Mapping of c-Fos Expression in Rat Brain Sub/Regions Following Chronic Social Isolation: Effective Treatments of Olanzapine, Clozapine or Fluoxetine
Abstract
:1. Introduction
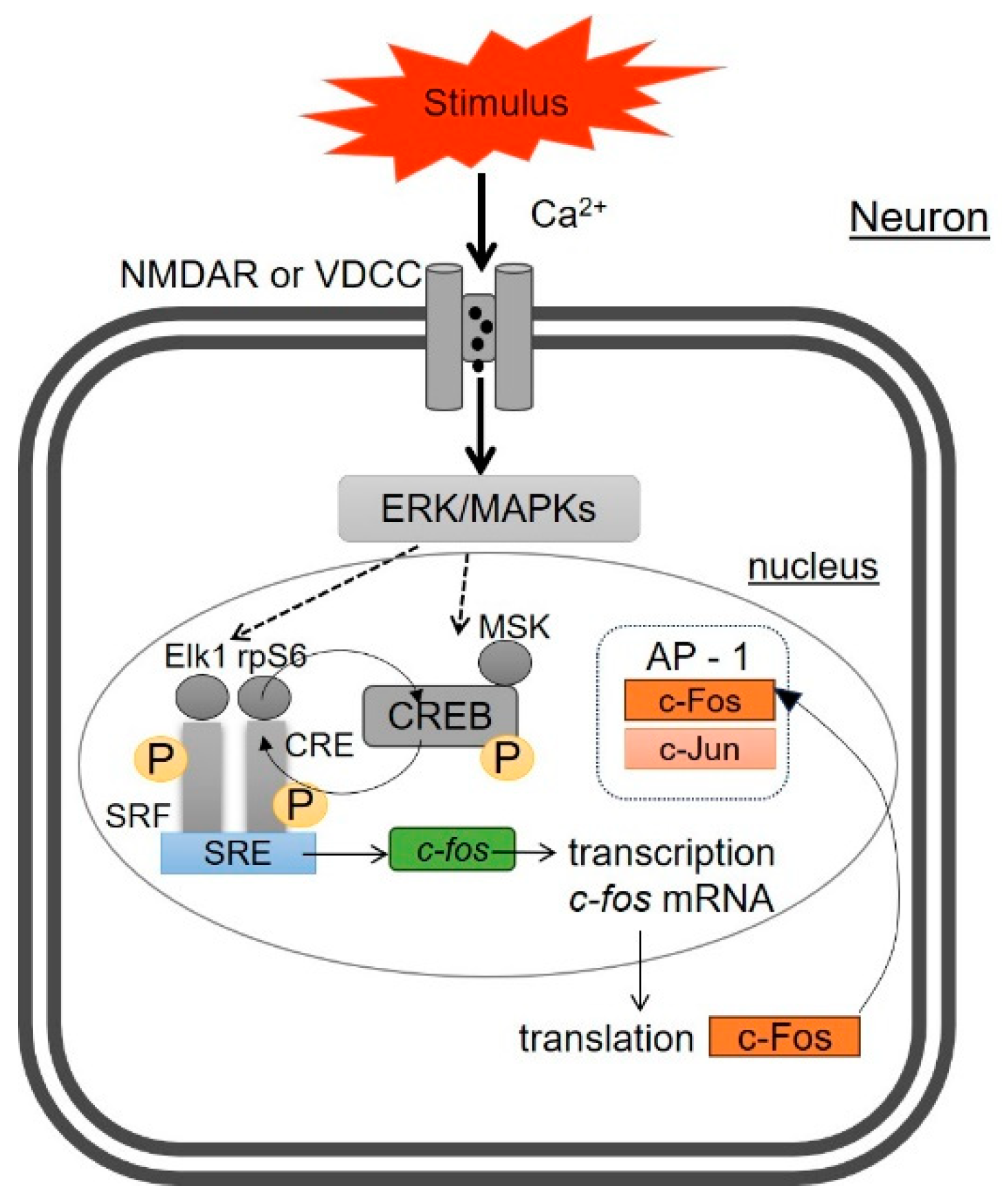
2. Molecular Mechanisms of c-Fos Expression in Neurons
3. c-Fos as a Marker of Cell Activation
4. Chronic Social Isolation as an Animal Model of Depression
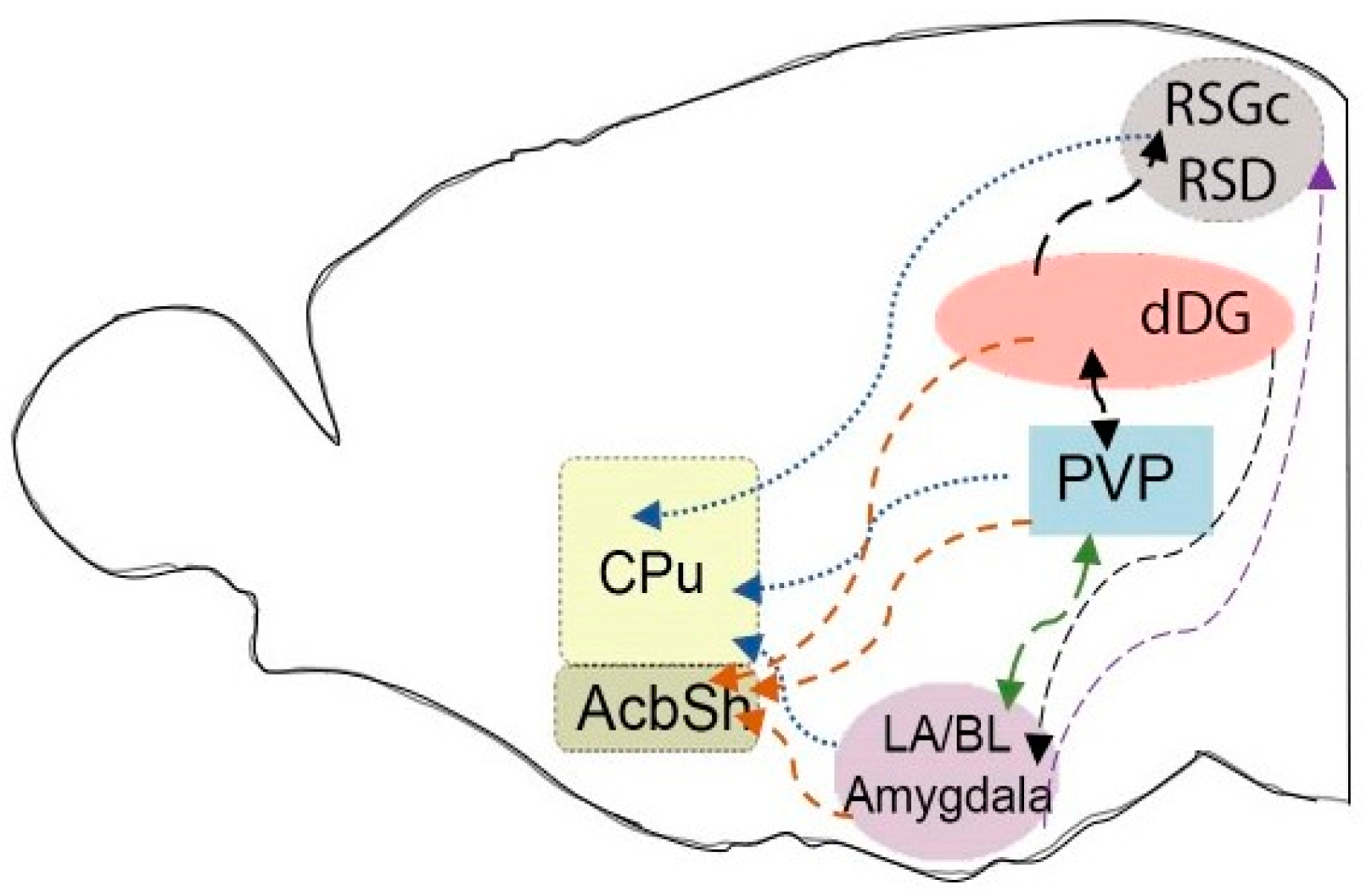
5. c-Fos Expression in Rat Brain Sub/Regions Following CSIS
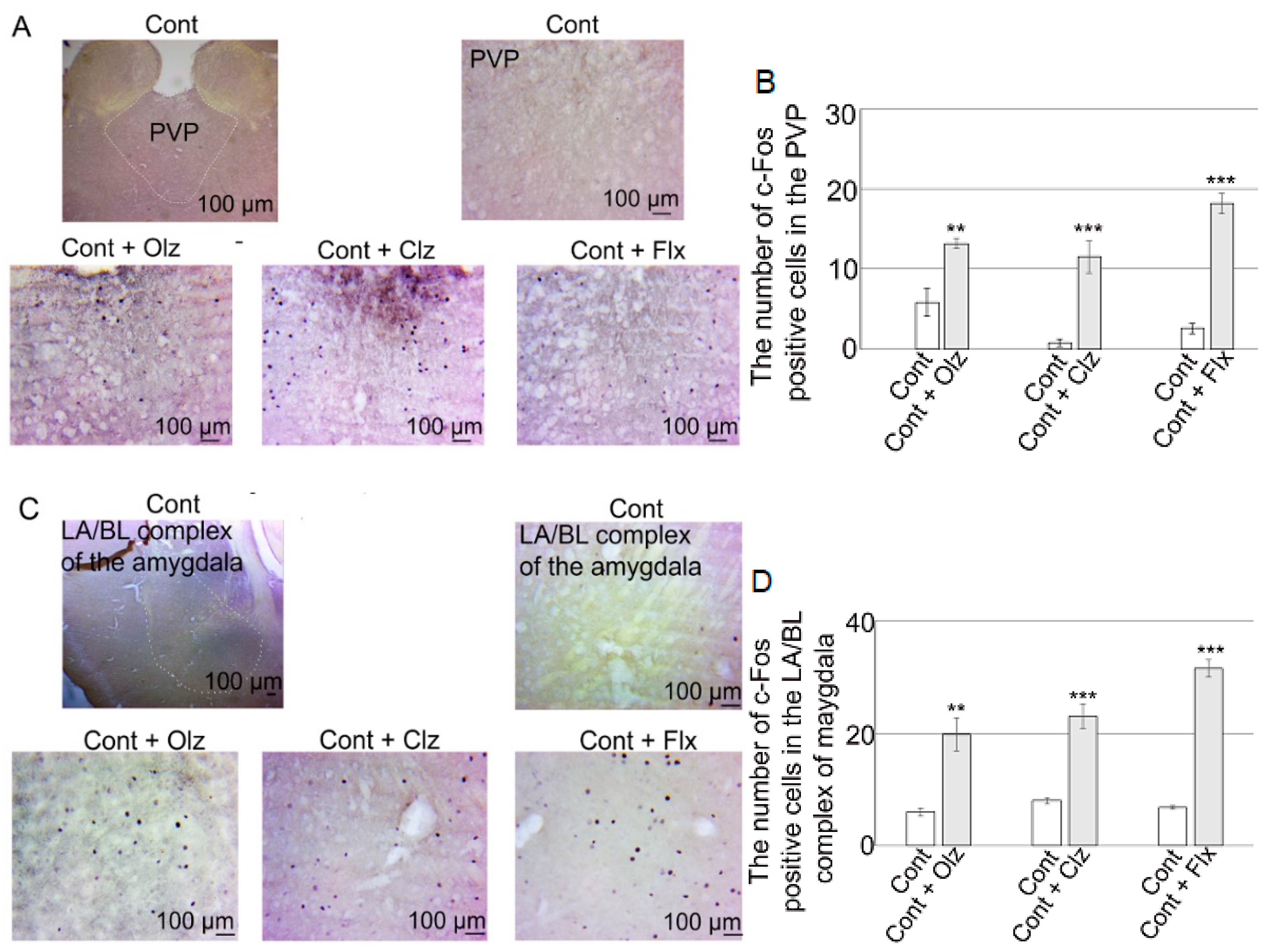
6. c-Fos Expression in Brain Sub/Regions in Control Rats Following Treatments with Olz, Clz, or Flx
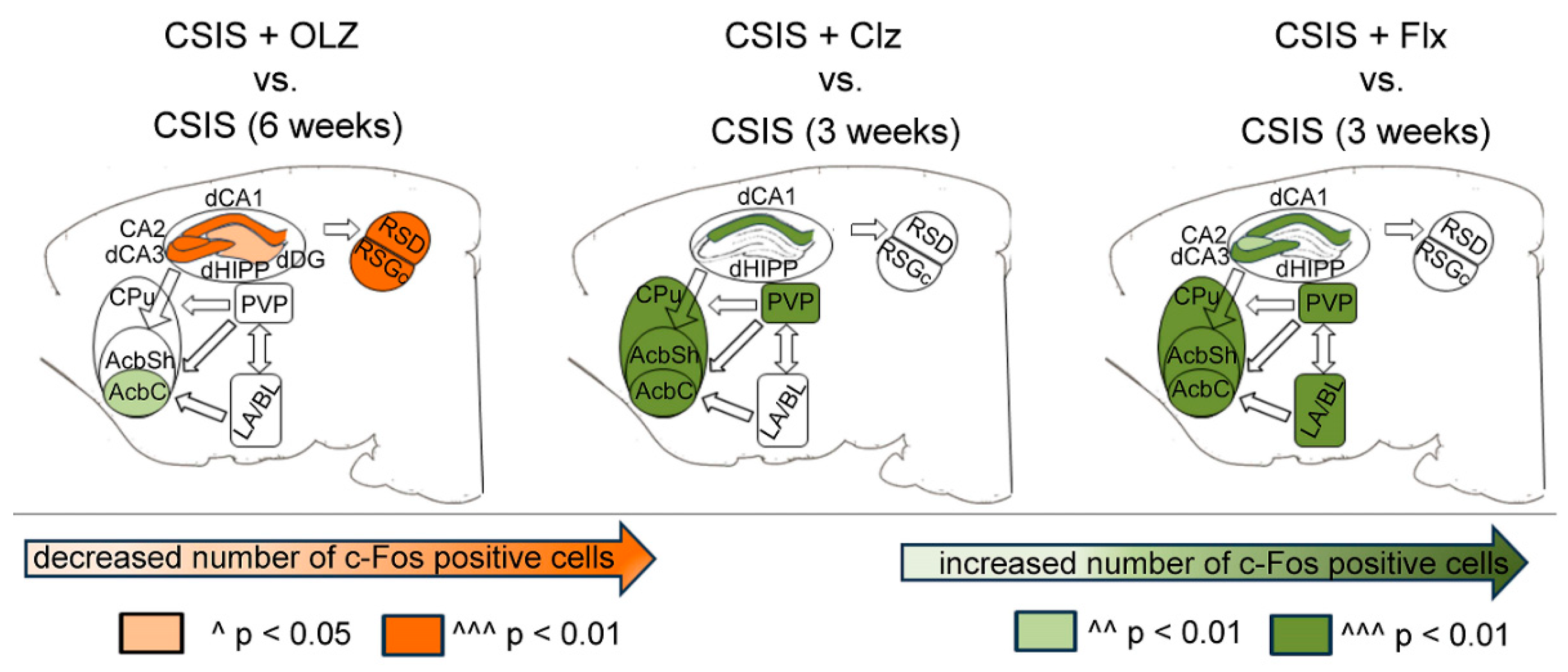
7. c-Fos Expression in Brain Sub/Regions in CSIS Rats After Effective Treatments with Olz, Clz, or Flx
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| 5HTT | 5-hydroxytryptamine transporter |
| AcbC | nucleus accumbens, core |
| AcbSh | nucleus accumbens, shell |
| AP-1 complex | activator protein-1 |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| CPu | caudate putamen |
| Clz | Clozapine |
| Cont | Controls |
| CA2 | cornu ammonis 2 |
| CSIS | chonic social isolation |
| cAMP | cyclic adenosine monophosphate |
| CRE | cAMP response element |
| CREB | cAMP response element binding protein |
| dCA | dorsal cornu ammonis |
| dCA1 | dorsal cornu ammonis 1 |
| dCA3 | dorsal cornu ammonis 3 |
| dDG | dorsal dentate gyrus |
| dHIPP | dorsal hippocampus |
| Elk1 | ETS like-1 protein |
| ERK | extracellular signal-regulated kinase |
| Flx | Fluoxetine |
| IL-6 | interleukin-6 |
| LA/BL | lateral/basolateral complex of the amygdala |
| mPFC | medial prefrontal cortex |
| MAPKS | mitogen-activated protein kinases |
| MSK | mitogen-stress activated protein kinases |
| NAc | nucleus accumbens |
| NF-κB | nuclear-factor kappa-B |
| NMDAR | N-methyl-D-aspartate receptor |
| Olz | Olanzapine |
| PKA | protein kinase A |
| PVT | paraventricular nucleus of thalamus |
| PVP | paraventricular nucleus of thalamus, posterior part |
| RSC | retrosplenial cortex |
| RSD | retrosplenial dysgranular cortex |
| RSGc | retrosplenial granular cortex, c subregion |
| ROS | reactive oxygen species |
| rpS6 | ribosomal protein kinsase S6 |
| SRF | serum response factor |
| VDCC | voltage-dependent calcium channel |
| vDG | ventral dentate gyrus |
| vHIPP | ventral hippocampus |
References
- Bullitt, E. Expression of C-Fos-like Protein as a Marker for Neuronal Activity Following Noxious Stimulation in the Rat. J. Comp. Neurol. 1990, 296, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Adams, D.H.; Simen, B.B. Transcription Factors as Modulators of Stress Responsivity. Tech. Behav. Neural Sci. 2005, 15, 679–698. [Google Scholar] [CrossRef]
- Herrera, D.G.; Robertson, H.A. Activation of C-Fos in the Brain. Prog. Neurobiol. 1996, 50, 83–107. [Google Scholar] [CrossRef] [PubMed]
- Angel, P.; Karin, M. The Role of Jun, Fos and the AP-1 Complex in Cell-Proliferation and Transformation. Biochim. Biophys. Acta 1991, 1072, 129–157. [Google Scholar] [CrossRef]
- Gius, D.; Cao, X.; Rauscher, F.J., III; Cohen, D.R.; Curran, T.; Sukhatme, V.P. Transcriptional Activation and Repression by Fos Are Independent Functions: The C Terminus Represses Immediate-Early Gene Expression via CArG Elements. Mol. Cell. Biol. 1990, 10, 4243. [Google Scholar] [CrossRef]
- Lucibello, F.C.; Lowag, C.; Neuberg, M.; Müller, R. Trans-Repression of the Mouse c-Fos Promoter: A Novel Mechanism of Fos-Mediated Trans-Regulation. Cell 1989, 59, 999–1007. [Google Scholar] [CrossRef]
- Kovács, K.J. C-Fos as a Transcription Factor: A Stressful (Re)View from a Functional Map. Neurochem. Int. 1998, 33, 287–297. [Google Scholar] [CrossRef]
- Matsuda, S.; Peng, H.; Yoshimura, H.; Wen, T.C.; Fukuda, T.; Sakanaka, M. Persistent C-Fos Expression in the Brains of Mice with Chronic Social Stress. Neurosci. Res. 1996, 26, 157–170. [Google Scholar] [CrossRef]
- Herdegen, T.; Kovary, K.; Buhl, A.; Bravo, R.; Zimmermann, M.; Gass, P. Basal Expression of the Inducible Transcription Factors C-Jun, JunB, JunD, c-Fos, FosB, and Krox-24 in the Adult Rat Brain. J. Comp. Neurol. 1995, 354, 39–56. [Google Scholar] [CrossRef]
- Cullinan, W.E.; Herman, J.P.; Battaglia, D.F.; Akil, H.; Watson, S.J. Pattern and Time Course of Immediate Early Gene Expression in Rat Brain Following Acute Stress. Neuroscience 1995, 64, 477–505. [Google Scholar] [CrossRef]
- Hughes, P.; Lawlor, P.; Dragunow, M. Basal Expression of Fos, Fos-Related, Jun, and Krox 24 Proteins in Rat Hippocampus. Brain Res. Mol. Brain Res. 1992, 13, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Campeau, S.; Dolan, D.; Akil, H.; Watson, S.J. C-Fos MRNA Induction in Acute and Chronic Audiogenic Stress: Possible Role of the Orbitofrontal Cortex in Habituation. Stress 2002, 5, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Chung, C.H. Brain-Wide Cellular Mapping of Acute Stress-Induced Activation in Male and Female Mice. FASEB J. 2021, 35, e22041. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Calvo-Torrent, A.; Herbert, J. Mapping Brain Response to Social Stress in Rodents with C-Fos Expression: A Review. Stress 2002, 5, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljević, A.; Perić, I.; Gass, P.; Inta, D.; Lang, U.E.; Borgwardt, S.; Filipović, D. Brain Sub/Region-Specific Effects of Olanzapine on c-Fos Expression of Chronically Socially Isolated Rats. Neuroscience 2019, 396, 46–65. [Google Scholar] [CrossRef]
