The Identification of New Pharmacological Targets for the Treatment of Glaucoma: A Network Pharmacology Approach
Abstract
:1. Introduction
2. Results
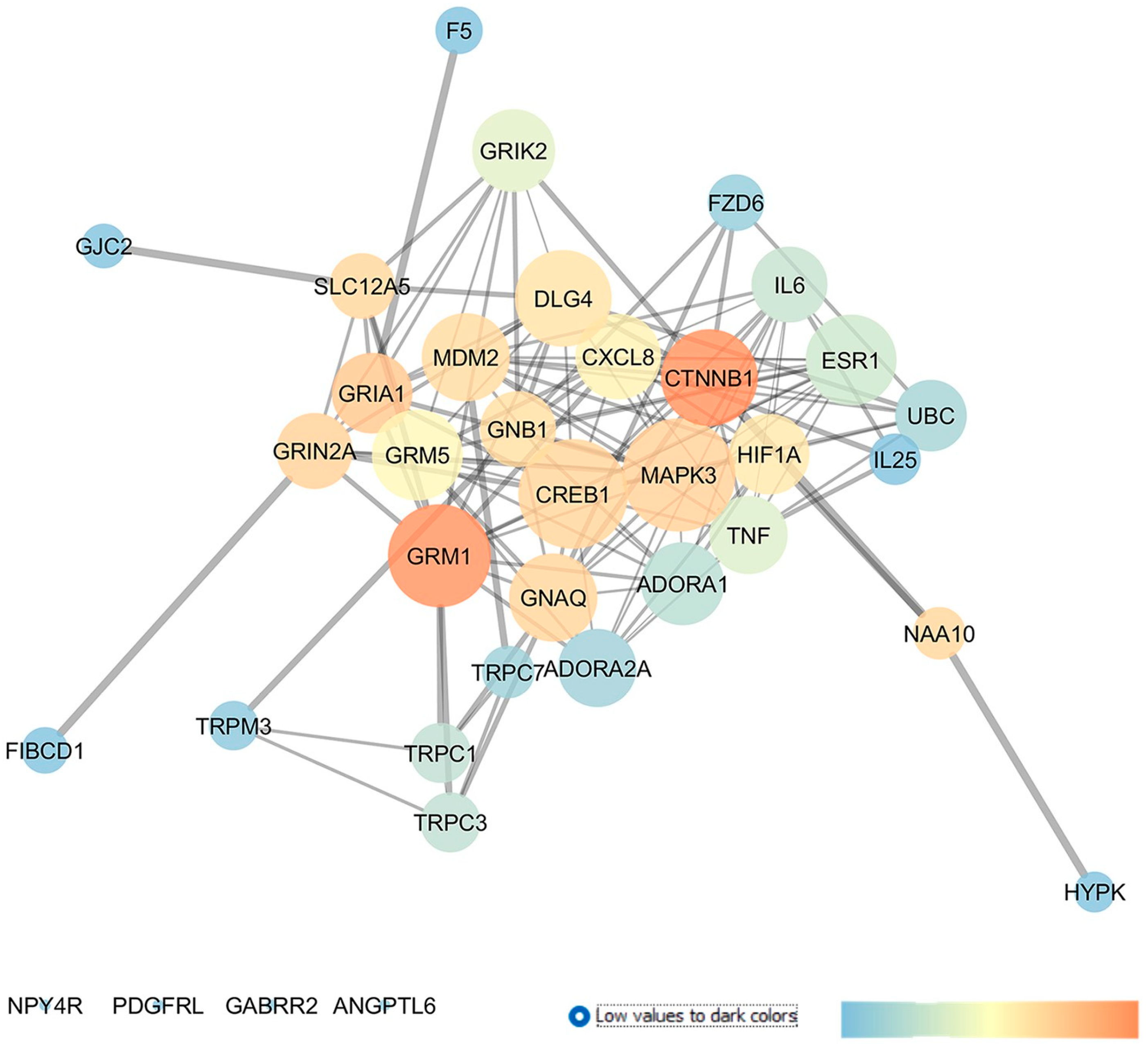
2.1. GSE133563: Analysis of Retinal Transcriptomic Data 2 Weeks after Optic Nerve Crush
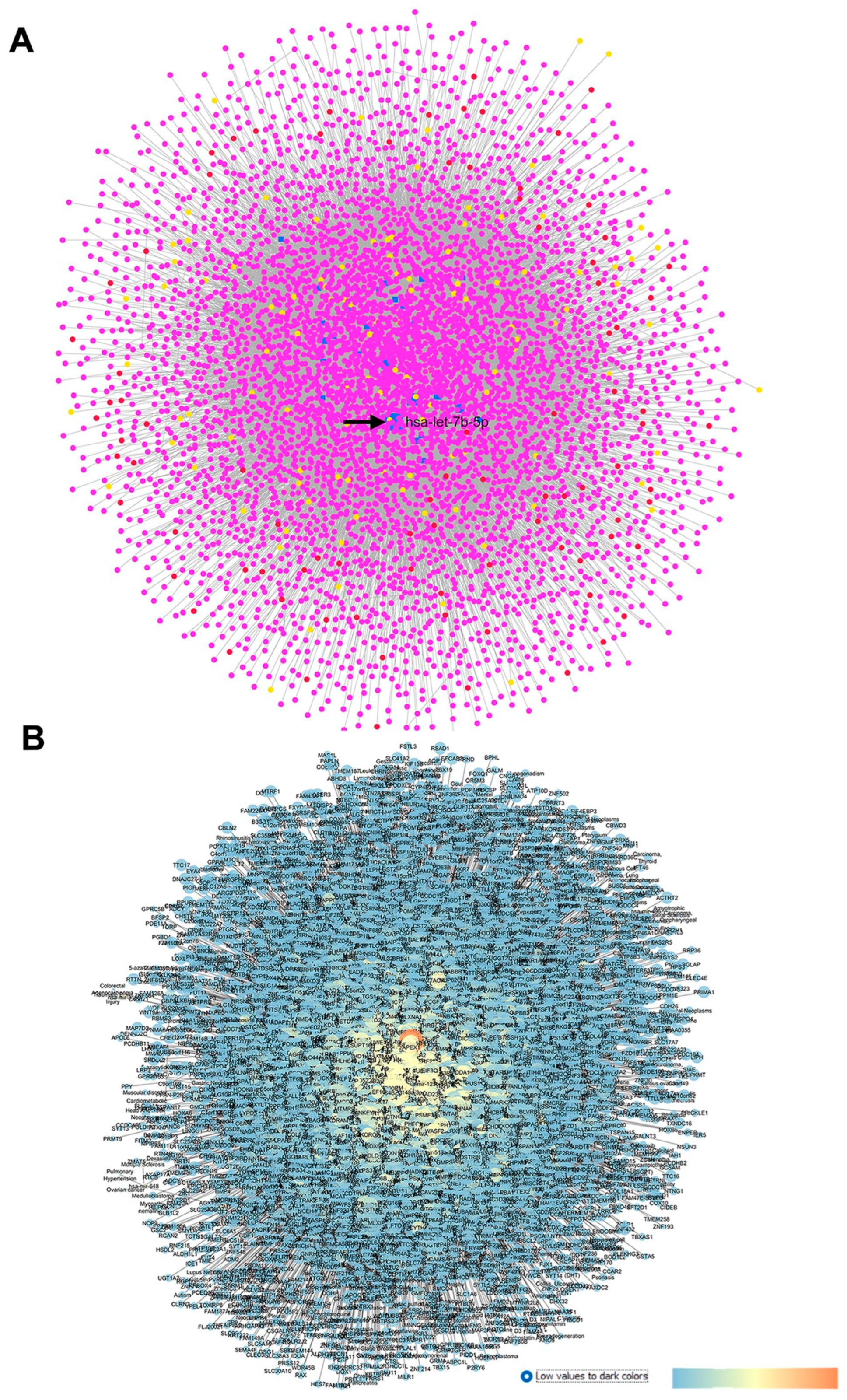
2.2. GSE105269: Analysis of Differentially Expressed miRNAs in Aqueous Humor of Exfoliation Glaucoma and Primary Open-Angle Glaucoma Patients
2.2.1. GSE105269 AH from Primary Open-Angle Glaucoma Patients
2.2.2. GSE105269 AH of Exfoliation Glaucoma
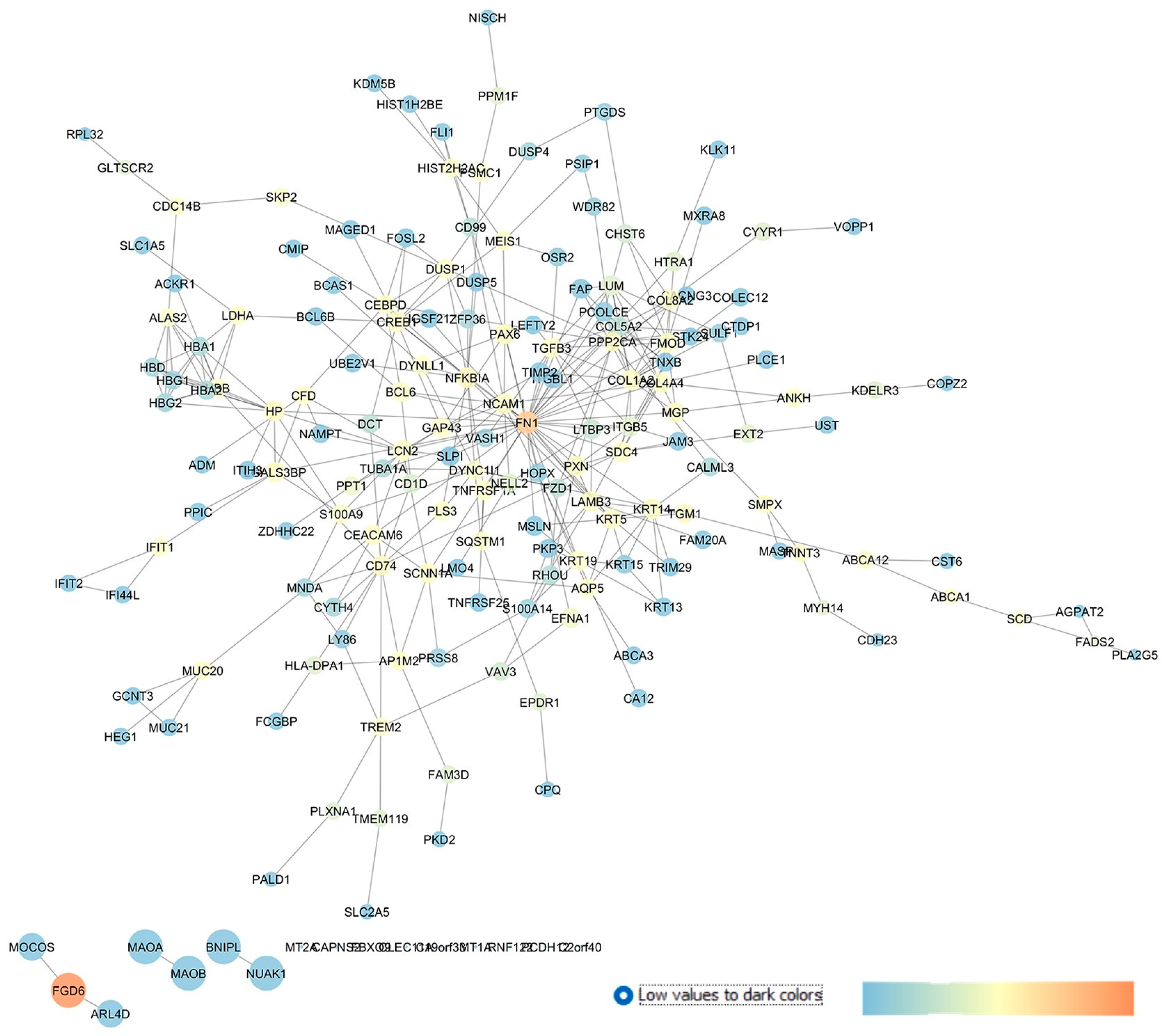
2.3. GSE27276: Analysis of Differentially Expressed Gene Profiles in Cultured Human Trabecular Meshwork Tissues from Primary Open-Angle Glaucoma Patients Compared to Cells Isolated from Control Healthy Subjects
2.4. GSE4316: Analysis of Differentially Expressed Gene Profiles in Trabecylar Meshwork Tissues of Primary Open-Angle Glaucoma Patients
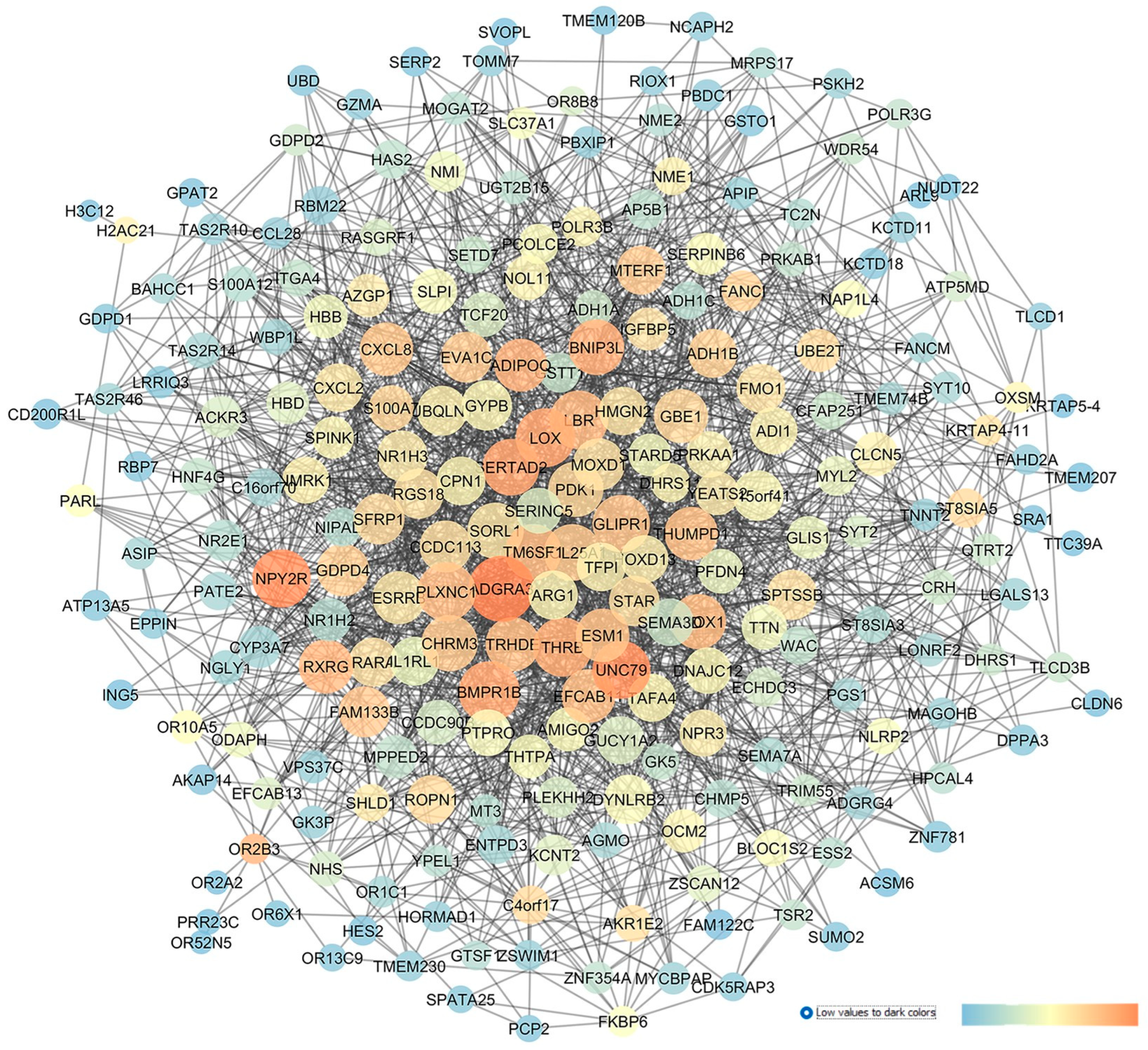
2.5. GSE45570: Analysis of Differentially Expressed Gene Profiles in Optic Nerve Head of Primary Open-Angle Glaucoma Patients and Ocular Hypertensive Patients without Glaucoma
2.6. GSE3554: Analysis of DBA/2J Mice Retinal Gene Expressions
2.7. Integration with Preclinical and Clinical Data
3. Discussion
4. Materials and Methods
4.1. GEO Dataset
4.2. Network Definition and Descriptor Computations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACG | Angle-closure glaucoma |
| AD | Alzheimer’s disease |
| AH | Aqueous humour |
| CTRL | Control |
| DE | Differentially expressed |
| DEGs | Differentially expressed genes |
| ECM | Extracellular matrix |
| EVC | Episcleral vein cauterization |
| GPCRs | G protein-coupled receptors |
| HTM | Human trabecular meshwork |
| IOP | Intraocular pressure |
| I/R | Ischemia/reperfusion |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LGN | Lateral geniculate nucleus |
| NF-kB | Nuclear factor kappa-B |
| NTG | Normal-tension glaucoma |
| NVG | Neovascular glaucoma |
| OHT | Ocular hypertension |
| ONC | Optic nerve crush |
| ONH | Optic nerve head |
| POAG | Primary open-angle glaucoma |
| RGCs | Retinal ganglion cells |
| TM | Trabecular meshwork |
| VEGF | Vascular endothelial growth factor |
| WGS | Whole-genome sequencing |
| XFG | Exfoliation glaucoma |
| XFS | Exfoliation syndrome |
References
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Chakrabarti, A.; Nazm, N.; Mehta, R.; Edward, D.P. Newer advances in medical management of glaucoma. Indian J. Ophthalmol. 2022, 70, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-H.; Musada, G.; Romano, G.; Bolton, E.; Porciatti, V. Anesthetic Preconditioning as Endogenous Neuroprotection in Glaucoma. Int. J. Mol. Sci. 2018, 19, 237. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Vargas, J.L.C.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Youngblood, H.; Schoenlein, P.V.; Pasquale, L.R.; Stamer, W.D.; Liu, Y. Estrogen dysregulation, intraocular pressure, and glaucoma risk. Exp. Eye Res. 2023, 237, 109725. [Google Scholar] [CrossRef]
- Liu, Y.; Allingham, R.R. Major review: Molecular genetics of primary open-angle glaucoma. Exp. Eye Res. 2017, 160, 62–84. [Google Scholar] [CrossRef] [PubMed]
- Casson, R.J. Medical therapy for glaucoma: A review. Clin. Exp. Ophthalmol. 2022, 50, 198–212. [Google Scholar] [CrossRef]
- Jayaram, H.; Kolko, M.; Friedman, D.S.; Gazzard, G. Glaucoma: Now and beyond. Lancet 2023, 402, 1788–1801. [Google Scholar] [CrossRef]
- Jeong, J.H.; Park, K.H.; Jeoung, J.W.; Kim, D.M. Preperimetric normal tension glaucoma study: Long-term clinical course and effect of therapeutic lowering of intraocular pressure. Acta Ophthalmol. 2014, 92, e185–e193. [Google Scholar] [CrossRef]
- Tribble, J.R.; Hui, F.; Quintero, H.; El Hajji, S.; Bell, K.; Di Polo, A.; Williams, P.A. Neuroprotection in glaucoma: Mechanisms beyond intraocular pressure lowering. Mol. Asp. Med. 2023, 92, 101193. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.A. Therapeutic Drugs and Devices for Tackling Ocular Hypertension and Glaucoma, and Need for Neuroprotection and Cytoprotective Therapies. Front. Pharmacol. 2021, 12, 729249. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-J.; Chung, M.-H.; Chen, B.-S. Investigating Pathogenetic Mechanisms of Alzheimer’s Disease by Systems Biology Approaches for Drug Discovery. Int. J. Mol. Sci. 2021, 22, 11280. [Google Scholar] [CrossRef] [PubMed]
