Association between Scabies Treatment and Parkinson’s Disease: A Nationwide, Population-Based Study
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics of Subjects and Comorbidities
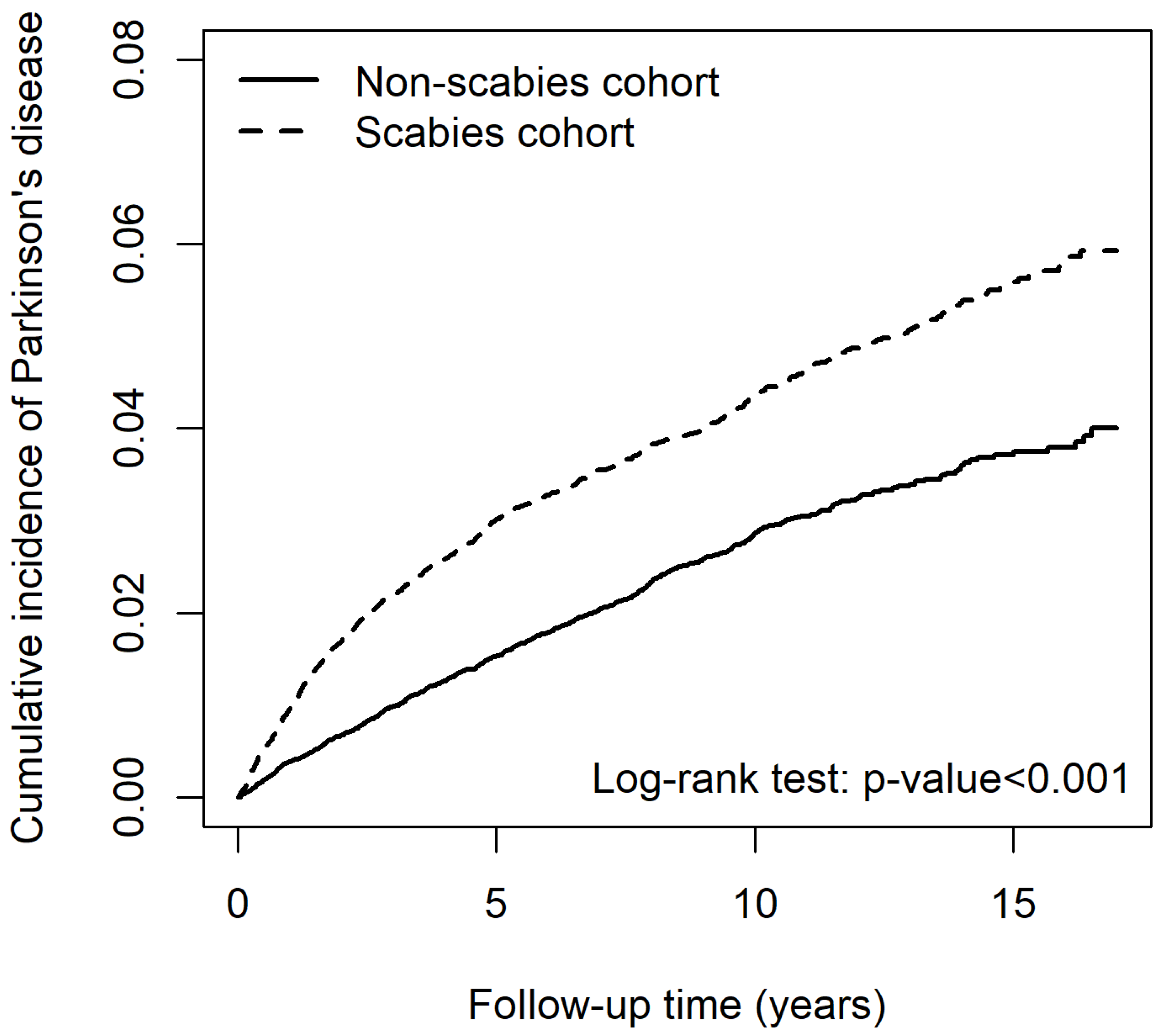
2.2. Risk for PD Associated with Scabies Infection
2.3. Baseline Characteristics of Subjects Treated for Scabies and Comorbidities
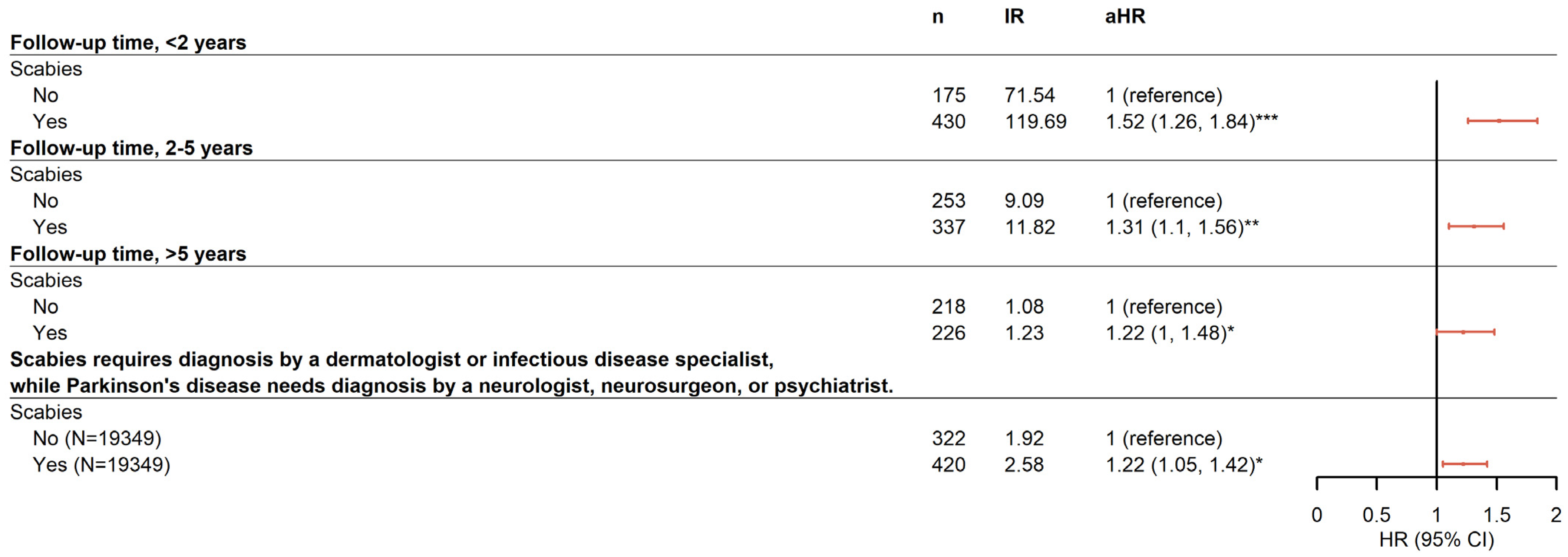
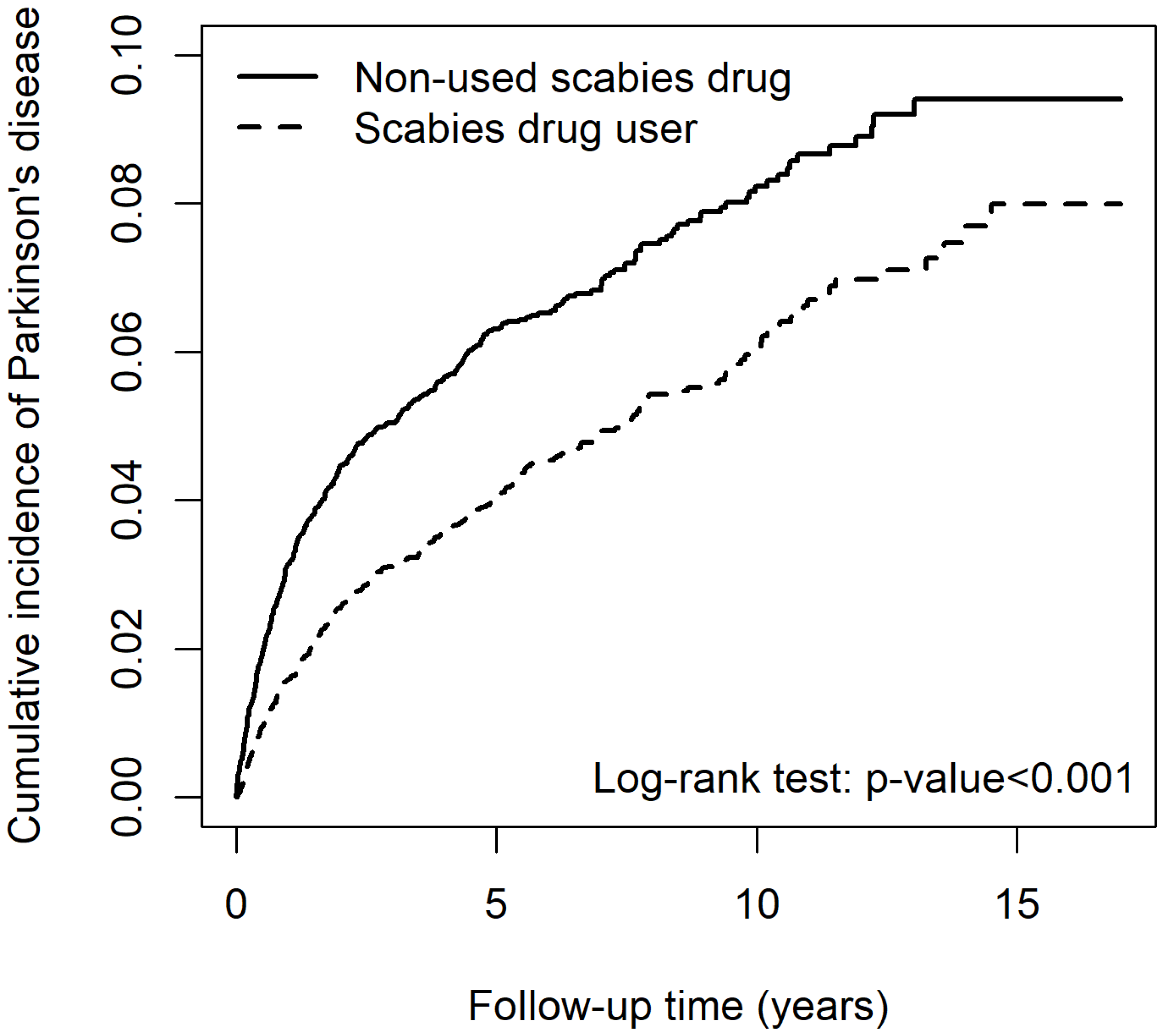
2.4. Risk for PD-Associated Lindane Use in Patients with Scabies
3. Discussion
4. Materials and Methods
4.1. Data Source and Collection
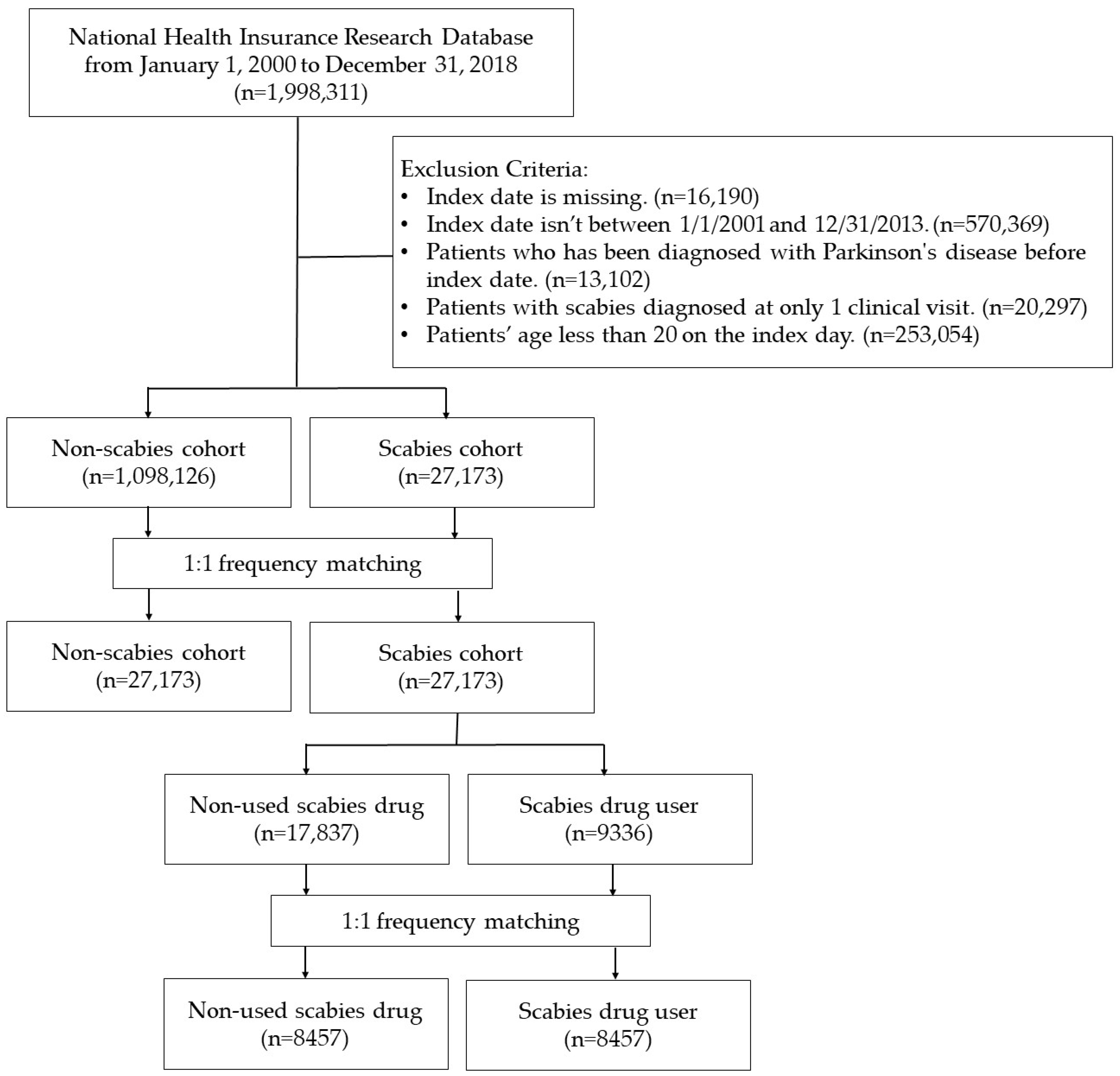
4.2. Study Design and Population
4.3. Comparison Group
4.4. Potential Confounders
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Isenbrandt, A.; Coulombe, K.; Morissette, M.; Bourque, M.; Lamontagne-Proulx, J.; Di Paolo, T.; Soulet, D. Three-Dimensional Analysis of Sex- and Gonadal Status- Dependent Microglial Activation in a Mouse Model of Parkinson’s Disease. Pharmaceuticals 2023, 16, 152. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Dhankhar, S.; Chauhan, S.; Garg, N.; Bhattacharya, T.; Ali, M.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, W.; et al. A Review on Natural Antioxidants for Their Role in the Treatment of Parkinson’s Disease. Pharmaceuticals 2023, 16, 908. [Google Scholar] [CrossRef] [PubMed]
- Vellingiri, B.; Chandrasekhar, M.; Sri Sabari, S.; Gopalakrishnan, A.V.; Narayanasamy, A.; Venkatesan, D.; Iyer, M.; Kesari, K.; Dey, A. Neurotoxicity of pesticides—A link to neurodegeneration. Ecotoxicol. Environ. Saf. 2022, 243, 113972. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Greco, A.; Fabbrini, G.; Inghilleri, M.; Rizzo, M.I.; Gallo, A.; Conte, M.; Rosato, C.; Ciniglio Appiani, M.; de Vincentiis, M. Parkinson’s disease: Autoimmunity and neuroinflammation. Autoimmun. Rev. 2016, 15, 1005–1011. [Google Scholar] [CrossRef]
- Liu, T.W.; Chen, C.M.; Chang, K.H. Biomarker of Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 4148. [Google Scholar] [CrossRef]
- Qu, Y.; Li, J.; Qin, Q.; Wang, D.; Zhao, J.; An, K.; Mao, Z.; Min, Z.; Xiong, Y.; Li, J.; et al. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 18. [Google Scholar] [CrossRef]
- Weiss, F.; Labrador-Garrido, A.; Dzamko, N.; Halliday, G. Immune responses in the Parkinson’s disease brain. Neurobiol. Dis. 2022, 168, 105700. [Google Scholar] [CrossRef]
- Thomas, C.; Coates, S.J.; Engelman, D.; Chosidow, O.; Chang, A.Y. Ectoparasites: Scabies. J. Am. Acad. Dermatol. 2020, 82, 533–548. [Google Scholar] [CrossRef]
