Salvia miltiorrhiza and Its Compounds as Complementary Therapy for Dyslipidemia: A Meta-Analysis of Clinical Efficacy and In Silico Mechanistic Insights
Abstract
1. Introduction
2. Results
2.1. Results of Meta-Analysis
2.1.1. Description of Studies
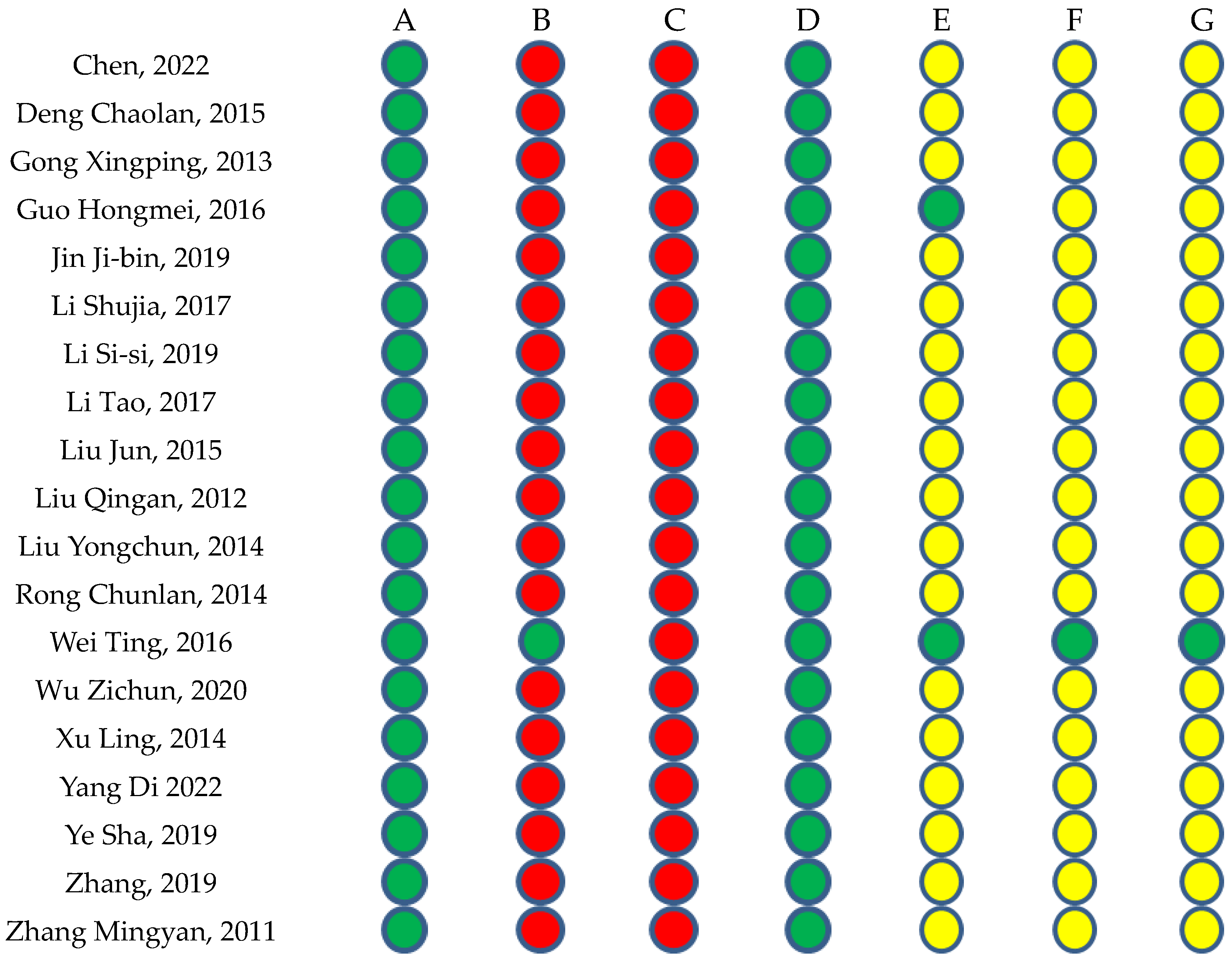
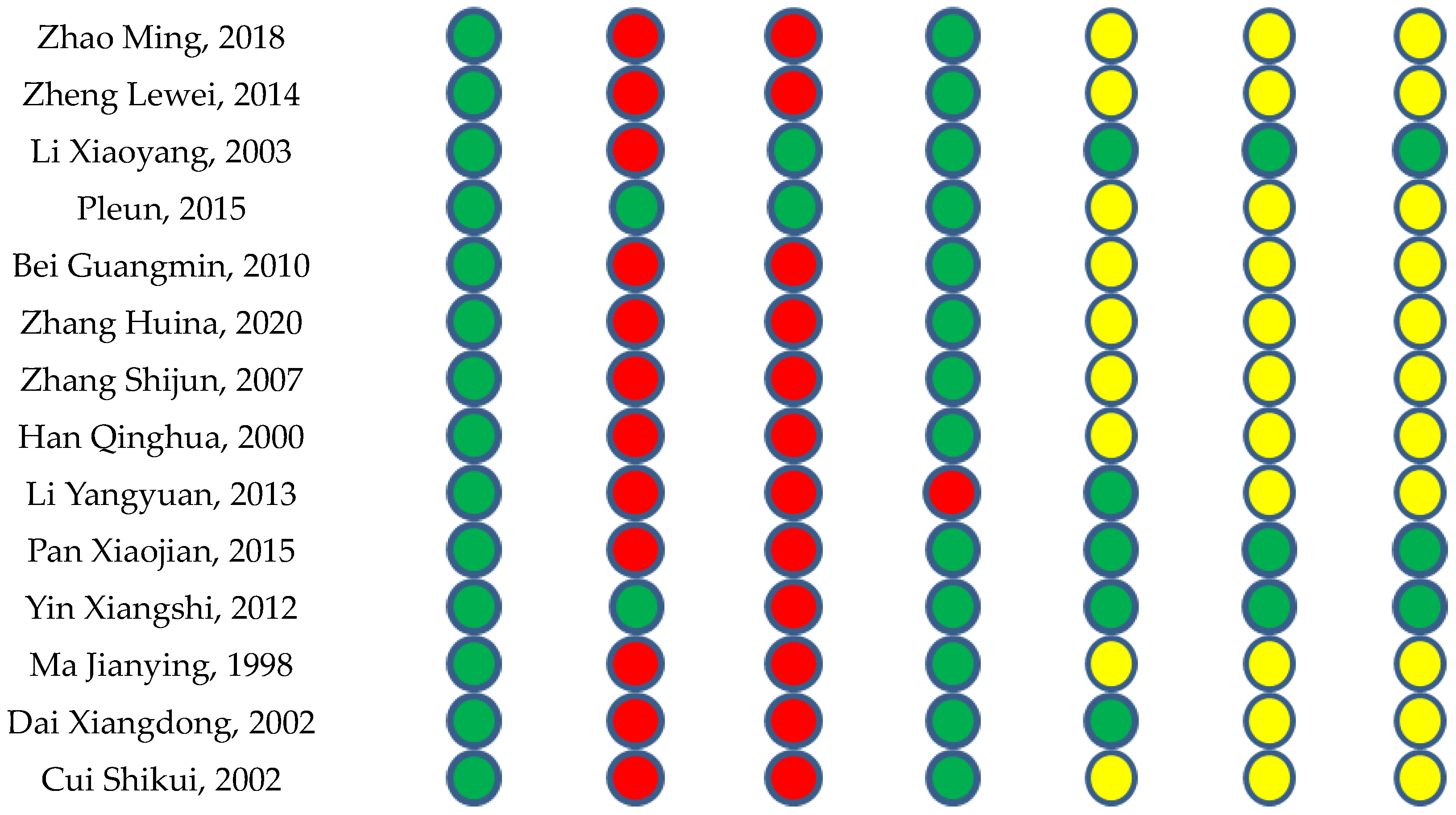
2.1.2. Risk of Bias in the Included Studies
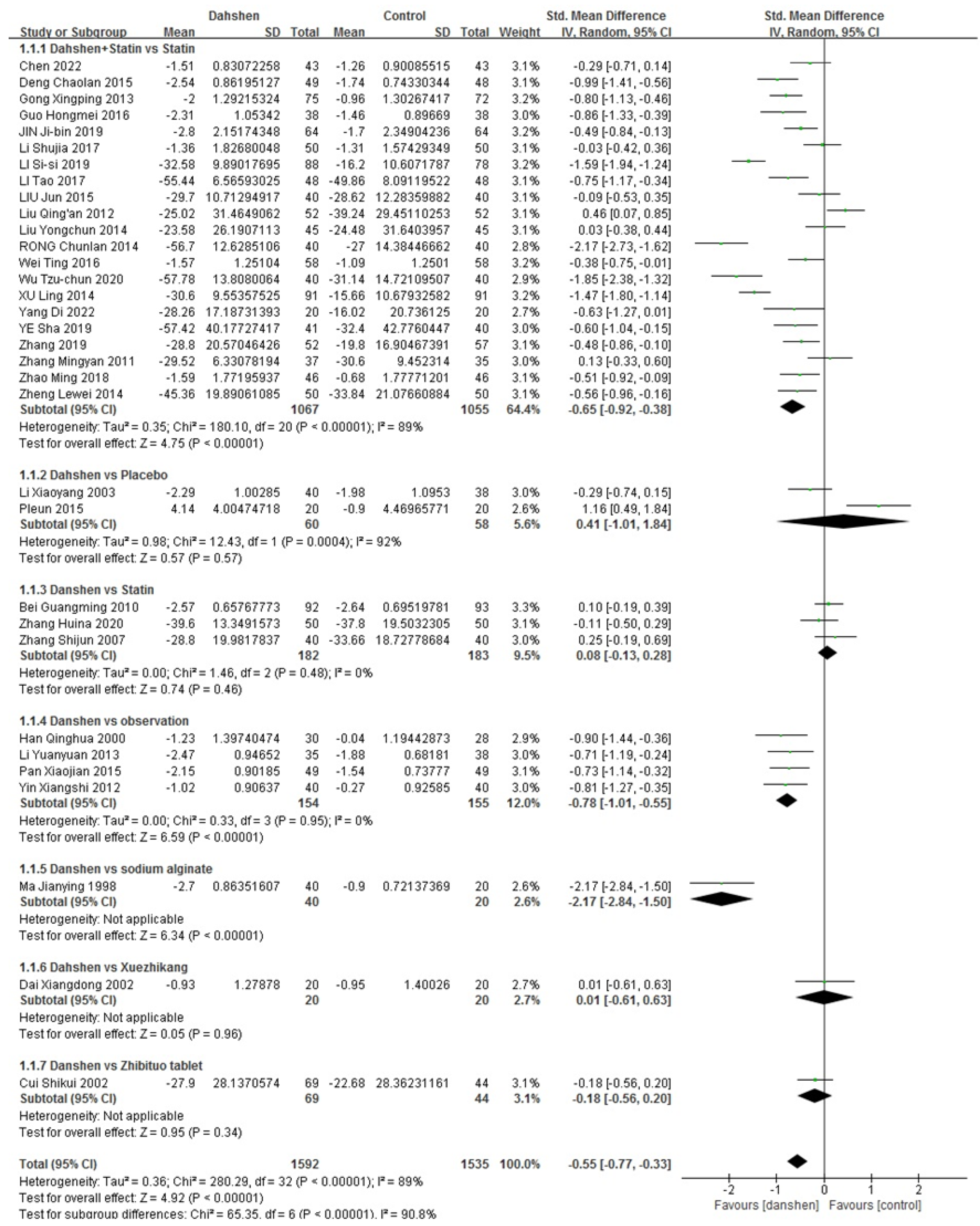
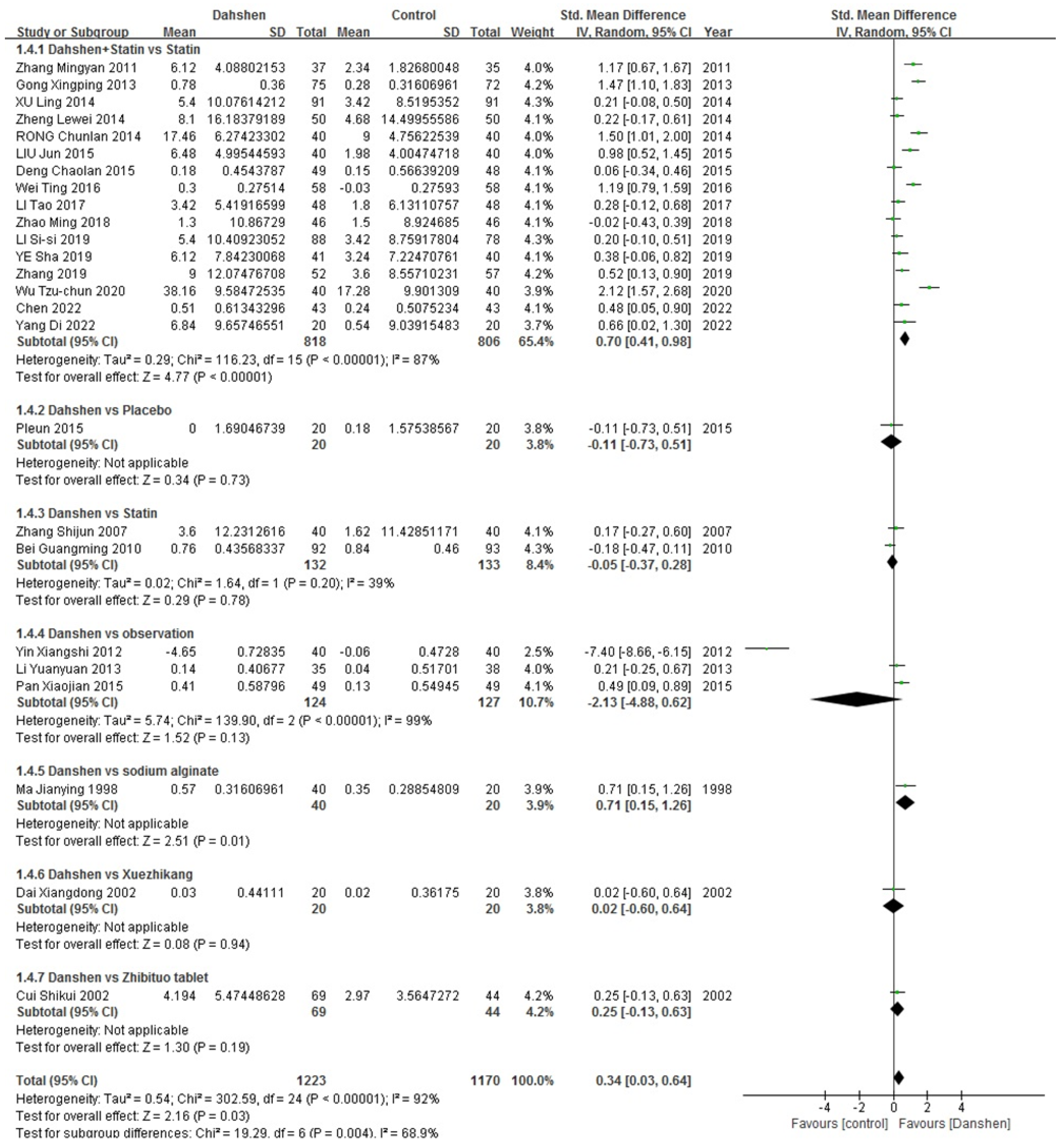
2.1.3. Effective Rate
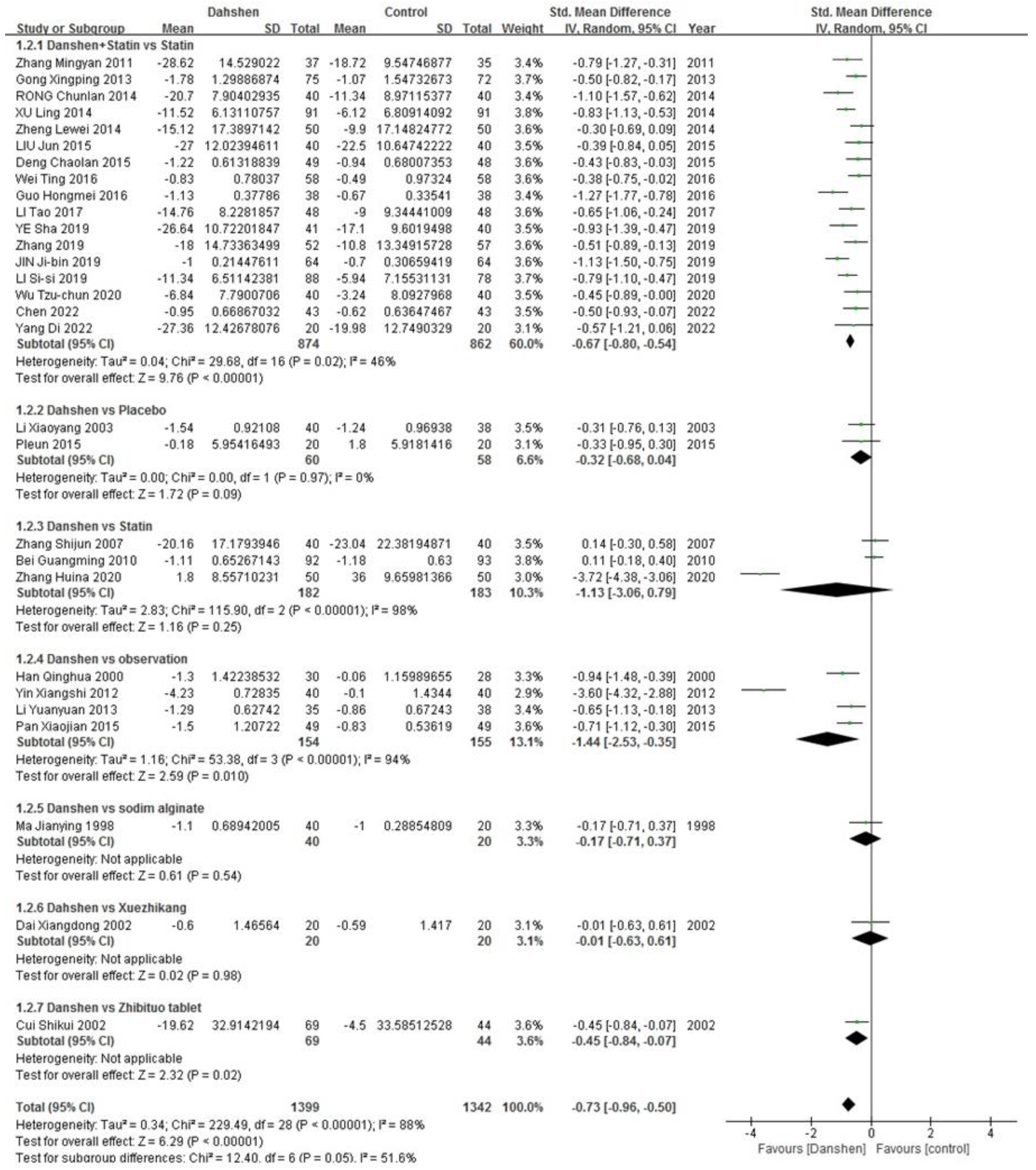
Total Cholesterol
Triglycerides
LDL-Cholesterols
HDL-Cholesterol
2.2. Results of In Silico Network Construction and Analysis
2.2.1. ADME of Active Compounds of Salvia miltiorrhiza
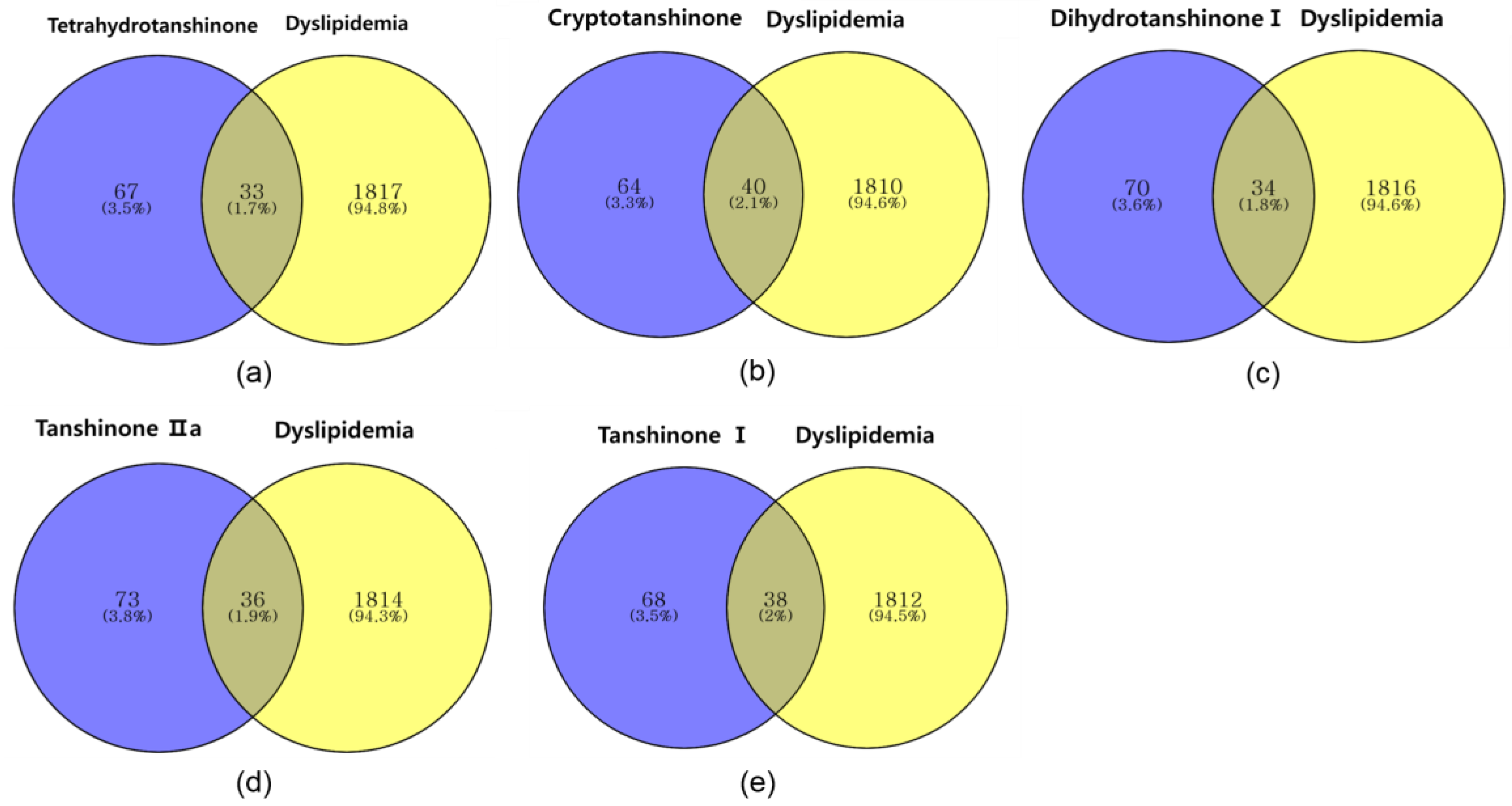
2.2.2. Identifying Overlapping Genes of Compounds and Dyslipidemia
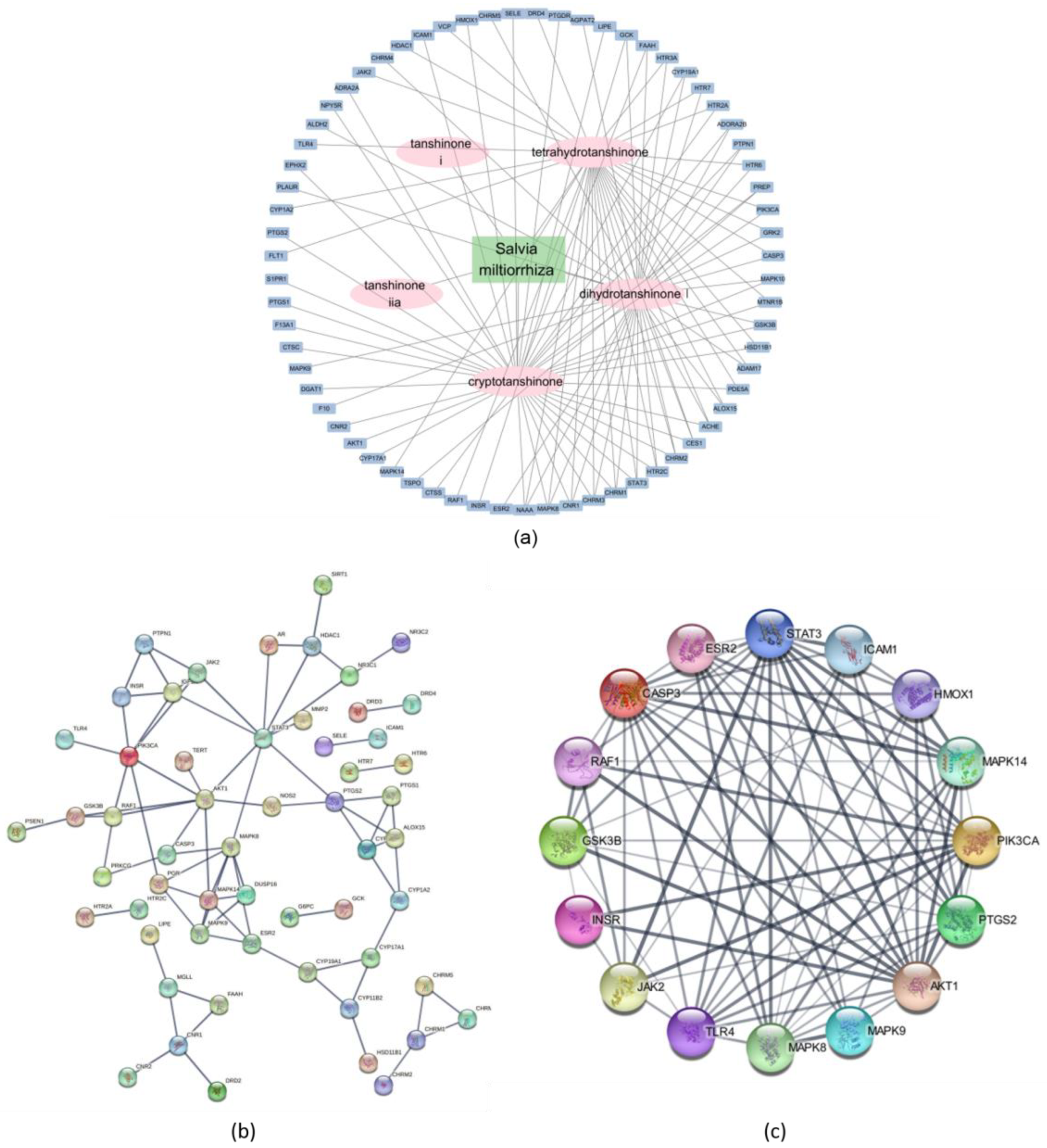
2.2.3. Network Description of Compounds and Dyslipidemia
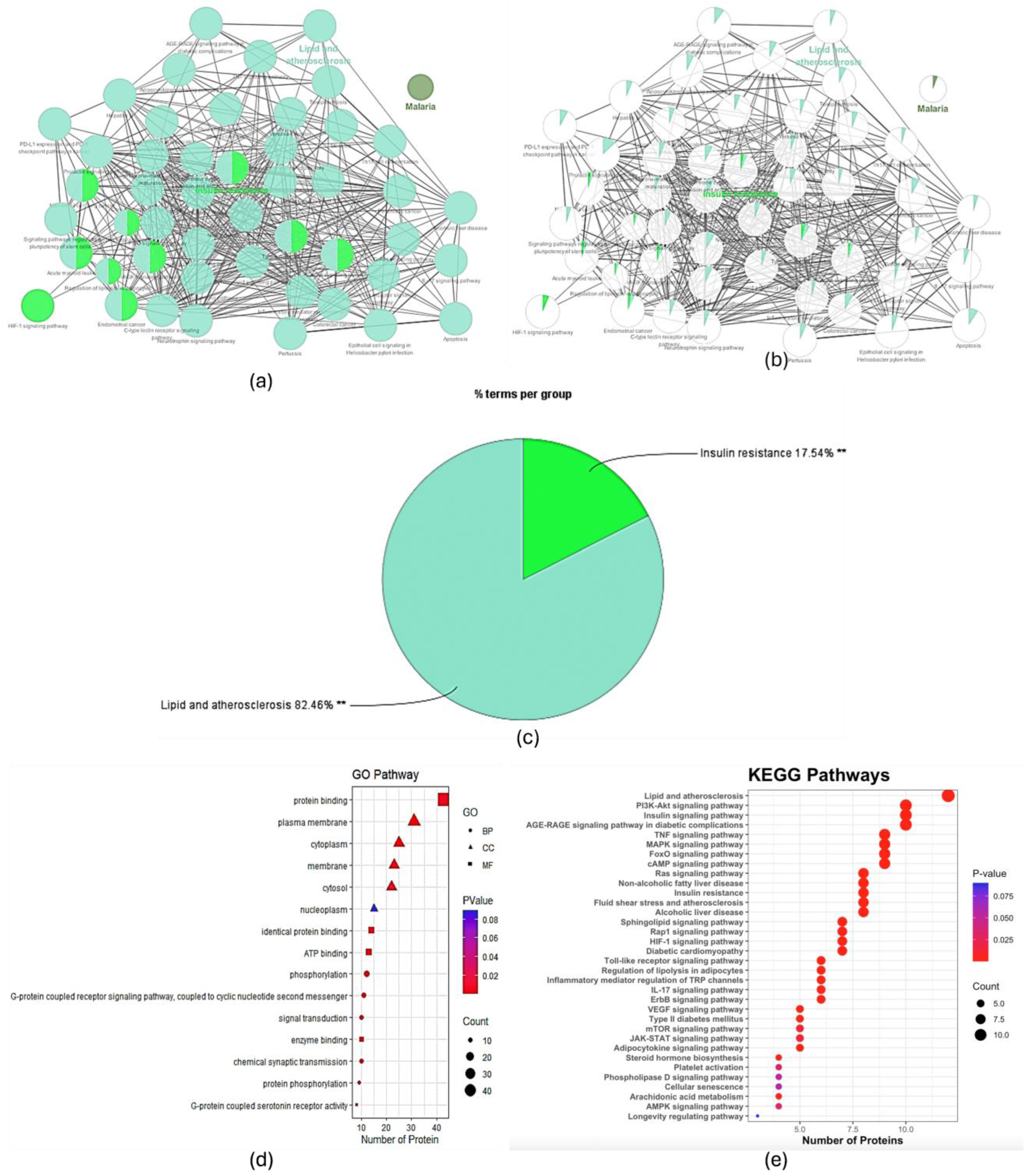
2.2.4. GO and KEGG Analyses
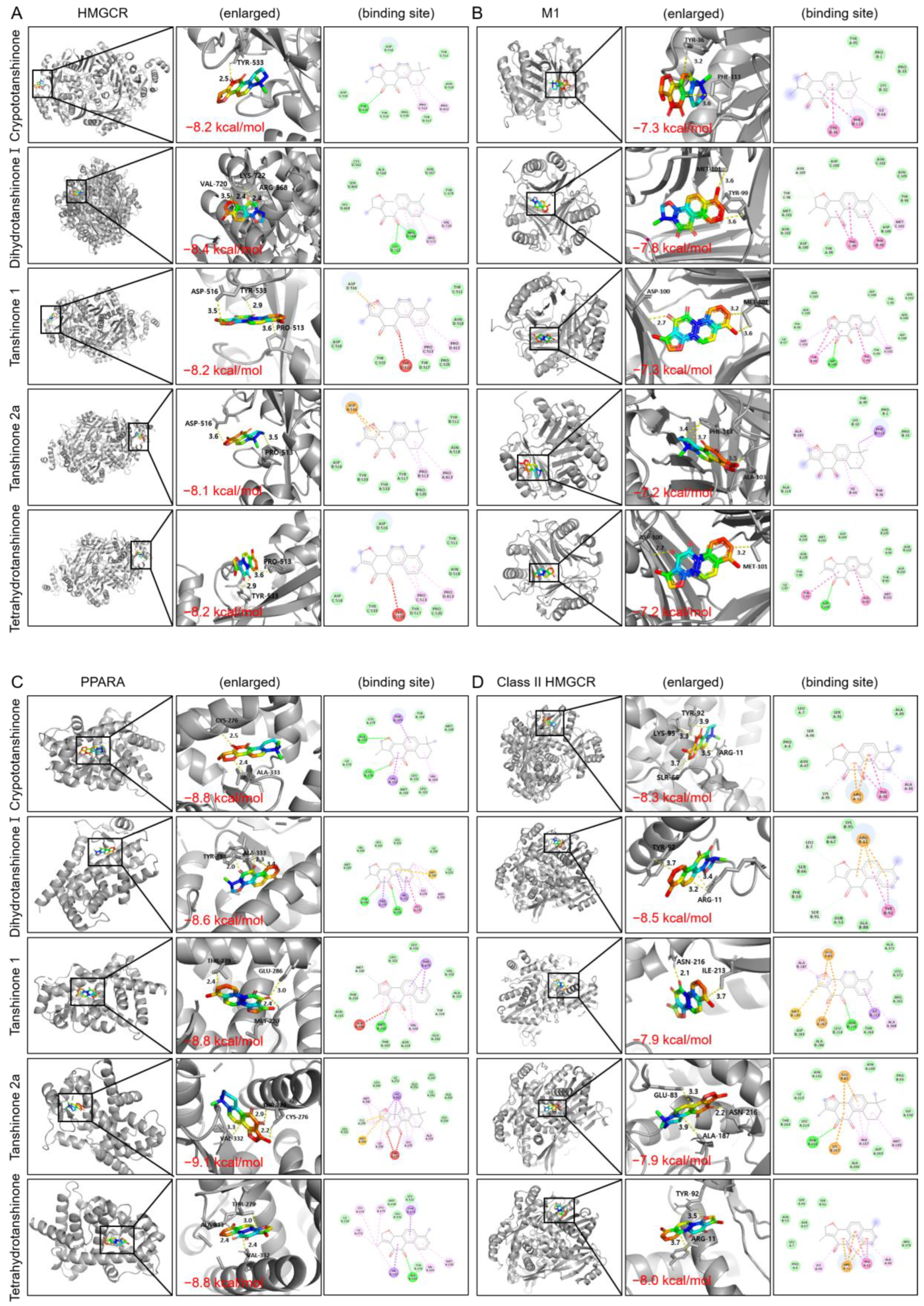
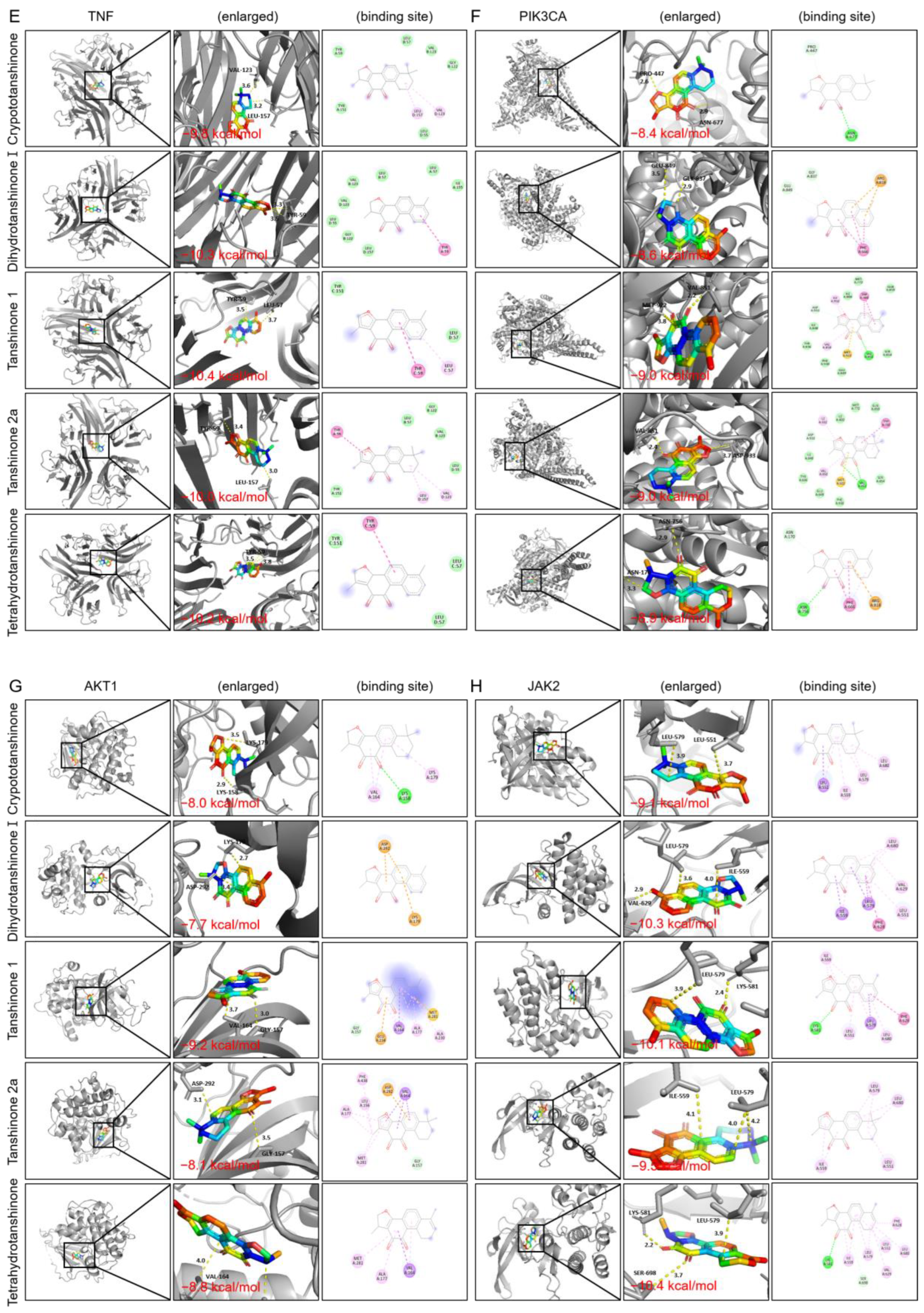
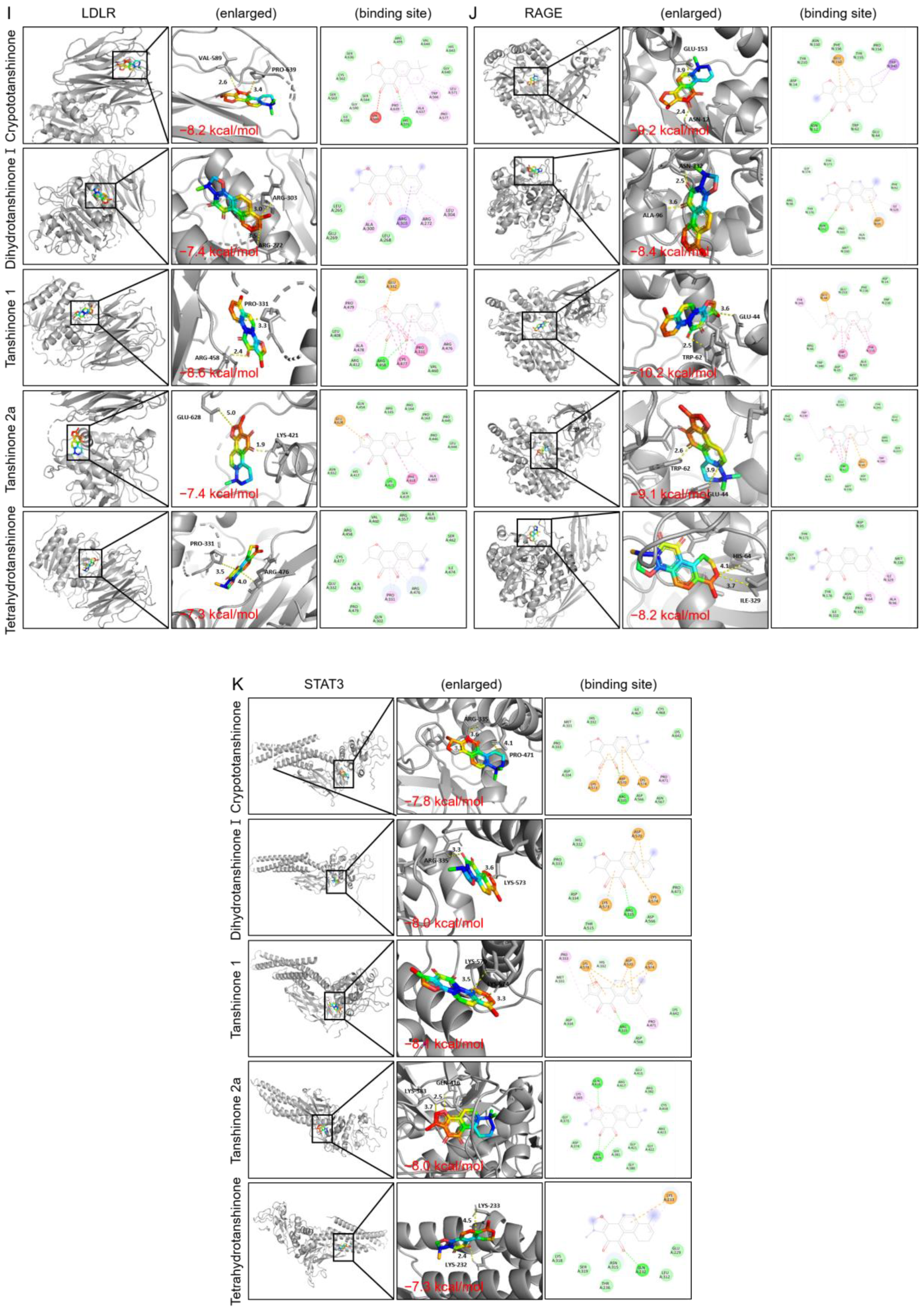
2.2.5. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Research Workflow Through Integrated Methodology
4.2. Data Sources and Search Strategy
4.3. Study Selection
4.3.1. Type of Studies
4.3.2. Type of Participants
4.3.3. Type of Interventions
4.3.4. Type of Outcome Measures
4.4. Methodological Quality Assessment
4.5. Quality of Evidence According to Outcome Measures
4.6. Statistical Analysis
4.7. Network Pharmacology Analysis of Anti-Dyslipidemia Mechanisms
4.8. Docking Interactions of Danshen Compounds and Target Proteins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Helkin, A.; Stein, J.J.; Lin, S.; Siddiqui, S.; Maier, K.G.; Gahtan, V. Dyslipidemia Part 1—Review of Lipid Metabolism and Vascular Cell Physiology. Vasc. Endovasc. Surg. 2016, 50, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.F. Lipid Oxidation Products on Inflammation-Mediated Hypertension and Atherosclerosis: A Mini Review. Front. Nutr. 2021, 8, 717740. [Google Scholar] [CrossRef]
- Du, Z.; Qin, Y. Dyslipidemia and Cardiovascular Disease: Current Knowledge, Existing Challenges, and New Opportunities for Management Strategies. J. Clin. Med. 2023, 12, 363. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef]
- Vinci, P.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Mearelli, F.; Biasinutto, C.; Fiotti, N.; Di Girolamo, F.G.; Biolo, G. Statin-Associated Myopathy: Emphasis on Mechanisms and Targeted Therapy. Int. J. Mol. Sci. 2021, 22, 11687. [Google Scholar] [CrossRef]
- Krahenbuhl, S.; Pavik-Mezzour, I.; von Eckardstein, A. Unmet Needs in LDL-C Lowering: When Statins Won’t Do! Drugs 2016, 76, 1175–1190. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, L.; Su, J.; Li, S.; Duncan, S.E.; Liu, Z.; Fan, G. Pharmacological Activity and Mechanism of Tanshinone IIA in Related Diseases. Drug Des. Devel Ther. 2020, 14, 4735–4748. [Google Scholar] [CrossRef]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G. Salvia miltiorrhiza in Treating Cardiovascular Diseases: A Review on Its Pharmacological and Clinical Applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Huang, C.Y.; Lee, S.D.; Maurya, N.; Kiefer, R.; Kumar, V.B. Review of Danshen: From its metabolism to possible mechanisms of its biological activities. J. Funct. Foods 2021, 85, 104613. [Google Scholar] [CrossRef]
- Chao, C. Clinical Efficacy of Compound Danshen Dropping Pills Combined with Atorvastatin in Treatment of Coronary Heart Disease Complicated with Hyperlipidemia and Its Effect on Serum TRAIL. Fujian J. Tradit. Chin. Med. 2022, 53, 7–9. [Google Scholar]