- Auwul, M.R.; Rahman, M.R.; Gov, E.; Shahjaman, M.; Moni, M.A. Bioinformatics and machine learning approach identifies potential drug targets and pathways in COVID-19. Brief. Bioinform. 2021, 22, bbab120. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Leggio, G.M.; Drago, F.; Salomone, S.; Bucolo, C. Computational systems biology approach to identify novel pharmacological targets for diabetic retinopathy. Biochem. Pharmacol. 2018, 158, 13–26. [Google Scholar] [CrossRef]
- Peng, H.; Tan, H.; Zhao, W.; Jin, G.; Sharma, S.; Xing, F.; Watabe, K.; Zhou, X. Computational systems biology in cancer brain metastasis. Front. Biosci. (Sch. Ed.) 2016, 8, 169–186. [Google Scholar] [CrossRef]
- Lazzara, F.; Conti, F.; Giuffrida, E.; Eandi, C.M.; Drago, F.; Platania, C.B.M.; Bucolo, C. Integrating network pharmacology: The next-generation approach in ocular drug discovery. Curr. Opin. Pharmacol. 2024, 74, 102425. [Google Scholar] [CrossRef]
- Yan, J.; Risacher, S.L.; Shen, L.; Saykin, A.J. Network approaches to systems biology analysis of complex disease: Integrative methods for multi-omics data. Brief. Bioinform. 2018, 19, 1370–1381. [Google Scholar] [CrossRef]
- Marbach, D.; Schaffter, T.; Mattiussi, C.; Floreano, D. Generating realistic in silico gene networks for performance assessment of reverse engineering methods. J. Comput. Biol. 2009, 16, 229–239. [Google Scholar] [CrossRef]
- Hu, G.; Di Paola, L.; Pullara, F.; Liang, Z.; Nookaew, I. Network Proteomics: From Protein Structure to Protein-Protein Interaction. Biomed. Res. Int. 2017, 2017, 8929613. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Di Paola, L.; Leggio, G.M.; Romano, G.L.; Drago, F.; Salomone, S.; Bucolo, C. Molecular features of interaction between VEGFA and anti-angiogenic drugs used in retinal diseases: A computational approach. Front. Pharmacol. 2015, 6, 248. [Google Scholar] [CrossRef] [PubMed]
- Clough, E.; Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; et al. NCBI GEO: Archive for gene expression and epigenomics data sets: 23-year update. Nucleic Acids Res. 2024, 52, D138–D144. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets--update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Sun, P.; Feng, S.; Yu, H.; Wang, X.; Fang, Y. Two hub genes of bipolar disorder, a bioinformatics study based on the GEO database. IBRO Neurosci. Rep. 2024, 17, 122–130. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Geiduschek, E.K.; McDowell, C.M. The Fibro-Inflammatory Response in the Glaucomatous Optic Nerve Head. Int. J. Mol. Sci. 2023, 24, 13240. [Google Scholar] [CrossRef]
- Mavlyutov, T.A.; Myrah, J.J.; Chauhan, A.K.; Liu, Y.; McDowell, C.M. Fibronectin extra domain A (FN-EDA) causes glaucomatous trabecular meshwork, retina, and optic nerve damage in mice. Cell Biosci. 2022, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Victorino, P.H.; Marra, C.; Iacobas, D.A.; Iacobas, S.; Spray, D.C.; Linden, R.; Adesse, D.; Petrs-Silva, H. Retinal Genomic Fabric Remodeling after Optic Nerve Injury. Genes 2021, 12, 403. [Google Scholar] [CrossRef]
- Drewry, M.D.; Challa, P.; Kuchtey, J.G.; Navarro, I.; Helwa, I.; Hu, Y.; Mu, H.; Stamer, W.D.; Kuchtey, R.W.; Liu, Y. Differentially expressed microRNAs in the aqueous humor of patients with exfoliation glaucoma or primary open-angle glaucoma. Hum. Mol. Genet. 2018, 27, 1263–1275. [Google Scholar] [CrossRef]
- Liu, Y.; Allingham, R.R.; Qin, X.; Layfield, D.; Dellinger, A.E.; Gibson, J.; Wheeler, J.; Ashley-Koch, A.E.; Stamer, W.D.; Hauser, M.A. Gene expression profile in human trabecular meshwork from patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6382–6389. [Google Scholar] [CrossRef]
- Liton, P.B.; Luna, C.; Challa, P.; Epstein, D.L.; Gonzalez, P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol. Vis. 2006, 12, 774–790. [Google Scholar] [PubMed]
