In Vivo HOXB7 Gene Silencing and Cotreatment with Tamoxifen for Luminal A Breast Cancer Therapy
Abstract
:1. Introduction
2. Results
2.1. Hybrid Nanoparticle Preparation, Characterization, and Purification
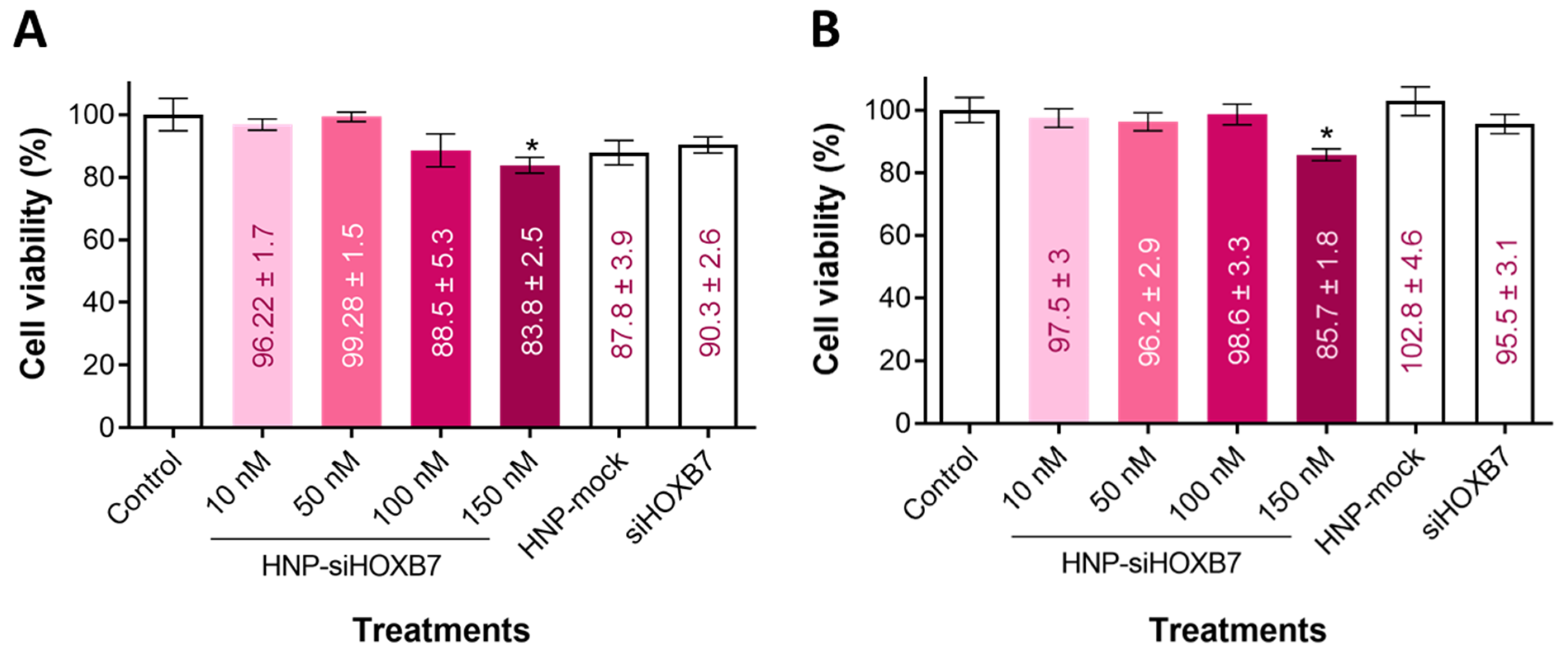
2.2. Cell Viability
2.3. Cell Migration
2.4. Hemolysis Assay
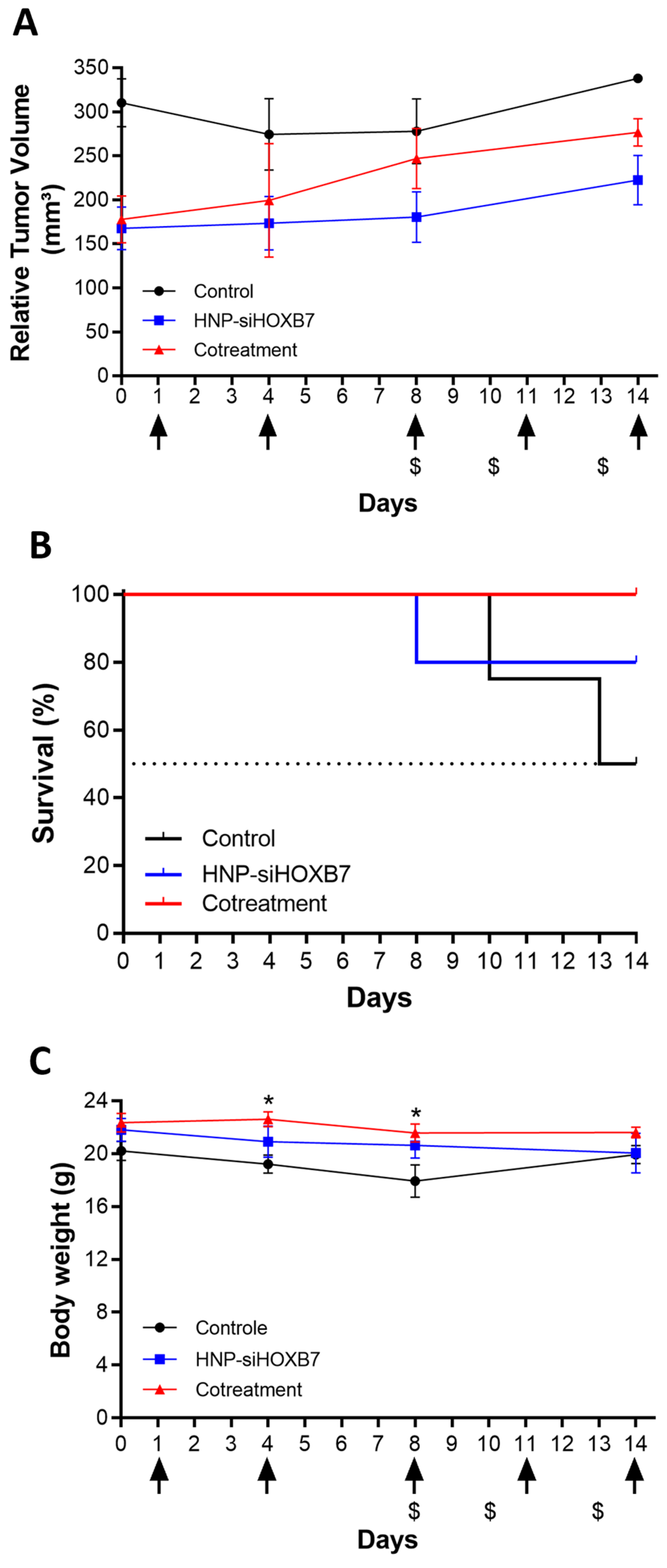
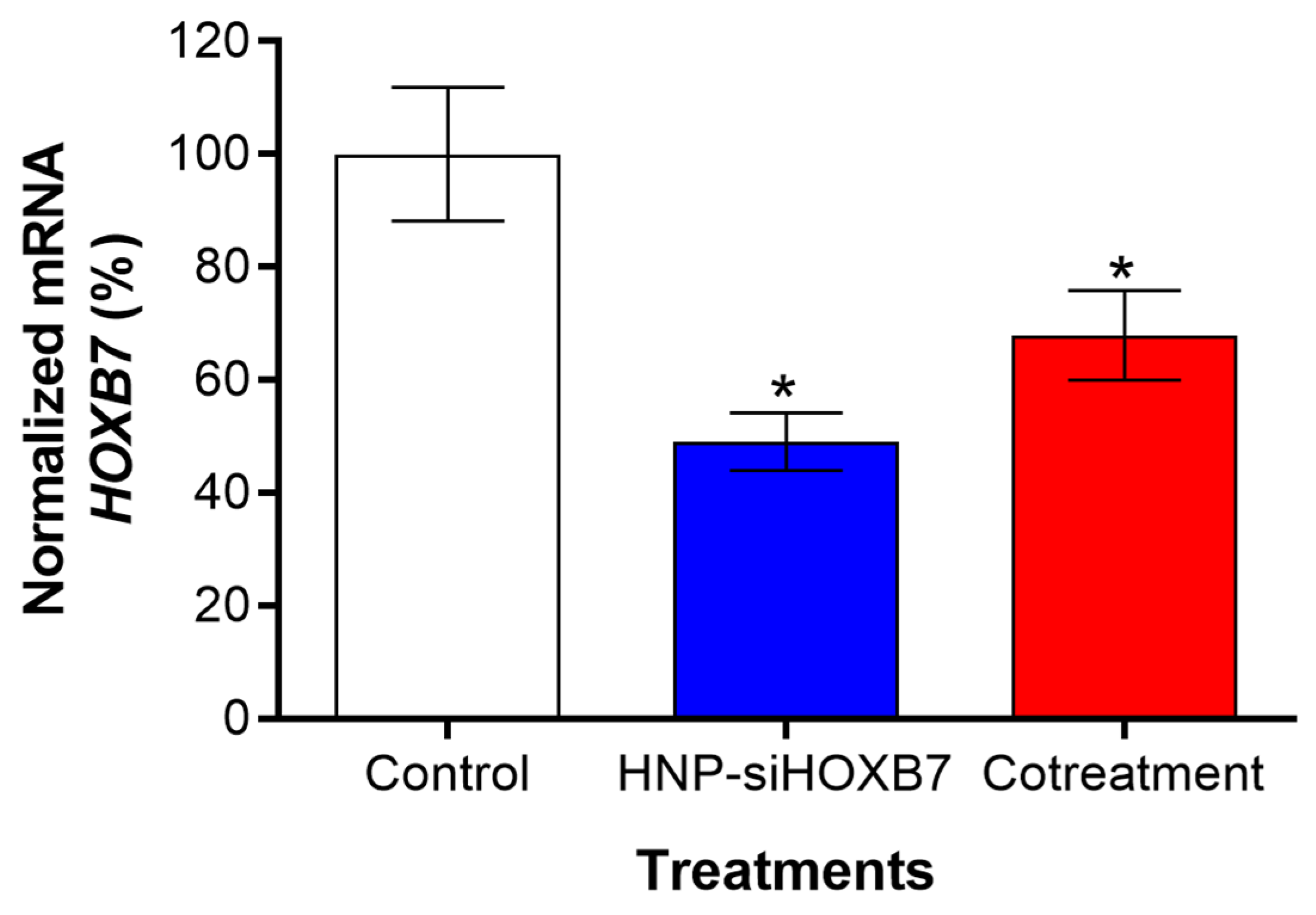
2.5. Antitumoral Activity and Gene Expression Evaluation
2.6. Histopathological Evaluation
2.7. Hematological and Biochemical Parameters Analysis
3. Discussion
4. Materials and Methods
4.1. Materials, Cell Line, and Animals
4.2. Preparation of Hybrid Nanoparticles
4.3. Physicochemical Characterization of Hybrid Nanoparticles
4.4. Colloidal Stability Studies
4.4.1. Long-Term Storage of the Dispersion
4.4.2. Lyophilization
4.5. Encapsulation Efficiency
4.6. In Vitro Assays
4.6.1. Cell Viability Assay
4.6.2. Scratch Assay
4.7. Hemolytic Activity
4.8. In Vivo Assay
4.8.1. Antitumoral Activity
4.8.2. HOXB7 Gene Expression Evaluation
4.8.3. Histopathological Analysis
4.8.4. Hematological and Biochemical Parameters Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286–287, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.G.; Otoni, K.M. Histological and molecular classification of breast cancer: What do we know? Mastology 2020, 30, e20200024. [Google Scholar] [CrossRef]
- Hoefnagel, L.D.; Moelans, C.B.; Meijer, S.L.; van Slooten, H.J.; Wesseling, P.; Wesseling, J.; Westenend, P.J.; Bart, J.; Seldenrijk, C.A.; Nagtegaal, I.D.; et al. Prognostic value of estrogen receptor α and progesterone receptor conversion in distant breast cancer metastases. Cancer 2012, 118, 4929–4935. [Google Scholar] [CrossRef]
