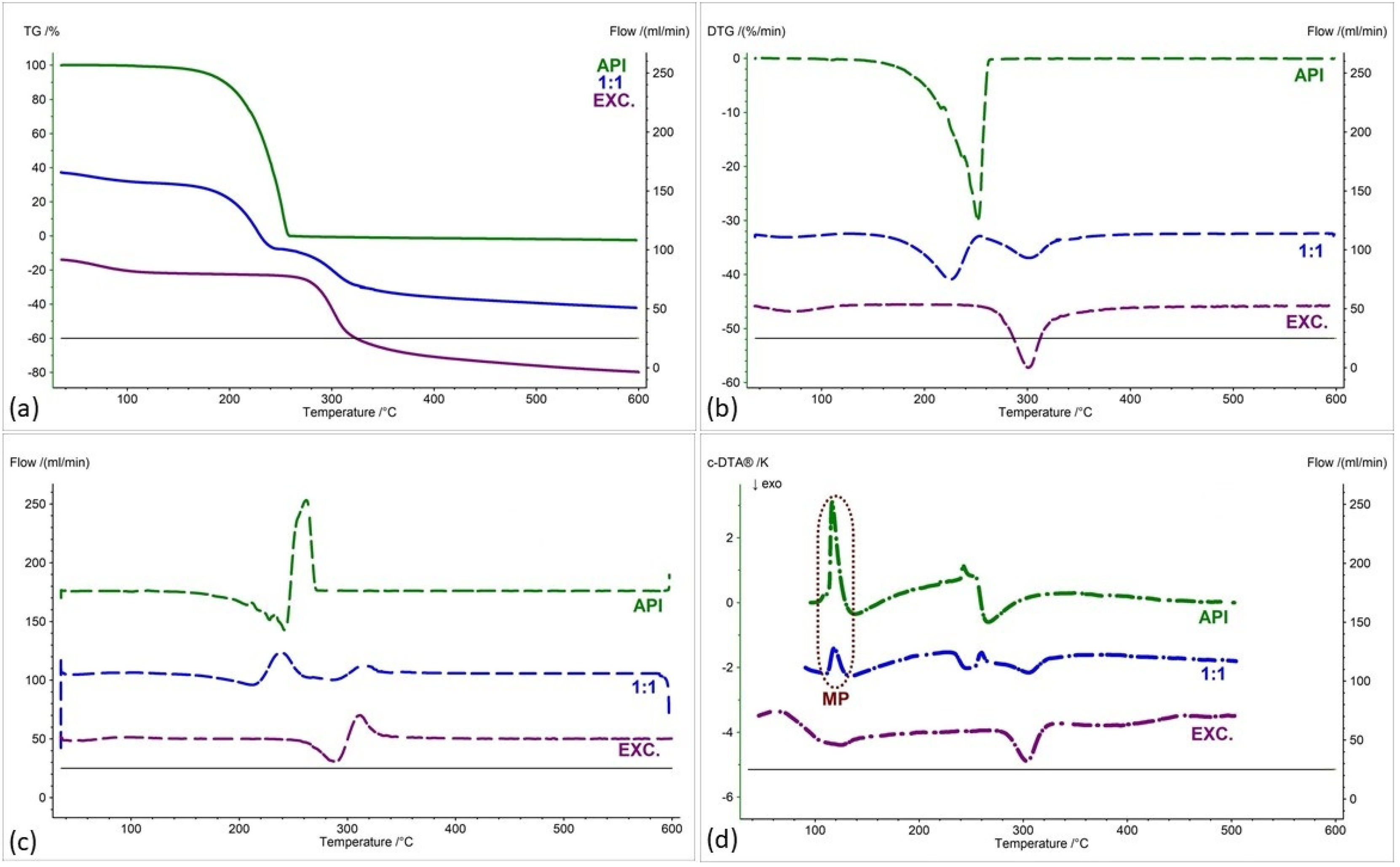
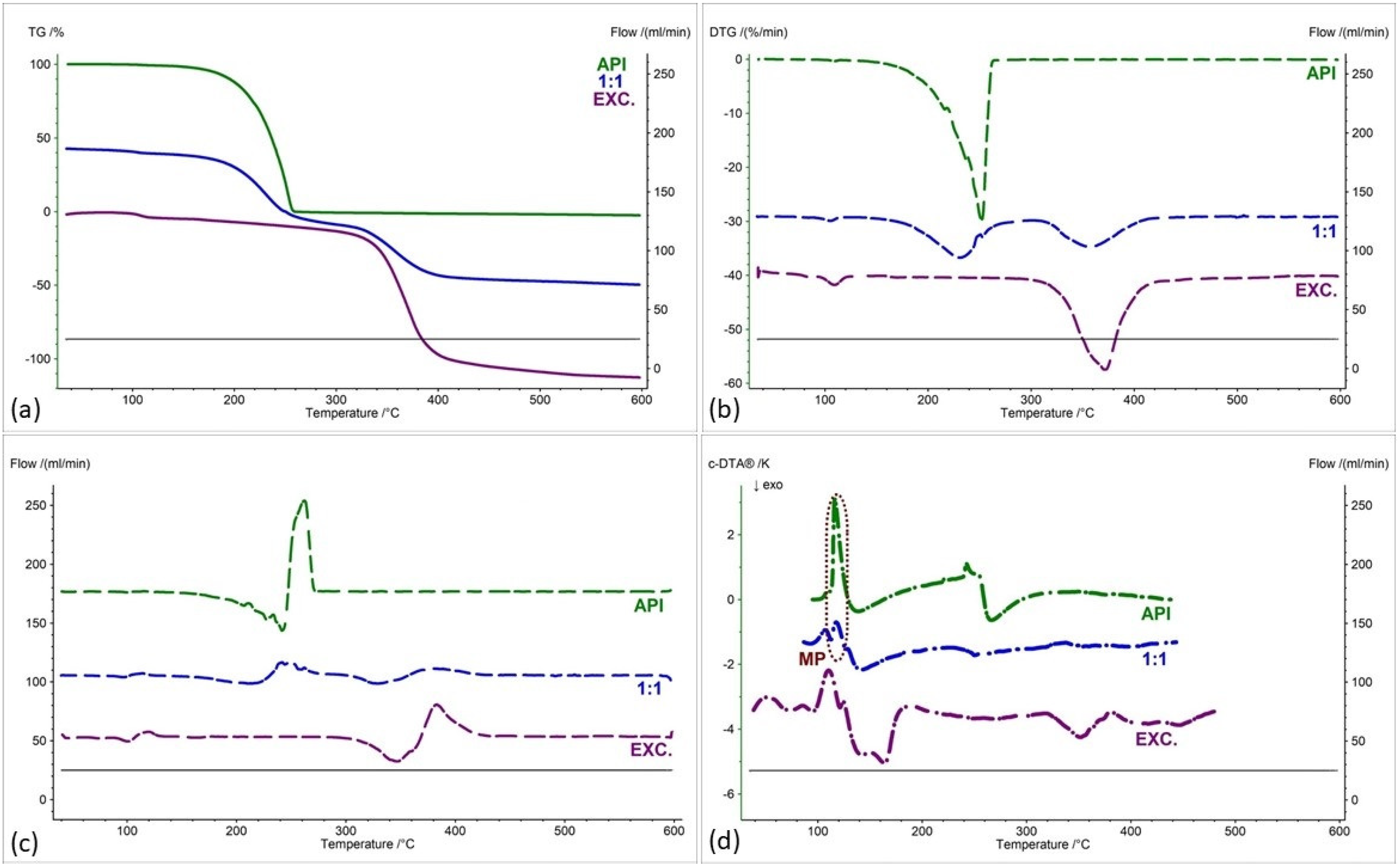
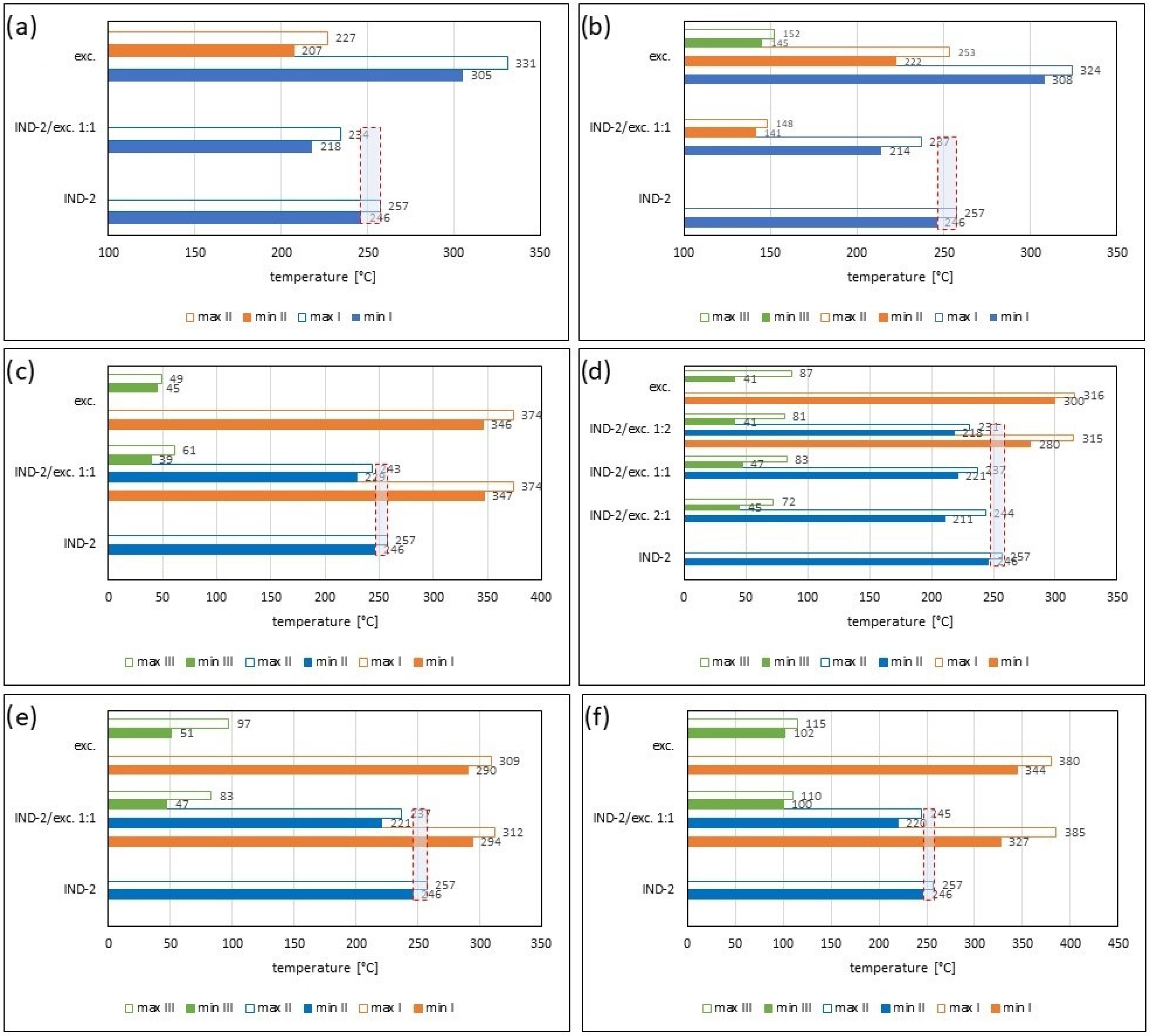


| Samples | Ratio (W:W) | Onset [°C] | Mid [°C] | Inflection [°C] | End [°C] | Weight Loss [%] |
|---|---|---|---|---|---|---|
| IND-1 | 275.5 | 306.0 | 304.9 | 336.4 | −76.68 | |
| Glucose anhydrous | 279.3 | 311.0 | 318.7 | 343.4 | −70.65 | |
| IND-1:EXC. | 2:1 | 247.0 | 288.8 | 315.5 | 340.4 | −58.14 |
| 1:1 | 180.2 | 268.7 | 197.4 | 268.8 | −60.93 | |
| 1:2 | 174.3 | 213.2 | 186.2 | 229.3 | −54.49 | |
| Lactose monohydrate | 259.2 | 299.9 | 310.0 | 338.2 | −48.63 | |
| IND-1:EXC. | 2:1 | 270.3 | 310.6 | 321.6 | 350.0 | −57.77 |
| 1:1 | 187.7 | 296.4 | 201.9 | 278.6 | −61.03 | |
| 1:2 | 192.4 | 252.1 | 206.4 | 250.2 | −45.99 | |
| Microcrystalline cellulose | 339.7 | 356.6 | 357.8 | 373.3 | −81.0 | |
| IND-1:EXC. | 1:1 | 266.2 | 309.8 | 320.1 | 352.8 | −69.26 |
| Starch from corn | 297.8 | 309.6 | 309.2 | 321.4 | −61.45 | |
| IND-1:EXC. | 1:1 | 260.0 | 296.0 | 290.9 | 333.2 | −69.60 |
| Chitosan | 283.0 | 302.4 | 300.4 | 322.3 | −42.60 | |
| IND-1:EXC. | 1:1 | 269.2 | 299.2 | 301.6 | 329.6 | −52.16 |
| Magnesium stearate | 339.8 | 365.5 | 372.0 | 391.1 | −84.73 | |
| IND-1:EXC. | 1:1 | 257.8 | 295.7 | 288.8 | 342.3 | −55.0 |
| Samples | Ratio (W:W) | Stage [First, Second, Third, Fourth] Peak [°C] Mass Change [%/min.] | |||||
|---|---|---|---|---|---|---|---|
| IND-1 | - | - | - | First 305.4 13.61 | - | - | |
| IND-1:EXC. | 2:1 | - | First 122.8 1.30 | Second 184.3 4.47 | Third 313.6 6.65 | - | - |
| 1:1 | - | First 118.0 0.60 | Second 197.7 7.68 | Third 314.9 5.68 | - | - | |
| 1:2 | - | First 126.1 0.67 | Second 187.1 10.79 | Third 308.7 3.84 | - | - | |
| Glucose anhydrous | - | - | First 219.9 3.74 | Second 319.2 11.61 | - | - | |
| IND-1:EXC. | 2:1 | First 134.1 1.37 | Second 145.1 1.37 | Third 194.6 5.10 | Fourth 322.2 8.52 | - | - |
| 1:1 | First 137.5 1.54 | Second 151.8 2.01 | Third 202.6 8.60 | Fourth 318.2 7.20 | - | - | |
| 1:2 | First 139.9 1.68 | Second 149.9 1.97 | Third 205.8 9.95 | Fourth 309.3 4.23 | - | - | |
| Lactose monohydrate | - | First 148.6 3.24 | Second 236.1 5.84 | Third 308.2 7.49 | - | - | |