- Chaolan, D. Clinical Efficacy of Coronary Heart Disease Combined Hyperlipidemia CSDP Joint Alto-generation Statin Therapy. Mod. Diagn. Treat. 2015, 26, 1754–1755. [Google Scholar]
- Xingping, G. Clinical Study on Compound Danshen Dropping Pill Combined with Simvastatin Treatment of Hyperlipidemia. China J. Chin. Med. 2013, 28, 1575–1576. [Google Scholar]
- Hongmei, G. Clinical Observation on Compound Danshen Dropping Pills Combined with Simvastatin in the Treatment of Coronary Heart Disease Combined with Hyperlipidemia. J. North. Pharm. 2016, 13, 47. [Google Scholar]
- Ji-bin, J.; Jing-dong, X. Study the Clinical 64 Cases Efficacy of Treatment of Coronary Heartdisease with Hyperlipidemia Using Simvastatin with Compound Danshen Dripping Pills. Smart Healthc. 2019, 5, 106–108. [Google Scholar]
- Shujia, L. Clinical Effiect of Compound Danshen Dropping Pills Combined with Rosuvastatin or Rosuvastatin Only in the Treatment of Coronary Heart Disease Complicated with Hyperlipidemia. J. Math. Med. 2017, 30, 1663–1664. [Google Scholar]
- Si-si, L.; Yi, Z.; Min, Z. Clinical analysis of salvia miltiorrhiza combined with rosuvastatin calcium in the treatment of coronary heart disease complicated with hyperlipidemia. J. North. Sichuan Med. Coll. 2019, 34, 448–451. [Google Scholar]
- Tao, L. A comparative study of combination therapy with Fufang Danshen drop pill and simvastatin and simvastatin alone oncoronary artery disease with hyperlipidemia. J. Chin. Res. Hosp. 2017, 4, 20–23. [Google Scholar]
- Jun, L.; Zhen, W.; Lingyue, K. Clinical assessment on treatment of hyperlipidemia by compositie salviae dropping pill combined with Simvastatin. Hebei J. Tradit. Chin. Med. 2015, 37, 93–94, 158. [Google Scholar]
- Qingan, L. Clinical analysis of 102 cases of coronary heart disease complicated with hyperlipidemia treated with Simvastatin (Shujiangzhi) combined with Compound Danshen Dropping Pills. J. Med. Theory Pract. 2012, 25, 1583–1584. [Google Scholar]
- Yongchun, L. Analysis of the clinical efficacy of Compound Danshen Dropping Pills combined with rosuvastatin calcium in the treatment of coronary heart disease complicated with hyperlipidemia. China Pract. Med. 2014, 9, 183–184. [Google Scholar]
- Chunlan, R. The clinical efficacy of compound Danshen dropping pill treatment on 80 cases with angina pectoris complicated with hyperlipidemia. China Med. Pharm. 2014, 4, 86–87, 97. [Google Scholar]
- Ting, W.; Jia, Z.; Jiang, L.; Kun, F.; Ye, S. Effect of simvastatin combined with Compound Danshen Dropping Pills on blood lipids and lipid ratio in patients with hyperlipidemia. Shaanxi Med. J. 2016, 45, 1228–1230. [Google Scholar]
- Zichun, W. Clinical efficacy of Compound Danshen Dropping Pills combined with rosuvastatin calcium in the treatment of coronary heart disease combined with hyperlipidemia. Chin. J. Clin. Ration. Drug Use 2020, 13, 32–33. [Google Scholar]
- Ling, X. Curative effect of Fufang Danshen Gutta Pills combining rosuvastatin on coronary heart diseasecomplicating hyperlipidemia. Chin. J. Evid. Based Cardiovasc. Med. 2014, 6, 99–101. [Google Scholar]
- Di, Y. Study on the improvement effect of Compound Danshen Dropping Pills combined with atorvastatin on vascular endothelial function and lipid metabolism disorders in patients with coronary heart disease and hyperlipidemia. Jilin Med. J. 2022, 43, 1266–1268. [Google Scholar]
- Sha, Y.; Ai-ping, J.; Jie, G.; Cui-ling, Y.; Qian-rong, Z. Effects of Compound Danshen Dripping Pills and Trimetazidine on the Hemorheology and Blood Lipid of Patients with Coronary Heart Disease Complicated with Hyperlipidemia. Progress. Mod. Biomed. 2019, 19, 868–871. [Google Scholar]