- Thomas, C.; Castillo Valladares, H.; Berger, T.G.; Chang, A.Y. Scabies, Bedbug, and Body Lice Infestations: A Review. JAMA 2024. published online. [Google Scholar] [CrossRef]
- Jannic, A.; Bernigaud, C.; Brenaut, E.; Chosidow, O. Scabies Itch. Dermatol. Clin. 2018, 36, 301–308. [Google Scholar] [CrossRef]
- Lake, S.J.; Engelman, D.; Sokana, O.; Nasi, T.; Boara, D.; Marks, M.; Whitfeld, M.J.; Romani, L.; Kaldor, J.M.; Steer, A.C.; et al. Health-related quality of life impact of scabies in the Solomon Islands. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 148–156. [Google Scholar] [CrossRef]
- Chen, J.Y.; Liu, J.M.; Chang, F.W.; Chang, H.; Cheng, K.C.; Yeh, C.L.; Wei, Y.F.; Hsu, R.J. Scabies increased the risk and severity of COPD: A nationwide population-based study. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.D.; Wang, K.H.; Huang, C.C.; Lin, H.C. Scabies increased the risk of chronic kidney disease: A 5-year follow-up study. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.P.; Chen, P.Y.; Hsu, C.Y.; Chang, R.; Hu, K.C.; Chiu, L.T.; Hung, Y.M.; Mar, G.Y. Scabies Infestation and Risk of Acute Myocardial Infarction: A Population-Based Cohort Study. J. Pers. Med. 2022, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Hsu, R.J.; Chang, F.W.; Yeh, C.L.; Huang, C.F.; Chang, S.T.; Chiu, N.C.; Chang, H.Y.; Chi, H.; Lin, C.Y. Increase the risk of intellectual disability in children with scabies: A nationwide population-based cohort study. Medicine 2017, 96, e7108. [Google Scholar] [CrossRef]
- Wu, M.H.; Li, C.Y.; Pan, H.; Lin, Y.C. The Relationship between Scabies and Stroke: A Population-Based Nationwide Study. Int. J. Environ. Res. Public Health 2019, 16, 3491. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, F.W.; Yang, J.J.; Chang, C.H.; Yeh, C.L.; Lei, W.T.; Huang, C.F.; Liu, J.M.; Hsu, R.J. Increased risk of bipolar disorder in patients with scabies: A nationwide population-based matched-cohort study. Psychiatry Res. 2017, 257, 14–20. [Google Scholar] [CrossRef]
- Naesborg-Nielsen, C.; Wilkinson, V.; Mejia-Pacheco, N.; Carver, S. Evidence underscoring immunological and clinical pathological changes associated with Sarcoptes scabiei infection: Synthesis and meta-analysis. BMC Infect. Dis. 2022, 22, 658. [Google Scholar] [CrossRef]
- Nolan, K.; Kamrath, J.; Levitt, J. Lindane toxicity: A comprehensive review of the medical literature. Pediatr. Dermatol. 2012, 29, 141–146. [Google Scholar] [CrossRef]