| IND-1:EXC. | 1:1 | - | - | - | First 320.5 9.43 | - | - |
| Microcrystalline cellulose | - | - | First 47.7 0.78 | Second 359.8 25.68 | - | - | |
| IND-1:EXC. | 1:1 | - | - | First 68.3 0.64 | Second 289.3 10.05 | - | - |
| Starch from corn | - | - | First 71.7 2.05 | Second 309.0 26.14 | - | - | |
| IND-1:EXC. | 1:1 | - | - | First 65.5 1.24 | Second 302.1 9.33 | - | - |
| Chitosan | - | - | First 72.6 1.39 | Second 301.1 11.62 | - | - | |
| IND-1:EXC. | 1:1 | First 107.0 0.79 | - | - | Second 287.0 7.66 | Third 374.2 6.63 | Fourth 456.1 3.49 |
| Magnesium stearate | First 109.3 1.89 | - | - | - | Second 372.0 17.59 | - | |
| Tested Samples | Parameter | Peak | |||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| IND-1 | Onset [°C] | 76.9 | 162.4 | - | - |
| Peak [°C] | 81.5 | 172.2 | - | - | |
| Type of reaction | En. | Ex. | - | - | |
| Area [K*s] | 53.9 | 33.5 | - | - | |
| Glucose anhydrous | Onset [°C] | 154.2 | 226.4 | 324.2 | - |
| Peak [°C] | 157.5 | 257.8 | 337.7 | - | |
| Type of reaction | En. | Ex. | Ex. | - | |
| Area [K*s] | 209.4 | 232.5 | 38.4 | - | |
| IND-1:EXC. 2:1 | Onset [°C] | 73.8 | 127.3 | - | - |
| Peak [°C] | 78.6 | 177.0 | - | - | |
| Type of reaction | En. | Ex. | - | - | |
| Area [K*s] | 21.6 | 79.8 | - | - | |
| IND-1:EXC. 1:1 | Onset [°C] | 69.2 | 138.5 | 193.8 | - |
| Peak [°C] | 80.1 | 169.8 | 207.3 | - | |
| Type of reaction | En. | Ex. | Ex. | - | |
| Area [K*s] | 19.3 | 123.2 | 52.1 | - | |
| IND-1:EXC. 1:2 | Onset [°C] | 70.0 | 144.6 | 187.7 | - |
| Peak [°C] | 80.1 | 167.3 | 197.6 | - | |
| Type of reaction | En. | Ex. | Ex. | - | |
| Area [K*s] | 8.4 | 75.4 | 25.3 | - | |
| Lactose monohydrate | Onset [°C] | 145.8 | 208.8 | 233.6 | - |
| Peak [°C] | 150.2 | 214.8 | 261.9 | - | |
| Type of reaction | En. | En. | Ex. | - | |
| Area [K*s] | 59.2 | 122.2 | 161.9 | - | |
| IND-1:EXC. 2:1 | Onset [°C] | 73.5 | 125.5 | 149.6 | - |
| Peak [°C] | 79.1 | 133.7 | 187.2 | - | |
| Type of reaction | En. | En. | Ex. | - | |
| Area [K*s] | 33.9 | 72.3 | 52.4 | - | |
| IND-1:EXC. 1:1 | Onset [°C] | 73.3 | 127.0 | 192.8 | - |
| Peak [°C] | 79.2 | 142.2 | 207.5 | - | |