- Hai-long, Z.; Bao-cheng, Z.; Yu-peng, L. Therapeutic effect of compound Danshen dripping pills combined atorvastatin on coronary heart disease complicated hyperlipidemia and its influence on serum TRAIL level. Chin. J. Cardiovasc. Rehabil. Med. 2019, 28, 360–364. [Google Scholar]
- Mingyan, Z.; Xiaogeng, Y.; Fanchao, M. Clinical Observation on Lovastatin Combined with Compound Danshen Dropping Pills in the Treatment of Hyperlipidemia. Chin. J. Pract. Nerv. Dis. 2011, 14, 75–76. [Google Scholar]
- Ming, Z. Clinical efficacy of Compound Danshen Dropping Pills combined with rosuvastatin in the treatment of patients with coronary heart disease and hyperlipidemia. World Latest Med. Inf. 2018, 18, 147–148. [Google Scholar]
- Lewei, Z. Curative Effect Observation of Atorvastatin and Fufang Danshen Diwan in the Treatment of Old-age and High Fat Blood Disease. Asia-Pac. Tradit. Med. 2014, 10, 102–103. [Google Scholar]
- Xiaoyang, L.; Xiaohua, G. Clinical analysis of 25 cases of hyperlipidemia treated with Compound Danshen Dropping Pills. J. Emerg. Tradit. Chin. Med. 2003, 12, 338. [Google Scholar]
- van Poppel, P.C.; Breedveld, P.; Abbink, E.J.; Roelofs, H.; van Heerde, W.; Smits, P.; Lin, W.; Tan, A.H.; Russel, F.G.; Donders, R. Salvia miltiorrhiza root water-extract (danshen) has no beneficial effect on cardiovascular risk factors. A randomized double-blind cross-over trial. PLoS ONE 2015, 10, e0128695. [Google Scholar] [CrossRef] [PubMed]
- Guangming, B.; Yuanlin, L.; Hongru, Z.; Shixiang, H.; Jie, H.; Yuhai, M.; Daohua, G.; Guíe, C. Observation on the long-term efficacy of Salvia miltiorrhiza on urinary microalbumin in patients with refractory hypertension combined with hyperlipidemia. Chin. J. Exp. Tradit. Med. Formulae 2010, 16, 244–245, 249. [Google Scholar]
- Huina, Z. Evaluation of the clinical effect of Compound Danshen Dropping Pills in the treatment of coronary heart disease and angina pectoris combined with hyperlipidemia and its effect on blood lipid levels. Cardiovasc. Dis. Electron. J. Integr. Tradit. Chin. West. Med. 2020, 8, 86, 89. [Google Scholar]
- Shi-jun, Z.; Ze-xiong, C.; You-wu, L.; Jian, Q.; Yong-hua, C.; Su-ling, L. Effection of compositie salviae dropping pill on hyperlipemia patients with phlegm and blood stasis syndrome. China J. Chin. Mater. Medica 2007, 32, 440–443. [Google Scholar]
- Qinghua, H.; Xiaohong, C.; Jiyuan, L. Effects of compound Danshen Dropping Pills on lipoprotein(a) and blood lipids in patients with coronary heart disease and hyperlipidemia. Chin. J. Integr. Tradit. West. Med. 2000, 20, 514. [Google Scholar]
- Yuanyuan, L.; Meng, Y. Observation on the efficacy of Compound Danshen Dropping Pills combined with atorvastatin in the treatment of hyperlipidemia. Guide China Med. 2013, 11, 517–518. [Google Scholar]
- Xiaojian, P. Clinical Observation on Compound Danshen Dropping Pills Combined with Atorvastatin Calcium Tablets in the Treatment of Elderly Patients with Hyperlipidemia. J. New Chin. Med. 2015, 47, 32–34. [Google Scholar]
- Xiangshi, Y.; Lihong, C. Observation on the efficacy of Compound Danshen Dropping Pills in the treatment of 80 cases of coronary heart disease, angina pectoris and hyperlipidemia. Chin. Community Dr. 2012, 14, 184–185. [Google Scholar]
- Jianying, M. Clinical Observation on the Treatment of Hyperlipidemia with Compound Danshen Dropping Pills. Gianjin J. Tradit. Chin. Med. 1998, 15, 24. [Google Scholar]
- Xiangdong, D.; Jingming, S. The efficacy of Compound Danshen Dropping Pills in the treatment of hyperlipidemia and its effect on platelet aggregation. Chin. J. Hemorheol. 2002, 12, 328–329. [Google Scholar]