- Steele, M.R.; Inman, D.M.; Calkins, D.J.; Horner, P.J.; Vetter, M.L. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Irnaten, M.; O’Malley, G.; Clark, A.F.; O’Brien, C.J. Transient receptor potential channels TRPC1/TRPC6 regulate lamina cribrosa cell extracellular matrix gene transcription and proliferation. Exp. Eye Res. 2020, 193, 107980. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Del-Castillo, J.; Cantu-Dibildox, J.; Sanz-González, S.M.; Zanón-Moreno, V.; Pinazo-Duran, M.D. Cytokine expression in tears of patients with glaucoma or dry eye disease: A prospective, observational cohort study. Eur. J. Ophthalmol. 2019, 29, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, G.; Chen, H.; Li, L.; Xu, Z.; Xu, L. Evaluations of aqueous humor protein markers in different types of glaucoma. Medicine 2022, 101, e31048. [Google Scholar] [CrossRef]
- Tezel, G.; Wax, M.B. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch. Ophthalmol. 2004, 122, 1348–1356. [Google Scholar] [CrossRef]
- Nsiah, N.Y.; Morgan, A.B.; Donkor, N.; Inman, D.M. Long-term HIF-1α stabilization reduces respiration, promotes mitophagy, and results in retinal cell death. Sci. Rep. 2023, 13, 20541. [Google Scholar] [CrossRef]
- Ergorul, C.; Ray, A.; Huang, W.; Wang, D.Y.; Ben, Y.; Cantuti-Castelvetri, I.; Grosskreutz, C.L. Hypoxia inducible factor-1α (HIF-1α) and some HIF-1 target genes are elevated in experimental glaucoma. J. Mol. Neurosci. 2010, 42, 183–191. [Google Scholar] [CrossRef]
- de Sousa, M.C.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering miRNAs’ Action through miRNA Editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef]
- Seong, H.; Cho, H.-K.; Kee, C.; Song, D.H.; Cho, M.-C.; Kang, S.S. Profiles of microRNA in aqueous humor of normal tension glaucoma patients using RNA sequencing. Sci. Rep. 2021, 11, 19024. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Smedowski, A.; Liu, X.; Podracka, L.; Akhtar, S.; Trzeciecka, A.; Pietrucha-Dutczak, M.; Lewin-Kowalik, J.; Urtti, A.; Ruponen, M.; Kaarniranta, K.; et al. Increased intraocular pressure alters the cellular distribution of HuR protein in retinal ganglion cells—A possible sign of endogenous neuroprotection failure. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Murphy, C.J.; Brandt, J.D.; Kiuchi, Y.; Russell, P. Altered stability of mRNAs associated with glaucoma progression in human trabecular meshwork cells following oxidative stress. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Salt, T.E.; Luong, V.; Wood, N.; Cheung, W.; Maass, A.; Ferrari, G.; Russo-Marie, F.; Sillito, A.M.; Cheetham, M.E.; et al. Targeting amyloid-beta in glaucoma treatment. Proc. Natl. Acad. Sci. USA 2007, 104, 13444–13449. [Google Scholar] [CrossRef]
- Goldblum, D.; Kipfer-Kauer, A.; Sarra, G.-M.; Wolf, S.; Frueh, B.E. Distribution of amyloid precursor protein and amyloid-beta immunoreactivity in DBA/2J glaucomatous mouse retinas. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5085–5090. [Google Scholar] [CrossRef]
- Yasuda, M.; Tanaka, Y.; Ryu, M.; Tsuda, S.; Nakazawa, T. RNA sequence reveals mouse retinal transcriptome changes early after axonal injury. PLoS ONE 2014, 9, e93258. [Google Scholar] [CrossRef]
- Kanamori, A.; Nakamura, M.; Nakanishi, Y.; Nagai, A.; Mukuno, H.; Yamada, Y.; Negi, A. Akt is activated via insulin/IGF-1 receptor in rat retina with episcleral vein cauterization. Brain Res. 2004, 1022, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.K.; Sykes, R.; Samples, J.; Edmunds, B.; Choi, D.; Keene, D.R.; Tufa, S.F.; Sun, Y.Y.; Keller, K.E. Identification of Missense Extracellular Matrix Gene Variants in a Large Glaucoma Pedigree and Investigation of the N700S Thrombospondin-1 Variant in Normal and Glaucomatous Trabecular Meshwork Cells. Curr. Eye Res. 2022, 47, 79–90. [Google Scholar] [CrossRef]
- Springelkamp, H.; Iglesias, A.I.; Mishra, A.; Höhn, R.; Wojciechowski, R.; Khawaja, A.P.; Nag, A.; Wang, Y.X.; Wang, J.J.; Cuellar-Partida, G.; et al. New insights into the genetics of primary open-angle glaucoma based on meta-analyses of intraocular pressure and optic disc characteristics. Hum. Mol. Genet. 2017, 26, 438–453. [Google Scholar] [CrossRef]
- AMartin-Gil, A.; de Lara, M.J.P.; Crooke, A.; Santano, C.; Peral, A.; Pintor, J. Silencing of P2Y(2) receptors reduces intraocular pressure in New Zealand rabbits. Br. J. Pharmacol. 2012, 165, 1163–1172. [Google Scholar] [CrossRef]