- Wang, Z.J.; Shwani, T.; Liu, J.; Zhong, P.; Yang, F.; Schatz, K.; Zhang, F.; Pralle, A.; Yan, Z. Molecular and Cellular Mechanisms for Differential Effects of Chronic Social Isolation Stress in Males and Females. Mol. Psychiatry 2022, 27, 3056–3068. [Google Scholar] [CrossRef]
- Groves, A.; Kihara, Y.; Jonnalagadda, D.; Rivera, R.; Kennedy, G.; Mayford, M.; Chun, J. A Functionally Defined In Vivo Astrocyte Population Identified by c-Fos Activation in a Mouse Model of Multiple Sclerosis Modulated by S1P Signaling: Immediate-Early Astrocytes (IeAstrocytes). eNeuro 2018, 5, ENEURO.0239-18.2018. [Google Scholar] [CrossRef]
- Eun, S.Y.; Hong, Y.H.; Kim, E.H.; Jeon, H.; Suh, Y.H.; Lee, J.E.; Jo, C.; Jo, S.A.; Kim, J. Glutamate Receptor-Mediated Regulation of c-Fos Expression in Cultured Microglia. Biochem. Biophys. Res. Commun. 2004, 325, 320–327. [Google Scholar] [CrossRef]
- Muir, D.A.; Compston, D.A.S. Growth Factor Stimulation Triggers Apoptotic Cell Death in Mature Oligodendrocytes. J. Neurosci. Res. 1996, 44, 1–11. [Google Scholar] [CrossRef]
- Sumner, B.E.H.; Cruise, L.A.; Slattery, D.A.; Hill, D.R.; Shahid, M.; Henry, B. Testing the Validity of C-Fos Expression Profiling to Aid the Therapeutic Classification of Psychoactive Drugs. Psychopharmacology 2004, 171, 306–321. [Google Scholar] [CrossRef]
- De Bartolomeis, A.; Buonaguro, E.F.; Latte, G.; Rossi, R.; Marmo, F.; Iasevoli, F.; Tomasetti, C. Immediate-Early Genes Modulation by Antipsychotics: Translational Implications for a Putative Gateway to Drug-Induced Long-Term Brain Changes. Front. Behav. Neurosci. 2017, 11, 284190. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Kosofsky, B.E.; Birnbaum, R.; Cohen, B.M.; Hyman, S.E. Differential Expression of C-Fos and Zif268 in Rat Striatum after Haloperidol, Clozapine, and Amphetamine. Proc. Natl. Acad. Sci. USA 1992, 89, 4270–4274. [Google Scholar] [CrossRef] [PubMed]
- Merchant, K.M.; Dobie, D.J.; Filloux, F.M.; Totzke, M.; Aravagiri, M.; Dorsa, D.M. Effects of Chronic Haloperidol and Clozapine Treatment on Neurotensin and C-Fos MRNA in Rat Neostriatal Subregions. Pharmacology 1994, 271, 460–471. [Google Scholar]
- Lino-de-Oliveira, C.; de Oliveira, R.M.W.; Pádua Carobrez, A.; de Lima, T.C.M.; Del Bel, E.A.; Guimarães, F.S. Antidepressant Treatment Reduces Fos-like Immunoreactivity Induced by Swim Stress in Different Columns of the Periaqueductal Gray Matter. Brain Res. Bull. 2006, 70, 414–421. [Google Scholar] [CrossRef]
- Inta, D.; Trusel, M.; Riva, M.A.; Sprengel, R.; Gass, P. Differential C-Fos Induction by Different NMDA Receptor Antagonists with Antidepressant Efficacy: Potential Clinical Implications. Int. J. Neuropsychopharmacol. 2009, 12, 1133–1136. [Google Scholar] [CrossRef]
- Vulink, N.C.C.; Figee, M.; Denys, D. Review of Atypical Antipsychotics in Anxiety. Eur. Neuropsychopharmacol. 2011, 21, 429–449. [Google Scholar] [CrossRef]
- Wang, P.; Si, T. Use of Antipsychotics in the Treatment of Depressive Disorders. Shanghai Arch. Psychiatry 2013, 25, 134. [Google Scholar] [CrossRef]
- Mauri, M.C.; Paletta, S.; Di Pace, C.; Reggiori, A.; Cirnigliaro, G.; Valli, I.; Altamura, A.C. Clinical Pharmacokinetics of Atypical Antipsychotics: An Update. Clin. Pharmacokinet. 2018, 57, 1493–1528. [Google Scholar] [CrossRef]
- Shahid, M.; Walker, G.B.; Zorn, S.H.; Wong, E.H.F. Asenapine: A Novel Psychopharmacologic Agent with a Unique Human Receptor Signature. J. Psychopharmacol. 2009, 23, 65–73. [Google Scholar] [CrossRef]
- Seeman, P. Clozapine, a Fast-off-D2 Antipsychotic. ACS Chem. Neurosci. 2014, 5, 24–29. [Google Scholar] [CrossRef]
- Kapur, S.; Seeman, P. Does Fast Dissociation from the Dopamine D2 Receptor Explain the Action of Atypical Antipsychotics?: A New Hypothesis. Am. J. Psychiatry 2001, 158, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Elbe, D. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, Third Edition. J. Can. Acad. Child Adolesc. Psychiatry 2010, 19, 230. [Google Scholar]
- Andrade, C. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Mens Sana Monogr. 2010, 8, 146. [Google Scholar] [CrossRef]
- Chen, J.; Gao, K.; Kemp, D.E. Second-Generation Antipsychotics in Major Depressive Disorder: Update and Clinical Perspective. Curr. Opin. Psychiatry 2011, 24, 10–17. [Google Scholar] [CrossRef]
- Li, Y.F. A Hypothesis of Monoamine (5-HT)-Glutamate/GABA Long Neural Circuit: Aiming for Fast-Onset Antidepressant Discovery. Pharmacol. Ther. 2020, 208, 107494. [Google Scholar] [CrossRef]
- Köhler, S.; Cierpinsky, K.; Kronenberg, G.; Adli, M. The Serotonergic System in the Neurobiology of Depression: Relevance for Novel Antidepressants. J. Psychopharmacol. 2016, 30, 13–22. [Google Scholar] [CrossRef]
- Sánchez, C.; Hyttel, J. Comparison of the Effects of Antidepressants and Their Metabolites on Reuptake of Biogenic Amines and on Receptor Binding. Cell. Mol. Neurobiol. 1999, 19, 467–489. [Google Scholar] [CrossRef]
- Ni, Y.G.; Miledi, R. Blockage of 5HT2C Serotonin Receptors by Fluoxetine (Prozac). Proc. Natl. Acad. Sci. USA 1997, 94, 2036–2040. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Torres-Sanchez, S.; Bravo, L.; Mico, J.A.; Berrocoso, E. Fluoxetine: A Case History of Its Discovery and Preclinical Development. Expert Opin. Drug Discov. 2014, 9, 567–578. [Google Scholar] [CrossRef]
- Becker, M.; Pinhasov, A.; Ornoy, A. Animal Models of Depression: What Can They Teach Us about the Human Disease? Diagnostics 2021, 11, 123. [Google Scholar] [CrossRef]
