Antimicrobial Activity of Origanum vulgare Essential Oil against Staphylococcus aureus and Escherichia coli
Abstract
1. Introduction
2. Results
2.1. Main OEO Compounds
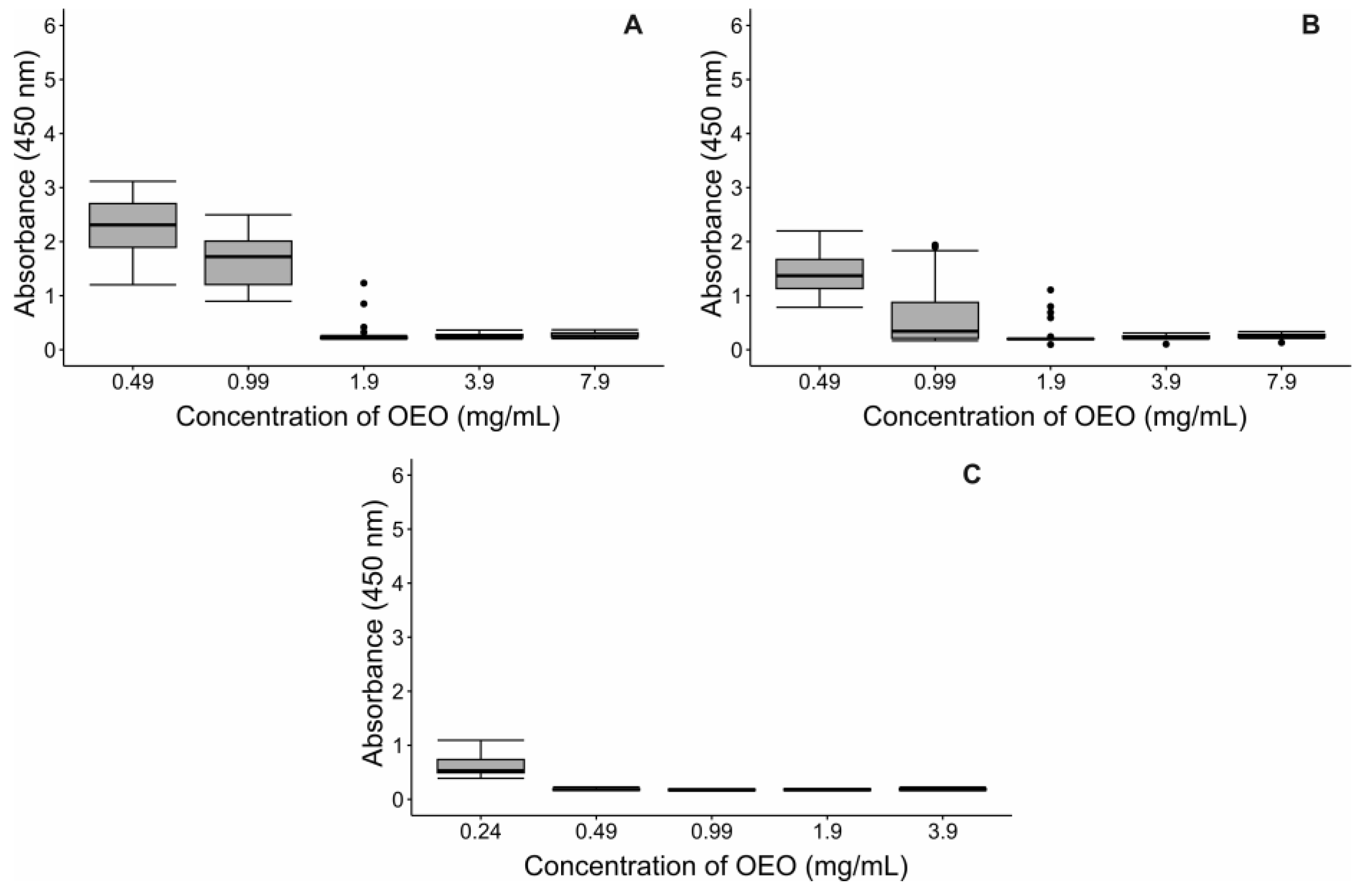
2.2. Bacterial Susceptibility to OEO
2.3. Minimum Inhibitory Concentration (MIC)
2.4. Minimum Bactericidal Concentration
3. Discussion
4. Materials and Methods
4.1. Plant Specimen
4.2. Essential Oil Extraction from Oregano
4.3. Chemical Composition by Gas Chromatography
4.4. Antimicrobial Activity
4.5. Susceptibility of Each Strain Against OEO
4.6. Determination of Minimum Inhibitory Concentration (MIC)
4.7. Determination of the Minimum Bactericidal Concentration (MBC)
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.A.T.; Elias, W.P.; Scaletsky, I.C.A.; Guth, B.E.C.; Rodrigues, J.F.; Piazza, R.M.F.; Ferreira, L.; Martinez, M.B. Escherichia coli diarreica. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Imre, K.; Ban-Cucerzan, A.; Herman, V.; Sallam, K.I.; Cristina, R.T.; Abd-Elghany, S.M.; Morar, D.; Popa, S.A.; Imre, M.; Morar, A. Occurrence, Pathogenic Potential and Antimicrobial Resistance of Escherichia coli Isolated from Raw Milk Cheese Commercialized in Banat Region, Romania. Antibiotics 2022, 11, 721. [Google Scholar] [CrossRef]
- Morar, A.; Ban-Cucerzan, A.; Herman, V.; Tîrziu, E.; Sallam, K.I.; Abd-Elghany, S.M.; Imre, K. Multidrug Resistant Coagulase-Positive Staphylococcus aureus and Their Enterotoxins Detection in Traditional Cheeses Marketed in Banat Region, Romania. Antibiotics 2021, 10, 1458. [Google Scholar] [CrossRef]
- Aslam, J.; Ali, H.M.; Hussain, S.; Ahmad, M.Z.; Siddique, A.B.; Shahid, M.; Shahzad, M.I.; Fatima, H.; Tariq, S.; Sadiq, F.; et al. Effectiveness of cephalosporins in hydrolysis and inhibition of Staphylococcus aureus and Escherichia coli biofilms. J. Veter. Sci. 2024, 25, e47. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, Z.; Li, S.; Fang, R.; Ono, H.K.; Hu, D.-L. Molecular Characteristics and Pathogenicity of Staphylococcus aureus Exotoxins. Int. J. Mol. Sci. 2023, 25, 395. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Lee, J.-H.; Ma, J.Y.; Tan, Y.; Lee, J. Antivirulence activities of retinoic acids against Staphylococcus aureus. Front. Microbiol. 2023, 14, e1224085. [Google Scholar] [CrossRef]
- da Rosa, C.G.; de Melo, A.P.Z.; Sganzerla, W.G.; Machado, M.H.; Nunes, M.R.; de Oliveira Brisola Maciel, M.V.; Bertoldi, F.C.; Barreto, P.L.M. Application in situ of zein nanocapsules loaded with Origanum vulgare Linneus and Thymus vulgaris as a preservative in bread. Food Hydrocoll. 2020, 99, 105339. [Google Scholar] [CrossRef]