- Jin, K.; Kong, X.; Shah, T.; Penet, M.F.; Wildes, F.; Sgroi, D.C.; Ma, X.J.; Huang, Y.; Kallioniemi, A.; Landberg, G.; et al. The HOXB7 protein renders breast cancer cells resistant to tamoxifen through activation of the EGFR pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 2736–2741. [Google Scholar] [CrossRef]
- Caré, A.; Silvani, A.; Meccia, E.; Mattia, G.; Peschle, C.; Colombo, M.P. Transduction of the SkBr3 breast cancer carcinoma cell line with the HOXB7 gene induces bFGF expression, increases cell proliferation, and reduces growth factor dependence. Oncogene 1998, 16, 3285–3289. [Google Scholar] [CrossRef]
- Wu, X.; Chen, H.; Parker, B.; Rubin, E.; Zhu, T.; Lee, J.S.; Argani, P.; Sukumar, S. HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res. 2006, 66, 9527–9534. [Google Scholar] [CrossRef]
- Rubin, E.; Wu, X.; Zhu, T.; Cheung, J.C.; Chen, H.; Lorincz, A.; Pandita, R.K.; Sharma, G.G.; Ha, H.C.; Gasson, J.; et al. A role for the HOXB7 homeodomain protein in DNA repair. Cancer Res. 2007, 67, 1527–1535. [Google Scholar] [CrossRef]
- Jin, K.; Park, S.; Teo, W.W.; Korangath, P.; Cho, S.S.; Yoshida, T.; Győrffy, B.; Goswami, C.P.; Nakshatri, H.; Cruz, L.A.; et al. HOXB7 is an ERα cofactor in the activation of HER2 and multiple ER target genes leading to endocrine resistance. Cancer Discov. 2015, 5, 944–959. [Google Scholar] [CrossRef]
- Jin, K.; Sukumar, S. A pivotal role for HOXB7 protein in endocrine-resistant breast cancer. Oncoscience 2015, 2, 917–919. [Google Scholar] [CrossRef]
- Urits, I.; Swanson, D.; Swett, M.C.; Patel, A.; Berardino, K.; Amgalan, A.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Viswanath, O. A Review of Patisiran (ONPATTRO®) for the Treatment of Polyneuropathy in People with Hereditary Transthyretin Amyloidosis. Neurol. Ther. 2020, 9, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Menck, C.F.M. A nova grande promessa da inovação em fármacos: RNA interferência saindo do laboratório para a clínica. Estud. Avançados 2010, 24, 99–108. [Google Scholar] [CrossRef]