| Type of reaction | En. | En. | Ex. | - | |
| Area [K*s] | 30.9 | 98.9 | 84.3 | - | |
| IND-1:EXC. 1:2 | Onset [°C] | 74.1 | 129.5 | 198.6 | - |
| Peak [°C] | 78.6 | 149.9 | 214.7 | - | |
| Type of reaction | En. | En. | Ex. | - | |
| Area [K*s] | 15.5 | 122.9 | 93.1 | - | |
| Microcrystalline cellulose | Onset [°C] | 41.1 | 358.0 | - | - |
| Peak [°C] | 50.1 | 382.5 | - | - | |
| Type of reaction | En. | Ex. | - | - | |
| Area [K*s] | 46.5 | 198.7 | - | - | |
| IND-1:EXC. 1:1 | Onset [°C] | - | 74.3 | 162.3 | 285.6 |
| Peak [°C] | 46.3 | 79.3 | 178.8 | 331.0 | |
| Type of reaction | En. | En. | Ex. | Ex. | |
| Area [K*s] | 7.1 | 23.2 | 10.7 | 48.7 | |
| Starch from corn | Onset [°C] | 42.0 | 283.0 | - | - |
| Peak [°C] | 57.6 | 308.5 | - | - | |
| Type of reaction | En. | Ex. | - | - | |
| Area [K*s] | 157.2 | 512.7 | - | - | |
| IND-1:EXC. 1:1 | Onset [°C] | - | 73.7 | 162.5 | 277.5 |
| Peak [°C] | 48.1 | 78.1 | 174.0 | 294.9 | |
| Type of reaction | En. | En. | Ex. | Ex. | |
| Area [K*s] | 12.9 | 11.1 | 32.3 | 23.4 | |
| Chitosan | Onset [°C] | 45.1 | 285.0 | - | - |
| Peak [°C] | 60.1 | 303.5 | - | - | |
| Type of reaction | En. | Ex. | - | - | |
| Area [K*s] | 65.7 | 141.3 | - | - | |
| IND-1:EXC. 1:1 | Onset [°C] | - | 76.1 | 171.4 | 279.6 |
| Peak [°C] | 44.4 | 79.7 | 185.7 | 302.7 | |
| Type of reaction | En. | En. | Ex. | Ex. | |
| Area [K*s] | 91.2 | 7.1 | 4.1 | 53.2 | |
| Magnesium stearate | Onset [°C] | 73.3 | 93.2 | 321.7 | - |
| Peak [°C] | 85.0 | 110.5 | 352.0 | - | |
| Type of reaction | En. | En. | Ex. | - | |
| Area [K*s] | 6.9 | 84.8 | 134.6 | - | |
| IND-1:EXC. 1:1 | Onset [°C] | 76.9 | 99.8 | 162.2 | 361.9 |
| Peak [°C] | 81.0 | 108.9 | 177.4 | 386.7 | |
| Type of reaction | En. | En. | Ex. | Ex. | |
| Area [K*s] | 16.3 | 30.5 | 21.5 | 126.4 | |
| Samples | Ratio (W:W) | Onset [°C] | Mid [°C] | Inflection [°C] | End [°C] | Weight Loss [%] |
|---|---|---|---|---|---|---|
| IND-2 | 224.7 | 238.9 | 252.1 | 257.3 | −92.08 | |
| Glucose anhydrous | 279.3 | 311.0 | 318.7 | 343.4 | −70.65 | |
| IND-2:EXC. | 1:1 | 199.2 | 220.4 | 227.7 | 243.3 | −61.59 |
| Lactose monohydrate | 259.2 | 299.9 | 310.0 | 338.2 | −48.63 | |