- Fonseca, B.; Martínez-Águila, A.; de Lara, M.J.P.; Miras-Portugal, M.T.; Gómez-Villafuertes, R.; Pintor, J. Changes in P2Y Purinergic Receptor Expression in the Ciliary Body in a Murine Model of Glaucoma. Front. Pharmacol. 2017, 8, 719. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, L.; Halder, N.; Singh, S.B.; Angmo, D.; Jayasundar, R.; Dada, T.; Velpandian, T. Evaluation of ADRB2 and OATP2A1 genetic polymorphisms in Indian patients with primary open-angle glaucoma. Pharmacogenet. Genom. 2024, 34, 20–24. [Google Scholar] [CrossRef]
- Sidjanin, D.J.; McCarty, C.A.; Patchett, R.; Smith, E.; Wilke, R.A. Pharmacogenetics of ophthalmic topical beta-blockers. Pers. Med. 2008, 5, 377–385. [Google Scholar] [CrossRef]
- Inagaki, Y.; Mashima, Y.; Fuse, N.; Funayama, T.; Ohtake, Y.; Yasuda, N.; Murakami, A.; Hotta, Y.; Fukuchi, T.; Tsubota, K. Polymorphism of beta-adrenergic receptors and susceptibility to open-angle glaucoma. Mol. Vis. 2006, 12, 673–680. [Google Scholar] [PubMed]
- McCarty, C.A.; Burmester, J.K.; Mukesh, B.N.; Patchett, R.B.; Wilke, R.A. Intraocular pressure response to topical beta-blockers associated with an ADRB2 single-nucleotide polymorphism. Arch. Ophthalmol. 2008, 126, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Aboobakar, I.F.; Allingham, R.R. Genetics of exfoliation syndrome and glaucoma. Int. Ophthalmol. Clin. 2014, 54, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Chakraborty, M.; Roy, A.; Sahay, P.; Pradhan, A.; Raj, N. Differential miRNA Expression: Signature for Glaucoma in Pseudoexfoliation. Clin. Ophthalmol. 2020, 14, 3025–3038. [Google Scholar] [CrossRef]
- Cho, H.-K.; Seong, H.; Kee, C.; Song, D.H.; Kim, S.J.; Seo, S.W.; Kang, S.S. MicroRNA profiles in aqueous humor between pseudoexfoliation glaucoma and normal tension glaucoma patients in a Korean population. Sci. Rep. 2022, 12, 6217. [Google Scholar] [CrossRef]
- Colak, D.; Morales, J.; Bosley, T.M.; Al-Bakheet, A.; AlYounes, B.; Kaya, N.; Abu-Amero, K.K. Genome-wide expression profiling of patients with primary open angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5899–5904. [Google Scholar] [CrossRef]
- Agapova, O.A.; Kaufman, P.L.; Hernandez, M.R. Androgen receptor and NFkB expression in human normal and glaucomatous optic nerve head astrocytes in vitro and in experimental glaucoma. Exp. Eye Res. 2006, 82, 1053–1059. [Google Scholar] [CrossRef]
- Hernandez, H.; Medina-Ortiz, W.E.; Luan, T.; Clark, A.F.; McDowell, C.M. Crosstalk Between Transforming Growth Factor Beta-2 and Toll-Like Receptor 4 in the Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Kase, S.; Kanda, A.; Shinmei, Y.; Noda, K.; Ishida, S. Expression of Vascular Endothelial Growth Factor-C in the Trabecular Meshwork of Patients with Neovascular Glaucoma and Primary Open-Angle Glaucoma. J. Clin. Med. 2021, 10, 2977. [Google Scholar] [CrossRef] [PubMed]
- Filla, M.S.; Faralli, J.A.; Desikan, H.; Peotter, J.L.; Wannow, A.C.; Peters, D.M. Activation of αvβ3 Integrin Alters Fibronectin Fibril Formation in Human Trabecular Meshwork Cells in a ROCK-Independent Manner. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3897–3913. [Google Scholar] [CrossRef] [PubMed]
- Yoneshige, A.; Hagiyama, M.; Takashima, Y.; Ueno, S.; Inoue, T.; Kimura, R.; Koriyama, Y.; Ito, A. Elevated Hydrostatic Pressure Causes Retinal Degeneration through Upregulating Lipocalin-2. Front. Cell Dev. Biol. 2021, 9, 664327. [Google Scholar] [CrossRef]
- Ueno, S.; Yoneshige, A.; Koriyama, Y.; Hagiyama, M.; Shimomura, Y.; Ito, A. Early Gene Expression Profile in Retinal Ganglion Cell Layer After Optic Nerve Crush in Mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 370–380. [Google Scholar] [CrossRef]
- Huang, C.; Cen, L.-P.; Liu, L.; Leaver, S.G.; Harvey, A.R.; Cui, Q.; Pang, C.P.; Zhang, M. Adeno-associated virus-mediated expression of growth-associated protein-43 aggravates retinal ganglion cell death in experimental chronic glaucomatous injury. Mol. Vis. 2013, 19, 1422–1432. [Google Scholar]
- Dreixler, J.C.; Bratton, A.; Du, E.; Shaikh, A.R.; Savoie, B.; Alexander, M.; Marcet, M.M.; Roth, S. Mitogen-activated protein kinase phosphatase-1 (MKP-1) in retinal ischemic preconditioning. Exp. Eye Res. 2011, 93, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Narta, K.; Teltumbade, M.R.; Vishal, M.; Sadaf, S.; Faruq, M.; Jama, H.; Waseem, N.; Rao, A.; Sen, A.; Ray, K.; et al. Whole Exome Sequencing Reveals Novel Candidate Genes in Familial Forms of Glaucomatous Neurodegeneration. Genes 2023, 14, 495. [Google Scholar] [CrossRef]
