Gentiopicroside Attenuates Lithium/Pilocarpine-Induced Epilepsy Seizures by Down-Regulating NR2B/CaMKII/CREB and TLR4/NF-κB Signaling Pathways in the Hippocampus of Mice
Abstract
1. Introduction
2. Results
2.1. Gent Normalized ECoG Measurements in Lithium/Pilo-Induced Seizures
2.2. Gent Improved Behavioral Measurements in Lithium/Pilo-Induced Seizures
2.3. Gent Pretreatment Decreased Lithium/Pilo-Induced Neurodegeneration
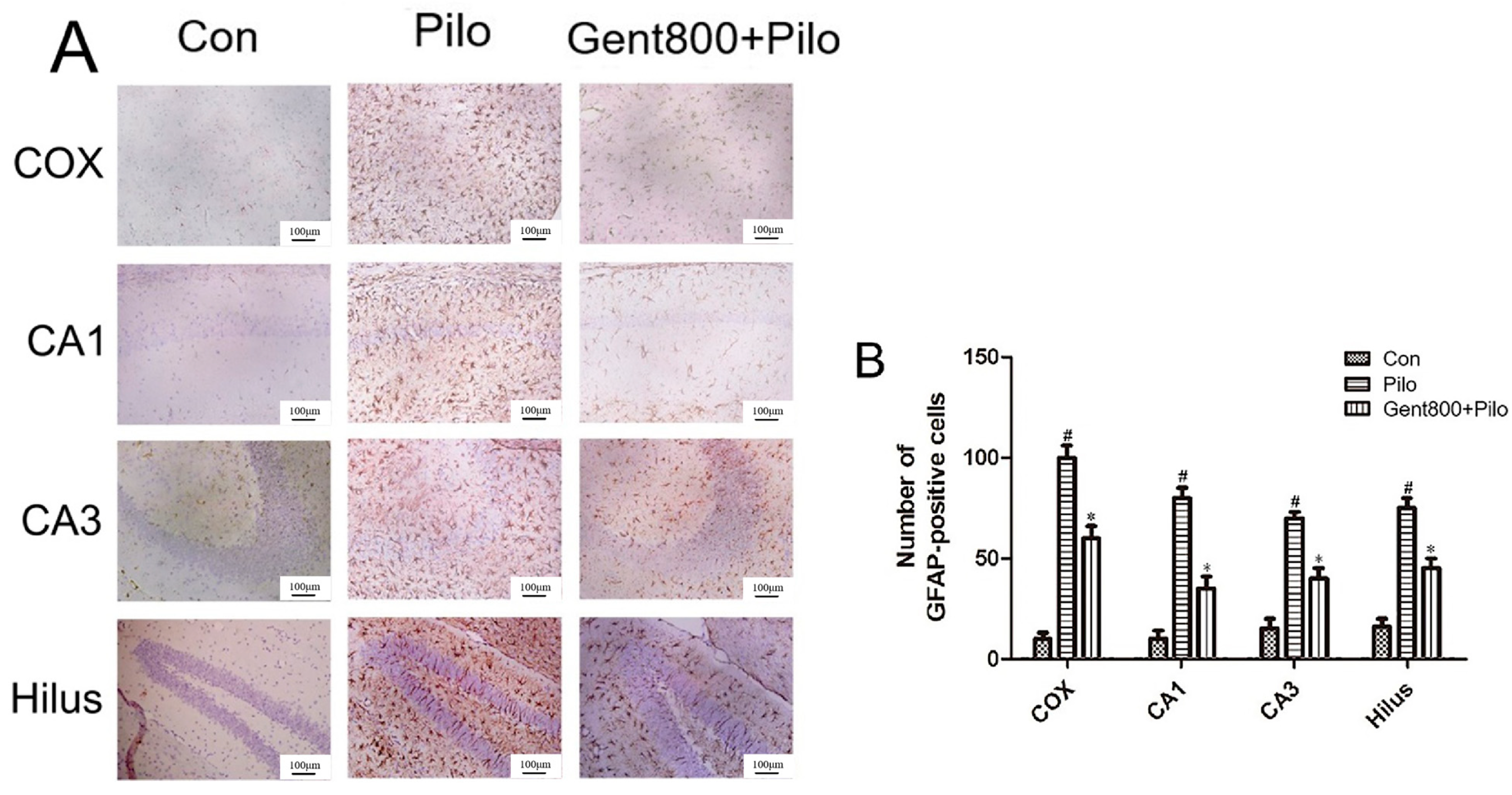
2.4. Gent Inhibited Lithium/Pilo-Induced Activation and Proliferation of Astrocytes
2.5. Gent Regulating Apoptosis-Related Factors
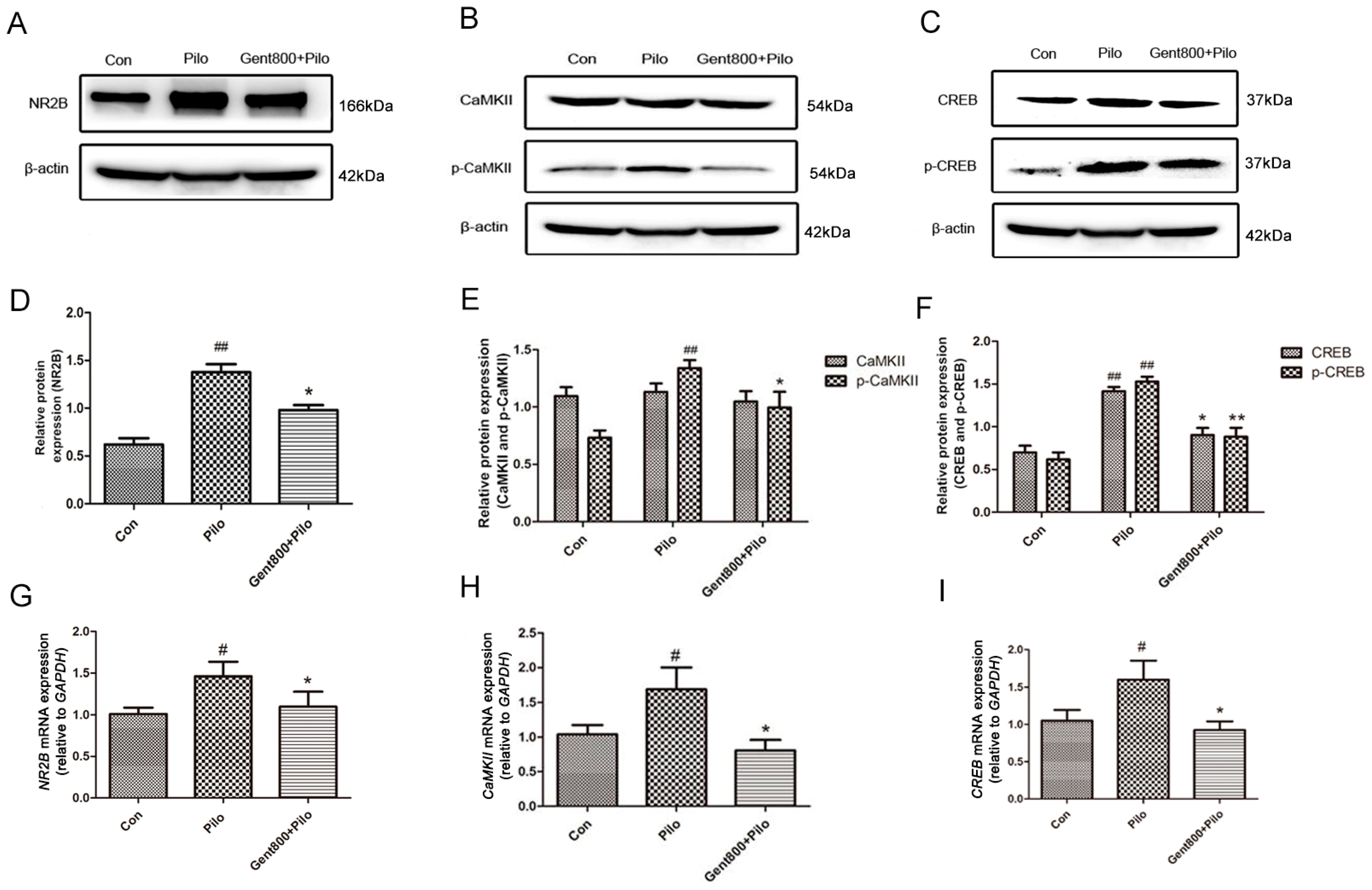