- Taglienti, A.; Donati, L.; Ferretti, L.; Tomassoli, L.; Sapienza, F.; Sabatino, M.; Di Massimo, G.; Fiorentino, S.; Vecchiarelli, V.; Nota, P.; et al. In vivo Antiphytoviral Activity of Essential Oils and Hydrosols From Origanum vulgare, Thymus vulgaris, and Rosmarinus officinalis to Control Zucchini Yellow Mosaic Virus and Tomato Leaf Curl New Delhi Virus in Cucurbita pepo L. Front. Microbiol. 2022, 13, 840893. [Google Scholar] [CrossRef]
- Vinicius de Oliveira Brisola Maciel, M.; da Rosa Almeida, A.; Machado, M.H.; Elias, W.C.; Gonçalves da Rosa, C.; Teixeira, G.L.; Noronha, C.M.; Bertoldi, F.C.; Nunes, M.R.; Dutra de Armas, R.; et al. Green synthesis, characteristics and antimicrobial activity of silver nanoparticles mediated by essential oils as reducing agents. Biocatal. Agric. Biotechnol. 2020, 28, 101746. [Google Scholar] [CrossRef]
- Taleb, M.H.; Abdeltawab, N.F.; Shamma, R.N.; AbdelGayed, S.S.; Mohamed, S.S.; Farag, M.A.; Ramadan, M.A. Origanum vulgare L. essential oil as a potential anti-acne topical nanoemulsion—In vitro and in vivo study. Molecules 2018, 23, 2164. [Google Scholar] [CrossRef] [PubMed]
- Brosset, A.; Blande, J.D. Volatile-mediated plant–plant interactions: Volatile organic compounds as modulators of receiver plant defence, growth, and reproduction. J. Exp. Bot. 2022, 73, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Effah, E.; Holopainen, J.K.; McCormick, A.C. Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 58–63. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Padilla-González, G.F.; Leuner, O.; Melnikovova, I.; Fernandez-Cusimamani, E. Pharmacology of Natural Volatiles and Essential Oils in Food, Therapy, and Disease Prophylaxis. Front. Pharmacol. 2021, 12, 740302. [Google Scholar] [CrossRef] [PubMed]
- Fikry, S.; Khalil, N.; Salama, O. Chemical profiling, biostatic and biocidal dynamics of Origanum vulgare L. essential oil. AMB Express 2019, 9, 41. [Google Scholar] [CrossRef]
- Caputo, L.; Amato, G.; de Bartolomeis, P.; De Martino, L.; Manna, F.; Nazzaro, F.; De Feo, V.; Barba, A.A. Impact of drying methods on the yield and chemistry of Origanum vulgare L. essential oil. Sci. Rep. 2022, 12, 3845. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Polito, G.; Semenzato, G.; Del Duca, S.; Castronovo, L.M.; Vassallo, A.; Chioccioli, S.; Borsetti, D.; Calabretta, V.; Puglia, A.M.; Fani, R.; et al. Endophytic Bacteria and Essential Oil from Origanum vulgare ssp. vulgare Share Some VOCs with an Antibacterial Activity. Microorganisms 2022, 10, 1424. [Google Scholar] [CrossRef]
- Ghazal, T.S.A.; Schelz, Z.; Vidács, L.; Szemerédi, N.; Veres, K.; Spengler, G.; Hohmann, J. Antimicrobial, Multidrug Resistance Reversal and Biofilm Formation Inhibitory Effect of Origanum majorana Extracts, Essential Oil and Monoterpenes. Plants 2022, 11, 1432. [Google Scholar] [CrossRef]
- Soltani, S.; Shakeri, A.; Iranshahi, M.; Boozari, M. A Review of the Phytochemistry and Antimicrobial Properties of Origanum vulgare L. and Subspecies. Iran. J. Pharm. Res. 2021, 20, 268–285. [Google Scholar]