- Reinehr, S.; Wulf, J.; Theile, J.; Schulte, K.K.; Peters, M.; Fuchshofer, R.; Dick, H.B.; Joachim, S.C. In a novel autoimmune and high-pressure glaucoma model a complex immune response is induced. Front. Immunol. 2024, 15, 1296178. [Google Scholar] [CrossRef]
- Diskin, S.; Cao, Z.; Leffler, H.; Panjwani, N. The role of integrin glycosylation in galectin-8-mediated trabecular meshwork cell adhesion and spreading. Glycobiology 2009, 19, 29–37. [Google Scholar] [CrossRef]
- Beggah, A.T.; Dours-Zimmermann, M.T.; Barras, F.M.; Brosius, A.; Zimmermann, D.R.; Zurn, A.D. Lesion-induced differential expression and cell association of Neurocan, Brevican, Versican V1 and V2 in the mouse dorsal root entry zone. Neuroscience 2005, 133, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.S.; Solano, A.G.; Tilve, S.M.; Mencio, C.P.; Martin, K.R.; Geller, H.M. Spatiotemporal distribution of chondroitin sulfate proteoglycans after optic nerve injury in rodents. Exp. Eye Res. 2020, 190, 107859. [Google Scholar] [CrossRef]
- Reinhard, J.; Renner, M.; Wiemann, S.; Shakoor, D.A.; Stute, G.; Dick, H.B.; Faissner, A.; Joachim, S.C. Ischemic injury leads to extracellular matrix alterations in retina and optic nerve. Sci. Rep. 2017, 7, 43470. [Google Scholar] [CrossRef]
- Samelska, K.; Zaleska-Żmijewska, A.; Bałan, B.; Grąbczewski, A.; Szaflik, J.P.; Kubiak, A.J.; Skopiński, P. Immunological and molecular basics of the primary open angle glaucoma pathomechanism. Cent. Eur. J. Immunol. 2021, 46, 111–117. [Google Scholar] [CrossRef]
- Howell, G.R.; MacNicoll, K.H.; Braine, C.E.; Soto, I.; Macalinao, D.G.; Sousa, G.L.; John, S.W.M. Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol. Dis. 2014, 71, 44–52. [Google Scholar] [CrossRef]
- Marola, O.J.; Howell, G.R.; Libby, R.T. Vascular derived endothelin receptor A controls endothelin-induced retinal ganglion cell death. Cell Death Discov. 2022, 8, 207. [Google Scholar] [CrossRef]
- Chiquet, C.; Vignal, C.; Gohier, P.; Heron, E.; Thuret, G.; Rougier, M.B.; Lehmann, A.; Flet, L.; Quesada, J.-L.; Roustit, M.; et al. Treatment of nonarteritic anterior ischemic optic neuropathy with an endothelin antagonist: ENDOTHELION (ENDOTHELin antagonist receptor in Ischemic Optic Neuropathy)-a multicentre randomised controlled trial protocol. Trials 2022, 23, 916. [Google Scholar] [CrossRef]
- Ivanov, D.; Dvoriantchikova, G.; Nathanson, L.; McKinnon, S.J.; Shestopalov, V.I. Microarray analysis of gene expression in adult retinal ganglion cells. FEBS Lett. 2006, 580, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; O’Neill, E.; Irnaten, M.; Wallace, D.; O’Brien, C. Corneal Stiffness and Collagen Cross-Linking Proteins in Glaucoma: Potential for Novel Therapeutic Strategy. J. Ocul. Pharmacol. Ther. 2020, 36, 582–594. [Google Scholar] [CrossRef]
- Yu, P.; Dong, W.-P.; Tang, Y.-B.; Chen, H.-Z.; Cui, Y.-Y.; Bian, X.-L. Huperzine A lowers intraocular pressure via the M3 mAChR and provides retinal neuroprotection via the M1 mAChR: A promising agent for the treatment of glaucoma. Ann. Transl. Med. 2021, 9, 332. [Google Scholar] [CrossRef]
- Cheng, L.; Sapieha, P.; Kittlerova, P.; Hauswirth, W.W.; Di Polo, A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J. Neurosci. 2002, 22, 3977–3986. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration. Int. J. Mol. Sci. 2016, 17, 1584. [Google Scholar] [CrossRef]
- Milbeck, S.M.; Bhattacharya, S.K. Alteration in Lysophospholipids and Converting Enzymes in Glaucomatous Optic Nerves. Investig. Ophthalmol. Vis. Sci. 2020, 61, 60. [Google Scholar] [CrossRef]
- Zukerman, R.; Harris, A.; Vercellin, A.V.; Siesky, B.; Pasquale, L.R.; Ciulla, T.A. Molecular Genetics of Glaucoma: Subtype and Ethnicity Considerations. Genes 2020, 12, 55. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Burdon, K.P.; Fogarty, R.; Sharma, S.; Hewitt, A.W.; Martin, S.; Law, M.H.; Cremin, K.; Bailey, J.N.C.; Loomis, S.J.; et al. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat. Genet. 2014, 46, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.A. Serotonin-2 receptor agonists as novel ocular hypotensive agents and their cellular and molecular mechanisms of action. Curr. Drug Targets 2010, 11, 978–993. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Leggio, G.M.; Drago, F.; Salomone, S.; Bucolo, C. Regulation of intraocular pressure in mice: Structural analysis of dopaminergic and serotonergic systems in response to cabergoline. Biochem. Pharmacol. 2013, 86, 1347–1356. [Google Scholar] [CrossRef]