- Shikui, C.; Guohong, L. 69 cases of hyperlipidemia treated with Compound Danshen Dropping Pills. Mod. J. Integr. Tradit. Chin. West. Med. 2002, 11, 1029–1030. [Google Scholar]
- Hu, P.; Liang, Q.L.; Luo, G.A.; Zhao, Z.Z.; Jiang, Z.H. Multi-component HPLC fingerprinting of Radix Salviae Miltiorrhizae and its LC-MS-MS identification. Chem. Pharm. Bull. 2005, 53, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Ridker, P.M.; Pradhan, A.; MacFadyen, J.G.; Libby, P.; Glynn, R.J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 2012, 380, 565–571. [Google Scholar] [CrossRef]
- Amarenco, P.; Kim, J.S.; Labreuche, J.; Charles, H.; Abtan, J.; Bejot, Y.; Cabrejo, L.; Cha, J.K.; Ducrocq, G.; Giroud, M.; et al. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N. Engl. J. Med. 2020, 382, 9. [Google Scholar] [CrossRef]
- Fox, K.M.; Tai, M.H.; Kostev, K.; Hatz, M.; Qian, Y.; Laufs, U. Treatment patterns and low-density lipoprotein cholesterol (LDL-C) goal attainment among patients receiving high- or moderate-intensity statins. Clin. Res. Cardiol. 2018, 107, 380–388. [Google Scholar] [CrossRef]
- Li, Z.M.; Xu, S.W.; Liu, P.Q. Salvia miltiorrhizaBurge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef]
- Shi, M.J.; Dong, B.S.; Yang, W.N.; Su, S.B.; Zhang, H. Preventive and therapeutic role of Tanshinone IIA in hepatology. Biomed. Pharmacother. 2019, 112, 108676. [Google Scholar] [CrossRef]
- Zhao, Y.; Qian, Y.; Sun, Z.; Shen, X.; Cai, Y.; Li, L.; Wang, Z. Role of PI3K in the Progression and Regression of Atherosclerosis. Front. Pharmacol. 2021, 12, 632378. [Google Scholar] [CrossRef]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Wang, X.; Wu, B.; Chen, K.; Deng, J. Modulation of the PI3K/Akt Pathway and Bcl-2 Family Proteins Involved in Chicken’s Tubular Apoptosis Induced by Nickel Chloride (NiCl2). Int. J. Mol. Sci. 2015, 16, 22989–23011. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Guh, J.Y.; Chen, H.C.; Hung, W.C.; Lai, Y.H.; Chuang, L.Y. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J. Cell Biochem. 2001, 81, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Yin, G.; Liang, P.; Feng, Y.; Yu, W.; Meng, D.; Liu, H.; Zhang, F. The Role of JAK/STAT Signaling Pathway and Its Downstream Influencing Factors in the Treatment of Atherosclerosis. J. Cardiovasc. Pharmacol. Ther. 2024, 29, 10742484241248046. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Receptor for AGE (RAGE): Signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci. 2011, 1243, 88–102. [Google Scholar] [CrossRef]
- El-Far, A.H.; Sroga, G.; Jaouni, S.K.A.; Mousa, S.A. Role and Mechanisms of RAGE-Ligand Complexes and RAGE-Inhibitors in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 3613. [Google Scholar] [CrossRef]
- Baldini, C.; Moriconi, F.R.; Galimberti, S.; Libby, P.; De Caterina, R. The JAK-STAT pathway: An emerging target for cardiovascular disease in rheumatoid arthritis and myeloproliferative neoplasms. Eur. Heart J. 2021, 42, 4389–4400. [Google Scholar] [CrossRef]
- Feingold, K.R. Lipid and Lipoprotein Metabolism. Endocrinol. Metab. Clin. N. Am. 2022, 51, 437–458. [Google Scholar] [CrossRef]
- Lennernas, H.; Fager, G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Clin. Pharmacokinet. 1997, 32, 403–425. [Google Scholar] [CrossRef]