2.5.1. Gent Diminished the NR2B/CaMKII/CREB Signaling Pathway in the Hippocampus of Lithium/Pilo-Induced Epilepsy-Seizure Mice
2.5.2. Gent Reduced the Expression of Apoptosis-Associated Proteins in the Hippocampus of Lithium/Pilo-Induced Epileptic-Seizure Mice
2.6. Gent Regulating Neuroinflammatory-Related Factors
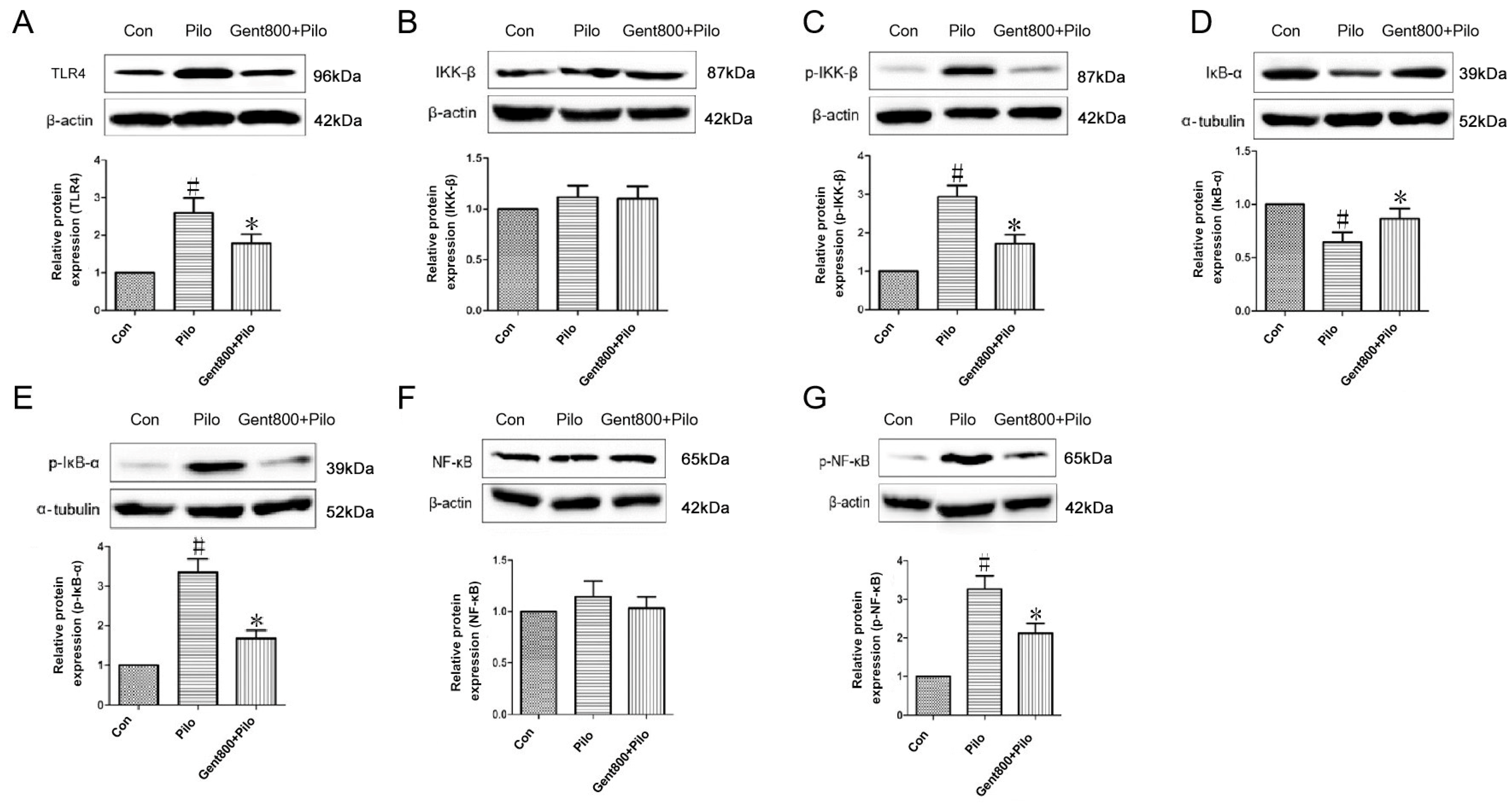
2.6.1. Gent Diminished Expression of the TLR4/NF-κB Signaling Pathway in the Hippocampus of Lithium/Pilo-Induced Epileptic-Seizure Mice
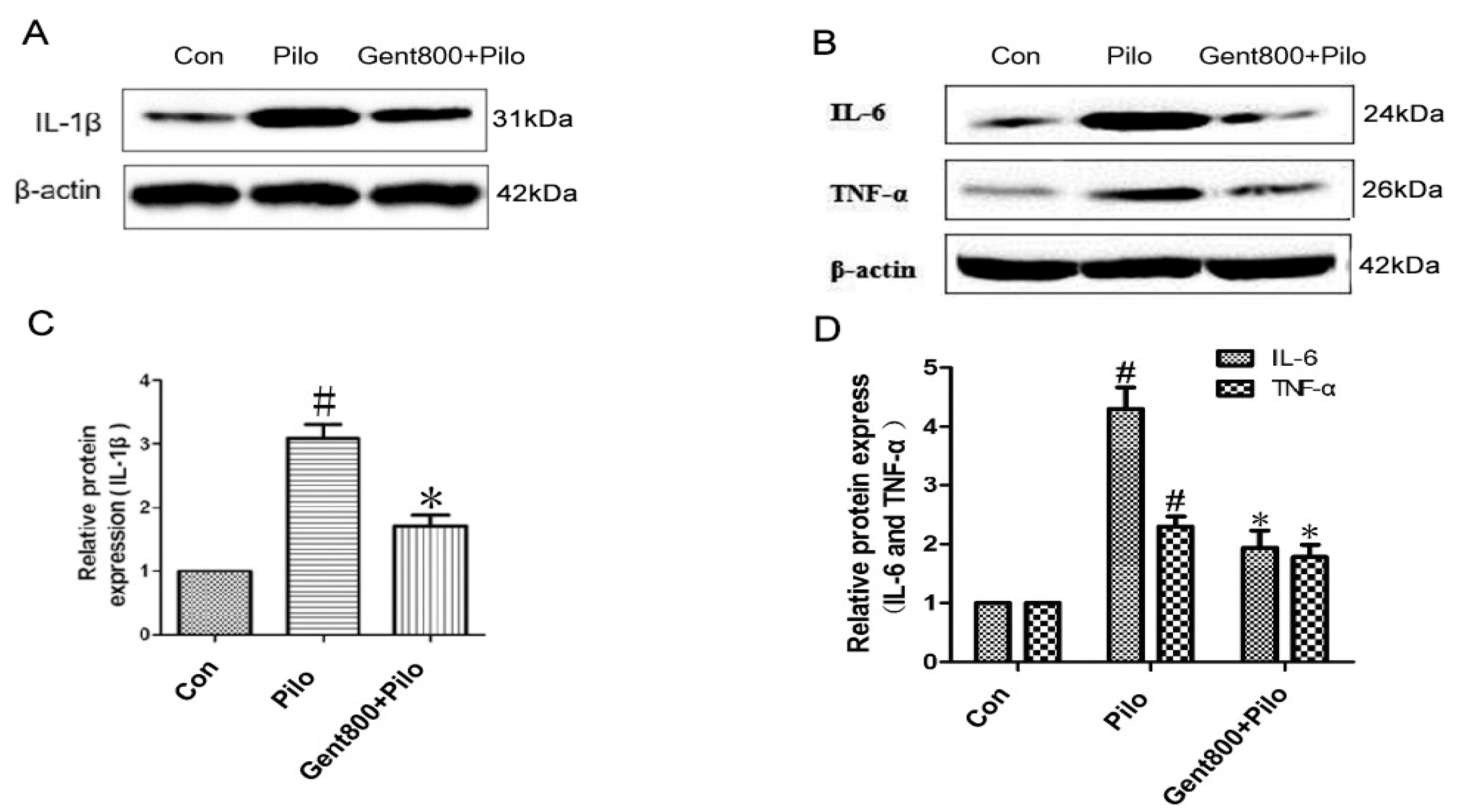
2.6.2. Gent Reduced the Expression of Pro-Inflammatory Cytokines in the Hippocampus of Lithium/Pilo-Induced Epileptic-Seizure Mice
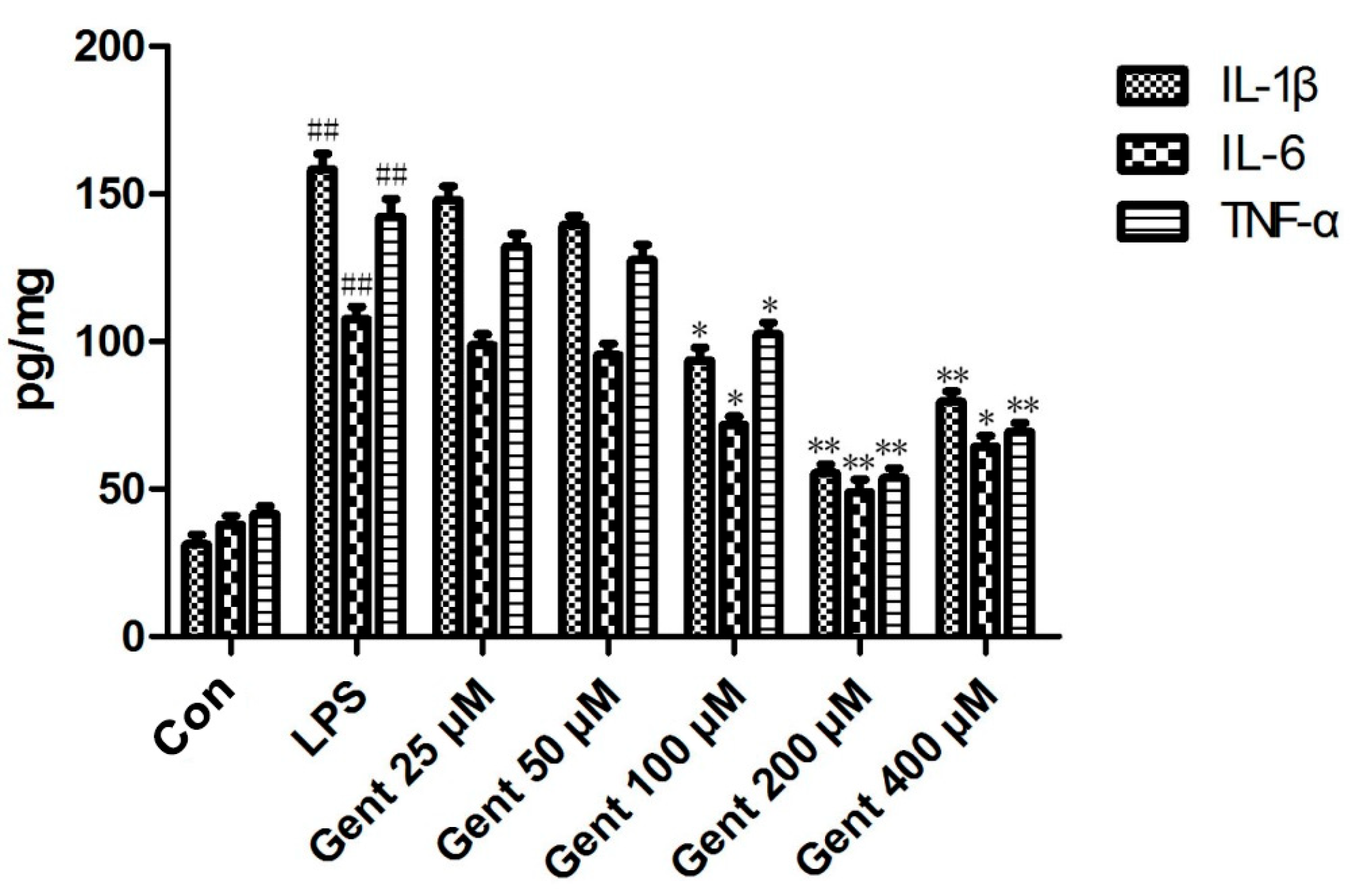