- Denoyer, A.; Godefroy, D.; Célérier, I.; Frugier, J.; Degardin, J.; Harrison, J.K.; Brignole-Baudouin, F.; Picaud, S.; Baleux, F.; Sahel, J.A.; et al. CXCR3 antagonism of SDF-1(5-67) restores trabecular function and prevents retinal neurodegeneration in a rat model of ocular hypertension. PLoS ONE 2012, 7, e37873. [Google Scholar] [CrossRef]
- Yu, N.; Zhang, Z.; Chen, P.; Zhong, Y.; Cai, X.; Hu, H.; Yang, Y.; Zhang, J.; Li, K.; Ge, J.; et al. Tetramethylpyrazine (TMP), an Active Ingredient of Chinese Herb Medicine Chuanxiong, Attenuates the Degeneration of Trabecular Meshwork through SDF-1/CXCR4 Axis. PLoS ONE 2015, 10, e0133055. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, X.; Yang, E.; Wang, Y.; Marra, K.G.; Ethier, C.R.; Schuman, J.S.; Du, Y. Adipose-derived stem cells integrate into trabecular meshwork with glaucoma treatment potential. FASEB J. 2020, 34, 7160–7177. [Google Scholar] [CrossRef]
- Kaur, I.; Kaur, J.; Sooraj, K.; Goswami, S.; Saxena, R.; Chauhan, V.S.; Sihota, R. Comparative evaluation of the aqueous humor proteome of primary angle closure and primary open angle glaucomas and age-related cataract eyes. Int. Ophthalmol. 2019, 39, 69–104. [Google Scholar] [CrossRef]
- Ramdas, W.D.; van Koolwijk, L.M.; Lemij, H.G.; Pasutto, F.; Cree, A.J.; Thorleifsson, G.; Janssen, S.F.; Jacoline, T.B.; Amin, N.; Rivadeneira, F.; et al. Common genetic variants associated with open-angle glaucoma. Hum. Mol. Genet. 2011, 20, 2464–2471. [Google Scholar] [CrossRef]
- Chai, X.; Low, K.Y.; Tham, Y.C.; Chee, M.L.; Thakur, S.; Zhang, L.; Tan, N.Y.; Khor, C.C.; Aung, T.; Wong, T.Y.; et al. Association of Glaucoma Risk Genes with Retinal Nerve Fiber Layer in a Multi-ethnic Asian Population: The Singapore Epidemiology of Eye Diseases Study. Investig. Ophthalmol. Vis. Sci. 2020, 61, 37. [Google Scholar] [CrossRef]
- Trikha, S.; Saffari, E.; Nongpiur, M.; Baskaran, M.; Ho, H.; Li, Z.; Tan, P.-Y.; Allen, J.; Khor, C.-C.; Perera, S.A.; et al. A Genetic Variant in TGFBR3-CDC7 Is Associated with Visual Field Progression in Primary Open-Angle Glaucoma Patients from Singapore. Ophthalmology 2015, 122, 2416–2422. [Google Scholar] [CrossRef]
- Lazzara, F.; Amato, R.; Platania, C.B.M.; Conti, F.; Chou, T.-H.; Porciatti, V.; Drago, F.; Bucolo, C. 1α,25-dihydroxyvitamin D3 protects retinal ganglion cells in glaucomatous mice. J. Neuroinflamm. 2021, 18, 206. [Google Scholar] [CrossRef]
- Quaranta, L.; Bruttini, C.; Micheletti, E.; Konstas, A.G.P.; Michelessi, M.; Oddone, F.; Katsanos, A.; Sbardella, D.; De Angelis, G.; Riva, I. Glaucoma and neuroinflammation: An overview. Surv. Ophthalmol. 2021, 66, 693–713. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; Matamoros, J.A.; Salobrar-García, E.; Elvira-Hurtado, L.; López-Cuenca, I.; Sánchez-Puebla, L.; Salazar, J.J.; Ramírez, J.M. Glaucoma: From pathogenic mechanisms to retinal glial cell response to damage. Front. Cell Neurosci. 2024, 18, 1354569. [Google Scholar] [CrossRef]
- Naguib, S.; Backstrom, J.R.; Gil, M.; Calkins, D.J.; Rex, T.S. Retinal oxidative stress activates the NRF2/ARE pathway: An early endogenous protective response to ocular hypertension. Redox Biol. 2021, 42, 101883. [Google Scholar] [CrossRef]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 2021, 83, 100916. [Google Scholar] [CrossRef]
- Chono, I.; Miyazaki, D.; Miyake, H.; Komatsu, N.; Ehara, F.; Nagase, D.; Kawamoto, Y.; Shimizu, Y.; Ideta, R.; Inoue, Y. High interleukin-8 level in aqueous humor is associated with poor prognosis in eyes with open angle glaucoma and neovascular glaucoma. Sci. Rep. 2018, 8, 14533. [Google Scholar] [CrossRef]
- Gramlich, O.W.; Beck, S.; von Thun Und Hohenstein-Blaul, N.; Boehm, N.; Ziegler, A.; Vetter, J.M.; Pfeiffer, N.; Grus, F.H. Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS ONE 2013, 8, e57557. [Google Scholar] [CrossRef] [PubMed]
