Phytochemicals from Passiflora coriacea Juss. Have Anti-Inflammatory and Neuroprotective Effects in Mouse Models
Abstract
:1. Introduction
2. Results
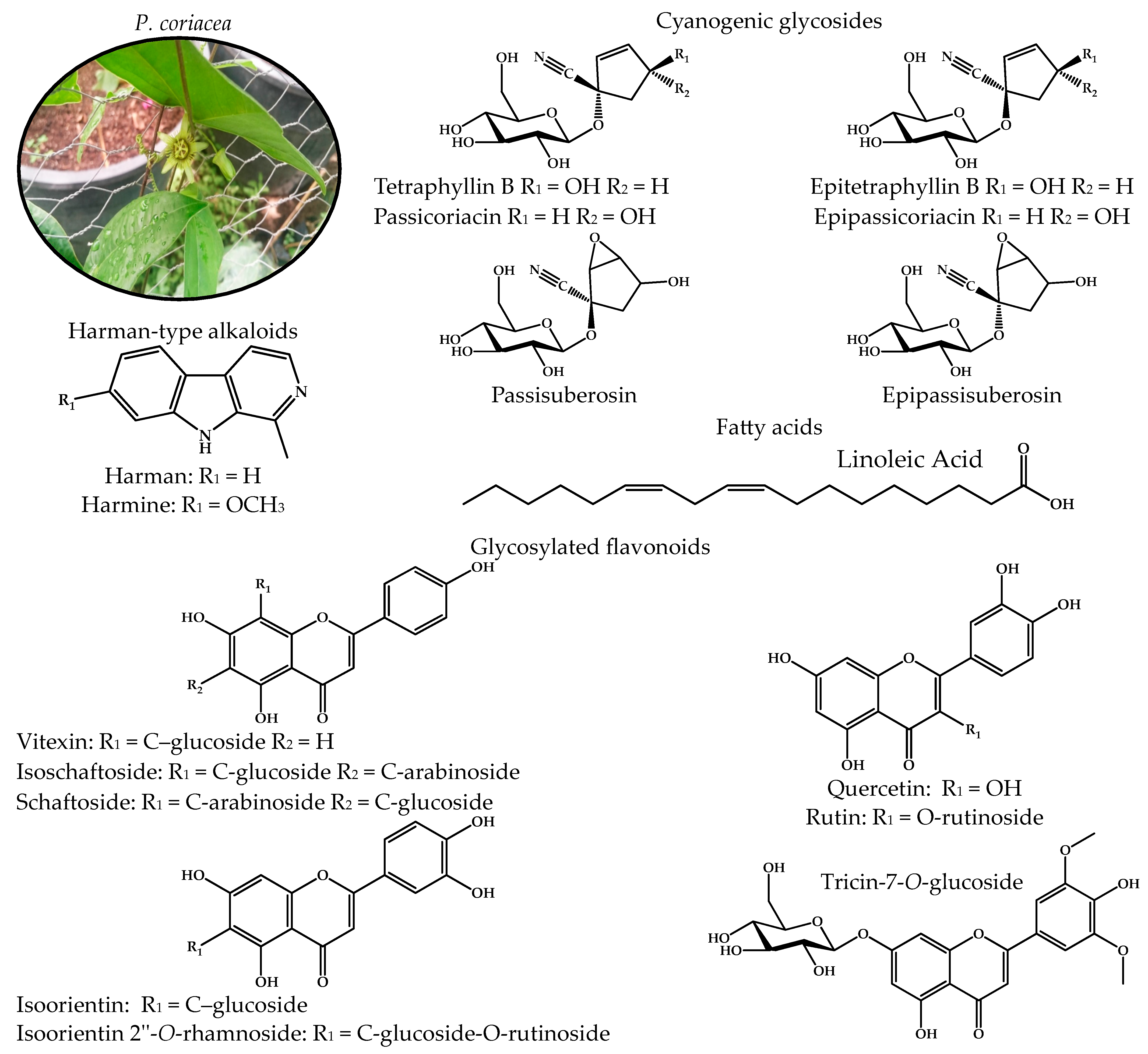
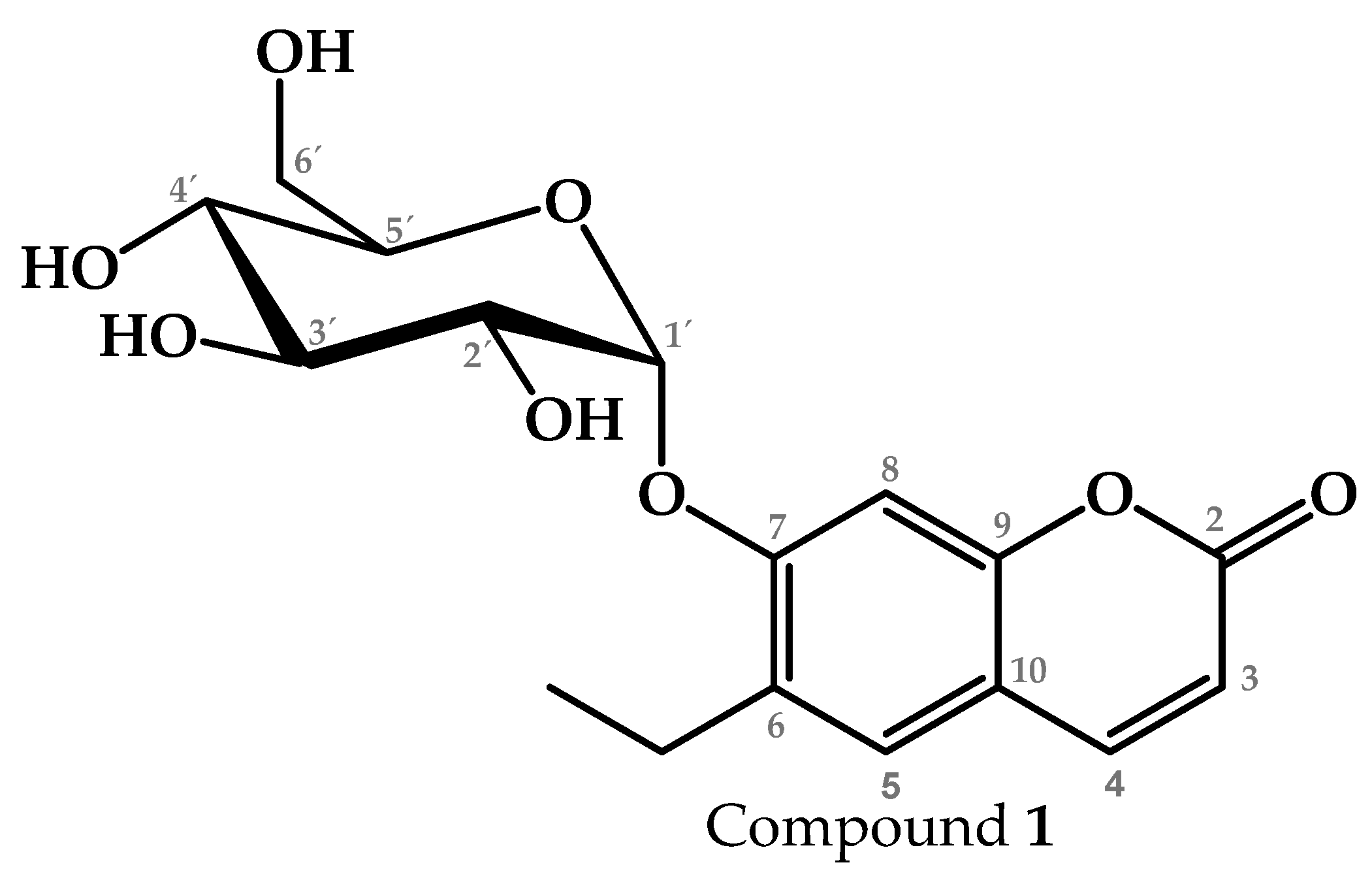
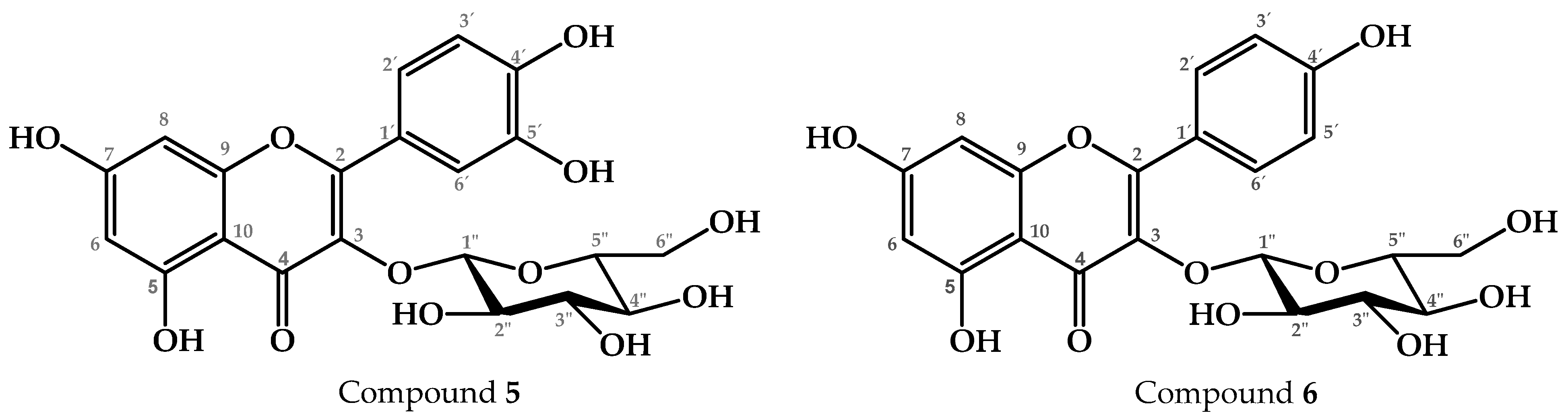
2.1. Chemical Profile of the Active Fractions of P. coriacea
2.2. Anti-Inflammatory Effect of P. coriacea Fractions
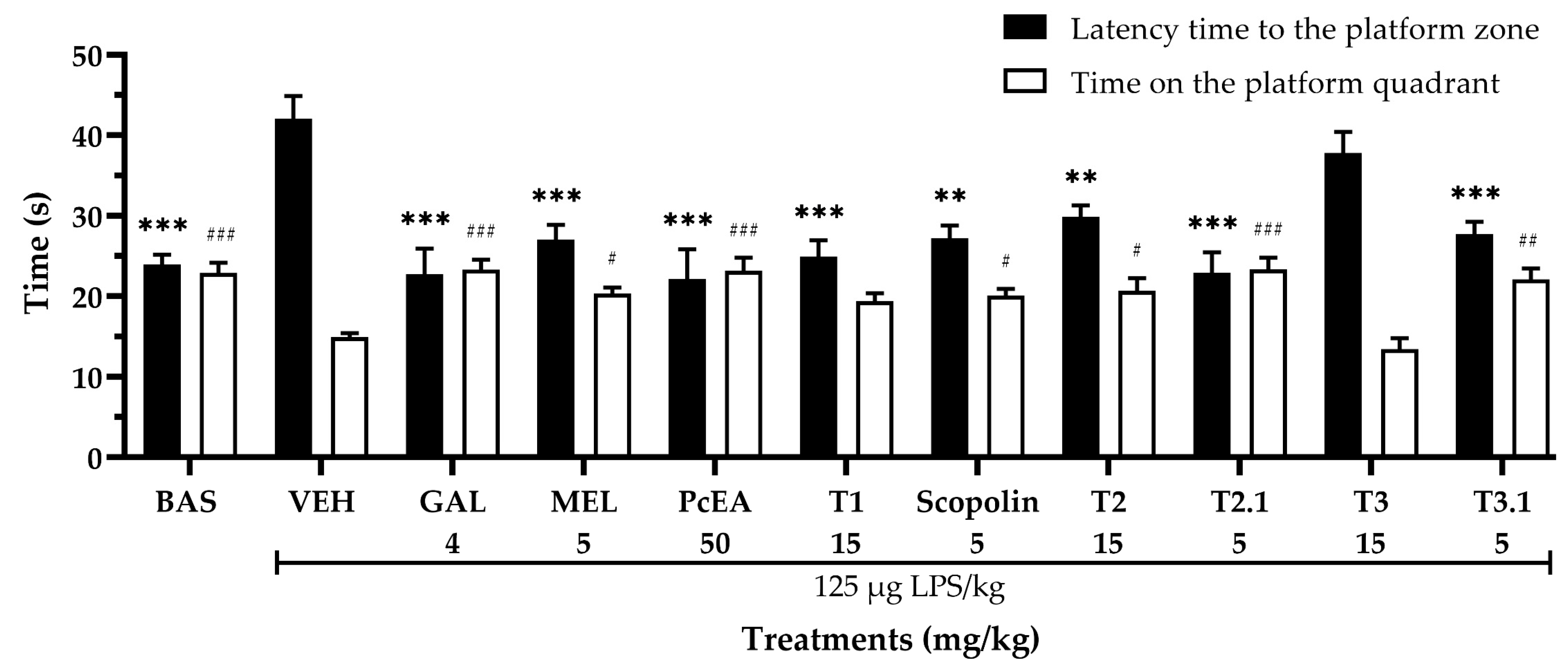
2.3. Neuroprotective Effect of P. coriacea Fractions
2.4. Sedative Effect of P. coriacea Fractions
