Evolving Clinical Features of Diabetic Ketoacidosis: The Impact of SGLT2 Inhibitors
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Data Collection
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karamichalakis, N.; Kolovos, V.; Paraskevaidis, I.; Tsougos, E. A New Hope: Sodium-Glucose Cotransporter-2 Inhibition to Prevent Atrial Fibrillation. J. Cardiovasc. Dev. Dis. 2022, 9, 236. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Ibanez, J.A.R.; Antonio, R.S.; Ishikawa, K.; Watanabe, S.; Picatoste Botija, M.B.; Salvo, A.J.S.; Hajjar, R.; Fuster, V.; Badimon, J. Empagliflozin Induces a Myocardial Metabolic Shift from Glucose Consumption to Ketone Metabolism that Mitigates Adverse Cardiac Remodeling and Improves Myocardial Contractility. J. Am. Coll. Cardiol. 2018, 71, A674. [Google Scholar] [CrossRef]
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Schönberger, E.; Mihaljević, V.; Steiner, K.; Šarić, S.; Kurevija, T.; Majnarić, L.T.; Bilić Ćurčić, I.; Canecki-Varžić, S. Immunomodulatory Effects of SGLT2 Inhibitors—Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public Health 2023, 20, 6671. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Butler, A.E.; Sahebkar, A. Sodium–glucose cotransporter inhibitors and oxidative stress: An update. J. Cell. Physiol. 2019, 234, 3231–3237. [Google Scholar] [CrossRef]
- Peng, W.K.; Chen, L.; Boehm, B.O.; Han, J.; Loh, T.P. Molecular phenotyping of oxidative stress in diabetes mellitus with point-of-care NMR system. npj Aging Mech. Dis. 2020, 6, 11. [Google Scholar] [CrossRef]
- Vadasz, B.; Arazi, M.; Shukha, Y.; Koren, O.; Taher, R. Sodium-glucose cotransporter-2-induced euglycemic diabetic ketoacidosis unmasks latent autoimmune diabetes in a patient misdiagnosed with type 2 diabetes mellitus: A case report. J. Med. Case Rep. 2021, 15, 62. [Google Scholar] [CrossRef]
- Morace, C.; Lorello, G.; Bellone, F.; Quartarone, C.; Ruggeri, D.; Giandalia, A.; Mandraffino, G.; Minutoli, L.; Squadrito, G.; Russo, G.T.; et al. Ketoacidosis and SGLT2 Inhibitors: A Narrative Review. Metabolites 2024, 14, 264. [Google Scholar] [CrossRef] [PubMed]
- Ata, F.; Yousaf, Z.; Khan, A.A.; Razok, A.; Akram, J.; Ali, E.A.H.; Abdalhadi, A.; Ibrahim, D.A.; Al Mohanadi, D.H.S.H.; Danjuma, M.I. SGLT-2 inhibitors associated euglycemic and hyperglycemic DKA in a multicentric cohort. Sci. Rep. 2021, 11, 110293. [Google Scholar] [CrossRef] [PubMed]
- Oriot, P.; Hermans, M.P. Euglycemic diabetic ketoacidosis in a patient with type 1 diabetes and SARS-CoV-2 pneumonia: Case-report and review of the literature. Acta Clin. Belgica Int. J. Clin. Lab. Med. 2022, 77, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Siebel, S.; Galderisi, A.; Patel, N.S.; Carria, L.R.; Tamborlane, W.V.; Sherr, J.L. Reversal of ketosis in type 1 diabetes is not adversely affected by SGLT2 inhibitor therapy. Diabetes Technol. Ther. 2019, 21, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Barski, L.; Eshkoli, T.; Brandstaetter, E.; Jotkowitz, A. Euglycemic diabetic ketoacidosis. Eur. J. Intern. Med. 2019, 63, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kapila, V.; Topf, J. Sodium-Glucose Co-transporter 2 Inhibitor-Associated Euglycemic Diabetic Ketoacidosis After Bariatric Surgery: A Case and Literature Review. Cureus 2021, 13, e17093. [Google Scholar] [CrossRef] [PubMed]
- Pishdad, R.; Auwaerter, P.G.; Kalyani, R.R. Diabetes, SGLT-2 Inhibitors, and Urinary Tract Infection: A Review. Curr. Diab. Rep. 2024, 24, 108–117. [Google Scholar] [CrossRef]
- Arshad, M.; Hoda, F.; Siddiqui, N.; Najmi, A.; Ahmad, M. Genito Urinary Infection and Urinary Tract Infection in Patients with Type 2 Diabetes Mellitus Receiving SGLT2 Inhibitors: Evidence from a Systematic Literature Review of Landmark Randomized Clinical Trial. Drug Res. 2024, 7, 307–313. [Google Scholar] [CrossRef]
- Somagutta, M.R.; Agadi, K.; Hange, N.; Jain, M.S.; Batti, E.; Emuze, B.O.; Amos-Arowoshegbe, E.O.; Popescu, S.; Hanan, S.; Kumar, V.R.; et al. Euglycemic Diabetic Ketoacidosis and Sodium-Glucose Cotransporter-2 Inhibitors: A Focused Review of Pathophysiology, Risk Factors, and Triggers. Cureus 2021, 13, e13665. [Google Scholar] [CrossRef]