- Torabian Kakhki, M.; Sedaghat, N.; Mohsenzadeh, M. Chemical composition, antioxidative, antibacterial, and time-kill activities of some selected plant essential oils against foodborne pathogenic and spoilage organisms. Vet. Res. Forum 2020, 11, 339–346. [Google Scholar] [PubMed]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental Chemistry of Essential Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Kerem, S.; Koşar, N.; Tekin, F.; Güreser, A.S.; Özbek, Ö. Investigation of antimicrobial activities and molecular characterization of the species belong to Origanum, Thymus and Thymbra genera by ISSR. Mol. Biol. Rep. 2023, 50, 289–298. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Kirpotina, L.N.; Schepetkin, I.A.; Khlebnikov, A.I.; Lisonbee, B.L.; Black, J.L.; Woolf, H.; Thurgood, T.L.; Graf, B.L.; Satyal, P.; et al. Volatile Composition, Antimicrobial Activity, and In Vitro Innate Immunomodulatory Activity of Echinacea purpurea (L.) Moench Essential Oils. Molecules 2023, 28, 7330. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and Antibacterial Capacities of Origanum vulgare L. Essential Oil from the Arid Andean Region of Chile and its Chemical Characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Delpit, B.; Lamy, J.; Holland, F.; Chalchat, J.C.; Garry, R.P. Clonal Selection of Sabinene Hydrate-Rich Thyme (Thymus vulgaris). Yield and Chemical Composition of Essential Oils. J. Essent. Oil Res. 2000, 12, 387–391. [Google Scholar] [CrossRef]
- Ortiz, Y.; García-Heredia, A.; Merino-Mascorro, A.; García, S.; Solís-Soto, L.; Heredia, N. Natural and synthetic antimicrobials reduce adherence of enteroaggregative and enterohemorrhagic Escherichia coli to epithelial cells. PLoS ONE 2021, 16, e0251096. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Tsitsilin, A.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef]
- Kosakowska, O.; Węglarz, Z.; Pióro-Jabrucka, E.; Przybył, J.L.; Kraśniewska, K.; Gniewosz, M.; Bączek, K. Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Li, S.; Xu, Y.; Wang, X.; Wang, C. Carvacrol methyl ether, a compound from the essential oil of Gardenia jasminoides fruits, exhibits antioxidant effects in the deep-frying of Chinese Youmotou using sunflower oil. LWT Food Sci. Technol. 2020, 128, 109502. [Google Scholar] [CrossRef]
- Krümmel, A.; Pagno, C.H.; Malheiros, P.D.S. Active Films of Cassava Starch Incorporated with Carvacrol Nanocapsules. Foods 2024, 13, 1141. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, L.; Zou, Y.; Luo, S.; Wang, X.; Liang, Y.; Du, Y.; Feng, R.; Wei, Q. Antibacterial activity and mechanism of three isomeric terpineols of Cinnamomum longepaniculatum leaf oil. Folia Microbiol. Praha 2021, 66, 59–67. [Google Scholar] [CrossRef]