| IND-2:EXC. | 1:1 | 204.5 | 226.2 | 227.1 | 246.6 | −57.50 |
| Microcrystalline cellulose | 339.7 | 356.6 | 357.8 | 373.3 | −81.0 | |
| IND-2:EXC. | 1:1 | 210.0 | 200.7 | 237.7 | 191.4 | −46.2 |
| Starch from corn | 297.8 | 309.6 | 309.2 | 321.4 | −61.45 | |
| IND-2:EXC. | 2:1 | 202.7 | 226.4 | 235.1 | 249.7 | −75.81 |
| 1:1 | 198.1 | 223.7 | 226.5 | 248.9 | −57.41 | |
| 1:2 | 261.3 | 287.0 | 304.5 | 323.0 | −65.19 | |
| Chitosan | 283.0 | 302.4 | 300.4 | 322.3 | −42.60 | |
| IND-2:EXC. | 1:1 | 199.6 | 204.6 | 227.6 | 209.7 | −43.23 |
| Magnesium stearate | 339.8 | 365.5 | 372.0 | 391.1 | −84.73 | |
| IND-2:EXC. | 1:1 | 198.8 | 251.6 | 230.7 | 295.9 | −67.96 |
| Samples | Ratio (W:W) | Stage [First, Second, Third, Fourth] Mass Change [%/min.] Peak [°C] | ||
|---|---|---|---|---|
| IND-2 | - | First 252.1 29.72 | - | |
| IND-2:EXC. | 1:1 | - | Third 227.2 15.28 | - |
| Glucose anhydrous | First 219.9 3.74 | - | Second 319.2 11.61 | |
| IND-2:EXC. | 1:1 | First 144.2 1.92 | Second 228.7 15.23 | |
| Lactose monohydrate | First 148.6 3.24 | Second 236.1 5.84 | Third 308.2 7.49 | |
| IND-2:EXC. | 1:1 | First 46.4 0.36 | Second 236.9 14.60 | Third 359.9 9.72 |
| Microcrystalline cellulose | First 47.7 0.78 | - | Second 359.8 25.68 | |
| IND-2:EXC. | 2:1 | First | Second | - |
| 65.5 | 232.9 | |||
| 0.62 | 17.64 | - | ||
| 1:1 | First 65.8 1.32 | Second 226.1 13.76 | - | |
| 1:2 | First 57.4 1.69 | Second 225.5 9.47 | Third 305.2 10.14 | |
| Starch from corn | First 71.7 2.05 | - | Second 309.0 26.14 | |
| IND-2:EXC. | 1:1 | First 67.9 1.01 | Second 231.5 10.74 | Third 302.8 5.49 |
| Chitosan | First 72.6 1.39 | - | Second 301.1 11.62 | |
| IND-2:EXC. | 1:1 | First 105.3 0.99 | Second 231.4 7.79 | Third 356.7 5.86 |
| Magnesium stearate | First 109.3 1.89 | - | Second 372.0 17.59 | |
| Tested Samples | Parameter | Peak | |||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| IND-2 | Onset [°C] | 114.4 | 247.0 | - | - |
| Peak [°C] | 116.4 | 266.3 | - | - | |
| Type of reaction | En. | Ex. | - | - | |
| Area [K*s] | 121.5 | 213.6 | - | - | |
| Glucose anhydrous | Onset [°C] | 154.2 | 226.4 | 324.2 | - |