- Mishra, A.; Srivastava, A.; Pateriya, A.; Tomar, M.S.; Mishra, A.K.; Shrivastava, A. Metabolic reprograming confers tamoxifen resistance in breast cancer. Chem.-Biol. Interact. 2021, 347, 109602. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, D.T.; Gualberto, A.C.M.; Valle, A.B.C.S.; Da Silva, L.C.; Ferreira, K.C.B.; Lemos, A.S.O.; Fabri, R.L.; Tavares, G.D.; Cazarim, M.S.; Gameiro, J.; et al. Macauba oil carried by polymeric micelles reduces migration and proliferation of triple-negative breast cancer cells. RSC Pharm. 2024, 1, 524–535. [Google Scholar] [CrossRef]
- Ferreira, K.C.B.; Valle, A.B.C.S.; Gualberto, A.C.M.; Aleixo, D.T.; Silva, L.M.; Santos, M.M.; Costa, D.S.; Oliveira, L.L.; Gameiro, J.; Tavares, G.D.; et al. Kaurenoic acid nanocarriers regulates cytokine production and inhibit breast cancer cell migration. J. Control. Release 2022, 352, 712–725. [Google Scholar] [CrossRef]
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Cruz, L.J.; Motiei, M. Redox-Sensitive and Hyaluronic Acid-Functionalized Nanoparticles for Improving Breast Cancer Treatment by Cytoplasmic 17α-Methyltestosterone Delivery. Molecules 2020, 25, 1181. [Google Scholar] [CrossRef]
- Gomhor, J.; Alqaraghuli, H.; Kashanian, S.; Rafipour, R.; Mahdavian, E.; Mansouri, K. Development and characterization of folic acid-functionalized apoferritin as a delivery vehicle for epirubicin against MCF-7 breast cancer cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 847–854. [Google Scholar] [CrossRef]