2.6.3. Gent Suppressed Lipopolysaccharide (LPS)-Induced Production of Pro-Inflammatory Cytokines in Astrocytes
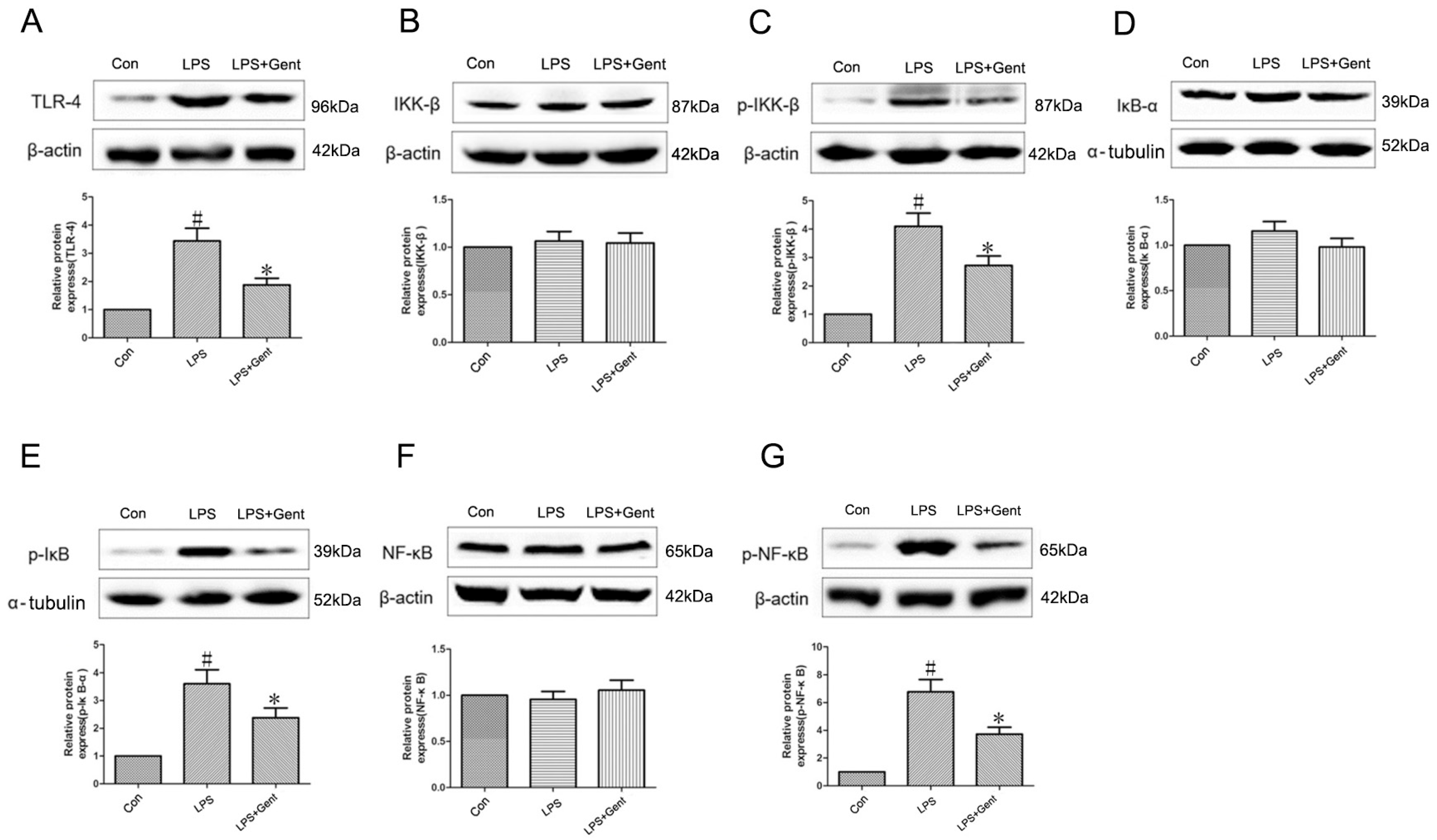
2.6.4. The Impact of Gent on Protein Levels Within the TLR-4/NF-κB Signaling Pathway in an LPS-Induced Astrocyte Inflammation Model
3. Discussion
4. Materials and Methods
4.1. Source of Drugs and Reagents
4.2. Animals
Animal Model
4.3. Cell Culture
4.4. Experimental Group
- Control group: 0.9% normal saline;
- Pilo group: 0.9% normal saline and Pilo (280 mg/kg);
- PB 30 mg/kg + Pilo group: phenobarbital dissolved in 0.9% normal saline;
- Gent 200 mg/kg + Pilo group: Gent dissolved in 0.9% normal saline;
- Gent 400 mg/kg + Pilo group;
- Gent 800 mg/kg + Pilo.