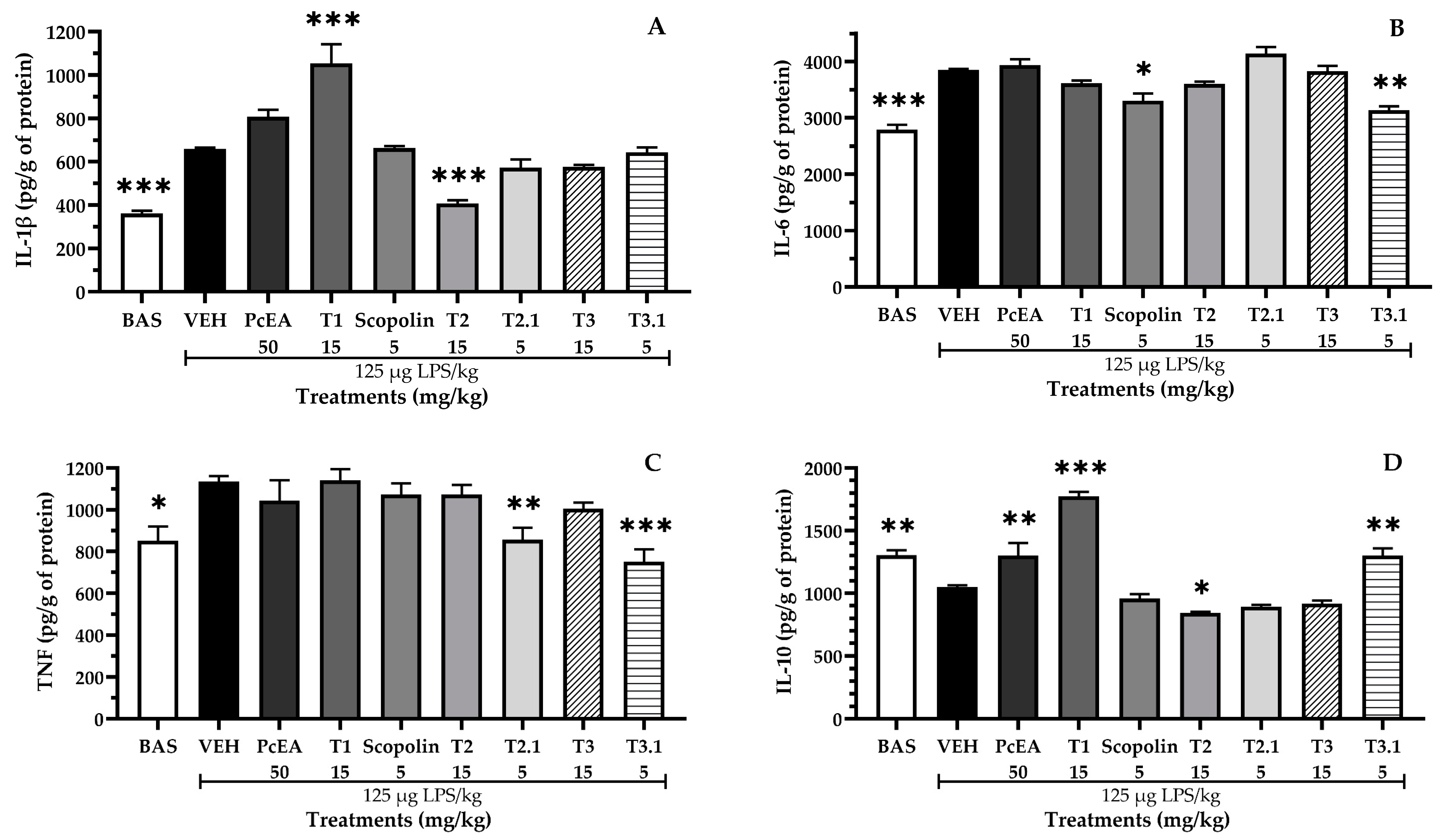
2.5. Immunomodulatory Effect of Fractions of P. coriacea
2.6. Effect of P. coriacea Fractions on Oxidative Stress Parameters
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation and Analysis of Extracts and Fractions
4.2.1. Chemical Fractionation of Active Extract (PcEA)
4.2.2. Isolation and Identification of Compound 1 from T1
4.2.3. NMR Analysis
4.2.4. Chemical Fractionation and Characterization of T2
4.2.5. CG–MS Analysis
4.2.6. Chemical Fractionation and Characterization of T3
4.2.7. HPLC Analysis