- Moazzam, M.; Zhang, M.; Hussain, A.; Yu, X.; Huang, J.; Huang, Y. The landscape of nanoparticle-based siRNA delivery and therapeutic development. Mol. Ther. 2024, 32, 284–312. [Google Scholar] [CrossRef]
- Whitehead, K.; Langer, R.; Anderson, D. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. 2009, 8, 129–138. [Google Scholar]
- Kakizawa, Y.; Kataoka, K. Block copolymer self-assembly into monodispersive nanoparticles with hybrid core of antisense DNA and calcium phosphate. Langmuir 2002, 18, 4539–4543. [Google Scholar] [CrossRef]
- Pittella, F.; Zhang, M.; Lee, Y.; Kim, H.J.; Tockary, T.; Osada, K.; Ishii, T.; Miyata, K.; Nishiyama, N.; Kataoka, K. Enhanced endosomal escape of siRNA-incorporating hybrid nanoparticles from calcium phosphate and PEG-block charge conversional polymer for efficient gene knockdown with negligible cytotoxicity. Biomaterials 2011, 32, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- de Mello, L.J.; Souza, G.R.R.; Winter, E.; Silva, A.H.; Pittella, F.; Creczynski-Pasa, T.B. Knockdown of antiapoptotic genes in breast cancer cells by siRNA loaded into hybrid nanoparticles. Nanotechnology 2017, 28, 175101. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.R.R.; Dalmina, M.; Restrepo, J.A.S.; de Mello Junior, L.J.; Silva, A.H.; Gualberto, A.; Gameiro, J.; Dittz, D.; Pasa, A.A.; Pittella, F.; et al. Short interfering RNA delivered by a hybrid nanoparticle targeting VEGF: Biodistribution and anti-tumor effect. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129938. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhang, X.; Wang, H.; Zhang, Q.; Li, H.; Li, Y.; Duan, Y. Biocompatible and colloidally stabilized mPEG-PE/calcium phosphate hybrid nanoparticles loaded with siRNAs targeting tumors. Oncotarget 2016, 7, 2855–2866. [Google Scholar] [CrossRef]