- Schmidt, E.; Wanner, J.; Höferl, M.; Jirovetz, L.; Buchbauer, G.; Gochev, V.; Girovam, T.; Stoyanova, A.; Geissler, M. Chemical Composition, Olfactory Analysis and Antibacterial Activity of Thymus vulgaris Chemotypes Geraniol, 4-Thujanol/Terpinen-4-ol, Thymol and Linalool Cultivated in Southern France. Nat. Prod. Commun. 2012, 7, e1934578X1200700833. [Google Scholar] [CrossRef]
- Torres, L.; Monteiro, M.L.G.; Da Silva, B.D.; Machado, M.A.M.; Mutz, Y.D.S.; Conte, C.A. Ultrasound-Assisted Nanoemulsion Loaded with Optimized Antibacterial Essential Oil Blend: A New Approach against Escherichia coli, Staphylococcus aureus, and Salmonella Enteritidis in Trout (Oncorhynchus mykiss) Fillets. Foods 2024, 13, 1569. [Google Scholar] [CrossRef] [PubMed]
- Luna-Solorza, J.M.; Ayala-Zavala, J.F.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Silva-Espinoza, B.A. Oregano Essential Oil versus Conventional Disinfectants against Salmonella Typhimurium and Escherichia coli O157:H7 Biofilms and Damage to Stainless-Steel Surfaces. Pathogens 2023, 12, 1245. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Ramani, R.; Jacob, B.M.; Ramaiah, S.; Mungoma, M.; Wanaina, S. In silico ATP Synthase Inhibition Activity and Antibacterial Activity of Selected Essential Oil against Escherichia coli and Resistant Acinetobacter baumannii. Int. J. Trop. Dis. Health 2022, 43, 1–11. [Google Scholar] [CrossRef]
- Pinto, L.; Cervellieri, S.; Netti, T.; Lippolis, V.; Baruzzi, F. Antibacterial Activity of Oregano (Origanum vulgare L.) Essential Oil Vapors against Microbial Contaminants of Food-Contact Surfaces. Antibiotics 2024, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zheng, K.; Lu, J.; Zeng, D.; Xiang, Q.; Ma, Y. Antibacterial characteristics of oregano essential oil and its mechanisms against Escherichia coli O157:H7. Food Meas. 2022, 16, 2989–2998. [Google Scholar] [CrossRef]
- Al-Hijazeen, M.; Mendonca, A.; Lee, E.J.; Ahn, D.U.; White, S. Fate of natural bacterial flora, and artificially inoculated Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella enterica in raw ground chicken meat with added oregano oil or tannic acid alone or combined. Food Control 2022, 139, 109059. [Google Scholar] [CrossRef]
- Castro–Alayo, E.M.; Chávez–Quintana, S.G.; Auquiñivín-Silva, E.A.; Fernández-Jeri, A.B.; Rodríguez-Hamamura, N.; Olivas-Orozco, G.; Sepúlveda-Ahumada, D.R. Aceites esenciales de plantas nativas del Perú: Efecto del lugar de cultivo en las características fisicoquímicas y actividad antioxidante. Sci. Agropecu. 2019, 10, 479–487. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol. Stream 2005, 16, 65–120. [Google Scholar]
- M07; Dilution AST for Aerobically Grown Bacteria—CLSI. Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume 38, p. 13.