| Peak [°C] | 157.5 | 257.8 | 337.7 | - | |
| Type of reaction | En. | Ex. | Ex. | - | |
| Area [K*s] | 209.4 | 232.5 | 38.4 | - | |
| IND-2:EXC. 1:1 | Onset [°C] | 112.2 | 149.0 | 230.2 | - |
| Peak [°C] | 117.0 | 154.2 | 240.9 | - | |
| Type of reaction | En. | En. | Ex. | - | |
| Area [K*s] | 57.4 | 90.3 | 93.6 | - | |
| Lactose monohydrate | Onset [°C] | 145.8 | 208.8 | 233.6 | - |
| Peak [°C] | 150.2 | 214.8 | 261.9 | - | |
| Type of reaction | En. | En. | Ex. | - | |
| Area [K*s] | 59.2 | 122.2 | 161.9 | - | |
| IND-2:EXC. 1:1 | Onset [°C] | 113.1 | 140.1 | 196.8 | 227.6 |
| Peak [°C] | 118.0 | 145.4 | 200.9 | 246.5 | |
| Type of reaction | En. | En. | En. | Ex. | |
| Area [K*s] | 53.3 | 27.1 | 95.0 | 116.2 | |
| Microcrystalline cellulose | Onset [°C] | 41.1 | 358.0 | - | - |
| Peak [°C] | 50.1 | 382.5 | - | - | |
| Type of reaction | En. | Ex. | - | - | |
| Area [K*s] | 46.5 | 198.7 | - | - | |
| IND-2:EXC. 1:1 | Onset [°C] | - | 113.1 | 241.7 | 361.4 |
| Peak [°C] | 37.6 | 118.3 | 251.1 | 382.1 | |
| Type of reaction | En. | En. | Ex. | Ex. | |
| Area [K*s] | 28.8 | 65.3 | 82.3 | 59.9 | |
| Starch from corn | Onset [°C] | 42.0 | 283.0 | - | - |
| Peak [°C] | 57.6 | 308.5 | - | - | |
| Type of reaction | En. | Ex. | - | - | |
| Area [K*s] | 157.2 | 512.7 | - | - | |
| IND-2:EXC. 2:1 | Onset [°C] | - | 112.5 | 237.5 | - |
| Peak [°C] | 53.4 | 116.0 | 253.3 | - | |
| Type of reaction | En. | En. | Ex. | - | |
| Area [K*s] | 72.4 | 90.4 | 90.1 | - | |
| IND-2:EXC. 1:1 | Onset [°C] | - | 112.3 | 233.2 | - |
| Peak [°C] | 47.6 | 116.8 | 249.2 | - | |
| Type of reaction | En. | En. | Ex. | - | |
| Area [K*s] | 125.8 | 51.9 | 142.8 | - | |
| IND-2:EXC. 1:2 | Onset [°C] | - | 113.4 | 230.2 | 294.2 |
| Peak [°C] | 38.5 | 118.5 | 238.6 | 318.8 | |
| Type of reaction | En. | En. | Ex. | Ex. | |
| Area [K*s] | 138.9 | 29.3 | 47.1 | 38.4 | |
| Chitosan | Onset [°C] | 45.1 | 285.0 | - | - |
| Peak [°C] | 60.1 | 303.5 | - | - | |
| Type of reaction | En. | Ex. | - | - | |
| Area [K*s] | 65.7 | 141.3 | - | - | |
| IND-2:EXC. 1:1 | Onset [°C] | - | 111.0 | 236.2 | 281.2 |
| Peak [°C] | 39.7 | 118.4 | 244.0 | 304.8 | |
| Type of reaction | En. | En. | Ex. | Ex. | |