4.5. Behavioural Observation
4.6. Cortical Electrocorticography Recording
4.7. Sample Preparation for Histological Staining
4.7.1. Nissl Staining
4.7.2. FJB Staining
4.7.3. Immunohistochemical Staining
4.8. qPCR
4.9. ELISA
4.10. Western Blot Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, D.C.; Tewari, B.P.; Chaunsali, L.; Sontheimer, H. Neuron-glia interactions in the pathophysiology of epilepsy. Nat. Rev. Neurosci. 2019, 20, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhou, D.; Sander, J.W.; Wang, W.; Li, S.; Hong, Z. Epilepsy in China: Major progress in the past two decades. Lancet Neurol. 2021, 20, 316–326. [Google Scholar] [CrossRef] [PubMed]
- National Health Family Planning Commission. National Medical Service and Quality Safety Report; Supervision and Quality Control Administration of National Health Commission: Beijing, China, 2018. (In Chinese) [Google Scholar]
- National Health Family Planning Commission. Healthcare Quality Report of the Department of Neurology; National Medical Quality Control Centre of the Department of Neurology: Beijing, China, 2018. (In Chinese) [Google Scholar]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Farber, N.B.; Olney, J.W. NMDA receptor function, memory, and brain aging. Dialogues Clin. Neurosci. 2000, 2, 219–232. [Google Scholar] [CrossRef]
- Ullah, I.; Badshah, H.; Naseer, M.I.; Lee, H.Y.; Kim, M.O. Thymoquinone and vitamin C attenuates pentylenetetrazole-induced seizures via activation of GABAB1 receptor in adult rats cortex and hippocampus. Neuromolecular Med. 2015, 17, 35–46. [Google Scholar] [CrossRef]
- Mikati, M.A.; Abi-Habib, R.J.; El Sabban, M.E.; Dbaibo, G.S.; Kurdi, R.M.; Kobeissi, M.; Farhat, F.; Asaad, W. Hippocampal programmed cell death after status epilepticus: Evidence for NMDA-receptor and ceramide-mediated mechanisms. Epilepsia 2003, 44, 282–291. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, R.; Zhu, X.Y.; Hao, Y.J.; Li, N.; Wang, J.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int. J. Mol. Med. 2015, 36, 633–644. [Google Scholar] [CrossRef]
- Liu, S.B.; Zhang, N.; Guo, Y.Y.; Zhao, R.; Shi, T.Y.; Feng, S.F.; Wang, S.Q.; Yang, Q.; Li, X.Q.; Wu, Y.M.; et al. G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. J. Neurosci. 2012, 32, 4887–4900. [Google Scholar] [CrossRef]
- Yang, J.; Dong, H.Q.; Liu, Y.H.; Ji, M.H.; Zhang, X.; Dai, H.Y.; Sun, Z.C.; Liu, L.; Zhou, J.; Sha, H.H.; et al. Laparotomy-Induced Peripheral Inflammation Activates NR2B Receptors on the Brain Mast Cells and Results in Neuroinflammation in a Vagus Nerve-Dependent Manner. Front. Cell Neurosci. 2022, 16, 771156. [Google Scholar] [CrossRef]
- Turski, W.A.; Cavalheiro, E.A.; Schwarz, M.; Czuczwar, S.J.; Kleinrok, Z.; Turski, L. Limbic seizures produced by pilocarpine in rats: Behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983, 9, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Duan, C.; Wang, R.; Chen, L.; Wang, Y. Inflammation-related genes and immune infiltration landscape identified in kainite-induced temporal lobe epilepsy based on integrated bioinformatics analysis. Front. Neurosci. 2022, 16, 996368. [Google Scholar] [CrossRef] [PubMed]
- Auzmendi, J.; González, N.; Girardi, E. The NMDAR subunit NR2B expression is modified in hippocampus after repetitive seizures. Neurochem. Res. 2009, 34, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Yang, Y.; Mi, Z.; Zhu, G.X.; Qi, A.P.; Ji, W.G.; Zhu, Z.R. Anticonvulsant effect of Rhynchophylline involved in the inhibition of persistent sodium current and NMDA receptor current in the pilocarpine rat model of temporal lobe epilepsy. Neuroscience 2016, 337, 355–369. [Google Scholar] [CrossRef]
- Hong, N.; Kim, H.J.; Kang, K.; Park, J.O.; Mun, S.; Kim, H.G.; Kang, B.H.; Chung, P.S.; Lee, M.Y.; Ahn, J.C. Photobiomodulation improves the synapses and cognitive function and ameliorates epileptic seizure by inhibiting downregulation of Nlgn3. Cell Biosci. 2023, 13, 8. [Google Scholar] [CrossRef]
- Dong, X.-X.; Wang, Y.; Qin, Z.-H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Chen, L.; Zhang, Y.; Xu, Z.; Liu, J.; Jiang, G.; Li, J.; Zhang, X.; Wang, K.; et al. The microRNA miR-124 suppresses seizure activity and regulates CREB1 activity. Expert Rev. Mol. Med. 2016, 18, e4. [Google Scholar] [CrossRef]