4.3. Experimental Animals
4.4. TPA-Induced Mouse Ear Edema
4.5. LPS-Induced Neuroinflammation Model
4.5.1. Morris Water Maze Test
4.5.2. Open Field Test
4.5.3. ELISA
4.5.4. Determination of SOD, GR, CAT, MDA, and NO
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, Y.-R.; Yang, W.-G.; Hou, X.-H.; Shen, D.-D.; Zhang, S.-N.; Li, Y.; Qiao, Y.-Y.; Wang, S.-Q.; Yuan, S.; Liu, H.-M. The Recent Advance of Interleukin-1 Receptor Associated Kinase 4 Inhibitors for the Treatment of Inflammation and Related Diseases. Eur. J. Med. Chem. 2023, 258, 115606. [Google Scholar] [CrossRef] [PubMed]
- Doty, K.R.; Guillot-Sestier, M.-V.; Town, T. The Role of the Immune System in Neurodegenerative Disorders: Adaptive or Maladaptive? Brain Res. 2015, 1617, 155–173. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Xia, Y.; Zhang, H.; Tian, J.; Shan, C.; Pang, F.; Wang, Y.; Shang, Y.; Chen, N. Effects of Multi-Mode Physical Stimulation on APP/PS1 Alzheimer’s Disease Model Mice. Heliyon 2022, 8, e12366. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 22 August 2024).