- Nangia, S.; Sureshkumar, R. Effects of nanoparticle charge and shape anisotropy on translocation through cell membranes. Langmuir 2012, 28, 17666–27671. [Google Scholar] [CrossRef]
- Matsumara, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Kakizawa, Y.; Furukawa, S.; Kataoka, K. Block copolymer-coated calcium phosphate nanoparticles sensing intracellular environment for oligodeoxynucleotide and siRNA delivery. J. Control. Release 2004, 97, 345–356. [Google Scholar] [CrossRef]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef]
- Statefeld, J.; Mckenna, S.; Patel, T. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Ver. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Winkler, A.W.; Hoff, H.E.; Smith, P.K. Cardiovascular effects of potassium, calcium, magnesium, and barium: An experimental study of toxicity and rationale of use in therapeutics. Yale J. Biol. Med. 1940, 13, 123–132. [Google Scholar]
- Zhou, Q.; Wang, Y.; Xiang, J.; Piao, Y.; Zhou, Z.; Tang, J.; Liu, X.; Shen, Y. Stabilized calcium phosphate hybrid nanocomposite using a benzoxaborole-containing polymer for pH-responsive siRNA delivery. Biomater. Sci. 2018, 6, 3178–3188. [Google Scholar] [CrossRef] [PubMed]
- Hosonuma, M.; Yoshimura, K. Association between pH regulation of the tumor microenvironment and immunological state. Front. Oncol. 2023, 13, 1175563. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.B.C.S.; Gualberto, A.C.; Ferreira, K.C.B.; Creczynski-Pasa, T.B.; Gameiro, J.; Tavares, G.D.; Pittella, F. HOXB7 siRNA Delivered by Hybrid Nanoparticles and the Co-Therapy with Tamoxifen: Promising Strategy against Hormone Receptor-Positive Breast Cancer. Mater. Proc. 2021, 4, 69. [Google Scholar]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef]