- Khalef, N.; Labib, H.; Helmy, H.; El Hamid, M.A.; Moemen, L.; Fahmy, I. Levels of cytokines in the aqueous humor of eyes with primary open angle glaucoma, pseudoexfoliation glaucoma and cataract. Electron. Physician 2017, 9, 3833–3837. [Google Scholar] [CrossRef]
- Tezel, G.; Li, L.Y.; Patil, R.V.; Wax, M.B. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1787–1794. [Google Scholar]
- Williams, P.A.; Tribble, J.R.; Pepper, K.W.; Cross, S.D.; Morgan, B.P.; Morgan, J.E.; John, S.W.M.; Howell, G.R. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol. Neurodegener. 2016, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, S.; Reinhard, J.; Gandej, M.; Kuehn, S.; Noristani, R.; Faissner, A.; Dick, H.B.; Joachim, S.C. Simultaneous Complement Response via Lectin Pathway in Retina and Optic Nerve in an Experimental Autoimmune Glaucoma Model. Front. Cell Neurosci. 2016, 10, 140. [Google Scholar] [CrossRef]
- Reinehr, S.; Reinhard, J.; Gandej, M.; Gottschalk, I.; Stute, G.; Faissner, A.; Dick, H.B.; Joachim, S.C. S100B immunization triggers NFκB and complement activation in an autoimmune glaucoma model. Sci. Rep. 2018, 8, 9821. [Google Scholar] [CrossRef]
- Amato, R.; Cammalleri, M.; Melecchi, A.; Bagnoli, P.; Porciatti, V. Natural History of Glaucoma Progression in the DBA/2J Model: Early Contribution of Müller Cell Gliosis. Cells 2023, 12, 1272. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Liu, H.; Mercieca, K.; Prinz, J.; Feng, Y.; Prokosch, V. The Association between Vascular Abnormalities and Glaucoma-What Comes First? Int. J. Mol. Sci. 2023, 24, 13211. [Google Scholar] [CrossRef]
- Liberatore, F.; Bucci, D.; Mascio, G.; Madonna, M.; Di Pietro, P.; Beneventano, M.; Puliti, A.M.; Battaglia, G.; Bruno, V.; Nicoletti, F.; et al. Permissive role for mGlu1 metabotropic glutamate receptors in excitotoxic retinal degeneration. Neuroscience 2017, 363, 142–149. [Google Scholar] [CrossRef]
- Ji, M.; Miao, Y.; Dong, L.-D.; Chen, J.; Mo, X.-F.; Jiang, S.-X.; Sun, X.-H.; Yang, X.-L.; Wang, Z. Group I mGluR-mediated inhibition of Kir channels contributes to retinal Müller cell gliosis in a rat chronic ocular hypertension model. J. Neurosci. 2012, 32, 12744–12755. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, G.-L.; Cheng, S.; Wang, Z.; Yang, X.-L. Activation of retinal glial cells contributes to the degeneration of ganglion cells in experimental glaucoma. Prog. Retin. Eye Res. 2023, 93, 101169. [Google Scholar] [CrossRef] [PubMed]
- Lambuk, L.; Lazaldin, M.A.M.; Ahmad, S.; Iezhitsa, I.; Agarwal, R.; Uskoković, V.; Mohamud, R. Brain-Derived Neurotrophic Factor-Mediated Neuroprotection in Glaucoma: A Review of Current State of the Art. Front. Pharmacol. 2022, 13, 875662. [Google Scholar] [CrossRef] [PubMed]
- Pacwa, A.; Machowicz, J.; Akhtar, S.; Rodak, P.; Liu, X.; Pietrucha-Dutczak, M.; Lewin-Kowalik, J.; Amadio, M.; Smedowski, A. Deficiency of the RNA-binding protein ELAVL1/HuR leads to the failure of endogenous and exogenous neuroprotection of retinal ganglion cells. Front. Cell Neurosci. 2023, 17, 1131356. [Google Scholar] [CrossRef]
- Burgaletto, C.; Platania, C.B.M.; Di Benedetto, G.; Munafò, A.; Giurdanella, G.; Federico, C.; Caltabiano, R.; Saccone, S.; Conti, F.; Bernardini, R.; et al. Targeting the miRNA-155/TNFSF10 network restrains inflammatory response in the retina in a mouse model of Alzheimer’s disease. Cell Death Dis. 2021, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Agarwal, P. Newer targets for modulation of intraocular pressure: Focus on adenosine receptor signaling pathways. Expert. Opin. Ther. Targets 2014, 18, 527–539. [Google Scholar] [CrossRef]
- Aires, I.D.; Boia, R.; Rodrigues-Neves, A.C.; Madeira, M.H.; Marques, C.; Ambrósio, A.F.; Santiago, A.R. Blockade of microglial adenosine A2A receptor suppresses elevated pressure-induced inflammation, oxidative stress, and cell death in retinal cells. Glia 2019, 67, 896–914. [Google Scholar] [CrossRef]
- Li, G.; Torrejon, K.Y.; Unser, A.M.; Ahmed, F.; Navarro, I.D.; Baumgartner, R.A.; Albers, D.S.; Stamer, W.D. Trabodenoson, an Adenosine Mimetic With A1 Receptor Selectivity Lowers Intraocular Pressure by Increasing Conventional Outflow Facility in Mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 383–392. [Google Scholar] [CrossRef]