- Vyas, S.; Kothari, S.L.; Kachhwaha, S. Nootropic Medicinal Plants: Therapeutic Alternatives for Alzheimer’s Disease. J. Herb. Med. 2019, 17–18, 100291. [Google Scholar] [CrossRef]
- John, A.; Reddy, P.H. Synaptic Basis of Alzheimer’s Disease: Focus on Synaptic Amyloid Beta, P-Tau and Mitochondria. Ageing Res. Rev. 2021, 65, 101208. [Google Scholar] [CrossRef]
- Yao, G.; Bai, Z.; Niu, J.; Zhang, R.; Lu, Y.; Gao, T.; Wang, H. Astragalin Attenuates Depression-like Behaviors and Memory Deficits and Promotes M2 Microglia Polarization by Regulating IL-4R/JAK1/STAT6 Signaling Pathway in a Murine Model of Perimenopausal Depression. Psychopharmacology 2022, 239, 2421–2443. [Google Scholar] [CrossRef]
- Patel, K.S.; Dharamsi, A.; Priya, M.; Jain, S.; Mandal, V.; Girme, A.; Modi, S.J.; Hingorani, L. Saffron (Crocus sativus L.) Extract Attenuates Chronic Scopolamine-Induced Cognitive Impairment, Amyloid Beta, and Neurofibrillary Tangles Accumulation in Rats. J. Ethnopharmacol. 2024, 326, 117898. [Google Scholar] [CrossRef] [PubMed]
- Dhapola, R.; Beura, S.K.; Sharma, P.; Singh, S.K.; HariKrishnaReddy, D. Oxidative Stress in Alzheimer’s Disease: Current Knowledge of Signaling Pathways and Therapeutics. Mol. Biol. Rep. 2024, 51, 48. [Google Scholar] [CrossRef]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s Disease: Insight into the Signaling Pathways and Therapeutic Avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef]
- Argueta, A.; Cano, L.; Rodarte, M.E. Atlas de Las Plantas de La Medicina Tradicional Mexicana; Instituto Nacional Indigenista: Mexico City, Mexico, 1994; Volume 1. [Google Scholar]
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A Review Update. J. Ethnopharmacol. 2004, 94, 1–23. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H. Assessment of Total Phenolic, Antioxidant, and Antibacterial Activities of Passiflora Species. Sci. World J. 2014, 10, 167309. [Google Scholar] [CrossRef]
- Kim, G.-H.; Lim, K.; Yang, H.S.; Lee, J.-K.; Kim, Y.; Park, S.-K.; Kim, S.-H.; Park, S.; Kim, T.-H.; Moon, J.-S.; et al. Improvement in Neurogenesis and Memory Function by Administration of Passiflora incarnata L. Extract Applied to Sleep Disorder in Rodent Models. J. Chem. Neuroanat. 2019, 98, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Aman, Y.; Fang, E.F.; Tencomnao, T.P. Edulis Extract Protects Against Amyloid-β Toxicity in Alzheimer’s Disease Models Through Maintenance of Mitochondrial Homeostasis via the FOXO3/DAF-16 Pathway. Mol. Neurobiol. 2022, 59, 5612–5629. [Google Scholar] [CrossRef]
- Raza, A.; Mallhi, A.I.; Ahmad, W.; Naz, D.Z.; Cheka, D.A.; Sampath, V. Examining the therapeutic impact of Passiflora nitida kunth extract on neurodegeneration in an experimental alzheimer’s model. J. Popul. Ther. Clin. Pharmacol. 2024, 31, 2112–2118. [Google Scholar] [CrossRef]
- Jaroszewski, J.W.; Olafsdottir, E.S.; Wellendorph, P.; Christensen, J.; Franzyk, H.; Somanadhan, B.; Budnik, B.A.; Bolt Jørgensen, L.; Clausen, V. Cyanohydrin Glycosides of Passiflora: Distribution Pattern, a Saturated Cyclopentane Derivative from P. Guatemalensis, and Formation of Pseudocyanogenic α-Hydroxyamides as Isolation Artefacts. Phytochemistry 2002, 59, 501–511. [Google Scholar] [CrossRef]
- Ghedira, K.; Goetz, P. Passiflora incarnate L.: La Passiflore Officinale (Passifloraceae). Phytothérapie 2013, 11, 252–257. [Google Scholar] [CrossRef]
- Castolo-Sanchez, S.; Trejo-Tapia, G.; Herrera-Ruiz, M.; Domínguez-Mendoza, B.E.; Vargas-Ruiz, R.; Zamilpa, A. Antidepressant Activity of Tricin-7-O-Glucoside and Anxiolytic-like Effect of Harmane from Passiflora coriacea Juss. On Mice. J. Ethnopharmacol. 2024, 335, 118624. [Google Scholar] [CrossRef]
- Kadyan, P.; Singh, L. Unraveling the Mechanistic Interplay of Mediators Orchestrating the Neuroprotective Potential of Harmine. Pharmacol. Rep. 2024, 76, 665–678. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.d.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic Insights and Perspectives Involved in Neuroprotective Action of Quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Mustapha, M.; Mat Taib, C.N. Beneficial Role of Vitexin in Parkinson’s Disease. Malays. J. Med. Sci. 2023, 30, 8–25. [Google Scholar] [CrossRef]