- Pizzo, J.S.; Pelvine, R.A.; da Silva, A.L.B.R.; Mikcha, J.M.G.; Visentainer, J.V.; Rodrigues, C. Use of Essential Oil Emulsions to Control Escherichia coli O157:H7 in the Postharvest Washing of Lettuce. Foods 2023, 12, 2571. [Google Scholar] [CrossRef]
- Paiano, R.B.; de Sousa, R.L.M.; Bonilla, J.; Moreno, L.Z.; de Souza, E.D.F.; Baruselli, P.S.; Moreno, A.M. In vitro effects of cinnamon, oregano, and thyme essential oils against Escherichia coli and Trueperella pyogenes isolated from dairy cows with clinical endometritis. Theriogenology 2023, 196, 106–111. [Google Scholar] [CrossRef]
- Heumann, C.; Schomaker, M.; Shalabh. Introduction to Statistics and Data Analysis; Springer: Cham, Switzerland, 2016; pp. 1–455. [Google Scholar]
- Becker, K.; Brunello, G.; Scotti, L.; Drescher, D.; John, G. Efficacy of 0.05% Chlorhexidine and 0.05% Cetylpyridinium Chloride Mouthwash to Eliminate Living Bacteria on In Situ Collected Biofilms: An In Vitro Study. Antibiotics 2021, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Izarra-Rojas, K.V.; Rojas-Palomino, N.; Gonzáles-Medrano, J.L.; Minaya-Gómez, G.; Berrocal-Huallpa, A.; Santiago-Contreras, J.; León-Quispe, J. In vitro inhibitory effect of chlorinated tetrasulfonated aluminum phthalocyanine against Leishmania (Viannia) Peruviana and Leishmania (Viannia) Braziliensis. Rev. Peru. Med. Exp. Salud Publica 2020, 37, 462–470. [Google Scholar] [CrossRef]
- Cobo-Angel, C.; Mosaddegh, A.; Aprea, M.; Guarino, C.; Cummings, K.J.; Cazer, C. Trends of feline Escherichia coli minimum inhibitory concentrations over 14 years illustrate the need for judicious antimicrobial use in cats. Am. J. Vet. Res. 2023, 84, 12. [Google Scholar] [CrossRef]
- Michael, A.; Kelman, T.; Pitesky, M. Overview of Quantitative Methodologies to Understand Antimicrobial Resistance via Minimum Inhibitory Concentration. Animals 2020, 10, 1405. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. 2023. Available online: https://www.r-project.org/ (accessed on 15 March 2023).