- Folkerts, M.M.; Parks, E.A.; Dedman, J.R.; Kaetzel, M.A.; Lyeth, B.G.; Berman, R.F. Phosphorylation of calcium calmodulin-dependent protein kinase II following lateral fluid percussion brain injury in rats. J. Neurotrauma 2007, 24, 638–650. [Google Scholar] [CrossRef]
- Schwarzbach, E.; Bonislawski, D.P.; Xiong, G.; Cohen, A.S. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus 2006, 16, 541–550. [Google Scholar] [CrossRef]
- Guo, J.; Wang, H.; Wang, Q.; Chen, Y.; Chen, S. Expression of p-CREB and activity-dependent miR-132 in temporal lobe epilepsy. Int. J. Clin. Exp. Med. 2014, 7, 1297–1306. [Google Scholar]
- Zhu, Y.; Li, C.S.; Wang, Y.Y.; Zhou, S.N. Change of MicroRNA-134, CREB and p-CREB expression in epileptic rat. Asian Pac. J. Trop. Med. 2015, 8, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Simon, R.P.; Taki, W.; Lan, J.Q.; Henshall, D.C. Characterization of neuronal death induced by focally evoked limbic seizures in the C57BL/6 mouse. J. Neurosci. Res. 2002, 69, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Huang, S.; Zhang, Y.; Zhang, H.; Zhao, H. Decrease of Cellular Communication Network Factor 1 (CCN1) Attenuates PTZ-Kindled Epilepsy in Mice. Cell Mol. Neurobiol. 2023, 43, 4279–4293. [Google Scholar] [CrossRef]
- Henneberger, C.; Steinhäuser, C. Astrocytic TLR-4 at the crossroads of inflammation and seizure susceptibility. J. Cell Biol. 2016, 215, 607–609. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Xu, C.; Liu, K.; Wang, Y.; Chen, L.; Wu, X.; Gao, F.; Guo, Y.; Zhu, J.; et al. Therapeutic potential of an anti-high mobility group box-1 monoclonal antibody in epilepsy. Brain Behav. Immun. 2017, 64, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Iori, V.; Iyer, A.M.; Ravizza, T.; Beltrame, L.; Paracchini, L.; Marchini, S.; Cerovic, M.; Hill, C.; Ferrari, M.; Zucchetti, M.; et al. Blockade of the IL-1R1/TLR-4 pathway mediates disease-modification therapeutic effects in a model of acquired epilepsy. Neurobiol. Dis. 2017, 99, 12–23. [Google Scholar] [CrossRef]
- Wang, F.; Xiong, X.; Zhong, Q.; Meng, Z.; Yang, H.; Yang, Q. Foxp exhibits antiepileptic effects in ictogenesis involved in TLR-4 signaling. FASEB J. 2017, 31, 2948–2962. [Google Scholar] [CrossRef]
- Lorigados Pedre, L.; Morales Chacón, L.M.; Pavón Fuentes, N. Follow-Up of Peripheral IL-1β and IL-6 and Relation with Apoptotic Death in Drug-Resistant Temporal Lobe Epilepsy Patients Submitted to Surgery. Behav. Sci. 2018, 8, 21. [Google Scholar] [CrossRef]
- Musto, A.; Gjorstrup, P.; Bazan, N. The omega-3 fatty acid-derived neuroprotectin D1 limits hippocampal hyperexcitability and seizure susceptibility in kindling epileptogenesis. Epilepsia 2011, 52, 1601–1608. [Google Scholar] [CrossRef]
- Musto, A.E.; Rosencrans, R.F.; Walker, C.P.; Bhattacharjee, S.; Raulji, C.M.; Belayev, L.; Fang, Z.; Gordon, W.C.; Bazan, N.G. Dysfunctional epileptic neuronal circuits and dysmorphic dendritic spines are mitigated by platelet-activating factor receptor antagonism. Sci. Rep. 2016, 6, 30298. [Google Scholar]
- Scorza, C.; Marques, M.; da Silva, G.S.; Naffah-Mazzacoratti, M.; Scorza, F.; Cavalheiro, E. Status epilepticus does not induce acute brain inflammatory response in the Amazon rodent Proechimys, an animal model resistant to epileptogenesis. Neurosci. Lett. 2018, 668, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Musto, A.E. The role of inflammation in the development of epilepsy. J. Neuroinflammation 2018, 15, 144. [Google Scholar]
- Oztürk, N.; Korkmaz, S.; Oztürk, Y.; Başer, K.H. Effects of gentiopicroside, sweroside and swertiamarine, secoiridoids from gentian (Gentiana lutea ssp. symphyandra), on cultured chicken embryonic fibroblasts. Planta Medica 2006, 72, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.H.; Wu, Y.L.; Wan, Y.; Li, X.; Xie, W.X.; Nan, J.X. Anti-apoptotic activity of gentiopicroside in D-galactosamine/lipopolysaccharide-induced murine fulminant hepatic failure. Chem. Biol. Interact. 2010, 188, 127–133. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.C.; Zhang, X.N.; Guo, Y.Y.; Xu, Z.H.; Cao, W.; Sun, X.L.; Sun, W.J.; Zhao, M.G. Down-regulation of NR2B receptors partially contributes to analgesic effects of Gentiopicroside in persistent inflammatory pain. Neuropharmacology 2008, 54, 1175–1181. [Google Scholar] [CrossRef]