- Putera, H.D.; Doewes, R.I.; Shalaby, M.N.; Ramírez-Coronel, A.A.; Clayton, Z.S.; Abdelbasset, W.K.; Murtazaev, S.S.; Jalil, A.T.; Rahimi, P.; Nattagh-Eshtivani, E.; et al. The Effect of Conjugated Linoleic Acids on Inflammation, Oxidative Stress, Body Composition and Physical Performance: A Comprehensive Review of Putative Molecular Mechanisms. Nutr. Metab. 2023, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Pizzolante, L.; Toschi, T.G.; Guzzo, F.; Ceoldo, S.; Marconi, A.M.; Andreetta, F.; Levi, M. Phenol Content Related to Antioxidant and Antimicrobial Activities of Passiflora spp. Extracts. Eur. Food Res. Technol. 2006, 223, 102–109. [Google Scholar] [CrossRef]
- Murillo, E.; Jiménez, Á.; Velásquez, A.; Clavijo, H.; Velásquez, C.E. Phenolic Components and Antioxidant Capacity of Six Wild Passiflora Species from the Andean Region of Colombia. J. Herbs Spices Med. Plants 2023, 29, 319–335. [Google Scholar] [CrossRef]
- Abourashed, E.A.; Vanderplank, J.R.; Khan, I.A. High-Speed Extraction and HPLC Fingerprinting of Medicinal Plants—II. Application to Harman Alkaloids of Genus Passiflora. Pharm. Biol. 2003, 41, 100–106. [Google Scholar] [CrossRef]
- Spencer, K.C.; Seigler, D.S. Passicoriacin and Epipassicoriacin: C-4 Epimers of Tetraphyllin B and Epitetraphyllin B from Passiflora coriacea. Phytochemistry 1987, 26, 1661–1663. [Google Scholar] [CrossRef]
- Farag, M.A.; Otify, A.; Porzel, A.; Michel, C.G.; Elsayed, A.; Wessjohann, L.A. Comparative Metabolite Profiling and Fingerprinting of Genus Passiflora Leaves Using a Multiplex Approach of UPLC-MS and NMR Analyzed by Chemometric Tools. Anal. Bioanal. Chem. 2016, 408, 3125–3143. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E.A.; Vanderplank, J.R.; Khan, I.A. High-Speed Extraction and HPLC Fingerprinting of Medicinal Plants—I. Application to Passiflora Flavonoids. Pharm. Biol. 2002, 40, 81–91. [Google Scholar] [CrossRef]
- Salihu, M.; Hassan, L.G.; Faruq, U.Z.; Yusuf, A.J. Deciphering the Interactions of Scopoletin and Scopolin from Catunaregam Nilotica Roots against Naja Nigricollis Phospholipase A2 Enzyme. Toxicon 2024, 243, 107732. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.; Kashyap, B.; Gurumayum, S.; Sarma, S.; Baruah, P.; Swargiary, D.; Saikia, A.; Deka, R.C.; Borah, J.C. Antihyperglycemic Potential of Quercetin-3-Glucoside Isolated from Leucaena leucocephala Seedpods via the SIRT1/AMPK/GLUT4 Signaling Cascade. ACS Omega 2024, 9, 32429–32443. [Google Scholar] [CrossRef]
- Lee, D.G.; Lee, J.S.; Quilantang, N.G.; Jacinto, S.D.; Lee, S. Determination of Afzelin and Astragalin from Lespedeza cuneata on Aldose Reductase Inhibition. J. Chromatogr. Sci. 2021, 59, 381–387. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, R.; Tong, Y.; Chen, P.; Shen, Y.; Miao, S.; Liu, X. Neuroprotection by Dihydrotestosterone in LPS-Induced Neuroinflammation. Neurobiol. Dis. 2020, 140, 104814. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.S.; Supalkar, K.V.; Sadar, S.S.; Vyawahare, N.S. Animal Models of Alzheimer’s Disease: An Origin of Innovative Treatments and Insight to the Disease’s Etiology. Brain Res. 2023, 1814, 148449. [Google Scholar] [CrossRef]
- Choe, K.; Park, J.S.; Park, H.Y.; Tahir, M.; Park, T.J.; Kim, M.O. Lupeol Protect against LPS-Induced Neuroinflammation and Amyloid Beta in Adult Mouse Hippocampus. Front. Nutr. 2024, 11, 1414696. [Google Scholar] [CrossRef]
- Rani, V.; Verma, R.; Kumar, K.; Chawla, R. Role of Pro-Inflammatory Cytokines in Alzheimer’s Disease and Neuroprotective Effects of Pegylated Self-Assembled Nanoscaffolds. Curr. Res. Pharmacol. Drug Discov. 2023, 4, 100149. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Panaro, M.A. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules 2020, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Harrison, F.E.; Hosseini, A.H.; McDonald, M.P. Endogenous Anxiety and Stress Responses in Water Maze and Barnes Maze Spatial Memory Tasks. Behav. Brain Res. 2009, 198, 247–251. [Google Scholar] [CrossRef]