| Area [K*s] | 111.1 | 30.9 | 18.3 | 45.6 | |
| Magnesium stearate | Onset [°C] | 73.3 | 93.2 | 321.7 | - |
| Peak [°C] | 85.0 | 110.5 | 352.0 | - | |
| Type of reaction | En. | En. | Ex. | - | |
| Area [K*s] | 6.9 | 84.8 | 134.6 | - | |
| IND-2:EXC. 1:1 | Onset [°C] | 93.4 | 111.6 | 246.6 | 338.2 |
| Peak [°C] | 107.2 | 117.7 | 250.2 | 360.1 | |
| Type of reaction | En. | En. | Ex. | Ex. | |
| Area [K*s] | 13.2 | 18.6 | 38.3 | 68.4 | |
| Samples | Starting Sample | 25 °C/45%RH/14 days | 60 °C/75%RH/14 days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* [±SD] | a* [±SD] | b* [±SD] | L* [±SD] | a* [±SD] | b* [±SD] | ΔE [±SD] | L* [±SD] | a* [±SD] | b* [±SD] | ΔE [±SD] | |
| IND-1 | 63.38 | 10.81 | 30.58 | 62.78 | 9.20 | 29.04 | 1.43 | 62.94 | 10.20 | 30.57 | 1.55 |
| [±0.30] | [±0.08] | [±0.16] | [±0.20] | [±0.07] | [±0.09] | [±0.30] | [±0.21] | [±0.38] | [±0.25] | [±0.38] | |
| IND-1/GA | 62.20 | 10.54 | 30.01 | 61.88 | 9.32 | 29.02 | 1.50 | 59.73 | 10.30 | 29.64 | 2.51 |
| [±0.19] | [±0.17] | [±0.09] | [±0.20] | [±0.02] | [±0.15] | [±0.20] | [±0.35] | [±0.50] | [±0.40] | [±0.50] | |
| IND-1/LM | 59.70 | 9.92 | 28.90 | 60.02 | 8.82 | 28.18 | 1.34 | 61.35 | 10.35 | 30.01 | 2.36 |
| [±0.03] | [±0.03] | [±0.02] | [±0.32] | [±0.03] | [±0.02] | [±0.32] | [±0.32] | [±0.13] | [±0.17] | [±0.32] | |
| IND-1/MC | 59.46 | 9.83 | 28.73 | 60.78 | 9.69 | 28.91 | 1.34 | 63.31 | 10.49 | 30.54 | 4.30 |
| [±0.08] | [±0.01] | [±0.08] | [±0.94] | [±0.04] | [±0.03] | [±0.94] | [±0.07] | [±0.13] | [±0.04] | [±0.13] | |
| IND-1/SC | 61.34 | 10.35 | 29.38 | 61.06 | 9.52 | 29.70 | 1.38 | 62.55 | 10.19 | 29.82 | 1.29 |
| [±0.15] | [±0.02] | [±0.05] | [±0.02] | [±0.07] | [±0.11] | [±0.15] | [±0.40] | [±0.66] | [±0.44] | [±0.66] | |
| IND-1/CH | 62.07 | 10.50 | 29.80 | 61.09 | 10.34 | 29.61 | 1.02 | 59.33 | 9.41 | 28.28 | 3.32 |
| [±0.14] | [±0.19] | [±0.18] | [±0.14] | [±0.15] | [±0.22] | [±0.22] | [±0.41] | [±0.54] | [±0.44] | [±0.54] | |
| IND-1/MS | 60.98 | 10.21 | 28.89 | 61.64 | 10.09 | 28.77 | 1.01 | 63.64 | 8.99 | 28.29 | 2.99 |
| [±0.78] | [±0.30] | [±0.34] | [±0.35] | [±0.01] | [±0.25] | [±0.78] | [±0.67] | [±0.02] | [±0.32] | [±0.78] | |