- Manaargadoo-Catin, M.; Ali-Cherif, A.; Pougnas, J.L.; Perrin, C. Hemolysis by surfactants—A review. Adv. Colloid Interface Sci. 2015, 228, 1–16. [Google Scholar] [CrossRef]
- Liu, H.; Dong, A.; Rasteh, A.M.; Wang, P.; Weng, J. Identification of the novel exhausted T cell CD8+ markers in breast cancer. Sci. Rep. 2024, 14, 19142. [Google Scholar] [CrossRef]
- Pittella, F.; Miyata, K.; Maeda, Y.; Suma, T.; Watanabe, S.; Chen, Q.; Christie, R.J.; Osada, K.; Nishiyama, N.; Kataoka, K. Pancreatic cancer therapy by systemic administration of VEGF siRNA contained in calcium phosphate/charge-conversional polymer hybrid nanoparticles. J. Control. Release 2012, 161, 868–874. [Google Scholar] [CrossRef]
- Silva, L.M.; Marconato, D.G.; Nascimento da Silva, M.P.; Barbosa Raposo, N.R.; Faria Silva Facchini, G.; Macedo, G.C.; Teixeira, F.S.; Barbosa da Silveira Salvadori, M.C.; Faria Pinto, P.; Moraes, J.; et al. Licochalcone A-loaded solid lipid nanoparticles improve antischistosomal activity in vitro and in vivo. Nanomedicine 2021, 16, 1641–1655. [Google Scholar] [CrossRef]
- Zhang, Y.; Leonard, M.; Shu, Y.; Yang, Y.; Shu, D.; Guo, P.; Zhang, X. Overcoming Tamoxifen Resistance of Human Breast Cancer by Targeted Gene Silencing Using Multifunctional pRNA Nanoparticles. ACS Nano 2016, 11, 335–346. [Google Scholar] [CrossRef]
- Raha, P.; Thomas, S.; Thurn, K.T.; Park, J.; Munster, P.N. Combined histone deacetylase inhibition and tamoxifen induces apoptosis in tamoxifen-resistant breast cancer models, by reversing Bcl-2 overexpression. Breast Cancer Res. 2015, 17, 26. [Google Scholar] [CrossRef]
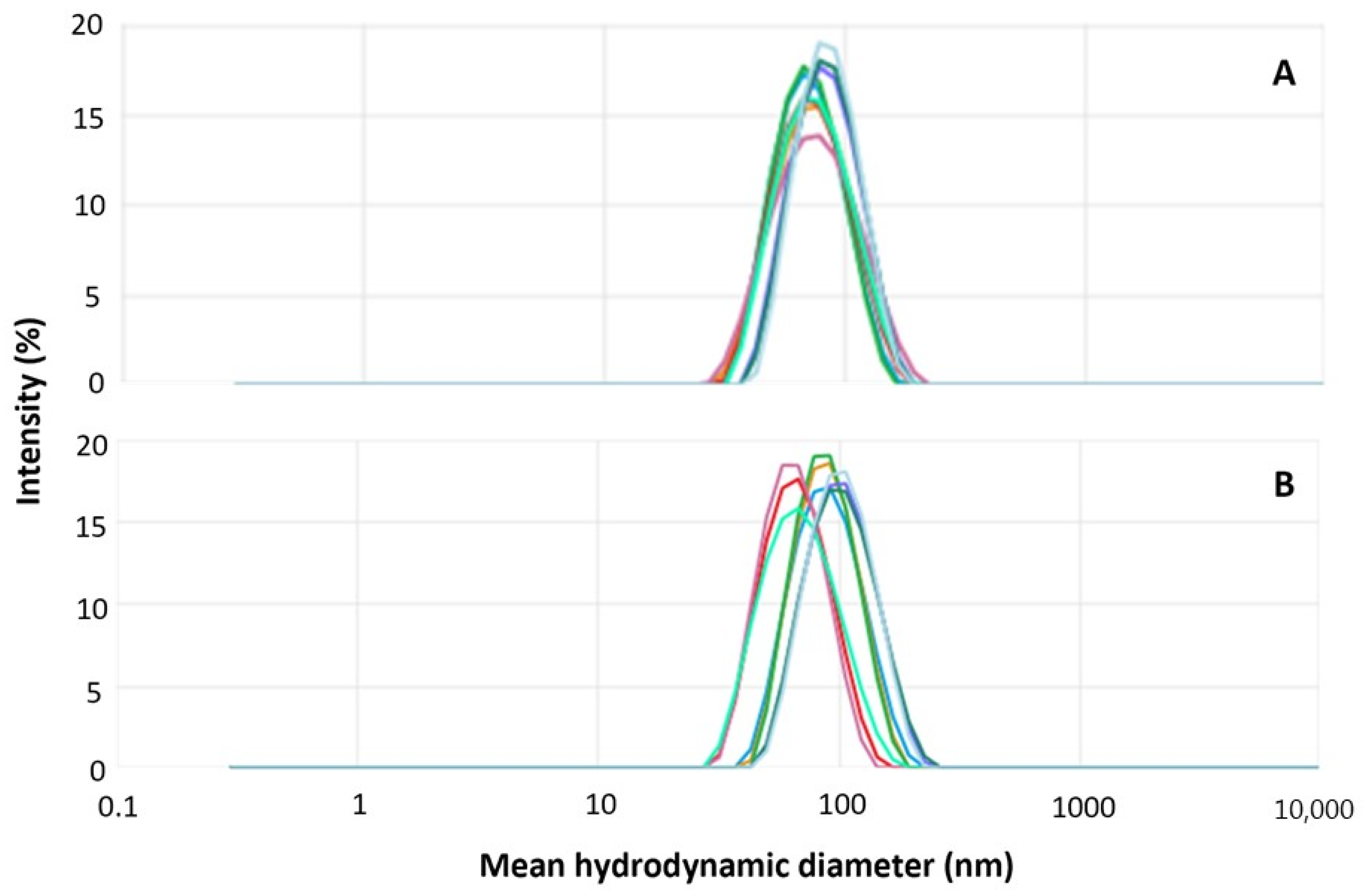