- Arlian, L.G.; Morgan, M.S.; Neal, J.S. Modulation of cytokine expression in human keratinocytes and fibroblasts by extracts of scabies mites. Am. J. Trop. Med. Hyg. 2003, 69, 652–656. [Google Scholar] [CrossRef]
- Walton, S.F. The immunology of susceptibility and resistance to scabies. Parasite Immunol. 2010, 32, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.Z.; Stevenson, A.W.; Prele, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Walton, S.F.; Murray, H.C.; King, M.; Kelly, A.; Holt, D.C.; Currie, B.J.; McCarthy, J.S.; Mounsey, K.E. Crusted scabies is associated with increased IL-17 secretion by skin T cells. Parasite Immunol. 2014, 36, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Shabgah, A.G.; Fattahi, E.; Shahneh, F.Z. Interleukin-17 in human inflammatory diseases. Postep. Dermatol. Alergol. 2014, 31, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Chen, S.; Zhu, J.; Ma, J.; Zhai, Y. The Emerging Role of Central and Peripheral Immune Systems in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 872134. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 2020, 21, 139–152. [Google Scholar] [CrossRef]
- Lysen, T.S.; Darweesh, S.K.L.; Ikram, M.K.; Luik, A.I.; Ikram, M.A. Sleep and risk of parkinsonism and Parkinson’s disease: A population-based study. Brain 2019, 142, 2013–2022. [Google Scholar] [CrossRef]
- Lin, W.; Lin, Y.K.; Yang, F.C.; Chung, C.H.; Hu, J.M.; Tsao, C.H.; Weng, Z.X.; Ko, C.A.; Chien, W.C. Risk of neurodegenerative diseases in patients with sleep disorders: A nationwide population-based case-control study. Sleep. Med. 2023, 107, 289–299. [Google Scholar] [CrossRef]
- Sohail, S.; Yu, L.; Schneider, J.A.; Bennett, D.A.; Buchman, A.S.; Lim, A.S.P. Sleep fragmentation and Parkinson’s disease pathology in older adults without Parkinson’s disease. Mov. Disord. 2017, 32, 1729–1737. [Google Scholar] [CrossRef]
- Villafuerte, G.; Miguel-Puga, A.; Rodriguez, E.M.; Machado, S.; Manjarrez, E.; Arias-Carrion, O. Sleep deprivation and oxidative stress in animal models: A systematic review. Oxid. Med. Cell. Longev. 2015, 2015, 234952. [Google Scholar] [CrossRef] [PubMed]