- Tabernero, L.; Rodwell, V.W.; Stauffacher, C.V. Crystal structure of a statin bound to a class II hydroxymethylglutaryl-CoA reductase. J. Biol. Chem. 2003, 278, 19933–19938. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, W.; Hargrove, J.L.; Greenspan, P.; Dean, R.G.; Taylor, E.W.; Hartle, D.K. Inhibition of TNF-alpha induced ICAM-1, VCAM-1 and E-selectin expression by selenium. Atherosclerosis 2002, 161, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Garlanda, C.; Locati, M. Macrophage diversity and polarization in atherosclerosis: A question of balance. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- van Raalte, D.H.; Li, M.; Pritchard, P.H.; Wasan, K.M. Peroxisome proliferator-activated receptor (PPAR)-alpha: A pharmacological target with a promising future. Pharm. Res. 2004, 21, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Chandran, U.; Mehendale, N.; Patil, S.; Chaguturu, R.; Patwardhan, B. Innovative Approaches in Drug Discovery. Ethnopharmacology, Systems Biology and Holistic Targeting Network Pharmacology, 1st ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 127–164. [Google Scholar] [CrossRef]


| First Author | Participants | Number of Cases/Age, Years | Intervention Methods | Observation Period | Outcome Indicators | |||
|---|---|---|---|---|---|---|---|---|
| Trial Group | Control Group | Trial Group | Control Group | |||||
| Chen, 2022 [10] | Patients aged 51–83 years | 63.97 ± 5.48 | 64.38 ± 5.79 | Standard treatment (anticoagulants, antiplatelet aggregation agents, blood sugar-lowering agents, antihypertensive drugs) with Danshen dripping tablets 10 T tid | Standard treatment (anticoagulants, antiplatelet aggregation agents, blood sugar-lowering agents, antihypertensive drugs) with Atorvastatin 20 mg qd | 12 weeks | Blood lipid levels via TC, TG, HDL-C, LDL-C | |
| Deng Chaolan, 2015 [11] | Patients aged 41–80 years | 49/61.58 ± 7.30 | 48/61.16 ± 7.15 | Compound Danshen soft capsules 27 mg tid | Atorvastatin 10 mg qd | 12 weeks | Blood lipid levels via TC, TG, HDL-C, LDL-C | |
| Gong Xingping, 2013 [12] | Patients aged 34–76 years | 77/ 45.7 ± 8.2 | 73/ 46.2 ± 8.6 | Simvastatin 20 mg qd with compound Danshen dripping tablet 10 T tid | Simvastatin 20 mg qd | 12 weeks | Blood lipid levels via TC, TG, HDL-C, LDL-C | |
| Guo Hongmei, 2016 [13] | 76 patients | 38/57.4 ± 5.2 | 38/56.1 ± 4.8 | Simvastatin 10 mg qd with compound Danshen dripping tablets 10 T tid | Simvastatin 10 mg qd | 12 weeks | Blood lipid levels via TC, TG, | |
| Jin Ji-bin, 2019 [14] | Patients aged 30–65 years | 64/45.8 ± 8.5 | 64/46.7 ± 7.9 | Simvastatin 5 mg qd with compound Danshen dripping tablets 10 T tid | Simvastatin 5 mg qd | 12 weeks | Blood lipid levels via TG, TC, LDL-C | |
| Li Shujia, 2017 [15] | Patients aged 45–85 years | 50/62.5 ± 5.1 | 50/61.9 ± 3.6 | Rosuvastatin 10 mg/20 mg/40 mg qd with compound Danshen dripping tablets 10 T tid | Rosuvastatin 10 mg/20 mg/40 mg qd | 8 weeks | Blood lipid levels via TG, TC, LDL-C | |
| Li Si-si, 2019 [16] | Patients average age of 56.4 ± 7.9 years | not mentioned | not mentioned | Baseline medications (enteric-coated aspirin 100 mg qd and isosorbide mononitrate 40 mg qd) with compound Danshen dripping tablets 270 mg tid | Baseline medications (enteric-coated aspirin 100 mg qd and isosorbide mononitrate 40 mg qd) with Rosuvastatin 10 mg qd | 24 weeks | Blood lipid levels via TG, LDL, HDL, TC | |
| Li Tao, 2017 [17] | Patients aged 42–81 years | 48/59.2 ± 6.1 | 48/60.4 ± 5.4 | Capsules containing compound Danshen dripping capsules 10 C tid | Simvastatin 20 mg/day | 12 weeks | Blood lipid levels via TC, TG, HDL-C, LDL-C | |
| Liu Jun, 2015 [18] | 80 patients | 40/54.8 ± 11.6 | 40/53.8 ± 9.4 | Simvastatin 20 mg qd with compound Danshen dripping pill 10 C tid | Simvastatin 20 mg qd | 8 weeks | Blood lipid levels via TG, TC, LDL-C | |
| Liu Qingan, 2012 [19] | 102 patients | 52/average age 51.7 | 50/average age 59.8 | Simvastatin 10 mg qd with complex Danshen preparation 25 mg tid | Simvastatin 10 mg qd | 20 weeks | Blood lipid levels via TG, LDL-C | |