- Conti, F.; Lazzara, F.; Romano, G.L.; Platania, C.B.M.; Drago, F.; Bucolo, C. Caffeine Protects Against Retinal Inflammation. Front. Pharmacol. 2021, 12, 824885. [Google Scholar] [CrossRef]
- Bucolo, C.; Campana, G.; Di Toro, R.; Cacciaguerra, S.; Spampinato, S. Sigma1 recognition sites in rabbit iris-ciliary body: Topical sigma1-site agonists lower intraocular pressure. J. Pharmacol. Exp. Ther. 1999, 289, 1362–1369. [Google Scholar]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Drago, F.; Bucolo, C. The P2X7 receptor as a new pharmacological target for retinal diseases. Biochem. Pharmacol. 2022, 198, 114942. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.L.; Amato, R.; Lazzara, F.; Porciatti, V.; Chou, T.-H.; Drago, F.; Bucolo, C. P2X7 receptor antagonism preserves retinal ganglion cells in glaucomatous mice. Biochem. Pharmacol. 2020, 180, 114199. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple. J. R. Stat. Society. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Koschützki, D.; Schreiber, F. Centrality analysis methods for biological networks and their application to gene regulatory networks. Gene Regul. Syst. Biol. 2008, 2, 193–201. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Kuhn, M.; Szklarczyk, D.; Pletscher-Frankild, S.; Blicher, T.H.; von Mering, C.; Jensen, L.J.; Bork, P. STITCH 4: Integration of protein-chemical interactions with user data. Nucleic Acids Res. 2014, 42, D401–D407. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Kostoulas, N.; Vergoulis, T.; Georgakilas, G.; Reczko, M.; Maragkakis, M.; Paraskevopoulou, M.D.; Prionidis, K.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA miRPath v.2.0: Investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012, 40, W498–W504. [Google Scholar] [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Stein, T.I.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef]


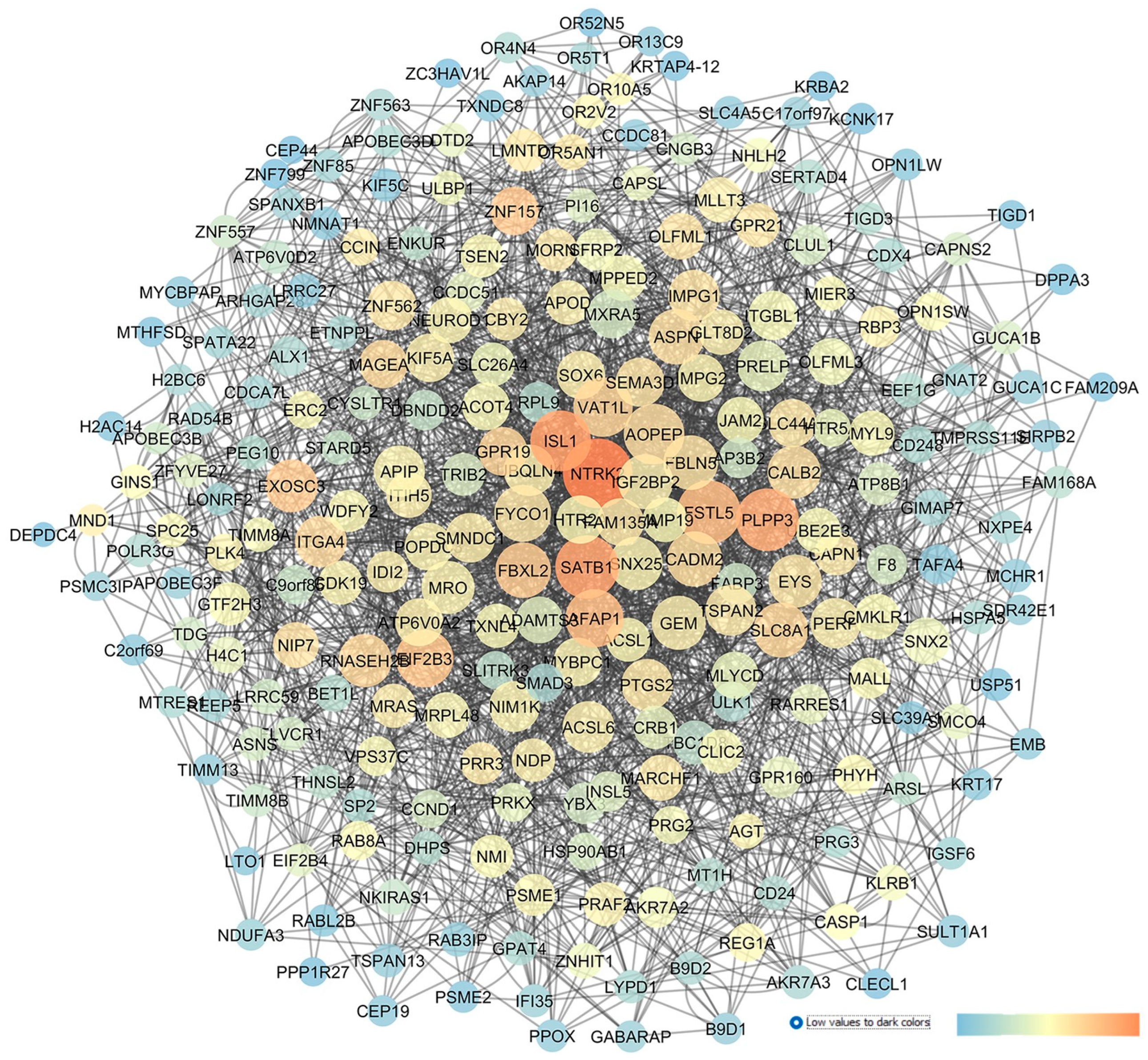
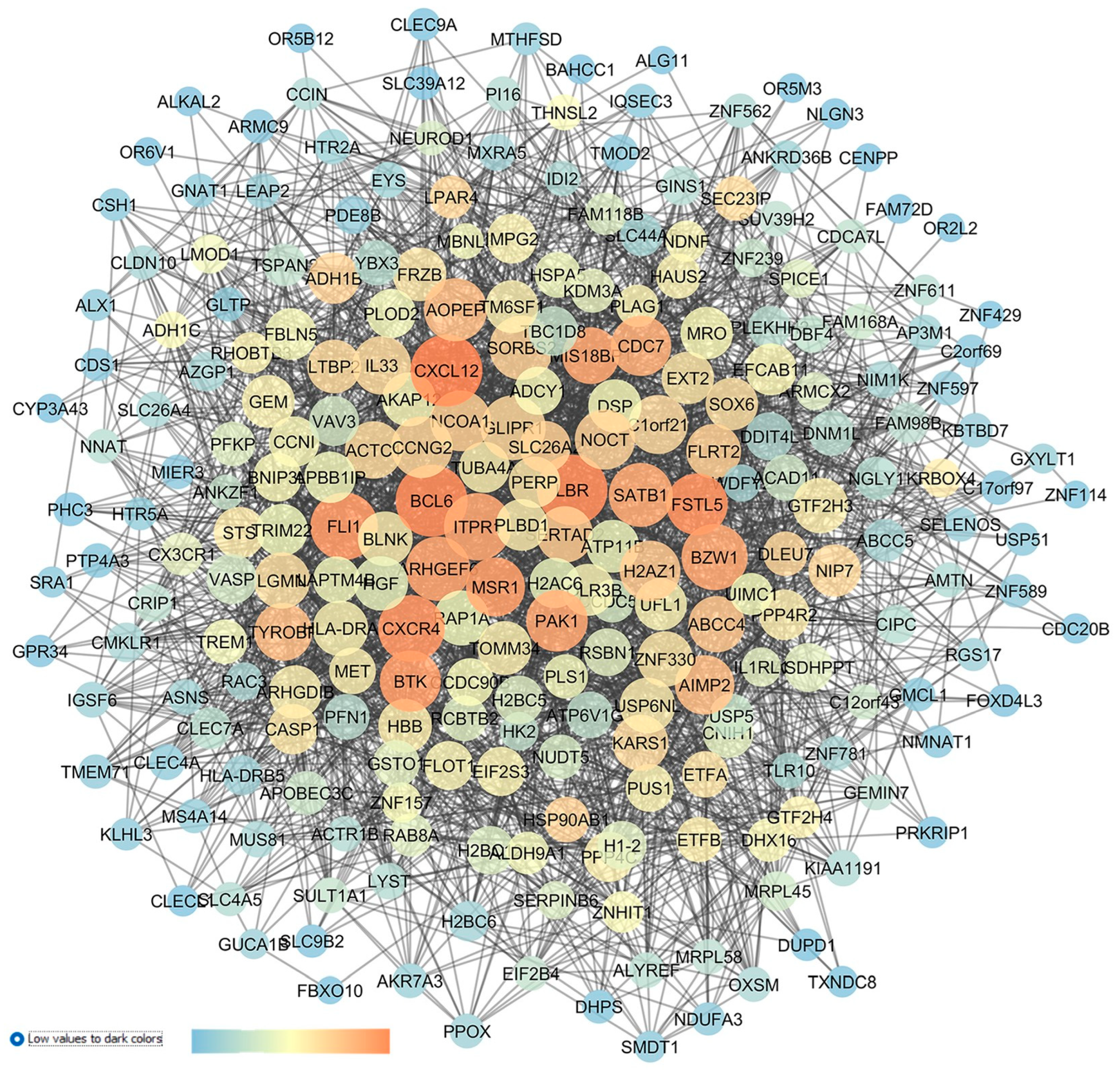
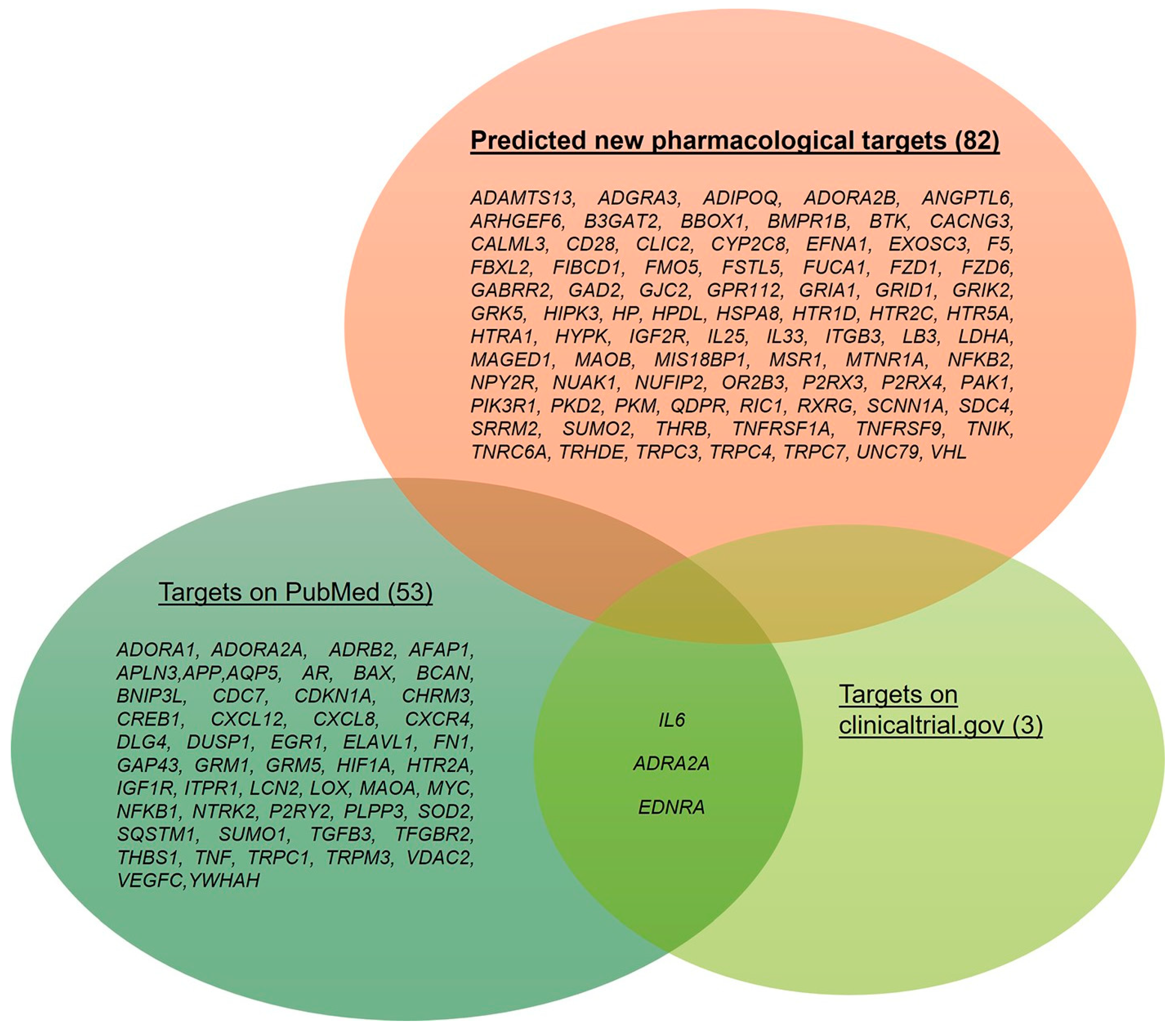
| GEO Dataset Code | Reference | Sample Group 1 | Sample Group 2 |
|---|---|---|---|
| GSE133563 | [28] | Optic nerve crush tissue (retina) of 8-week-old Lister Hooded rats | Sham-operated tissue (retina) of 8-week-old Lister Hooded rats |
| GSE105269 | [29] | Aqueous humor of POAG or XFG patients | Aqueous humor of control patients |
| GSE27276 | [30] | Trabecular meshwork cell culture, whose cells were isolated from POAG patients | Trabecular meshwork cell culture, whose cells were isolated from control subjects |
| GSE4316 | [31] | POAG human trabecular meshwork tissues | Human trabecular meshwork tissues of control donors |
| GSE45570 | Optic nerve head of POAG or OHT patients | Optic nerve head of control subjects | |
| GSE3554 | [32] | Tissues (retina) of 8-month-old DBA/2J mice of (with an elevated IOP) | Tissues (retina) of DBA/2J 3-month-old mice (with a normal IOP) |
| Node | Closeness Centrality | Stress | Degree |
|---|---|---|---|
| Hsa-let-7b-5p | 0.62 | 2,455,969,486 | 4489 |
| Elavl1 | 0.51 | 123,020,754 | 1244 |
| Myc | 0.49 | 18,006,672 | 512 |
| Parp1 | 0.49 | 10,943,968 | 122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuffrida, E.; Platania, C.B.M.; Lazzara, F.; Conti, F.; Marcantonio, N.; Drago, F.; Bucolo, C. The Identification of New Pharmacological Targets for the Treatment of Glaucoma: A Network Pharmacology Approach. Pharmaceuticals 2024, 17, 1333. https://doi.org/10.3390/ph17101333
Giuffrida E, Platania CBM, Lazzara F, Conti F, Marcantonio N, Drago F, Bucolo C. The Identification of New Pharmacological Targets for the Treatment of Glaucoma: A Network Pharmacology Approach. Pharmaceuticals. 2024; 17(10):1333. https://doi.org/10.3390/ph17101333
Chicago/Turabian StyleGiuffrida, Erika, Chiara Bianca Maria Platania, Francesca Lazzara, Federica Conti, Nicoletta Marcantonio, Filippo Drago, and Claudio Bucolo. 2024. "The Identification of New Pharmacological Targets for the Treatment of Glaucoma: A Network Pharmacology Approach" Pharmaceuticals 17, no. 10: 1333. https://doi.org/10.3390/ph17101333
APA StyleGiuffrida, E., Platania, C. B. M., Lazzara, F., Conti, F., Marcantonio, N., Drago, F., & Bucolo, C. (2024). The Identification of New Pharmacological Targets for the Treatment of Glaucoma: A Network Pharmacology Approach. Pharmaceuticals, 17(10), 1333. https://doi.org/10.3390/ph17101333