- Czéh, B.; Simon, M. Benefits of Animal Models to Understand the Pathophysiology of Depressive Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110049. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Berton, O.; McClung, C.A.; DiLeone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential Role of BDNF in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Bagot, R.C.; Parise, E.M.; Peña, C.J.; Zhang, H.X.; Maze, I.; Chaudhury, D.; Persaud, B.; Cachope, R.; Bolaños-Guzmán, C.A.; Cheer, J.; et al. Ventral Hippocampal Afferents to the Nucleus Accumbens Regulate Susceptibility to Depression. Nat. Commun. 2015, 6, 7062. [Google Scholar] [CrossRef]
- Friedman, A.K.; Juarez, B.; Ku, S.M.; Zhang, H.; Calizo, R.C.; Walsh, J.J.; Chaudhury, D.; Zhang, S.; Hawkins, A.; Dietz, D.M.; et al. KCNQ Channel Openers Reverse Depressive Symptoms via an Active Resilience Mechanism. Nat. Commun. 2016, 7, 11671. [Google Scholar] [CrossRef]
- Zhang, H.; Chaudhury, D.; Nectow, A.R.; Friedman, A.K.; Zhang, S.; Juarez, B.; Liu, H.; Pfau, M.L.; Aleyasin, H.; Jiang, C.; et al. A1- and Β3-Adrenergic Receptor–Mediated Mesolimbic Homeostatic Plasticity Confers Resilience to Social Stress in Susceptible Mice. Biol. Psychiatry 2019, 85, 226–236. [Google Scholar] [CrossRef]
- Harris, A.Z.; Atsak, P.; Bretton, Z.H.; Holt, E.S.; Alam, R.; Morton, M.P.; Abbas, A.I.; Leonardo, E.D.; Bolkan, S.S.; Hen, R.; et al. A Novel Method for Chronic Social Defeat Stress in Female Mice. Neuropsychopharmacology 2018, 43, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ai, L.; Chen, D.; Zhou, D.; Han, Y.; Ji, R.; Hu, M.; Wang, Q.; Zhang, M.; Wang, Y.; et al. Multiple Integrated Social Stress Induces Depressive-like Behavioral and Neural Adaptations in Female C57BL/6J Mice. Neurobiol. Dis. 2024, 190, 106374. [Google Scholar] [CrossRef]
- Bagot, R.C.; Cates, H.M.; Purushothaman, I.; Vialou, V.; Heller, E.A.; Yieh, L.; LaBonté, B.; Peña, C.J.; Shen, L.; Wittenberg, G.M.; et al. Ketamine and Imipramine Reverse Transcriptional Signatures of Susceptibility and Induce Resilience-Specific Gene Expression Profiles. Biol. Psychiatry 2017, 81, 285–295. [Google Scholar] [CrossRef]
- Cao, J.L.; Covington, H.E.; Friedman, A.K.; Wilkinson, M.B.; Walsh, J.J.; Cooper, D.C.; Nestler, E.J.; Han, M.H. Mesolimbic Dopamine Neurons in the Brain Reward Circuit Mediate Susceptibility to Social Defeat and Antidepressant Action. J. Neurosci. 2010, 30, 16453–16458. [Google Scholar] [CrossRef]
- Donahue, R.J.; Muschamp, J.W.; Russo, S.J.; Nestler, E.J.; Carlezon, W.A. Effects of Striatal ΔFosB over Expression and Ketamine on Social Defeat Stress-Induced Anhedonia in Mice. Biol. Psychiatry 2014, 76, 550–558. [Google Scholar] [CrossRef]
- Goñi-Balentziaga, O.; Perez-Tejada, J.; Renteria-Dominguez, A.; Lebeña, A.; Labaka, A. Social Instability in Female Rodents as a Model of Stress Related Disorders: A Systematic Review. Physiol. Behav. 2018, 196, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.L.; Han, M.H.; Graham, D.L.; Green, T.A.; Vialou, V.; Iñiguez, S.D.; Cao, J.L.; Kirk, A.; Chakravarty, S.; Kumar, A.; et al. CREB Regulation of Nucleus Accumbens Excitability Mediates Social Isolation-Induced Behavioral Deficits. Nat. Neurosci. 2009, 12, 200–209. [Google Scholar] [CrossRef]
- Filipović, D.; Todorović, N.; Bernardi, R.E.; Gass, P. Oxidative and Nitrosative Stress Pathways in the Brain of Socially Isolated Adult Male Rats Demonstrating Depressive- and Anxiety-like Symptoms. Brain Struct. Funct. 2017, 222, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Khalil, D.; Gould, E. Social Isolation Delays the Positive Effects of Running on Adult Neurogenesis. Nat. Neurosci. 2006, 9, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Wall, V.L.; Fischer, E.K.; Bland, S.T. Isolation Rearing Attenuates Social Interaction-Induced Expression of Immediate Early Gene Protein Products in the Medial Prefrontal Cortex of Male and Female Rats. Physiol. Behav. 2012, 107, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, Z.S.; Vaz, A.; Bordes, J.; Ünlü, S.; Sep, M.S.C.; Vinkers, C.H.; Pinto, L.; Yapici-Eser, H. Translational Models of Stress and Resilience: An Applied Neuroscience Methodology Review. Neurosci. Appl. 2024, 3, 104064. [Google Scholar] [CrossRef]
- Stanisavljević, A.; Perić, I.; Gass, P.; Inta, D.; Lang, U.E.; Borgwardt, S.; Filipović, D. Fluoxetine Modulates Neuronal Activity in Stress-Related Limbic Areas of Adult Rats Subjected to the Chronic Social Isolation. Brain Res. Bull. 2020, 163, 95–108. [Google Scholar] [CrossRef]
- Todorović, N.; Filipović, D. The Antidepressant- and Anxiolytic-like Effects of Fluoxetine and Clozapine in Chronically Isolated Rats Involve Inhibition of Hippocampal TNF-α. Pharmacol. Biochem. Behav. 2017, 163, 57–65. [Google Scholar] [CrossRef]
- Stanisavljević, A.; Perić, I.; Bernardi, R.E.; Gass, P.; Filipović, D. Clozapine Increased C-Fos Protein Expression in Several Brain Subregions of Socially Isolated Rats. Brain Res. Bull. 2019, 152, 35–44. [Google Scholar] [CrossRef]
- Aparicio, L.; Fierro, L.; Abreu, A.; Cárdenas, T.; Hernández, G.; Ávila, C.; Durán, R.; Aguilar, H.; Denes, M.; Chi-Castañeda, D.; et al. Current Opinion on the Use of C-Fos in Neuroscience. NeuroSci 2022, 3, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, S.M. Lighting Up Neuronal Pathways: The Development of a Novel Transgenic Rat That Identifies Fos-Activated Neurons Using a Red Fluorescent Protein. Endocrinology 2009, 150, 5199. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, Z.; Ho, K.P.; Qian, Z. Effects of Spinal Cord Injury on C-Fos Expression in Hypothalamic Paraventricular Nucleus and Supraoptic Nucleus in Rats. Brain Res. 2006, 1087, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Lerea, L.S.; Butler, L.S.; McNamara, J.O. NMDA and Non-NMDA Receptor-Mediated Increase of c-Fos MRNA in Dentate Gyrus Neurons Involves Calcium Influx via Different Routes. J. Neurosci. 1992, 12, 2973–2981. [Google Scholar] [CrossRef]