| Retention Time | Compound | Abundance (%) | RI Cal a | RI Lit b | Fragments m/z |
|---|---|---|---|---|---|
| 31.06 | 2-Menthen-1-ol | 36.33 | 1117 | 1126 | 43.0; 71.0; 81.0 |
| 37.89 | Linalyl acetate | 9.26 | 1250 | 1257 | 93.0; 43.0; 121.0 |
| 35.33 | Terpinen-4-ol | 9.01 | 1198 | 1177 | 71.1; 93.2; 111.2 |
| 29.24 | 4-Thujanol | 6.33 | 1083 | 1075 | 93.2; 71.1; 43.1 |
| 26.26 | Menthene | 5.81 | 1028 | 1017 | 121.0; 93.0; 136.0 |
| 23.85 | Sabinene | 5.18 | 984 | 974 | 93.2; 91.2; 77.1 |
| 37.73 | Carvacrol methyl ether | 5.14 | 1247 | 1244 | 149.0; 164.0; 91.0 |
| 26.66 | o-Cymene | 3.37 | 1036 | 1022 | 119.0; 134.0; 117.0 |
| 35.92 | L-.alpha.-Terpineol | 2.67 | 1210 | 1190 | 59.0; 93.0; 121.0 |
| 37.19 | Thymol methyl ether | 2.36 | 1236 | 1235 | 149.0; 164.0; 91.0 |
| 30.32 | Linalool | 2.27 | 1103 | 1099 | 93,2; 71,1; 41,1 |
| 40.53 | Thymol | 1.93 | 1304 | 1291 | 135.0; 150.0; 91.0 |
| 24.21 | .beta.-Myrcene | 1.76 | 991 | 991 | 93.0; 69.0; 41.0 |
| 30.03 | Terpinolene | 1.71 | 1097 | 1088 | 121.2; 93.2; 136.2 |
| 27.21 | .beta.-Phellandrene | 1.44 | 1046 | 1031 | 93,0; 91,0; 77,0 |
| 26.97 | D-Limonene | 1.36 | 1041 | 1018 | 68.0; 93.0; 67.0 |
| 21.11 | alpha-Thujene | 1.01 | 934 | 929 | 93.0; 91.0; 77.0 |
| Other compounds | 0.65 | - | - | - | |
| 38.26 | Pulegone | 0.56 | 1258 | 1237 | 81.1; 152.2; 67.2 |
| 27.35 | Z-beta-Ocimene | 0.44 | 1048 | 1038 | 93.0; 91.0; 79.0 |
| 25.71 | .alpha.-Phellandrene | 0.36 | 1018 | 1005 | 93.0; 91.0; 77.0 |
| 36.1 | trans-Piperitol | 0.27 | 1214 | 1208 | 84.2; 93.2; 91.2 |
| 35.09 | endo-Borneol | 0.25 | 1194 | 1167 | 95.2; 110.2; 41.1 |
| 23.52 | 1-Octen-3-ol | 0.15 | 978 | 980 | 57.0, 43.0; 72.0 |
| 33.99 | (-)-Menthone | 0.14 | 1173 | 1154 | 112.0; 69.0; 139.0 |
| 51.21 | delta-Cadinene | 0.14 | 1542 | 1524 | 161.0; 119.0; 134.0 |
| 34.64 | Levomenthol | 0.1 | 1185 | 1175 | 71.1; 95.2; 81.1 |
| Density Dilution (mg/mL) [%] | S. aureus ATCC 25923 | S. aureus Isolate | E. coli ATCC 25922 |
|---|---|---|---|
| (χ2 = 16.31; p = 0.006) | (χ2 = 16.59; p = 0.005) | (χ2 = 16.18; p = 0.006) | |
| 957 [100] | 16.7 (1.1) * | 19.7 (0.6) * | 20.7 (1.2) * |
| 765.6 [80] | 14.3 (1.5) * | 18.3 (1.2) * | 19.0 (2.0) * |
| 574.2 [60] | 11.3 (0.6) * | 15.3 (0.6) * | 14.7 (1.2) * |
| 382.8 [40] | 9.3 (1.2) ⍏ | 10.7 (0.6) * | 11.0 (1.0) ⍏ |
| 191.4 [20] | 7.7 (1.5) ⍏ | 8.3 (0.6) ⍏ | 10 (0.3) ⍏ |
| Susceptibility control | 26 (0.1) (a) | 26 (0.1) (a) | 29 (1.5) (b) |
| Microorganism | Time | % Inhibition | IC 50% | CI 95% | |||||
|---|---|---|---|---|---|---|---|---|---|
| 7.9 mg/mL | 3.9 mg/mL | 1.9 mg/mL | 1.0 mg/mL | 0.5 mg/mL | 0.2 mg/mL | ||||