- Meltan, S.; Panuganti, B.; Tarbox, M. Evaluation and Management of Pruritus and Scabies in the Elderly Population. Clin. Geriatr. Med. 2024, 40, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Mbuagbaw, L.; Sadeghirad, B.; Morgan, R.L.; Mertz, D.; Motaghi, S.; Ghadimi, M.; Babatunde, I.; Zani, B.; Pasumarthi, T.; Derby, M.; et al. Failure of scabies treatment: A systematic review and meta-analysis. Br. J. Dermatol. 2024, 190, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Meyersburg, D.; Hoellwerth, M.; Brandlmaier, M.; Handisurya, A.; Kaiser, A.; Prodinger, C.; Bauer, J.W. Comparison of topical permethrin 5% vs. benzyl benzoate 25% treatment in scabies: A double-blinded randomized controlled trial. Br. J. Dermatol. 2024, 190, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Agarwal, S. Comparison of the effects of ivermectin, permethrin, and gamma benzene hexachloride alone and with that of combination therapy for the management of scabies. J. Popul. Ther. Clin. Pharmacol. 2022, 29, e87–e96. [Google Scholar] [CrossRef]
- Tanaka, K. gamma-BHC: Its history and mystery—Why is only gamma-BHC insecticidal? Pestic. Biochem. Physiol. 2015, 120, 91–100. [Google Scholar] [CrossRef]
- Corrigan, F.M.; Wienburg, C.L.; Shore, R.F.; Daniel, S.E.; Mann, D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J. Toxicol. Environ. Health A 2000, 59, 229–234. [Google Scholar] [CrossRef]
- Mudawal, A.; Singh, A.; Yadav, S.; Mishra, M.; Singh, P.K.; Chandravanshi, L.P.; Mishra, J.; Khanna, V.K.; Bandyopadhyay, S.; Parmar, D. Similarities in lindane induced alterations in protein expression profiling in different brain regions with neurodegenerative diseases. Proteomics 2015, 15, 3875–3882. [Google Scholar] [CrossRef]
- Kamel, F.; Hoppin, J.A. Association of pesticide exposure with neurologic dysfunction and disease. Environ. Health Perspect. 2004, 112, 950–958. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Lai, S.W.; Liao, K.F.; Lin, C.L.; Lin, C.H. Association between Parkinson’s disease and proton pump inhibitors therapy in older people. Biomedicine 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Grinan-Ferre, C.; Bellver-Sanchis, A.; Guerrero, A.; Pallas, M. Advancing personalized medicine in neurodegenerative diseases: The role of epigenetics and pharmacoepigenomics in pharmacotherapy. Pharmacol. Res. 2024, 205, 107247. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, X.; Qian, Y.; Luo, Q.; Song, Y.; Xiao, Q. Analysis of serum levels of organochlorine pesticides and related factors in Parkinson’s disease. Neurotoxicology 2022, 88, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Z.D.; Yi, L.X.; Tan, B.J.; Tan, E.K. Interaction between Caffeine Consumption & Genetic susceptibility in Parkinson’s disease: A systematic review. Ageing Res. Rev. 2024, 99, 102381. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, A.; Newell, M.E.; Halden, R.U. Literature review and meta-analysis of environmental toxins associated with increased risk of Parkinson’s disease. Sci. Total Environ. 2024, 931, 172838. [Google Scholar] [CrossRef]