| IND-2 | 59.99 | 10.75 | 28.92 | 60.85 | 10.06 | 29.37 | 1.19 | 60.13 | 9.88 | 28.64 | 0.93 |
| [±0.17] | [±0.29] | [±0.21] | [±0.49] | [±0.07] | [±0.18] | [±0.49] | [±0.03] | [±0.04] | [±0.07] | [±0.29] | |
| IND-2/GA | 59.82 | 10.07 | 28.57 | 61.10 | 10.67 | 29.98 [±0.09] | 1.99 | 60.60 | 9.48 | 28.56 | 0.97 |
| [±0.05] | [±0.18] | [±0.08] | [±0.11] | [±0.02] | [±0.18] | [±0.42] | [±0.14] | [±0.27] | [±0.42] | ||
| IND-2/LM | 62.13 | 10.91 | 29.18 | 61.68 | 10.72 | 29.85 | 0.83 | 60.65 | 9.15 | 28.25 | 2.49 |
| [±0.86] | [±0.25] | [±0.20] | [±0.30] | [±0.18] | [±0.24] | [±0.86] | [±0.26] | [±0.14] | [±0.08] | [±0.86] | |
| IND-2/MC | 62.09 | 11.01 | 29.20 | 61.73 | 10.17 | 29.66 | 1.55 | 61.62 | 9.11 | 28.37 | 2.13 |
| [±0.04] | [±0.05] | [±0.07] | [±0.83] | [±0.33] | [±0.73] | [±0.83] | [±0.01] | [±0.02] | [±0.02] | [±0.07] | |
| IND-2/SC | 62.66 | 10.90 | 29.38 | 62.08 | 10.07 | 28.59 | 1.26 | 65.17 | 9.58 | 30.38 | 3.00 |
| [±0.04] | [±0.07] | [±0.18] | [±0.33] | [±0.09] | [±0.46] | [±0.46] | [±0.03] | [±0.03] | [±0.02] | [±0.18] | |
| IND-2/CH | 59.81 | 10.22 | 28.77 | 59.99 | 9.56 | 28.75 | 1.27 | 63.11 | 9.99 | 28.69 | 3.30 |
| [±0.23] | [±0.11] | [±0.13] | [±0.24] | [±0.03] | [±0.18] | [±0.24] | [±0.05] | [±0.03] | [±0.06] | [±0.23] | |
| IND-2/MS | 61.73 | 10.55 | 28.96 | 61.94 | 10.26 | 28.28 | 1.48 | 60.90 | 10.76 | 69.78 | 0.92 |
| [±0.24] | [±0.08] | [±0.13] | [±0.41] | [±0.32] | [±0.47] | [±0.47] | [±0.06] | [±0.03] | [±0.04] | [±0.24] | |
| Samples | λ1 [nm] | λ2 [nm] | λ3 [nm] |
|---|---|---|---|
| IND-1 | 206 | 252 | 281 |
| IND-1–glucose anhydrous | 206 | 252 | 281 |
| IND-1–lactose monohydrate | 206 | 252 | 281 |
| IND-1–microcrystalline cellulose | 206 | 252 | 281 |
| IND-1–starch from corn | 206 | 257 | 279 |
| IND-1–chitosan | 206 | 252 | 281 |
| IND-1–magnesium stearate | 206 | 253 | 279 |
| IND-2 | 208 | 260 | 290 |
| IND-2–glucose anhydrous | 208 | 260 | 290 |
| IND-2–lactose monohydrate | 208 | 260 | 290 |
| IND-2–microcrystalline cellulose | 208 | 260 | 290 |
| IND-2–starch from corn | 207 | 252 | 290 |
| IND-2–chitosan | 206 | 258 | 289 |
| IND-2–magnesium stearate | 209 | 260 | 290 |