| Liu Yongchun, 2014 [20] | 90 patients | 45/65.4 ± 5.1 | 45/64.8 ± 3.7 | Rosuvastatin calcium 10 mg qd with compound Danshen dripping pill 270 mg tid | Rosuvastatin 10 mg qd | 12 weeks | Blood lipid levels via TG, TC, LDL-C | |
| Rong Chunlan, 2014 [21] | Patients aged 45–77 years | 40/57.33 ± 3.29 | 40/57.33 ± 3.29 | Standard treatment with compound Danshen dripping capsule 10 C tid | Standard treatment (β-blockers, calcium channel blockers, antiplatelet agents, statins) | 4 weeks | Blood lipid levels via TC, TG, HDL-C, LDL-C | |
| Wei Ting, 2016 [22] | Patients aged 45–86 years | 58/62.8 ± 11.5 | 58/64.7 ± 12.8 | Simvastatin 10 mg qd with compound Danshen dripping capsules 10 C tid | Simvastatin 10 mg qd | 12 weeks | Blood lipid levels via TG, TC, LDL-C | |
| Wu Zichun, 2020 [23] | Patients aged 52–81 years | 65.42 ± 2.44 | 66.42 ± 3.21 years | Conventional treatment with Danshen dripping capsules 10 C tid | Conventional treatment with Rosuvastatin Calcium 10 mg qd | 8 weeks | Blood lipid levels via TC, TG, HDL-C, LDL-C | |
| Xu Ling, 2014 [24] | 182 patients | 91 (randomized) | 91 (randomized) | Rosuvastatin calcium 10 mg qd with compound Danshen dripping capsule 10 C tid | Rosuvastatin 10 mg qd | 8 weeks | Blood lipid levels via TC, TG, HDL-C, LDL-C | |
| Yang Di 2022 [25] | Patients aged 36–82 years | 63. 18 ± 4. 10 | 62. 38 ± 4. 28 | Danshen dripping tablets 270 mg tid | Atorvastatin 20 mg qd | 12 weeks | Blood lipid levels via TC, TG, HDL-C, LDL-C | |
| Ye Sha, 2019 [26] | Patients aged <75 years | 41/59.2 ± 6.1 | 40/60.4 ± 5.4 | Compound Danshen dripping capsules 10 C tid | Trimetazidine 20 mg tid | 12 weeks | Blood lipid levels via TC, TG, HDL-C, LDL-C | |
| Zhang, 2019 [27] | Patients aged 52–89 years | 65.8 ± 9.7 | 67.5 ± 7.6 | Danshen dripping pills 27 mg bid | Atorvastatin 10 mg qd | 12 weeks | Blood lipid levels via TG, TC, LDL-C | |
| Zhang Mingyan, 2011 [28] | Patients aged 31–69 | 37/56 ± 11.45 | 35/55 ± 10.78 | Lovastatin 20 mg qd with compound Salvia miltiorrhiza dripping tablets 10 T tid | Lovastatin 20 mg qd | 8 weeks | Blood lipid levels via TG, TC, LDL-C, HDL-C | |
| Zhao Ming, 2018 [29] | Patients aged 46–83 years | 46/61.82 ± 4.90 | 46/61.95 ± 4.92 | Rosuvastatin 10–40 mg qd with compound Danshen driping tablets 10 T tid | Rosuvastatin 10–40 mg qd | 8 weeks | Blood lipid levels via TG, LDL-C, HDL-C | |
| Zheng Lewei, 2014 [30] | 100 patients aged 60~86(72 ± 2.6) | 50 (randomized) | 50 (randomized) | Atorvastatin calcium 10 mg qd with compound Danshen dripping capsules 10 C tid | Atorvastatin Calcium 20 mg qd | 8 weeks | TC, TG, HDL-C | |
| Li Xiaoyang, 2003 [31] | Patients average age under 60 | 20 | 20 | Complex Salvia ginseng preparation 30 C tid | Inositol nicotinate 0.6~1.2 g tid | 8 weeks | Blood lipid levels via TG, TC, | |
| Pleun, 2015 [32] | Patients aged 40–70 years | Randomized double-blind placebo-controlled crossover study n= 20 | Danshen capsules 500 mg (4 capsules) tid | Placebo capsules 500 mg (4 capsules) tid | 4 weeks | Blood lipid levels via TG, TC, LDL-C, HDL-C | ||
| Bei Guangmin, 2010 [33] | Patients aged 42–65 | 92/52.9 ± 7 | 93/54.3 ± 6.4 | Candesartan ester capsules 8 mg qd, Hydrochlorothiazide 10 mg qd, long-acting nifedipine 10 mg bid with Danshen tablets 4 T tid | Candesartan ester capsules 8 mg qd, Hydrochlorothiazide 10 mg qd, Long-acting nifedipine 10 mg bid, Atorvastatin 10 mg qd | 24 weeks | Blood lipid levels via TG, TC, LDL-C, HDL-C | |