- Tsai, K.S.; Yen, C.S.; Wu, P.Y.; Chiang, J.H.; Shen, J.L.; Yang, C.H.; Chen, H.Y.; Chen, Y.H.; Chen, W.C. Traditional Chinese Medicine Decreases the Stroke Risk of Systemic Corticosteroid Treatment in Dermatitis: A Nationwide Population-Based Study. Evid. Based Complement. Alternat. Med. 2015, 2015, 543517. [Google Scholar] [CrossRef]
- Wacholder, S.; McLaughlin, J.K.; Silverman, D.T.; Mandel, J.S. Selection of controls in case-control studies. I. Principles. Am. J. Epidemiol. 1992, 135, 1019–1028. [Google Scholar] [CrossRef]

| Variables | Scabies | p-Value | |||
|---|---|---|---|---|---|
| No (n = 27,173) | Yes (n = 27,173) | ||||
| n | % | n | % | ||
| Age, years | >0.999 | ||||
| 20–49 | 11,770 | 43.32 | 11,770 | 43.32 | |
| 50–69 | 7009 | 25.79 | 7009 | 25.79 | |
| ≥70 | 8394 | 30.89 | 8394 | 30.89 | |
| Mean ± SD a | 54.51 | 20.84 | 54.55 | 20.92 | 0.823 |
| Sex | >0.999 | ||||
| Female | 11,732 | 43.18 | 11,732 | 43.18 | |
| Male | 15,441 | 56.82 | 15,441 | 56.82 | |
| Comorbidities | |||||
| Cerebrovascular accident | 3404 | 12.53 | 7423 | 27.32 | <0.001 |
| Hypertension | 9544 | 35.12 | 12,328 | 45.37 | <0.001 |
| Diabetes | 4472 | 16.46 | 6977 | 25.68 | <0.001 |
| Chronic obstructive pulmonary disease | 4549 | 16.74 | 7372 | 27.13 | <0.001 |
| Coronary heart disease | 4971 | 18.29 | 6823 | 25.11 | <0.001 |
| Dementia | 809 | 2.98 | 2917 | 10.73 | <0.001 |
| Alzheimer’s disease/Lewy body dementia | 300 | 1.10 | 651 | 2.40 | <0.001 |
| Chronic kidney disease | 1078 | 3.97 | 2097 | 7.72 | <0.001 |
| Anxiety/Depressive disorder | 5314 | 19.56 | 7529 | 27.71 | <0.001 |
| Sleep disorders | 5881 | 21.64 | 8380 | 30.84 | <0.001 |
| Follow-up time, years | |||||
| Mean ± SD a | 8.54 | 4.59 | 7.96 | 4.89 | <0.001 |
| Variables | Parkinson’s Disease | cHR | (95% CI) | p-Value | aHR | (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| n | PY | IR | |||||||
| Scabies | |||||||||
| No | 646 | 232,173.69 | 2.78 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 993 | 216,256.68 | 4.59 | 1.64 | (1.48, 1.81) *** | <0.001 | 1.46 | (1.32, 1.63) *** | <0.001 |
| Age, years | |||||||||
| 20–49 | 156 | 245,166.57 | 0.64 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| 50–69 | 452 | 119,717.56 | 3.78 | 5.90 | (4.92, 7.08) *** | <0.001 | 4.46 | (3.68, 5.41) *** | <0.001 |