- Johansen, F.-E.; Prywes, R. Two Pathways for Serum Regulation of the C-Fos Serum Response Element Require Specific Sequence Elements and a Minimal Domain of Serum Response Factor. Mol. Cell. Biol. 1994, 14, 5920. [Google Scholar] [CrossRef]
- Tu, Y.C.; Huang, D.Y.; Shiah, S.G.; Wang, J.S.; Lin, W.W. Regulation of C-Fos Gene Expression by NF-ΚB: A P65 Homodimer Binding Site in Mouse Embryonic Fibroblasts but Not Human HEK293 Cells. PLoS ONE 2013, 8, e84062. [Google Scholar] [CrossRef]
- Sheng, M.; Greenberg, M.E. The Regulation and Function of C-Fos and Other Immediate Early Genes in the Nervous System. Neuron 1990, 4, 477–485. [Google Scholar] [CrossRef]
- Hudson, A.E. Genetic Reporters of Neuronal Activity: C-Fos and G-CaMP6. Methods Enzymol. 2018, 603, 197–220. [Google Scholar] [CrossRef]
- Sheng, M.; McFadden, G.; Greenberg, M.E. Membrane Depolarization and Calcium Induce C-Fos Transcription via Phosphorylation of Transcription Factor CREB. Neuron 1990, 4, 571–582. [Google Scholar] [CrossRef]
- Cruz-Mendoza, F.; Jauregui-Huerta, F.; Luquin, S.; Aguilar-Delgadillo, A.; García-Estrada, J. Immediate Early Gene C-Fos in the Brain: Focus on Glial Cells. Brain Sci. 2022, 12, 687. [Google Scholar] [CrossRef]
- Edling, Y.; Ingelman-Sundberg, M.; Simi, A. Glutamate Activates C-Fos in Glial Cells via a Novel Mechanism Involving the Glutamate Receptor Subtype MGlu5 and the Transcriptional Repressor DREAM. Glia 2007, 55, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Pecháň, P.A.; Chowdhury, K.; Gerdes, W.; Seifert, W. Glutamate Induces the Growth Factors NGF, BFGF, the Receptor FGF-R1 and c-Fos MRNA Expression in Rat Astrocyte Culture. Neurosci. Lett. 1993, 153, 111–114. [Google Scholar] [CrossRef]
- Liu, H.-N.; Almazan, G. Glutamate Induces C-Fos Proto-Oncogene Expression and Inhibits Proliferation in Oligodendrocyte Progenitors: Receptor Characterization. Eur. J. Neurosci. 1995, 7, 2355–2363. [Google Scholar] [CrossRef]
- Reul, J.M.H.M. Making Memories of Stressful Events: A Journey Along Epigenetic, Gene Transcription, and Signaling Pathways. Front. Psychiatry 2014, 5, 5. [Google Scholar] [CrossRef]
- Ishida, H.; Mitsui, K.; Nukaya, H.; Matsumoto, K.; Tsuji, K. Study of Active Substances Involved in Skin Dysfunction Induced by Crowding Stress. I. Effect of Crowding and Isolation on Some Physiological Variables, Skin Function and Skin Blood Perfusion in Hairless Mice. Biol. Pharm. Bull. 2003, 26, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Garzón, J.; Del Río, J. Hyperactivity Induced in Rats by Long-Term Isolation: Further Studies on a New Animal Model for the Detection of Antidepressants. Eur. J. Pharmacol. 1981, 74, 287–294. [Google Scholar] [CrossRef]
- Filipović, D.; Novak, B.; Xiao, J.; Yan, Y.; Yeoh, K.; Turck, C.W. Chronic Fluoxetine Treatment of Socially Isolated Rats Modulates Prefrontal Cortex Proteome. Neuroscience 2022, 501, 52–71. [Google Scholar] [CrossRef]
- Zlatković, J.; Todorović, N.; Bošković, M.; Pajović, S.B.; Demajo, M.; Filipović, D. Different Susceptibility of Prefrontal Cortex and Hippocampus to Oxidative Stress Following Chronic Social Isolation Stress. Mol. Cell. Biochem. 2014, 393, 43–57. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.P.; de Andrade, J.S.; Spina, M.; Chamon, J.V.; Silva, P.H.D.; Werder, A.K.; Ortolani, D.; de Santana Cardoso Thomaz, L.; Romariz, S.; Ribeiro, D.A.; et al. Clozapine Prevented Social Interaction Deficits and Reduced C-Fos Immunoreactivity Expression in Several Brain Areas of Rats Exposed to Acute Restraint Stress. PLoS ONE 2022, 17, e0262728. [Google Scholar] [CrossRef]
- Pinna, A.; Costa, G.; Contu, L.; Morelli, M. Fos Expression Induced by Olanzapine and Risperidone in the Central Extended Amygdala. Eur. J. Pharmacol. 2019, 865, 172764. [Google Scholar] [CrossRef]
- Perić, I.; Stanisavljević, A.; Gass, P.; Filipović, D. Fluoxetine Reverses Behavior Changes in Socially Isolated Rats: Role of the Hippocampal GSH-Dependent Defense System and Proinflammatory Cytokines. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, L.M.; Gullone, E. The Clinical Significance of Loneliness: A Literature Review. Clin. Psychol. Rev. 2006, 26, 695–718. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Du Preez, J.L.; Viljoen, F.P.; Berk, M.; Emsley, R.; Harvey, B.H. Social Isolation Rearing Induces Mitochondrial, Immunological, Neurochemical and Behavioural Deficits in Rats, and Is Reversed by Clozapine or N-Acetyl Cysteine. Brain Behav. Immun. 2013, 30, 156–167. [Google Scholar] [CrossRef]
- Filipović, D.; Gavrilović, L.; Dronjak, S.; Radojčić, M.B. Brain Glucocorticoid Receptor and Heat Shock Protein 70 Levels in Rats Exposed to Acute, Chronic or Combined Stress. Neuropsychobiology 2005, 51, 107–114. [Google Scholar] [CrossRef]
- Todorović, N.; Filipović, D. Prefrontal Cortical Glutathione-Dependent Defense and Proinflammatory Mediators in Chronically Isolated Rats: Modulation by Fluoxetine or Clozapine. Neuroscience 2017, 355, 49–60. [Google Scholar] [CrossRef]
- Zlatković, J.; Filipović, D. Chronic Social Isolation Induces NF-ΚB Activation and Upregulation of INOS Protein Expression in Rat Prefrontal Cortex. Neurochem. Int. 2013, 63, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Novak, B.; Xiao, J.; Yan, Y.; Bernardi, R.E.; Turck, C.W. Chronic Fluoxetine Treatment in Socially-Isolated Rats Modulates the Prefrontal Cortex Synaptoproteome. J. Proteom. 2023, 282, 104925. [Google Scholar] [CrossRef]