| Zhang Huina, 2020 [34] | No information | 63.74 ± 3.28 | 63.52 ± 3.79 | Danshen dripping capsules 10 mg tid | Conventional symptomatic treatment (Betaloc (metoprolol), aspirin, lifestyle adjustment, low-sodium, low-fat diet, metformin, nifedipine, simvastatin 10 mg qd) | 4 weeks | Blood lipid levels via TC, TG, HDL-C, LDL-C | |
| Zhang Shijun, 2007 [35] | Patients aged 26–63 | 40/38 ± 10.2 | 41/39 ± 11.8 | Composite Salviae dropping pills 10 C tid | Simvastatin 20 mg qd | 12 weeks | Blood lipid levels via TG, TC, LDL-C, HDL-C | |
| Han Qinghua, 2000 [36] | Patients aged 50–70 | 30/64.2 ± 0.3 | 28/63.8 ± 0.3 | Compound Danshen dropping capsules 10 C tid | No treatment | 4 weeks | Blood lipid levels via TG, TC, LDL-C | |
| Li Yangyuan, 2013 [37] | 73 patients | 35/60.7 ± 13.6 | 38/59.2 ± 11.4 | Atorvastatin 10 mg qd with compound Danshen dripping capsules 10 C tid | Atorvastatin 10 mg qd | 4 weeks | Blood lipid levels via TG, TC, LDL-C, HDL-C | |
| Pan Xiaojian, 2015 [38] | 98 patients | 49/67.3 ± 3.2 | 49/67.1 ± 4.2 | Atorvastatin 10 mg qd with compound Danshen tablets 10 T tid | Atorvastatin 10 mg qd | 20 weeks | Blood lipid levels via TG, TC, LDL-C, HDL-C | |
| Yin Xiangshi, 2012 [39] | 80 patients aged 58–75 | 40 | 40 | Standard treatment (aspirin, β-blockers, calcium channel blockers, nitrates with compound Danshen preparation 10 T tid | Standard treatment (aspirin, β-blockers, calcium channel blockers, nitrates) | 4 weeks | Blood lipid levels via TG, TC, LDL-C, HDL-C | |
| Ma Jianying, 1998 [40] | Patients aged 40–45 years | 40 | 20 | Complex Salvia ginseng preparation 10 T tid | Sodium alginate double ester capsule 50 mg tid | 8 weeks | Blood lipid levels via TG, TC, LDL-C, HDL-C | |
| Dai Xiangdong, 2002 [41] | No information | 20/56.3 ± 8.5 | 20/58.7 ± 7.5 | Danshen capsules 10 C tid | Xuezhikang 0.6 g bid | 4 weeks | Blood lipid levels via TG, TC, LDL-C, HDL-C | |
| Cui Shikui, 2002 [42] | Patients aged 36–79 | 69/58 | 44/51 | Compound Danshen preparation 10 T tid | Zhibitu capsule 1 C bid | 4 weeks | Blood lipid levels via TG, TC, LDL-C | |
| Std. Mean Difference | Heterogeneity | |
|---|---|---|
| Danshen + Statin vs. Statin (n = 21) | −0.65 [−0.92, −0.38] | p < 0.00001, I2 = 89% |
| Danshen vs. Placebo (n = 2) | 0.41 [−1.01, 1.84] | p = 0.0004, I2 = 92% |
| Danshen vs. Statin (n = 3) | 0.08 [−0.13, 0.28] | p = 0.48, I2 = 0% |
| Danshen vs. Observation (n = 4) | −0.78 [−1.01, −0.55] | p = 0.95, I2 = 0% |
| Danshen vs. Sodium Alginate (n = 1)/ Xuezhikang (n = 1)/ Zhibituo Tablet (n = 1) | ||
| Total | −0.55 [−0.77, −0.33] | p < 0.00001, I2 = 89% |
| Std. Mean Difference | Heterogeneity | |
|---|---|---|
| Danshen + Statin vs. Statin (n = 17) | −0.67 [−0.80, −0.54] | p = 0.02, I2 = 46% |
| Danshen vs. Placebo (n = 2) | −0.32 [−0.68, 0.04] | p = 0.97, I2 = 0% |
| Danshen vs. Statin (n = 3) | −1.13 [−3.06, 0.79] | p < 0.00001, I2 = 98% |
| Danshen vs. Observation (n = 4) | −1.44 [−2.53, −0.35] | p < 0.00001, I2 = 94% |
| Danshen vs. Sodium Alginate (n = 1)/ Xuezhikang (n = 1)/Zhibituo Tablet (n = 1) | ||
| Total | −0.73 [−0.96, −0.50] | p < 0.00001, I2 = 88% |
| Std. Mean Difference | Heterogeneity | |
|---|---|---|
| Danshen + Statin vs. Statin (n = 19) | −0.56 [−0.78, −0.34] | p < 0.00001, I2 = 82% |
| Danshen vs. Statin (n = 2) | 0.18 [−0.07, 0.43] | p = 0.31, I2 = 5% |
| Danshen vs. Observation (n = 4) | −1.39 [−2.51, −0.28] | p < 0.00001, I2 = 95% |
| Danshen vs. Sodium Alginate (n = 1)/ Xuezhikang (n = 1)/Placebo (n = 1) | ||
| Total | −0.58 [−0.83, −0.33] | p < 0.00001, I2 = 89% |
| Std. Mean Difference | Heterogeneity | |
|---|---|---|
| Danshen + Statin vs. Statin (n = 16) | 0.70 [0.41, 0.98] | p < 0.00001, I2 = 87% |