| ≥70 | 1031 | 83,546.24 | 12.34 | 18.50 | (15.61, 21.97) *** | <0.001 | 10.49 | (8.59, 12.82) *** | <0.001 |
| Sex | |||||||||
| Female | 738 | 201,356.80 | 3.67 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Male | 901 | 247,073.58 | 3.65 | 0.98 | (0.89, 1.08) | 0.648 | 1.15 | (1.04, 1.27) ** | 0.005 |
| Comorbidities | |||||||||
| CVA | |||||||||
| No | 940 | 395,558.29 | 2.38 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 699 | 52,872.09 | 13.22 | 5.07 | (4.58, 5.6) *** | <0.001 | 1.53 | (1.36, 1.71) *** | <0.001 |
| Hypertension | |||||||||
| No | 494 | 315,302.97 | 1.57 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 1145 | 133,127.41 | 8.60 | 5.14 | (4.62, 5.72) *** | <0.001 | 1.28 | (1.12, 1.46) *** | <0.001 |
| Diabetes | |||||||||
| No | 1058 | 381,136.81 | 2.78 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 581 | 67,293.57 | 8.63 | 2.86 | (2.59, 3.17) *** | <0.001 | 1.11 | (1, 1.24) | 0.057 |
| COPD | |||||||||
| No | 980 | 380,942.81 | 2.57 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 659 | 67,487.57 | 9.76 | 3.50 | (3.16, 3.86) *** | <0.001 | 1.17 | (1.05, 1.31) ** | 0.005 |
| CHD | |||||||||
| No | 958 | 381,549.46 | 2.51 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 681 | 66,880.92 | 10.18 | 3.73 | (3.38, 4.13) *** | <0.001 | 1.06 | (0.94, 1.18) | 0.337 |
| Dementia | |||||||||
| No | 1361 | 435,390.77 | 3.13 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 278 | 13,039.61 | 21.32 | 5.73 | (5.02, 6.54) *** | <0.001 | 1.38 | (1.19, 1.61) *** | <0.001 |
| AD/Lewy body dementia | |||||||||
| No | 1563 | 444,024.68 | 3.52 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 76 | 4405.70 | 17.25 | 4.29 | (3.41, 5.4) *** | <0.001 | 1.33 | (1.05, 1.69) * | 0.02 |
| CKD | |||||||||
| No | 1504 | 434,047.31 | 3.47 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 135 | 14,383.07 | 9.39 | 2.35 | (1.97, 2.81) *** | <0.001 | 0.88 | (0.73, 1.05) | 0.163 |
| Anxiety/Depressive disorder | |||||||||
| No | 975 | 357,061.40 | 2.73 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 664 | 91,368.97 | 7.27 | 2.54 | (2.3, 2.8) *** | <0.001 | 1.26 | (1.13, 1.41) *** | <0.001 |
| Sleep disorders | |||||||||
| No | 963 | 352,130.28 | 2.73 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 676 | 96,300.10 | 7.02 | 2.41 | (2.18, 2.66) *** | <0.001 | 1.19 | (1.06, 1.33) ** | 0.002 |
| Variables | Scabies Drug Used | p-Value | |||
|---|---|---|---|---|---|
| No (n = 8457) | Yes (n = 8457) | ||||