- Unal, G. Social Isolation as a Laboratory Model of Depression. In Mental Health Effects of COVID-19; Elsevier: Amsterdam, The Netherlands, 2021; pp. 133–151. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Lv, Q.Y.; Chen, M.M.; Li, Y.; Yu, Y.; Liao, H. Brain Circuit Dysfunction in Specific Symptoms of Depression. Eur. J. Neurosci. 2022, 55, 2393–2403. [Google Scholar] [CrossRef]
- Felippe, R.M.; Oliveira, G.M.; Barbosa, R.S.; Esteves, B.D.; Gonzaga, B.M.S.; Horita, S.I.M.; Garzoni, L.R.; Beghini, D.G.; Araújo-Jorge, T.C.; Fragoso, V.M.S. Experimental Social Stress: Dopaminergic Receptors, Oxidative Stress, and c-Fos Protein Are Involved in Highly Aggressive Behavior. Front. Cell. Neurosci. 2021, 15, 696834. [Google Scholar] [CrossRef]
- Perić, I.; Costina, V.; Stanisavljević, A.; Findeisen, P.; Filipović, D. Proteomic Characterization of Hippocampus of Chronically Socially Isolated Rats Treated with Fluoxetine: Depression-like Behaviour and Fluoxetine Mechanism of Action. Neuropharmacology 2018, 135, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Yan, G.; Xuan, Y.; Peng, H.; Huang, Q.J.; Wu, R.; Xu, H. Chronic Social Isolation Decreases Glutamate and Glutamine Levels and Induces Oxidative Stress in the Rat Hippocampus. Behav. Brain Res. 2015, 282, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Nasir, B.; ul Haq, I.; Kim, S.J. Oxidative Stress, Consequences and ROS Mediated Cellular Signaling in Rheumatoid Arthritis. Chem. Biol. Interact. 2018, 281, 121–136. [Google Scholar] [CrossRef] [PubMed]
- März, P.; Herget, T.; Lang, E.; Otten, U.; Rose-John, S. Activation of Gp130 by IL-6/Soluble IL-6 Receptor Induces Neuronal Differentiation. Eur. J. Neurosci. 1997, 9, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Bongartz, H.; Seiß, E.A.; Bock, J.; Schaper, F. Glucocorticoids Attenuate Interleukin-6-Induced c-Fos and Egr1 Expression and Impair Neuritogenesis in PC12 Cells. J. Neurochem. 2021, 157, 532–549. [Google Scholar] [CrossRef]
- Stanisavljević Ilić, A.; Đorđević, S.; Inta, D.; Borgwardt, S.; Filipović, D. Olanzapine Effects on Parvalbumin/GAD67 Cell Numbers in Layers/Subregions of Dorsal Hippocampus of Chronically Socially Isolated Rats. Int. J. Mol. Sci. 2023, 24, 17181. [Google Scholar] [CrossRef]
- Musardo, S.; Contestabile, A.; Knoop, M.; Baud, O.; Bellone, C. Oxytocin Neurons Mediate the Effect of Social Isolation via the VTA Circuits. Elife 2022, 11, 73421. [Google Scholar] [CrossRef]
- Ieraci, A.; Mallei, A.; Popoli, M. Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast. 2016, 2016, 6212983. [Google Scholar] [CrossRef]
- Guven, E.B.; Pranic, N.M.; Unal, G. The Differential Effects of Brief Environmental Enrichment Following Social Isolation in Rats. Cogn. Affect. Behav. Neurosci. 2022, 22, 818–832. [Google Scholar] [CrossRef]
- Zorzo, C.; Méndez-López, M.; Méndez, M.; Arias, J.L. Adult Social Isolation Leads to Anxiety and Spatial Memory Impairment: Brain Activity Pattern of COx and c-Fos. Behav. Brain Res. 2019, 365, 170–177. [Google Scholar] [CrossRef]
- Opalka, A.N.; Wang, D.V. Hippocampal Efferents to Retrosplenial Cortex and Lateral Septum Are Required for Memory Acquisition. Learn. Mem. 2020, 27, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, N.; Corcoran, K.A.; Guedea, A.L.; Shepherd, G.M.G.; Radulovic, J. Differential Contributions of Glutamatergic Hippocampal→Retrosplenial Cortical Projections to the Formation and Persistence of Context Memories. Cereb. Cortex 2019, 29, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T.; Rockland, K.S. GABAergic Projections from the Hippocampus to the Retrosplenial Cortex in the Rat. Eur. J. Neurosci. 2007, 26, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Schumann, C.M.; Van Hoesen, G.W. Evidence for Direct Projections from the Basal Nucleus of the Amygdala to Retrosplenial Cortex in the Macaque Monkey. Exp. Brain Res. 2008, 186, 47–57. [Google Scholar] [CrossRef]
- Dziewiątkowski, J.; Spodnik, J.H.; Biranowska, J.; Kowiański, P.; Majak, K.; Moryś, J. The Projection of the Amygdaloid Nuclei to Various Areas of the Limbic Cortex in the Rat. Folia Morphol. 1997, 57, 301–308. [Google Scholar]
- Mitchell, A.S.; Czajkowski, R.; Zhang, N.; Jeffery, K.; Nelson, A.J.D. Retrosplenial Cortex and Its Role in Spatial Cognition. Brain Neurosci. Adv. 2018, 2, 239821281875709. [Google Scholar] [CrossRef]
- Vertes, R.P.; Hoover, W.B. Projections of the Paraventricular and Paratenial Nuclei of the Dorsal Midline Thalamus in the Rat. J. Comp. Neurol. 2008, 508, 212–237. [Google Scholar] [CrossRef]
- Li, S.; Kirouac, G.J. Projections from the Paraventricular Nucleus of the Thalamus to the Forebrain, with Special Emphasis on the Extended Amygdala. J. Comp. Neurol. 2008, 506, 263–287. [Google Scholar] [CrossRef]
- Barson, J.R.; Mack, N.R.; Gao, W.J. The Paraventricular Nucleus of the Thalamus Is an Important Node in the Emotional Processing Network. Front. Behav. Neurosci. 2020, 14, 598469. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wei, C.; Wang, H.; Sui, N.; Kirouac, G.J. Orexins in the Paraventricular Nucleus of the Thalamus Mediate Anxiety-like Responses in Rats. Psychopharmacology 2010, 212, 251–265. [Google Scholar] [CrossRef]