| Danshen vs. Statin (n = 2) | −0.05 [−0.37, 0.28] | p = 0.20, I2 = 39% |
| Danshen vs. Observation (n = 3) | −2.13 [−4.88, 0.62] | p < 0.00001, I2 = 99% |
| Danshen vs. Sodium Alginate (n = 1)/ Xuezhikang (n = 1)/ Zhibituo Tablet (n = 1)/Placebo (n = 1) | ||
| Total | 0.34 [0.03, 0.64] | p < 0.00001, I2 = 92% |
| Molecular Name | Tetrahydrotanshinone | Cryptotanshinone | Dihydrotanshinone I | Tanshinone IIa | Tanshinone I | |
|---|---|---|---|---|---|---|
| MW | 280.34 | 296.39 | 278.32 | 294.37 | 276.3 | |
| OB (%) | 38.75 | 52.34 | 45.04 | 49.89 | 29.27 | |
| DL | 0.36 | 0.4 | 0.36 | 0.4 | 0.36 | |
| Lipinski | Yes; 0 violations | Yes; 0 violations | Yes; 0 violations | Yes; 0 violations | Yes; 0 violations | |
| Absorption | Caco-2 | 0.96 | 0.95 | 0.95 | 1.05 | 1.05 |
| Distribution | PPB | 82.42 | 88.37 | 86.71 | 89.13 | 87.91 |
| BBB | 0.39 | 0.51 | 0.43 | 0.7 | 0.53 | |
| Metabolism | CYP1A2 inhibitor | Yes | Yes | Yes | Yes | Yes |
| CYP2C19 inhibitor | Yes | Yes | Yes | Yes | Yes | |
| CYP2C9 inhibitor | Yes | Yes | Yes | Yes | No | |
| CYP2D6 inhibitor | No | No | Yes | Yes | No | |
| CYP3A4 inhibitor | Yes | Yes | Yes | Yes | Yes | |
| Excretion | T 1/2 | 1.729 | 1.886 | 1.919 | 2.084 | 2.145 |
| Toxicity | Human hepatoxicity | 0.758 | 0.73 | 0.826 | 0.776 | 0.83 |
| LD50 | 2.583 | 2.605 | 2.562 | 2.712 | 2.525 | |
| Target Name | PBD ID | Binding Affinity (kcal/mol) | ||||
|---|---|---|---|---|---|---|
| Crypto- Tanshinone | Dihydro- Tanshinone I | Tanshinone I | Tanshinone IIa | Tetrahydro- Tanshinone | ||
| Lipid and atherosclerosis-related proteins | ||||||
| HMGCR | 1dqa | −8.2 | −8.4 | −8.2 | −8.1 | −8.2 |
| Class II HMGCR | 1t02 | −8.3 | −8.5 | −7.9 | −7.9 | −8 |
| LDLR | 3p5b | −8.2 | −7.4 | −8.6 | −7.4 | −7.3 |
| PPARA | 1i7g | −8.8 | −8.6 | −8.8 | −9.1 | −8.8 |
| JAK2 | 4fvq | −9.1 | −10.3 | −10.1 | −9.5 | −10.4 |
| RAGE | 3o3u | −9.2 | −8.4 | −10.2 | −9.1 | −8.2 |
| Insulin resistance-related proteins | ||||||
| STAT3 | 1bg1 | −7.9 | −8 | −8.1 | −8 | −7.3 |
| Macrophage | 1gd0 | −7.3 | −7.8 | −7.3 | −7.2 | −7.2 |
| TNF | 2az5 | −9.8 | −10.3 | −10.4 | −10 | −10.2 |
| PI3K-Akt-related proteins | ||||||
| PIK3CA | 8tsa | −8.4 | −8.6 | −9 | −9 | −8.9 |
| AKT1 | 4gv1 | −8 | −7.7 | −9.2 | −8.1 | −8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-S.; Lee, H.-Y.; Oh, S.-H.; Kim, C.-B.; Kim, J.-H.; Yoo, S.-H.; Yoo, Y.-J.; Lee, S.-Y.; Lee, B.-C. Salvia miltiorrhiza and Its Compounds as Complementary Therapy for Dyslipidemia: A Meta-Analysis of Clinical Efficacy and In Silico Mechanistic Insights. Pharmaceuticals 2024, 17, 1426. https://doi.org/10.3390/ph17111426
Lee M-S, Lee H-Y, Oh S-H, Kim C-B, Kim J-H, Yoo S-H, Yoo Y-J, Lee S-Y, Lee B-C. Salvia miltiorrhiza and Its Compounds as Complementary Therapy for Dyslipidemia: A Meta-Analysis of Clinical Efficacy and In Silico Mechanistic Insights. Pharmaceuticals. 2024; 17(11):1426. https://doi.org/10.3390/ph17111426
Chicago/Turabian StyleLee, Min-Seong, Han-Young Lee, Seung-Hyun Oh, Chang-Bum Kim, Ji-Han Kim, Seung-Hoon Yoo, Yeon-Joo Yoo, Su-Yeon Lee, and Byung-Cheol Lee. 2024. "Salvia miltiorrhiza and Its Compounds as Complementary Therapy for Dyslipidemia: A Meta-Analysis of Clinical Efficacy and In Silico Mechanistic Insights" Pharmaceuticals 17, no. 11: 1426. https://doi.org/10.3390/ph17111426
APA StyleLee, M.-S., Lee, H.-Y., Oh, S.-H., Kim, C.-B., Kim, J.-H., Yoo, S.-H., Yoo, Y.-J., Lee, S.-Y., & Lee, B.-C. (2024). Salvia miltiorrhiza and Its Compounds as Complementary Therapy for Dyslipidemia: A Meta-Analysis of Clinical Efficacy and In Silico Mechanistic Insights. Pharmaceuticals, 17(11), 1426. https://doi.org/10.3390/ph17111426