- Yong, Q.; Huang, C.; Chen, B.; An, J.; Zheng, Y.; Zhao, L.; Peng, C.; Liu, F. Gentiopicroside improves NASH and liver fibrosis by suppressing TLR4 and NLRP3 signaling pathways. Biomed. Pharmacother. 2024, 177, 116952. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, L.; Xu, S.; Zheng, P.; Du, J.; Wu, J.; Yu, J.; Sun, T. Gentiopicroside alleviated epileptogenesis in immature rats through inactivation of NLRP3 inflammasome by inhibiting P2X7R expression. Int. J. Dev. Neurosci. 2022, 83, 53–66. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Stoops, E.; Goozee, K.; Villemagne, V.L.; Asih, P.R.; Verberk, I.M.W.; Dave, P.; Taddei, K.; Sohrabi, H.R.; et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl. Psychiatry 2021, 11, 27. [Google Scholar] [CrossRef]
- Rostas, J.A.P.; Skelding, K.A. Calcium/Calmodulin-Stimulated Protein Kinase II (CaMKII): Different Functional Outcomes from Activation, Depending on the Cellular Microenvironment. Cells 2023, 12, 401. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Jin, W.-D.; Liu, H.-L.; Fu, W.-L.; Zhang, Y. Regulation and mechanism of CREB signaling pathway in nervous system diseases. Chin. J. Gen. Pract. 2019, 17, 644–648. [Google Scholar]
- Coulter, D.A.; Steinhäuser, C. Role of astrocytes in epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022434. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Sun, X.; Lin, Z.; Yang, Y.; Zhang, M.; Xu, Z.; Liu, P.; Liu, Z.; Huang, H. Gentiopicroside targets PAQR3 to activate the PI3K/AKT signaling pathway and ameliorate disordered glucose and lipid metabolism. Acta Pharm. Sin. B 2022, 12, 2887–2904. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A. Pilocarpine-induced seizures revisited: What does the model mimic? Epilepsy Curr. 2009, 9, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, E.A.; Silva, D.F.; Turski, W.A.; Calderazzo-Filho, L.S.; Bortolotto, Z.A.; Turski, L. The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Brain Res. 1987, 465, 43–58. [Google Scholar] [CrossRef]
- Fujikawa, D.G. Prolonged seizures and cellular injury: Understanding the connection. Epilepsy Behav. EB 2005, 7 (Suppl. S3), S3–S11. [Google Scholar] [CrossRef]
- Weise, J.; Engelhorn, T.; Dörfler, A.; Aker, S.; Bähr, M.; Hufnagel, A. Expression time course and spatial distribution of activated caspase-3 after experimental status epilepticus: Contribution of delayed neuronal cell death to seizure-induced neuronal injury. Neurobiol. Dis. 2005, 18, 582–590. [Google Scholar] [CrossRef]
- Chen, S.; Su, H.; Yue, C.; Remy, S.; Royeck, M.; Sochivko, D.; Opitz, T.; Beck, H.; Yaari, Y. An increase in persistent sodium current contributes to intrinsic neuronal bursting after status epilepticus. J. Neurophysiol. 2011, 105, 117–129. [Google Scholar] [CrossRef]
- Kumbhare, D.; Chaniary, K.D.; Baron, M.S. Preserved dichotomy but highly irregular and burst discharge in the basal ganglia in alert dystonic rats at rest. Brain Res. 2015, 1624, 297–313. [Google Scholar] [CrossRef]
- Xie, W.; Strong, J.A.; Kim, D.; Shahrestani, S.; Zhang, J.M. Bursting activity in myelinated sensory neurons plays a key role in pain behavior induced by localized inflammation of the rat sensory ganglion. Neuroscience 2012, 206, 212–223. [Google Scholar] [CrossRef]
- Blaise, M.C.; Sowdhamini, R.; Pradhan, N. Comparative analysis of different competitive antagonists interaction with NR2A and NR2B subunits of N-methyl-D-aspartate (NMDA) ionotropic glutamate receptor. J. Mol. Model. 2005, 11, 489–502. [Google Scholar] [CrossRef]