- Gao, C.; Gohel, C.A.; Leng, Y.; Ma, J.; Goldman, D.; Levine, A.J.; Penzo, M.A. Molecular and Spatial Profiling of the Paraventricular Nucleus of the Thalamus. Elife 2023, 12, e81818. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.T.; Kirouac, G.J.; Zubieta, J.K.; Bhatnagar, S. Contributions of the Paraventricular Thalamic Nucleus in the Regulation of Stress, Motivation, and Mood. Front. Behav. Neurosci. 2014, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Terranova, J.I.; Yokose, J.; Osanai, H.; Marks, W.D.; Yamamoto, J.; Ogawa, S.K.; Kitamura, T. Hippocampal-Amygdala Memory Circuits Govern Experience-Dependent Observational Fear. Neuron 2022, 110, 1416–1431.e13. [Google Scholar] [CrossRef]
- Rei, D.; Mason, X.; Seo, J.; Gräff, J.; Rudenko, A.; Wang, J.; Rueda, R.; Siegert, S.; Cho, S.; Canter, R.G.; et al. Basolateral Amygdala Bidirectionally Modulates Stress-Induced Hippocampal Learning and Memory Deficits through a P25/Cdk5-Dependent Pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 7291–7296. [Google Scholar] [CrossRef]
- Maren, S.; Fanselow, M.S. Synaptic Plasticity in the Basolateral Amygdala Induced by Hippocampal Formation Stimulation in Vivo. J. Neurosci. 1995, 15, 7548–7564. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.-W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- McDonald, A.J.; Mott, D.D. Functional Neuroanatomy of Amygdalohippocampal Interconnections and Their Role in Learning and Memory. J. Neurosci. Res. 2017, 95, 797. [Google Scholar] [CrossRef] [PubMed]
- Kerfoot, E.C.; Williams, C.L. Contributions of the Nucleus Accumbens Shell in Mediating the Enhancement in Memory Following Noradrenergic Activation of Either the Amygdala or Hippocampus. Front. Pharmacol. 2018, 9, 47. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, D.; He, X.; Feng, Q.; Lin, R.; Xu, F.; Fu, L.; Luo, M. Whole-Brain Mapping of Inputs to Projection Neurons and Cholinergic Interneurons in the Dorsal Striatum. PLoS ONE 2015, 10, e0123381. [Google Scholar] [CrossRef]
- Monko, M.E.; Heilbronner, S.R. Retrosplenial Cortical Connectivity with Frontal Basal Ganglia Networks. J. Cogn. Neurosci. 2021, 33, 1096–1105. [Google Scholar] [CrossRef]
- Jacobson, L.; Sapolsky, R. The Role of the Hippocampus in Feedback Regulation of the Hypothalamic-Pituitary-Adrenocortical Axis. Endocr. Rev. 1991, 12, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; MacQueen, G. The Role of the Hippocampus in the Pathophysiology of Major Depression. J. Psychiatry Neurosci. 2004, 29, 417. [Google Scholar] [PubMed]
- Tzakis, N.; Holahan, M.R. Social Memory and the Role of the Hippocampal CA2 Region. Front. Behav. Neurosci. 2019, 13, 233. [Google Scholar] [CrossRef]
- Janak, P.H.; Tye, K.M. From Circuits to Behaviour in the Amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef]
- Drevets, W.C. Neuroimaging Abnormalities in the Amygdala in Mood Disorders. Ann. N. Y. Acad. Sci. 2003, 985, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.; Chen, X.; Zhang, Z.; Xiao, L.; Zhou, Y. Anhedonia Correlates with Functional Connectivity of the Nucleus Accumbens Subregions in Patients with Major Depressive Disorder. Neuroimage 2021, 30, 102599. [Google Scholar] [CrossRef] [PubMed]
- Britt, J.P.; Benaliouad, F.; McDevitt, R.A.; Stuber, G.D.; Wise, R.A.; Bonci, A. Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron 2012, 76, 790–803. [Google Scholar] [CrossRef]
- LeGates, T.A.; Kvarta, M.D.; Tooley, J.R.; Francis, T.C.; Lobo, M.K.; Creed, M.C.; Thompson, S.M. Reward Behaviour Is Regulated by the Strength of Hippocampus-Nucleus Accumbens Synapses. Nature 2018, 564, 258–262. [Google Scholar] [CrossRef]
- Deutch, A.Y.; Öngür, D.; Duman, R.S. Antipsychotic Drugs Induce Fos Protein in the Thalamic Paraventricular Nucleus: A Novel Locus of Antipsychotic Drug Action. Neuroscience 1995, 66, 337–346. [Google Scholar] [CrossRef]
- Noseda, R.; Borsook, D.; Burstein, R. Neuropeptides and Neurotransmitters That Modulate Thalamo-Cortical Pathways Relevant to Migraine Headache. Headache 2017, 57, 97. [Google Scholar] [CrossRef]
- Yoshino, Y.; Ochi, S.; Yamazaki, K.; Nakata, S.; Iga, J.I.; Ueno, S.I. Endothelial Nitric Oxide Synthase in Rat Brain Is Downregulated by Sub-Chronic Antidepressant Treatment. Psychopharmacology 2017, 234, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Leroy, F.; Martyniuk, K.M.; Feng, W.; McManus, E.; Bailey, M.R.; Javitch, J.A.; Balsam, P.D.; Kellendonk, C. Dopamine D2 Receptors in the Paraventricular Thalamus Attenuate Cocaine Locomotor Sensitization. eNeuro 2017, 4, ENEURO.0227-17.2017. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P. Antipsychotic Drugs, Dopamine Receptors, and Schizophrenia. Clin. Neurosci. Res. 2001, 1, 53–60. [Google Scholar] [CrossRef]
- León, L.A.; Cardenas, F.P. Contribution of the Dopaminergic System to the Effect of Chronic Fluoxetine in the Rat Forced Swim Test. Psychol. Neurosci. 2008, 1, 81–86. [Google Scholar] [CrossRef]
- Collu, M.; Poggiu, A.S.; Devoto, P.; Serra, G. Behavioural Sensitization of Mesolimbic Dopamine D2 Receptors in Chronic Fluoxetine-Treated Rats. Eur. J. Pharmacol. 1997, 322, 123–127. [Google Scholar] [CrossRef]
- Ferrara, N.C.; Trask, S.; Rosenkranz, J.A. Maturation of Amygdala Inputs Regulate Shifts in Social and Fear Behaviors: A Substrate for Developmental Effects of Stress. Neurosci. Biobehav. Rev. 2021, 125, 11–25. [Google Scholar] [CrossRef]