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the Regulation of Neuronal Development, Survival and Function. Cytokine 2021, 144, 155582. [Google Scholar] [CrossRef]
- Pan, R.; Dai, Y.; Gao, X.; Xia, Y. Scopolin Isolated from Erycibe Obtusifolia Benth Stems Suppresses Adjuvant-Induced Rat Arthritis by Inhibiting Inflammation and Angiogenesis. Int. Immunopharmacol. 2009, 9, 859–869. [Google Scholar] [CrossRef]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent Advances in Molecular Pathways and Therapeutic Implications Targeting Neuroinflammation for Alzheimer’s Disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kim, S. Oxidative Stress: Culprit or Consequence in Alzheimer’s Amyloidopathy. Neural Regen. Res. 2023, 18, 1861–1868. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Hornick, A.; Langer, T.; Stuppner, H.; Prast, H. Acetylcholinesterase Inhibitory Activity of Scopolin and Scopoletin Discovered by Virtual Screening of Natural Products. J. Med. Chem. 2004, 47, 6248–6254. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Serrano, C.E.; Jiménez-Ferrer, E.; Herrera-Ruiz, M.; Zamilpa, A.; Vargas-Villa, G.; Ramírez-Carreto, R.J.; Chavarría, A.; Tortoriello, J.; Pedraza-Alva, G.; Pérez-Martínez, L. A Malva Parviflora’s Fraction Prevents the Deleterious Effects Resulting from Neuroinflammation. Biomed. Pharmacother. 2019, 118, 109349. [Google Scholar] [CrossRef]
- Jerom, J.P.; Jalal, A.; Sajan, A.L.; Soman, R.; Nair, R.H.; Narayanan, S.P. In-Vitro Neuro-2a Cytotoxicity Analysis and Molecular Docking Investigation on Potential Anti-Amyloid Agents from Adiantum lunulatum. Heliyon 2024, 10, e38127. [Google Scholar] [CrossRef]
- Gupta, D.D.; Mishra, S.; Verma, S.S.; Shekher, A.; Rai, V.; Awasthee, N.; Das, T.J.; Paul, D.; Das, S.K.; Tag, H.; et al. Evaluation of Antioxidant, Anti-Inflammatory and Anticancer Activities of Diosgenin Enriched Paris Polyphylla Rhizome Extract of Indian Himalayan Landraces. J. Ethnopharmacol. 2021, 270, 113842. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X. Diosgenin and Its Analogs: Potential Protective Agents Against Atherosclerosis. Drug Des. Devel Ther. 2022, 16, 2305–2323. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, Y.W.; Park, M.K.; Byun, S.H.; Kim, S.C.; Lee, J.R. Anti-Inflammatory Steroid from Phragmitis Rhizoma Modulates LPS-Mediated Signaling Through Inhibition of NF-ΚB Pathway. Inflammation 2016, 39, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Modak, D.; Haydar, M.S.; Georrge, J.J.; Bhattacharjee, S. Exploring the Ameliorative Role of Equisetum diffusum D. Don Whole-Plant Methanolic-Extract in Acute Inflammation and Molecular Docking Analysis of GC-MS-Identified Phytocompounds with Few Prominent Inflammatory Markers/Cytokines for Inspecting the Potent. Pharmacogn. Res. 2023, 16, 82–97. [Google Scholar] [CrossRef]
- Yang, Q.; Kang, Z.H.; Zhang, J.; Qu, F.; Song, B. Neuroprotective Effects of Isoquercetin: An In Vitro and In Vivo Study. Cell J. 2021, 23, 355–365. [Google Scholar] [CrossRef]
- Du, R.; Pei, H.; He, Z.; Wang, J.; Zhou, X.; Li, W.; Zhu, D.; Zhang, C. Astragalin Improves Cognitive Disorder in Alzheimer’s Disease: Based on Network Pharmacology and Molecular Docking Simulation. CNS Neurosci. Ther. 2024, 30, e14799. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Z.; Wang, S.-H.; Zhang, R.-H.; Lin, J.-H.; Tian, Y.-H.; Yang, Y.-Q.; Liu, J.; Ma, Y.-X. Neuroprotective Effect of Astragalin via Activating PI3K/Akt-MTOR-Mediated Autophagy on APP/PS1 Mice. Cell Death Discov. 2023, 9, 15. [Google Scholar] [CrossRef]
- Kim, E.H.; Shim, Y.Y.; Lee, H.I.; Lee, S.; Reaney, M.J.T.; Chung, M.J. Astragalin and Isoquercitrin Isolated from Aster Scaber Suppress LPS-Induced Neuroinflammatory Responses in Microglia and Mice. Foods 2022, 11, 1505. [Google Scholar] [CrossRef]