- Di Maio, R.; Mastroberardino, P.G.; Hu, X.; Montero, L.M.; Greenamyre, J.T. Thiol oxidation and altered NR2B/NMDA receptor functions in in vitro and in vivo pilocarpine models: Implications for epileptogenesis. Neurobiol. Dis. 2013, 49, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dong, J.; Shen, K.; Bai, Y.; Zhang, Y.; Lv, X.; Chao, J.; Yao, H. NMDA receptor NR2B subunits contribute to PTZ-kindling-induced hippocampal astrocytosis and oxidative stress. Brain Res. Bull. 2015, 114, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, G.; Pagliardini, S.; Ferrario, A.; Gardoni, F.; Tassi, L.; Setola, V.; Garbelli, R.; LoRusso, G.; Spreafico, R.; Di Luca, M.; et al. AlphaCaMKII and NMDA-receptor subunit expression in epileptogenic cortex from human periventricular nodular heterotopia. Epilepsia 2002, 43 (Suppl. S5), 209–216. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Rosenberg, H.C. Brief seizure activity alters Ca2+/calmodulin dependent protein kinase II dephosphorylation and subcellular distribution in rat brain for several hours. Neurosci. Lett. 2004, 357, 95–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, G.; Gao, B.; Li, Y.; Liang, S.; Wang, X.; Wang, X.; Zhu, B. NR4A1 Knockdown Suppresses Seizure Activity by Regulating Surface Expression of NR2B. Sci. Rep. 2016, 6, 37713. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, S.; Chen, G.; Cai, M.; Liu, R.; Deng, J.; Liu, J.; Zhang, T.; Tan, Q.; Hai, C. Gastrodin alleviates cerebral ischemic damage in mice by improving anti-oxidant and anti-inflammation activities and inhibiting apoptosis pathway. Neurochem. Res. 2015, 40, 661–673. [Google Scholar] [CrossRef]
- Sherafat, M.A.; Ronaghi, A.; Ahmad-Molaei, L.; Nejadhoseynian, M.; Ghasemi, R.; Hosseini, A.; Naderi, N.; Motamedi, F. Kindling-induced learning deficiency and possible cellular and molecular involved mechanisms. Neurol. Sci. 2013, 34, 883–890. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef]
- Uludag, I.F.; Duksal, T.; Tiftikcioglu, B.I.; Zorlu, Y.; Ozkaya, F.; Kirkali, G. IL-1β, IL-6 and IL-1Ra levels in temporal lobe epilepsy. Seizure 2015, 26, 22–25. [Google Scholar] [CrossRef]
- Vezzani, A.; Aronica, E.; Mazarati, A.; Pittman, Q.J. Epilepsy and brain inflammation. Exp. Neurol. 2013, 244, 11–21. [Google Scholar] [CrossRef]
- Xiao, Z.; Peng, J.; Yang, L.; Kong, H.; Yin, F. Interleukin-1β plays a role in the pathogenesis of mesial temporal lobe epilepsy through the PI3K/Akt/mTOR signaling pathway in hippocampal neurons. J. Neuroimmunol. 2015, 282, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Kang, X.; Qiu, J.; Du, Y.; Jiang, J. Anti-Inflammatory Small Molecules to Treat Seizures and Epilepsy: From Bench to Bedside. Trends Pharmacol. Sci. 2016, 37, 463–484. [Google Scholar] [PubMed]
- Casas-Grajales, S.; Ramos-Tovar, E.; Chávez-Estrada, E.; Alvarez-Suarez, D.; Hernández-Aquino, E.; Reyes-Gordillo, K.; Cerda-García-Rojas, C.M.; Camacho, J.; Tsutsumi, V.; Lakshman, M.R.; et al. Antioxidant and immunomodulatory activity induced by stevioside in liver damage: In vivo, in vitro and in silico assays. Life Sci. 2019, 224, 187–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Korgaonkar, A.A.; Swietek, B.; Wang, J.; Elgammal, F.S.; Elkabes, S.; Santhakumar, V. Toll-like receptor 4 enhancement of non-NMDA synaptic currents increases dentate excitability after brain injury. Neurobiol. Dis. 2015, 74, 240–253. [Google Scholar]
- Leal, B.; Chaves, J.; Carvalho, C.; Rangel, R.; Santos, A.; Bettencourt, A.; Lopes, J.; Ramalheira, J.; Silva, B.M.; da Silva, A.M.; et al. Brain expression of inflammatory mediators in Mesial Temporal Lobe Epilepsy patients. J. Neuroimmunol. 2017, 313, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Pernhorst, K.; Herms, S.; Hoffmann, P.; Cichon, S.; Schulz, H.; Sander, T.; Schoch, S.; Becker, A.J.; Grote, A. TLR-4, ATF-3 and IL8 inflammation mediator expression correlates with seizure frequency in human epileptic brain tissue. Seizure 2013, 22, 675–678. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Xie, N.; Wang, C.; Lian, Y.; Wu, C.; Zhang, H.; Zhang, Q. Puerarin protects hippocampal neurons against cell death in pilocarpine-induced seizures through antioxidant and anti-apoptotic mechanisms. Cell. Mol. Neurobiol. 2014, 34, 1175–1182. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Deng, X.H.; Ma, P.S.; Li, F.M.; Peng, X.D.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. The Anticonvulsant and Neuroprotective Effects of Oxysophocarpine on Pilocarpine-Induced Convulsions in Adult Male Mice. Cell. Mol. Neurobiol. 2017, 37, 339–349. [Google Scholar] [CrossRef]