- Young, K.D.; Friedman, E.S.; Collier, A.; Berman, S.R.; Feldmiller, J.; Haggerty, A.E.; Thase, M.E.; Siegle, G.J. Response to SSRI Intervention and Amygdala Activity during Self-Referential Processing in Major Depressive Disorder. Neuroimage 2020, 28, 102388. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Lan, X.; Li, W.; Zhang, F.; Fu, L.; Ye, Y.; Ning, Y.; Zhou, Y. Functional Connectivity of the Amygdala and the Antidepressant and Antisuicidal Effects of Repeated Ketamine Infusions in Major Depressive Disorder. Front. Neurosci. 2023, 17, 1123797. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Suckling, J.; Ooi, C.; Fu, C.H.Y.; Williams, S.C.R.; Walsh, N.D.; Mitterschiffthaler, M.T.; Pich, E.M.; Bullmore, E. Functional Coupling of the Amygdala in Depressed Patients Treated with Antidepressant Medication. Neuropsychopharmacology 2007, 33, 1909–1918. [Google Scholar] [CrossRef]
- Pinna, A.; Morelli, M. Differential Induction of Fos-Like-Immunoreactivity in the Extended Amygdala after Haloperidol and Clozapine. Neuropsychopharmacology 1999, 21, 93–100. [Google Scholar] [CrossRef]
- Hall, H.; Sedvall, G.; Magnusson, O.; Kopp, J.; Halldin, C.; Farde, L. Distribution of D1- and D2-Dopamine Receptors, and Dopamine and Its Metabolites in the Human Brain. Neuropsychopharmacology 1994, 11, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.F. Disentangling the Diverse Roles of Dopamine D2 Receptors in Striatal Function and Behavior. Neurochem. Int. 2019, 125, 35. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Zipursky, R.B.; Remington, G.; Jones, C.; Dasilva, J.; Wilson, A.A.; Houle, S. 5-HT2 and D2 Receptor Occupancy of Olanzapine in Schizophrenia: A PET Investigation. Am. J. Psychiatry 1998, 155, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.S.; Panicker, M.M. Identifying the In Vivo Cellular Correlates of Antipsychotic Drugs. eNeuro 2018, 5, ENEURO.0220-18.2018. [Google Scholar] [CrossRef]
- Wyass, J.M.; Van Groen, T. Connections between the Retrosplenial Cortex and the Hippocampal Formation in the Rat: A Review. Hippocampus 1992, 2, 1–11. [Google Scholar] [CrossRef]
- Palomero-Gallagher, N.; Zilles, K. Isocortex. In The Rat Nervous System; Elsevier Inc.: Amsterdam, The Netherlands, 2004; pp. 729–757. ISBN 9780080542614. [Google Scholar]
- Cho, D.I.; Quan, W.Y.; Oak, M.H.; Choi, H.J.; Lee, K.Y.; Kim, K.M. Functional Interaction between Dopamine Receptor Subtypes for the Regulation of C-Fos Expression. Biochem. Biophys. Res. Commun. 2007, 357, 1113–1118. [Google Scholar] [CrossRef]
- Meltzer, H.Y. An Overview of the Mechanism of Action of Clozapine. J. Clin. Psychiatry 1994, 55, 47–52. [Google Scholar]
- Ferreira-Fernandes, E.; Pinto-Correia, B.; Quintino, C.; Remondes, M. A Gradient of Hippocampal Inputs to the Medial Mesocortex. Cell Rep. 2019, 29, 3266–3279.e3. [Google Scholar] [CrossRef]
- Simpson, E.H.; Gallo, E.F.; Balsam, P.D.; Javitch, J.A.; Kellendonk, C. How Changes in Dopamine D2 Receptor Levels Alter Striatal Circuit Function and Motivation. Mol. Psychiatry 2022, 27, 436. [Google Scholar] [CrossRef]
- Gerfen, C.R. Segregation of D1 and D2 Dopamine Receptors in the Striatal Direct and Indirect Pathways: An Historical Perspective. Front. Synaptic Neurosci. 2022, 14, 1002960. [Google Scholar] [CrossRef]
- Goto, Y.; Grace, A.A. Dopaminergic Modulation of Limbic and Cortical Drive of Nucleus Accumbens in Goal-Directed Behavior. Nat. Neurosci. 2005, 8, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.N.; Abbas, A.I.; Farrell, M.S.; Setola, V.; Sciaky, N.; Huang, X.P.; Kroeze, W.K.; Crawford, L.K.; Piel, D.A.; Keiser, M.J.; et al. The Presynaptic Component of the Serotonergic System Is Required for Clozapine’s Efficacy. Neuropsychopharmacology 2011, 36, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, K.; Smith, S.E.; Zetterström, T.S.C.; Pei, Q.; Franklin, M.; Sharp, T. Effect of Antidepressant Drugs on Dopamine D1 and D2 Receptor Expression and Dopamine Release in the Nucleus Accumbens of the Rat. Psychopharmacology 1998, 140, 470–477. [Google Scholar] [CrossRef]
- Alex, K.D.; Pehek, E.A. Pharmacologic Mechanisms of Serotonergic Regulation of Dopamine Neurotransmission. Pharmacol. Ther. 2007, 113, 296–320. [Google Scholar] [CrossRef]
- Yoshimoto, K.; McBride, W.J. Regulation of Nucleus Accumbens Dopamine Release by the Dorsal Raphe Nucleus in the Rat. Neurochem. Res. 1992, 17, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Reed, M.C.; Nijhout, H.F. Computational Studies of the Role of Serotonin in the Basal Ganglia. Front. Integr. Neurosci. 2013, 7, 46737. [Google Scholar] [CrossRef]
- Voorn, P.; Vanderschuren, L.J.M.J.; Groenewegen, H.J.; Robbins, T.W.; Pennartz, C.M.A. Putting a Spin on the Dorsal-Ventral Divide of the Striatum. Trends Neurosci. 2004, 27, 468–474. [Google Scholar] [CrossRef]
- French, S.J.; Totterdell, S. Individual Nucleus Accumbens-Projection Neurons Receive Both Basolateral Amygdala and Ventral Subicular Afferents in Rats. Neuroscience 2003, 119, 19–31. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Fan, G.; Li, A.; Yuan, J.; Xu, T. Cell-Type-Specific Afferent Innervation of the Nucleus Accumbens Core and Shell. Front. Neuroanat. 2018, 12, 412069. [Google Scholar] [CrossRef]
- Penzo, M.A.; Gao, C. The Paraventricular Nucleus of the Thalamus: An Integrative Node Underlying Homeostatic Behavior. Trends Neurosci. 2021, 44, 538–549. [Google Scholar] [CrossRef]
- Pisa, M. Motor Functions of the Striatum in the Rat: Critical Role of the Lateral Region in Tongue and Forelimb Reaching. Neuroscience 1988, 24, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Hamamura, T.; Lee, Y.; Miyata, S.; Habara, T.; Endo, S.; Taoka, H.; Kuroda, S. Atypical Properties of Several Classes of Antipsychotic Drugs on the Basis of Differential Induction of Fos-like Immunoreactivity in the Rat Brain. Life Sci. 2004, 76, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.S.; Fibiger, H.C. Effects of Olanzapine on Regional C-Fos Expression in Rat Forebrain. Neuropsychopharmacology 1996, 14, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.K.; Kim, K.O.; Lee, S.H.; Lee, Y.S.; Son, H. C-Fos Expression by Dopaminergic Receptor Activation in Rat Hippocampal Neurons. Mol. Cells 2000, 10, 546–551. [Google Scholar] [CrossRef]