| S. aureus ATCC | 18 | 91.4 | 91.1 | 93.3 | 54.0 | 27.7 | - | 0.77 | 0.72–0.82 |
| 24 | 92.2 | 92.8 | 94.1 | 53.6 | 31.1 | - | 0.76 | 0.69–0.84 | |
| 36 | 93.7 | 94.7 | 95.0 | 57.1 | 54.4 | - | 0.51 | 0.41–0.61 | |
| 42 | 94.4 | 94.7 | 90.9 | 67.8 | 49.3 | - | 0.51 | 0.45–0.56 | |
| S. aureus isolate | 18 | 86.1 | 86.4 | 90.3 | 86.4 | 40.3 | - | 0.5 | 0.49–0.52 |
| 24 | 88.8 | 89.6 | 91.9 | 90.7 | 49.6 | - | 0.48 | 0.44–0.53 | |
| 36 | 91.1 | 92.7 | 93.2 | 83.0 | 48.5 | - | 0.47 | 0.44–0.50 | |
| 42 | 94.1 | 94.6 | 89.5 | 59.3 | 52.6 | - | 0.53 | 0.43–0.63 | |
| E. coli ATCC | 18 | - | 95.0 | 95.2 | 95.3 | 94.9 | 77.5 | 0.19 | 0.13–0.24 |
| 24 | - | 95.0 | 95.1 | 95.2 | 95.0 | 85.6 | 0.17 | 0.07–0.27 | |
| 36 | - | 95.0 | 95.2 | 95.3 | 95.1 | 86.5 | 0.17 | 0.11–0.24 | |
| 42 | - | 95.6 | 95.6 | 95.6 | 95.5 | 85.7 | 0.18 | 0.05–0.29 | |
| Differences Among Time Points | |
|---|---|
| Time | Median |
| 18 | 89.593 a |
| 24 | 91.450 a,b |
| 36 | 92.921 b,c |
| 48 | 94.243 c |
| Differences Among Bacteria | |
| Strains | Median |
| S. aureus isolated | 89.334 a |
| S. aureus ATCC | 91.827 a |
| E. coli ATCC | 95.141 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada-Muñoz, S.; Cortez, D.; Rascón, J.; Chavez, S.G.; Caetano, A.C.; Díaz-Manchay, R.J.; Sandoval-Bances, J.; Huyhua-Gutierrez, S.; Gonzales, L.; Chenet, S.M.; et al. Antimicrobial Activity of Origanum vulgare Essential Oil against Staphylococcus aureus and Escherichia coli. Pharmaceuticals 2024, 17, 1430. https://doi.org/10.3390/ph17111430
Tejada-Muñoz S, Cortez D, Rascón J, Chavez SG, Caetano AC, Díaz-Manchay RJ, Sandoval-Bances J, Huyhua-Gutierrez S, Gonzales L, Chenet SM, et al. Antimicrobial Activity of Origanum vulgare Essential Oil against Staphylococcus aureus and Escherichia coli. Pharmaceuticals. 2024; 17(11):1430. https://doi.org/10.3390/ph17111430
Chicago/Turabian StyleTejada-Muñoz, Sonia, Denny Cortez, Jesús Rascón, Segundo G. Chavez, Aline C. Caetano, Rosa J. Díaz-Manchay, Julio Sandoval-Bances, Sonia Huyhua-Gutierrez, Lizandro Gonzales, Stella M. Chenet, and et al. 2024. "Antimicrobial Activity of Origanum vulgare Essential Oil against Staphylococcus aureus and Escherichia coli" Pharmaceuticals 17, no. 11: 1430. https://doi.org/10.3390/ph17111430
APA StyleTejada-Muñoz, S., Cortez, D., Rascón, J., Chavez, S. G., Caetano, A. C., Díaz-Manchay, R. J., Sandoval-Bances, J., Huyhua-Gutierrez, S., Gonzales, L., Chenet, S. M., & Tapia-Limonchi, R. (2024). Antimicrobial Activity of Origanum vulgare Essential Oil against Staphylococcus aureus and Escherichia coli. Pharmaceuticals, 17(11), 1430. https://doi.org/10.3390/ph17111430