- Hu, Y.; Fang, X.; Wang, J.; Ren, T.-T.; Zhao, Y.-Y.; Dai, J.-F.; Qin, X.-Y.; Lan, R. Astragalin Attenuates AlCl3/D-Galactose-Induced Aging-like Disorders by Inhibiting Oxidative Stress and Neuroinflammation. Neurotoxicology 2022, 91, 60–68. [Google Scholar] [CrossRef]
- Gutiérrez-Román, A.S.; Trejo-Tapia, G.; Herrera-Ruiz, M.; Monterrosas-Brisson, N.; Trejo-Espino, J.L.; Zamilpa, A.; González-Cortazar, M. Effect of Terpenoids and Flavonoids Isolated from Baccharis Conferta Kunth on TPA-Induced Ear Edema in Mice. Molecules 2020, 25, 1379. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-Inflammation Induced by Lipopolysaccharide Causes Cognitive Impairment through Enhancement of Beta-Amyloid Generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Monterrosas-Brisson, N.; Jiménez-Ferrer, E.; Bahena-Pérez, R.; González-Cortazar, M.; Porras-Dávila, S.L.; Tapia-Maruri, D.; Martínez-Duncker, I.; Herrera-Ruiz, M. Nootropic Effects of 7-Isoprenyloxycoumarin and Herniarin from Tagetes Lucida on Lipopolysaccharide-Induced Neuroinflammation. Rev. Bras. De Farmacogn. 2024, 34, 738–749. [Google Scholar] [CrossRef]
- Porras-Dávila, S.; Jiménez-Ferrer, E.; Román Ramos, R.; González-Cortazar, M.; Almanza-Pérez, J.C.; Herrera-Ruiz, M. Herniarin, Dimethylfraxetin and Extracts from Tagetes Lucida, in Psychosis Secondary to Ketamine and Its Interaction with Haloperidol. Plants 2022, 11, 2789. [Google Scholar] [CrossRef]
- Castro-Martínez, G.; Herrera-Ruiz, M.; González-Cortázar, M.; Porras-Dávila, S.L.; Almanza Pérez, J.C.; Jimenez-Ferrer, E. Effects of Five Coumarins and Standardized Extracts from Tagetes lucida Cav. on Motor Impairment and Neuroinflammation Induced with Cuprizone. Pharmaceuticals 2023, 16, 1391. [Google Scholar] [CrossRef]
- Vargas-Maya, N.I.; Padilla-Vaca, F.; Romero-González, O.E.; Rosales-Castillo, E.A.S.; Rangel-Serrano, Á.; Arias-Negrete, S.; Franco, B. Refinement of the Griess Method for Measuring Nitrite in Biological Samples. J. Microbiol. Methods 2021, 187, 106260. [Google Scholar] [CrossRef]



| Name | Structure | Retention Time (min) |
|---|---|---|
| Fitone | 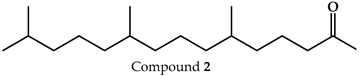 | 17.66 |
| 7-dehydrodiosgenin |  | 31.79 |
| Tremulone | 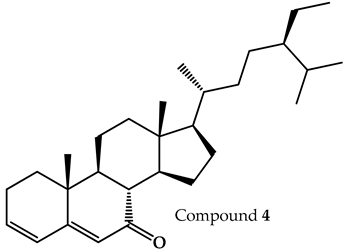 | 37.41 |
| Treatment | Edema (mg) Mean ± SEM | Edema Inhibition (%) |
|---|---|---|
| VEH | 10.90 ± 0.56 | ̶ |
| INDO | 1.14 ± 0.22 *** | 89.5 |
| PcEA | 1.81 ± 0.22 *** | 83.3 |
| PcAq | 8.86 ± 0.50 | 18.6 |
| T1 | 3.76 ± 0.46 *** | 65.5 |
| Scopolin | 0.82 ± 0.28 *** | 92.5 |
| T2 | 1.72 ± 0.37 *** | 84.2 |
| T2.1 (terpene mixture) | 1.27 ± 0.16 *** | 88.3 |
| T3 | 3.30 ± 0.58 *** | 69.7 |
| T3.1 (flavonoids glycosides mixture) | 3.84 ± 0.28 *** | 64.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castolo-Sanchez, S.; Zamilpa, A.; Herrera-Ruiz, M.; Trejo-Espino, J.L.; Domínguez-Mendoza, B.E.; González-Cortazar, M.; Trejo-Tapia, G. Phytochemicals from Passiflora coriacea Juss. Have Anti-Inflammatory and Neuroprotective Effects in Mouse Models. Pharmaceuticals 2024, 17, 1534. https://doi.org/10.3390/ph17111534
Castolo-Sanchez S, Zamilpa A, Herrera-Ruiz M, Trejo-Espino JL, Domínguez-Mendoza BE, González-Cortazar M, Trejo-Tapia G. Phytochemicals from Passiflora coriacea Juss. Have Anti-Inflammatory and Neuroprotective Effects in Mouse Models. Pharmaceuticals. 2024; 17(11):1534. https://doi.org/10.3390/ph17111534
Chicago/Turabian StyleCastolo-Sanchez, Samir, Alejandro Zamilpa, Maribel Herrera-Ruiz, José Luis Trejo-Espino, Blanca Eda Domínguez-Mendoza, Manasés González-Cortazar, and Gabriela Trejo-Tapia. 2024. "Phytochemicals from Passiflora coriacea Juss. Have Anti-Inflammatory and Neuroprotective Effects in Mouse Models" Pharmaceuticals 17, no. 11: 1534. https://doi.org/10.3390/ph17111534
APA StyleCastolo-Sanchez, S., Zamilpa, A., Herrera-Ruiz, M., Trejo-Espino, J. L., Domínguez-Mendoza, B. E., González-Cortazar, M., & Trejo-Tapia, G. (2024). Phytochemicals from Passiflora coriacea Juss. Have Anti-Inflammatory and Neuroprotective Effects in Mouse Models. Pharmaceuticals, 17(11), 1534. https://doi.org/10.3390/ph17111534