| Hydrodynamic Diameter (nm) | PDI | Zeta Potential (mV) | |
|---|---|---|---|
| HNP-siHOXB7 | 74.4 ± 5.5 | 0.1 ± 0.03 | −1.16 ± 0.95 |
| HNP-mock | 82.2 ± 14.5 | 0.1 ± 0.02 | −0.86 ± 0.1 |
| Hydrodynamic Diameter (nm) | PDI | |||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | 6 d.a. | 17 d.a. | Before | After | 6 d.a. | 17 d.a. | |
| HNP-mock | 79.83 ± 0.03 | 78.38 ± 0.2 | 82.57 ± 0.33 | 88.44 ± 0.46 * | 0.08 ± 0 | 0.08 ± 0 | 0.08 ± 0 | 0.07 ± 0 |
| HNP-siHOXB7 | 70.78 ± 0.03 | 68.08 ± 044 | 71.4 ± 0.25 | 73.66 ± 0.27 * | 0.07 ± 0 | 0.06 ± 0 | 0.06 ± 0 | 0.06 ± 0 |
| Hydrodynamic Diameter (nm) | PDI | |||||
|---|---|---|---|---|---|---|
| Before | After | 10 d.a. | Before | After. | 10 d.a. | |
| HNP | 68.51 ± 0.2 | 74.33 ± 1.2 | 80.1 ± 0.55 | 0.03 ± 0 | 0.15 ± 0.03 | 0.17 ±0.02 |
| HNP + Sucrose 10% | 81.21 ± 0.1 | 79.4 ± 0.4 | 151.6 ± 7 * | 0.18 ± 0 | 0.19 ± 0 | 0.66 ± 0.07 |
| Hematological Parameters | |||||||
| Parameters | Control | HNP + TMX | TMX | Parameters | Control | HNP + TMX | TMX |
| RBC (×106/mm3) | 7.9 ± 0.2 | 7.82 ± 0.2 | 7.5 ± 0.1 | Banded neutrophils (mm3) | 0 | 0 | 0 |
| HGB (g/dL) | 12.05 ± 0.2 | 12.23 ± 0.4 | 11.53 ± 0.1 | Segmented neutrophils (mm3) | 2582 ± 101.4 | 1343 ± 182.3 * | 1184 ± 274 * |
| HCT (%) | 46 ± 1 | 46.25 ± 0.9 | 43 ± 0.4 | Eosinophil (mm3) | 16 ± 10.8 | 8.5 ± 5.5 | 9 ± 9 |
| MCV (fL) | 57.75 ± 0.3 | 59.13 ± 0.4 | 57.26 ± 1.1 | Lymphocyte (mm3) | 284.3 ± 178.2 | 224.3 ± 101.8 | 246 ± 5 |
| MCHC (g/dL) | 26.2 ± 0.2 | 26.41 ± 0.3 | 26.81 ± 0.4 | Monocyte (mm3) | 284.5 ± 93.5 | 144 ± 38.2 | 161 ± 3 |
| WBC (mm3) | 3100 ± 601.4 | 2300 ± 288.7 | 1175 ± 265.8 * | Basophil (mm3) | 0 | 0 | 0 |
| Metamyelocyte (mm3) | 0 | 0 | 0 | PLT (mil/mm3) | 472.3 ± 23.2 | 558.3 ± 57.2 | 612.5 ± 51.1 |