- Kobayashi, K.; Haneda, E.; Higuchi, M.; Suhara, T.; Suzuki, H. Chronic Fluoxetine Selectively Upregulates Dopamine D1-Like Receptors in the Hippocampus. Neuropsychopharmacology 2012, 37, 1500. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ikeda, Y.; Sakai, A.; Yamasaki, N.; Haneda, E.; Miyakawa, T.; Suzuki, H. Reversal of Hippocampal Neuronal Maturation by Serotonergic Antidepressants. Proc. Natl. Acad. Sci. USA 2010, 107, 8434. [Google Scholar] [CrossRef]
- Cherubini, E.; Miles, R. The CA3 Region of the Hippocampus: How Is It? What Is It for? How Does It Do It? Front. Cell. Neurosci. 2015, 9, 19. [Google Scholar] [CrossRef]
- Li, X.; Liang, S.; Li, Z.; Li, S.; Xia, M.; Verkhratsky, A.; Li, B. Leptin Increases Expression of 5-HT2B Receptors in Astrocytes Thus Enhancing Action of Fluoxetine on the Depressive Behavior Induced by Sleep Deprivation. Front. Psychiatry 2019, 10, 430121. [Google Scholar] [CrossRef]
- Guo, N.; Klitenick, M.A.; Tham, C.S.; Fibiger, H.C. Receptor Mechanisms Mediating Clozapine-Induced c-Fos Expression in the Forebrain. Neuroscience 1995, 65, 747–756. [Google Scholar] [CrossRef]
- Bymaster, F.; Perry, K.W.; Nelson, D.L.; Wong, D.T.; Rasmussen, K.; Moore, N.A.; Calligaro, D.O. Olanzapine: A Basic Science Update. Br. J. Psychiatry 1999, 174, 36–40. [Google Scholar] [CrossRef]
- Andrabi, S.S.; Vishnoi, S.; Madan, R.; Bhardwaj, N.; Tabassum, H.; Akram, M.; Parvez, S. Clozapine Improves Behavioral and Biochemical Outcomes in a MK-801-Induced Mouse Model of Schizophrenia. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Osacka, J.; Szelle Cernackova, A.; Horvathova, L.; Majercikova, Z.; Pirnik, Z.; Kiss, A. Clozapine Impact on C-Fos Expression in Mild Stress Preconditioned Male Rats Exposed to a Novelty Stressor. J. Neurosci. Res. 2018, 96, 1786–1797. [Google Scholar] [CrossRef] [PubMed]
- Tunc-Ozcan, E.; Peng, C.Y.; Zhu, Y.; Dunlop, S.R.; Contractor, A.; Kessler, J.A. Activating Newborn Neurons Suppresses Depression and Anxiety-like Behaviors. Nat. Commun. 2019, 10, 3768. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.M.; Goyal, A.; Ramdeen, N.; Hallas, B.H.; Horowitz, A.T.; Torres, G. Characterization of Fluoxetine plus Olanzapine Treatment in Rats: A Behavior, Endocrine, and Immediate-Early Gene Expression Analysis. Synapse 2003, 50, 353–364. [Google Scholar] [CrossRef]
- Sykes, D.A.; Moore, H.; Stott, L.; Holliday, N.; Javitch, J.A.; Robert Lane, J.; Charlton, S.J. Extrapyramidal Side Effects of Antipsychotics Are Linked to Their Association Kinetics at Dopamine D2 Receptors. Nat. Commun. 2017, 8, 763. [Google Scholar] [CrossRef]
| CSIS (Three and Six Weeks) vs. Controls | ||||
|---|---|---|---|---|
| Brain Region | Brain Subregion | ↑c-Fos Expression | ||
| CSIS (3 Weeks) [60] | CSIS (3 Weeks) [58] | CSIS (6 Weeks) [15] | ||
| RSC | RSGc/RSD | ** p < 0.01/ *** p < 0.001 | *** p < 0.001 | *** p < 0.001 |
| dHIPP | dDG, | *** p < 0.001 | * p < 0.05 | *** p < 0.001 |
| thalamus | PVP | ** p < 0.01 | *** p < 0.001 | *** p < 0.001 |
| amygdala | LA/BL complex of the amygdala | *** p < 0.001 | *** p < 0.001 | *** p < 0.001 |
| dorsal striatum/NAc | CPu/AcbSh | * p < 0.05/ *** p < 0.001 | * p < 0.05/ *** p < 0.001 | *** p < 0.001 |
| Olz-, Clz- or Flx-Treated Controls vs. Controls | |||
|---|---|---|---|
| Brain Region | Brain Subregion | Treatments | c-Fos Expression |
| thalamus | PVP | Olz, Clz or Flx | ↑ |
| amygdala | LA/BL complex of amygdala | Olz, Clz or Flx | ↑ |
| dorsal striatum | CPu | Olz or Clz | ↑ |
| NAc | AcbC | Olz or Clz | ↑ |
| NAc | AcbSh | Olz or Clz | ↑ |
| dHPP | dDG | Clz or Flx | ↑ |
| RSC | RSD | Clz or Flx | ↑ |
| RSC | RSGc | Clz or Flx | ↑ |
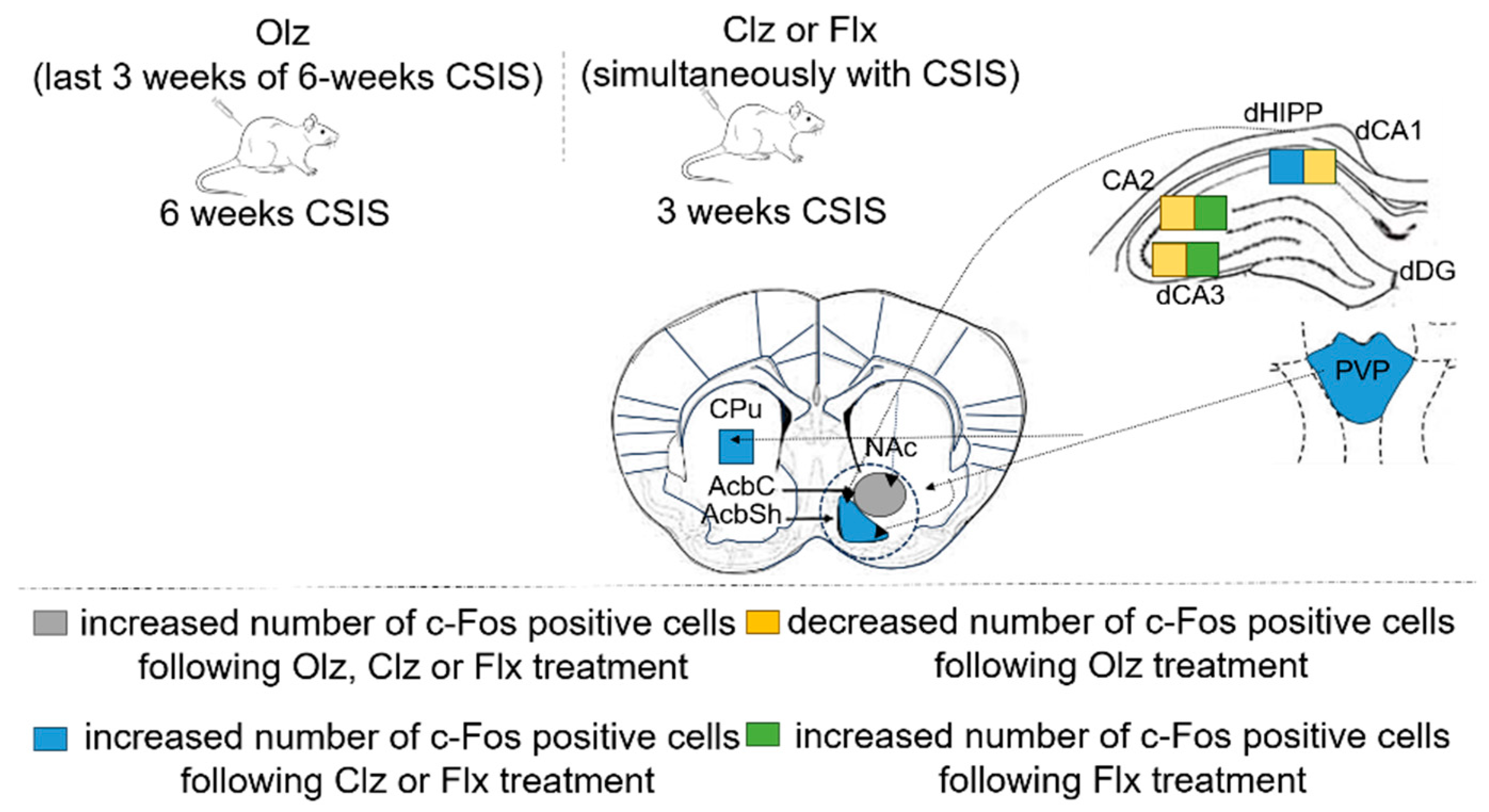
| Olz-, Clz- or Flx-Treated CSIS vs. CSIS | |||
|---|---|---|---|
| Brain Region | Brain Subregion | Treatments | c-Fos Expression |
| NAc | AcbC | Olz, Clz or Flx | ↑ |
| dHIPP | dCA1 | Clz or Flx/Olz | ↑/↓ |
| thalamus | PVP | Clz or Flx | ↑ |
| dorsal striatum/NAc | CPu/AcbSh | Clz or Flx | ↑ |
| dHIPP | CA2 | Flx/Olz | ↑/↓ |
| dHIPP | dCA3 | Flx/Olz | ↑/↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanisavljević Ilić, A.; Filipović, D. Mapping of c-Fos Expression in Rat Brain Sub/Regions Following Chronic Social Isolation: Effective Treatments of Olanzapine, Clozapine or Fluoxetine. Pharmaceuticals 2024, 17, 1527. https://doi.org/10.3390/ph17111527
Stanisavljević Ilić A, Filipović D. Mapping of c-Fos Expression in Rat Brain Sub/Regions Following Chronic Social Isolation: Effective Treatments of Olanzapine, Clozapine or Fluoxetine. Pharmaceuticals. 2024; 17(11):1527. https://doi.org/10.3390/ph17111527
Chicago/Turabian StyleStanisavljević Ilić, Andrijana, and Dragana Filipović. 2024. "Mapping of c-Fos Expression in Rat Brain Sub/Regions Following Chronic Social Isolation: Effective Treatments of Olanzapine, Clozapine or Fluoxetine" Pharmaceuticals 17, no. 11: 1527. https://doi.org/10.3390/ph17111527
APA StyleStanisavljević Ilić, A., & Filipović, D. (2024). Mapping of c-Fos Expression in Rat Brain Sub/Regions Following Chronic Social Isolation: Effective Treatments of Olanzapine, Clozapine or Fluoxetine. Pharmaceuticals, 17(11), 1527. https://doi.org/10.3390/ph17111527