| Mixtures | TGA | c-DTA | FTIR | Colorimetry | UV–Vis | |
|---|---|---|---|---|---|---|
| Room Conditions | Stress Conditions | |||||
| IND-1/GA | - | + | + | + | - | + |
| IND-1/LM | - | + | + | + | - | + |
| IND-1/MC | + | + | 0 | + | - | + |
| IND-1/SC | + | + | 0 | + | + | + |
| IND-1/CH | + | + | 0 | + | - | + |
| IND-1/MS | + | + | 0 | + | - | + |
| IND-2/GA | + | + | + | + | + | + |
| IND-2/LM | + | + | + | + | - | + |
| IND-2/MC | + | + | 0 | + | - | + |
| IND-2/SC | + | + | 0 | + | - | + |
| IND-2/CH | + | + | 0 | + | - | + |
| IND-2/MS | + | + | 0 | + | + | + |
| Value of Parameter ΔE | Color Change Visible to the Human Eye |
|---|---|
| <0.5 | Not visible |
| From 0.5 to 1.5 | Barely visible |
| From 1.5 to 3.0 | Poorly visible |
| From 3.0 to 6.0 | Visible |
| >6.0 | Very visible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broncel, M.; Juszczak, A.; Szczolko, W.; Silvestri, D.; Białek-Dratwa, A.; Wacławek, S.; Kowalski, O.; Ramos, P. Thermal Compatibility of New ACEI Derivatives with Popular Excipients Used to Produce Solid Pharmaceutical Formulations. Pharmaceuticals 2024, 17, 1323. https://doi.org/10.3390/ph17101323
Broncel M, Juszczak A, Szczolko W, Silvestri D, Białek-Dratwa A, Wacławek S, Kowalski O, Ramos P. Thermal Compatibility of New ACEI Derivatives with Popular Excipients Used to Produce Solid Pharmaceutical Formulations. Pharmaceuticals. 2024; 17(10):1323. https://doi.org/10.3390/ph17101323
Chicago/Turabian StyleBroncel, Mateusz, Anna Juszczak, Wojciech Szczolko, Daniele Silvestri, Agnieszka Białek-Dratwa, Stanisław Wacławek, Oskar Kowalski, and Paweł Ramos. 2024. "Thermal Compatibility of New ACEI Derivatives with Popular Excipients Used to Produce Solid Pharmaceutical Formulations" Pharmaceuticals 17, no. 10: 1323. https://doi.org/10.3390/ph17101323
APA StyleBroncel, M., Juszczak, A., Szczolko, W., Silvestri, D., Białek-Dratwa, A., Wacławek, S., Kowalski, O., & Ramos, P. (2024). Thermal Compatibility of New ACEI Derivatives with Popular Excipients Used to Produce Solid Pharmaceutical Formulations. Pharmaceuticals, 17(10), 1323. https://doi.org/10.3390/ph17101323