- Uyanikgil, Y.; Özkeşkek, K.; Çavuşoğlu, T.; Solmaz, V.; Tümer, M.K.; Erbas, O. Positive effects of ceftriaxone on pentylenetetrazol-induced convulsion model in rats. Int. J. Neurosci. 2016, 126, 70–75. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Ma, P.S.; Wu, W.; Zhou, R.; Hao, Y.J.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Neuroprotective actions of taurine on hypoxic-ischemic brain damage in neonatal rats. Brain Res. Bull. 2016, 124, 295–305. [Google Scholar] [CrossRef] [PubMed]
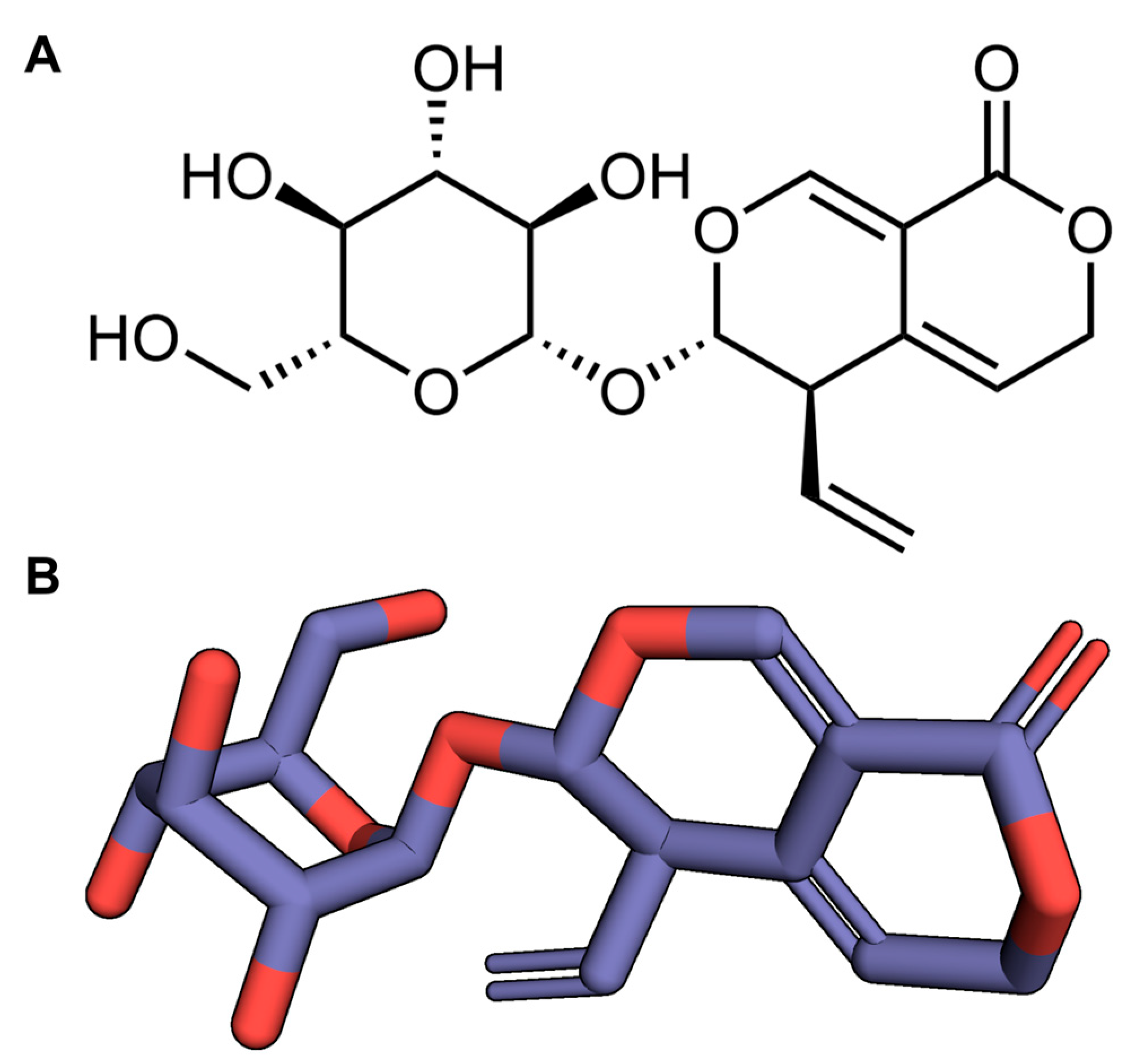
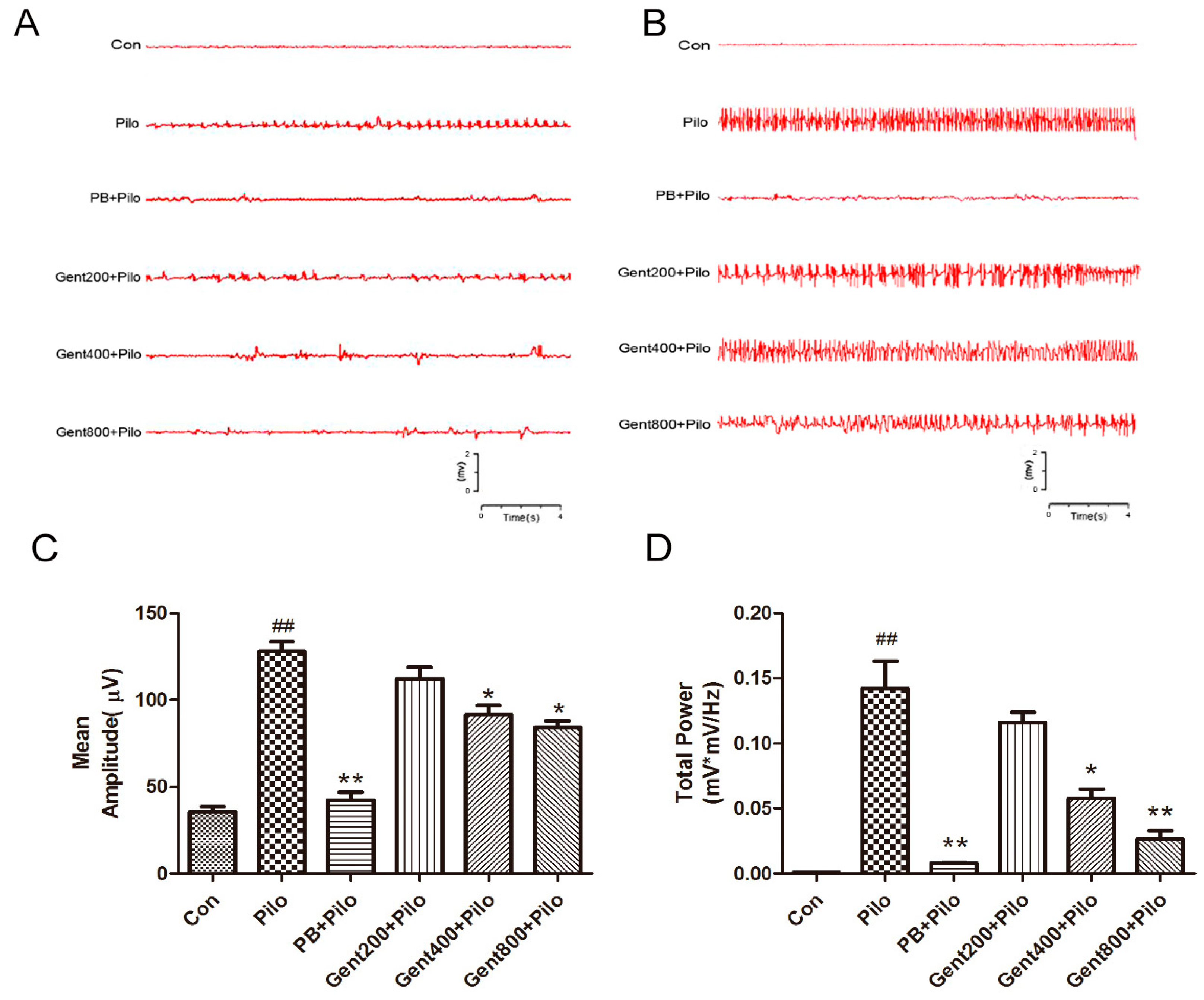
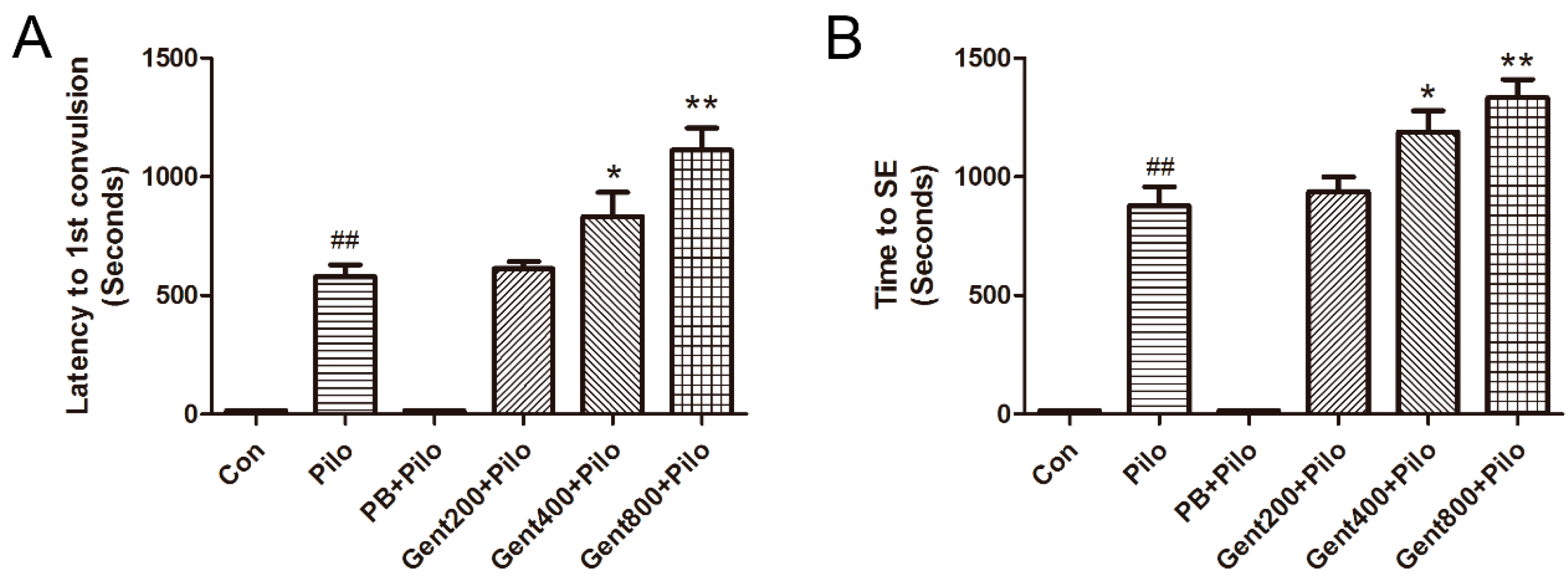
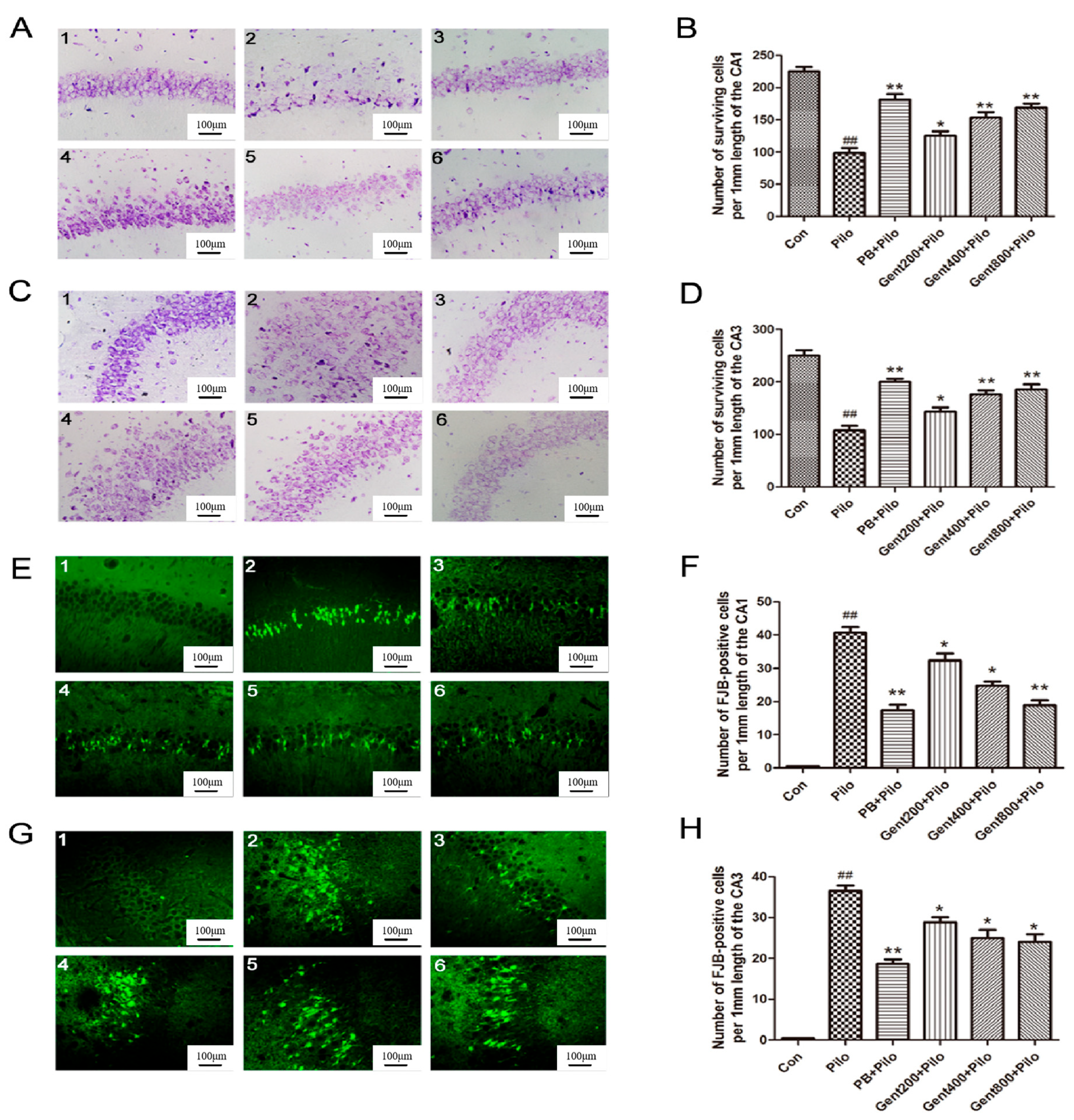

| Groups | Number of Animals/Group | Percentage Convulsion (%) | Percentage SE (%) | Percentage Survival (%) |
|---|---|---|---|---|
| Con | 16 | 0 (0 of 16) | 0 (0 of 16) | 100 (16 of 16) |
| Pilo | 16 | 100 (16 of 16) ## | 93.7 (15 of 16) ## | 62.5 (10 of 16) |
| PB + Pilo | 16 | 0 (0 of 16) | 0 (0 of 16) | 100 (16 of 16) |
| Gent200 + Pilo | 16 | 100 (16 of 16) | 100 (16 of 16) | 87.5 (14 of 16) |
| Gent400 + Pilo | 16 | 100 (16 of 16) | 75.0 (12 of 16) | 100 (16 of 16) * |
| Gent800 + Pilo | 16 | 100 (16 of 16) | 56.2 (9 of 16) * | 100 (16 of 16) * |
| Gene | Primer | Sequences |
|---|---|---|
| NR2B | Forward | 5′-TGCTGCTCATTGTCTCTGCT -3′ |
| Reverse | 5′-CTTTGCCGATGGTGAAAGAT-3′ | |
| CaMKII | Forward | 5′-TTCAATGCCAGGAGGAAACT-3′ |
| Reverse | 5′-TCACACCATCGCTCTTCTTG-3′ | |
| CREB | Forward | 5′-CTGCCCACTGCTAGTTTGGT-3′ |
| Reverse | 5′-CTGCCCACTGCTAGTTTGGT-3′ | |
| β-actin | Forward | 5′-GAGACCTTCAACACCCCAGC-3′ |
| Reverse | 5′-ATGTCACGCACGATTTCCCC-3′ |
| Antibody Type | Antibody Target | Dilution | Company | Country | Catalog Number |
|---|---|---|---|---|---|
| Rabbit | Bax | 1:2000 | Protein-Tech | China | 50599-2-Ig |
| Bcl-2 | 26593-1-AP | ||||
| TLR4 | 19811-1-AP | ||||
| NF-κB p65 | Abcam | USA | Ab32536 | ||
| IL-1β | 1:1500 | Protein-Tech | China | 26048-1-AP | |
| NR2B | 1:1000 | Abcam | USA | Ab254356 | |
| CaMKII | Ab134041 | ||||
| CREB | Ab32515 | ||||
| p-NF-κB p65 | Ab239882 | ||||
| Caspase-3 | Ab32351 | ||||
| IKK-β | SAB | USA | #24063 | ||
| IKB-α | #13376 | ||||
| IL-6 | Protein-Tech | USA | 26404-1-AP | ||
| p-CaMKII (Thr 286) | 1:500 | Abcam | USA | Ab171095 | |
| p-CREB (Ser 133) | Ab32096 | ||||
| TNF-α | Ab183218 | ||||
| p-IKK-β (Tyr188) | SAB | USA | #11929 | ||
| p-IKB-α (Ser32/Ser36) | #11152 | ||||
| β-actin | 1:2000 | Protein-Tech | China | 20536-1-AP | |
| α-tubulin | 1:20,000 | Protein-Tech | China | 11224-1-AP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.-M.; Liu, G.; Du, J.; Liu, Y.; Wei, W.; Lan, X.-B.; Hai, D.-M.; Ma, L.; Yu, J.-Q.; Liu, N. Gentiopicroside Attenuates Lithium/Pilocarpine-Induced Epilepsy Seizures by Down-Regulating NR2B/CaMKII/CREB and TLR4/NF-κB Signaling Pathways in the Hippocampus of Mice. Pharmaceuticals 2024, 17, 1413. https://doi.org/10.3390/ph17111413
Tian M-M, Liu G, Du J, Liu Y, Wei W, Lan X-B, Hai D-M, Ma L, Yu J-Q, Liu N. Gentiopicroside Attenuates Lithium/Pilocarpine-Induced Epilepsy Seizures by Down-Regulating NR2B/CaMKII/CREB and TLR4/NF-κB Signaling Pathways in the Hippocampus of Mice. Pharmaceuticals. 2024; 17(11):1413. https://doi.org/10.3390/ph17111413
Chicago/Turabian StyleTian, Miao-Miao, Gang Liu, Juan Du, Yue Liu, Wei Wei, Xiao-Bing Lan, Dong-Mei Hai, Lin Ma, Jian-Qiang Yu, and Ning Liu. 2024. "Gentiopicroside Attenuates Lithium/Pilocarpine-Induced Epilepsy Seizures by Down-Regulating NR2B/CaMKII/CREB and TLR4/NF-κB Signaling Pathways in the Hippocampus of Mice" Pharmaceuticals 17, no. 11: 1413. https://doi.org/10.3390/ph17111413
APA StyleTian, M.-M., Liu, G., Du, J., Liu, Y., Wei, W., Lan, X.-B., Hai, D.-M., Ma, L., Yu, J.-Q., & Liu, N. (2024). Gentiopicroside Attenuates Lithium/Pilocarpine-Induced Epilepsy Seizures by Down-Regulating NR2B/CaMKII/CREB and TLR4/NF-κB Signaling Pathways in the Hippocampus of Mice. Pharmaceuticals, 17(11), 1413. https://doi.org/10.3390/ph17111413