| n | % | n | % | ||
| Scabies drug | |||||
| Lindane | - | - | 8399 | 99.31 | |
| Permethrin | - | - | 27 | 0.32 | |
| Lindane + Permethrin | - | - | 31 | 0.37 | |
| Age, years | >0.999 | ||||
| 20–49 | 2592 | 30.65 | 2592 | 30.65 | |
| 50–69 | 2275 | 26.90 | 2275 | 26.90 | |
| ≥70 | 3590 | 42.45 | 3590 | 42.45 | |
| mean ± SD a | 61.13 | 20.14 | 61.11 | 20.22 | 0.959 |
| Sex | >0.999 | ||||
| Female | 3533 | 41.78 | 3533 | 41.78 | |
| Male | 4924 | 58.22 | 4924 | 58.22 | |
| Comorbidities | |||||
| CVA | 204 | 2.41 | 3430 | 40.56 | <0.001 |
| Hypertension | 258 | 3.05 | 5005 | 59.18 | <0.001 |
| Diabetes | 143 | 1.69 | 2895 | 34.23 | <0.001 |
| COPD | 195 | 2.31 | 3366 | 39.80 | <0.001 |
| Coronary heart disease | 171 | 2.02 | 2879 | 34.04 | <0.001 |
| Dementia | 107 | 1.27 | 1571 | 18.58 | <0.001 |
| AD/Lewy body dementia | 28 | 0.33 | 319 | 3.77 | <0.001 |
| CKD | 58 | 0.69 | 954 | 11.28 | <0.001 |
| Anxiety/Depressive disorder | 146 | 1.73 | 2952 | 34.91 | <0.001 |
| Sleep disorders | 149 | 1.76 | 3386 | 40.04 | <0.001 |
| Follow-up time, years | |||||
| mean ± SD a | 4.83 | 4.27 | 5.68 | 4.29 | <0.001 |
| Variables | Parkinson’s Disease | cHR | (95% CI) | p-Value | aHR | (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| n | PY | IR | |||||||
| Scabies drugs | |||||||||
| No | 453 | 40,839.15 | 11.09 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 349 | 48,020.43 | 7.27 | 0.68 | (0.59, 0.78) *** | <0.001 | 0.15 | (0.12, 0.19) *** | <0.001 |
| Age, years | |||||||||
| 20–49 | 79 | 41,582.54 | 1.90 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| 50–69 | 227 | 26,759.53 | 8.48 | 4.17 | (3.22, 5.38) *** | <0.001 | 3.31 | (2.55, 4.3) *** | <0.001 |
| ≥70 | 496 | 20,517.51 | 24.17 | 9.70 | (7.61, 12.36) *** | <0.001 | 5.90 | (4.56, 7.63) *** | <0.001 |
| Sex | |||||||||
| Female | 368 | 38,791.69 | 9.49 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Male | 434 | 50,067.89 | 8.67 | 0.88 | (0.77, 1.01) | 0.076 | 1.03 | (0.89, 1.18) | 0.708 |
| Comorbidities | |||||||||
| CVA | |||||||||
| No | 500 | 75,868.65 | 6.59 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 302 | 12,990.93 | 23.25 | 2.90 | (2.51, 3.35) *** | <0.001 | 1.84 | (1.45, 2.34) *** | <0.001 |
| Hypertension | |||||||||
| No | 427 | 66,920.55 | 6.38 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 375 | 21,939.03 | 17.09 | 2.32 | (2.02, 2.67) *** | <0.001 | 2.32 | (1.72, 3.13) *** | <0.001 |
| Diabetes | |||||||||