| Biochemical Parameters | |||||||
| Parameters | Control | HNP + TMX | TMX | Parameters | Control | HNP + TMX | TMX |
| Urea (mg/dL) | 47.51 ± 7.6 | 41.3 ± 3.5 | 46.78 ± 2.9 | ALT (U/L) | 60.4 ± 2.8 | 55.65 ± 8.6 | 40.35 ± 0.6 |
| Creatinine (mg/dL) | 0.4 ± 0.02 | 0.57 ± 0.04 * | 0.42 ± 0.03 | AST (U/L) | 218 ± 28.6 | 175.5 ± 13.3 | 170.5 ± 4.2 |
| Plasma protein | 6.1 ± 0.1 | 5.65 ± 0.1 * | 5.3 ± 0.1 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle, A.B.C.d.S.; da Silva, F.F.A.; Carneiro, M.Â.P.; Espuche, B.; Tavares, G.D.; Bernardes, E.S.; Moya, S.E.; Pittella, F. In Vivo HOXB7 Gene Silencing and Cotreatment with Tamoxifen for Luminal A Breast Cancer Therapy. Pharmaceuticals 2024, 17, 1325. https://doi.org/10.3390/ph17101325
Valle ABCdS, da Silva FFA, Carneiro MÂP, Espuche B, Tavares GD, Bernardes ES, Moya SE, Pittella F. In Vivo HOXB7 Gene Silencing and Cotreatment with Tamoxifen for Luminal A Breast Cancer Therapy. Pharmaceuticals. 2024; 17(10):1325. https://doi.org/10.3390/ph17101325
Chicago/Turabian StyleValle, Ana Beatriz Caribé dos Santos, Fábio Fernando Alves da Silva, Maria Ângela Pepe Carneiro, Bruno Espuche, Guilherme Diniz Tavares, Emerson Soares Bernardes, Sergio Enrique Moya, and Frederico Pittella. 2024. "In Vivo HOXB7 Gene Silencing and Cotreatment with Tamoxifen for Luminal A Breast Cancer Therapy" Pharmaceuticals 17, no. 10: 1325. https://doi.org/10.3390/ph17101325
APA StyleValle, A. B. C. d. S., da Silva, F. F. A., Carneiro, M. Â. P., Espuche, B., Tavares, G. D., Bernardes, E. S., Moya, S. E., & Pittella, F. (2024). In Vivo HOXB7 Gene Silencing and Cotreatment with Tamoxifen for Luminal A Breast Cancer Therapy. Pharmaceuticals, 17(10), 1325. https://doi.org/10.3390/ph17101325