| No | 604 | 76,695.44 | 7.88 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 198 | 12,164.13 | 16.28 | 1.78 | (1.52, 2.09) *** | <0.001 | 1.09 | (0.89, 1.33) | 0.418 |
| COPD | |||||||||
| No | 532 | 74,984.21 | 7.09 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 270 | 13,875.37 | 19.46 | 2.36 | (2.03, 2.73) *** | <0.001 | 1.36 | (1.1, 1.69) ** | 0.005 |
| CHD | |||||||||
| No | 586 | 77,127.12 | 7.60 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 216 | 11,732.46 | 18.41 | 2.06 | (1.76, 2.41) *** | <0.001 | 0.85 | (0.69, 1.05) | 0.129 |
| Dementia | |||||||||
| No | 632 | 84,315.75 | 7.50 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 170 | 4543.83 | 37.41 | 3.68 | (3.1, 4.37) *** | <0.001 | 1.48 | (1.19, 1.84) *** | <0.001 |
| AD/Lewy body dementia | |||||||||
| No | 758 | 87,734.30 | 8.64 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 44 | 1125.28 | 39.10 | 3.56 | (2.63, 4.83) *** | <0.001 | 1.50 | (1.08, 2.07) * | 0.015 |
| CKD | |||||||||
| No | 748 | 85,480.57 | 8.75 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 54 | 3379.01 | 15.98 | 1.47 | (1.12, 1.94) ** | 0.006 | 0.72 | (0.54, 0.96) * | 0.027 |
| Anxiety/Depressive disorder | |||||||||
| No | 575 | 73,425.78 | 7.83 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 227 | 15,433.80 | 14.71 | 1.79 | (1.53, 2.08) *** | <0.001 | 1.37 | (1.11, 1.68) ** | 0.003 |
| Sleep disorders | |||||||||
| No | 559 | 71,771.41 | 7.79 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Yes | 243 | 17,088.16 | 14.22 | 1.71 | (1.47, 1.98) *** | <0.001 | 1.36 | (1.11, 1.67) ** | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, K.-S.; Lu, M.-K.; Liu, C.-H.; Tsai, F.-J.; Chen, W.-C.; Chen, H.-Y.; Lin, H.-J.; Lin, C.-L.; Lee, J.-C.; Man, K.-M.; et al. Association between Scabies Treatment and Parkinson’s Disease: A Nationwide, Population-Based Study. Pharmaceuticals 2024, 17, 1342. https://doi.org/10.3390/ph17101342
Tsai K-S, Lu M-K, Liu C-H, Tsai F-J, Chen W-C, Chen H-Y, Lin H-J, Lin C-L, Lee J-C, Man K-M, et al. Association between Scabies Treatment and Parkinson’s Disease: A Nationwide, Population-Based Study. Pharmaceuticals. 2024; 17(10):1342. https://doi.org/10.3390/ph17101342
Chicago/Turabian StyleTsai, Kao-Sung, Ming-Kuei Lu, Chao-Hong Liu, Fuu-Jen Tsai, Wen-Chi Chen, Huey-Yi Chen, Heng-Jun Lin, Cheng-Li Lin, Jen-Chih Lee, Kee-Ming Man, and et al. 2024. "Association between Scabies Treatment and Parkinson’s Disease: A Nationwide, Population-Based Study" Pharmaceuticals 17, no. 10: 1342. https://doi.org/10.3390/ph17101342