- Juneja, D.; Nasa, P.; Jain, R.; Singh, O. Sodium-glucose Cotransporter-2 Inhibitors induced euglycemic diabetic ketoacidosis: A meta summary of case reports. World J. Diabetes 2023, 14, 1314–1322. [Google Scholar] [CrossRef]
- Bonora, B.M.; Avogaro, A.; Fadini, G.P. Euglycemic Ketoacidosis. Curr. Diab. Rep. 2020, 20, 25. [Google Scholar] [CrossRef]
- He, Z.; Lam, K.; Zhao, W.; Yang, S.; Li, Y.; Mo, J.; Gao, S.; Liang, D.; Qiu, K.; Huang, M.; et al. SGLT-2 inhibitors and euglycemic diabetic ketoacidosis/diabetic ketoacidosis in FAERS: A pharmacovigilance assessment. Acta Diabetol. 2023, 60, 401–411. [Google Scholar] [CrossRef]
- Puls, H.A.; Haas, N.L.; Franklin, B.J.; Theyyunni, N.; Harvey, C.E. Euglycemic diabetic ketoacidosis associated with SGLT2i use: Case series. Am. J. Emerg. Med. 2021, 44, 11–13. [Google Scholar] [CrossRef] [PubMed]
- El Ess, M.S.; ElRishi, M.A. Severe euglycemic diabetic ketoacidosis secondary to sodium-glucose co-transporter 2 inhibitor: Case report and literature review. Ann. Med. Surg. 2023, 85, 2097–2101. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, G.; De Pascalis, A.; Capelli, I.; Gasperoni, L.; Di Lullo, L.; Bellasi, A.; La Manna, G. Mineral and Electrolyte Disorders with SGLT2i Therapy. JBMR Plus 2019, 3, e10242. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, G.; De Pascalis, A.; Gasperoni, L.; Tondolo, F.; Zappulo, F.; Capelli, I.; Cappuccilli, M.; Manna, G. La The Off Target Effects, Electrolyte and Mineral Disorders of SGLT2i. Molecules 2020, 25, 2757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huan, Y.; Leibensperger, M.; Seo, B.; Song, Y. Comparative Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Serum Electrolyte Levels in Patients with Type 2 Diabetes: A Pairwise and Network Meta-Analysis of Randomized Controlled Trials. Kidney360 2022, 3, 477–487. [Google Scholar] [CrossRef]
- Kataoka, H.; Yoshida, Y. Enhancement of the serum chloride concentration by administration of sodium-glucose cotransporter-2 inhibitor and its mechanisms and clinical significance in type 2 diabetic patients: A pilot study. Diabetol. Metab. Syndr. 2020, 12, 5. [Google Scholar] [CrossRef]
- León Jiménez, D.; Gómez Huelgas, R.; Miramontes González, J.P. The mechanism of action of sodium–glucose co-transporter 2 inhibitors is similar to carbonic anhydrase inhibitors. Eur. J. Heart Fail. 2018, 20, 409. [Google Scholar] [CrossRef]
- Stamatiades, G.A.; D’Silva, P.; Elahee, M.; Viana, G.M.; Sideri-Gugger, A.; Majumdar, S.K. Diabetic Ketoacidosis Associated with Sodium-Glucose Cotransporter 2 Inhibitors: Clinical and Biochemical Characteristics of 29 Cases. Int. J. Endocrinol. 2023, 2023, 6615624. [Google Scholar] [CrossRef]
- Koufakis, T.; Pavlidis, A.N.; Metallidis, S.; Kotsa, K. Sodium-glucose co-transporter 2 inhibitors in COVID-19: Meeting at the crossroads between heart, diabetes and infectious diseases. Int. J. Clin. Pharm. 2021, 43, 764–767. [Google Scholar] [CrossRef]
- Fernandez Felix, D.A.; Madrigal Loria, G.; Sharma, S.; Sharma, S.; Arias Morales, C.E. A Rare Case of Empagliflozin-Induced Euglycemic Diabetic Ketoacidosis Obscured by Alkalosis. Cureus 2022, 14, e25818. [Google Scholar] [CrossRef]
- Banakh, I.; Kung, R.; Gupta, S.; Matthiesson, K.; Tiruvoipati, R. Euglycemic diabetic ketoacidosis in association with dapagliflozin use after gastric sleeve surgery in a patient with type II diabetes mellitus. Clin. Case Rep. 2019, 7, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Penttilä, I.; Penttilä, K.; Holm, P.; Laitinen, H.; Ranta, P.; Törrönen, J.; Rauramaa, R. Methods, units and quality requirements for the analysis of haemoglobin A 1c in diabetes mellitus. World J. Methodol. 2016, 6, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Mercantepe, F.; Baydur Sahin, S.; Cumhur Cure, M.; Karadag, Z. Relationship Between Serum Endocan Levels and Other Predictors of Endothelial Dysfunction in Obese Women. Angiology 2022, 74, 948–957. [Google Scholar] [CrossRef] [PubMed]
 : sodium loss,
: sodium loss,  : potassium gain,
: potassium gain,  : chloride gain,
: chloride gain,  : bicarbonate loss,
: bicarbonate loss,  : SGLT2 inhibitions by SGLT2-is.
: SGLT2 inhibitions by SGLT2-is.
 : sodium loss,
: sodium loss,  : potassium gain,
: potassium gain,  : chloride gain,
: chloride gain,  : bicarbonate loss,
: bicarbonate loss,  : SGLT2 inhibitions by SGLT2-is.
: SGLT2 inhibitions by SGLT2-is.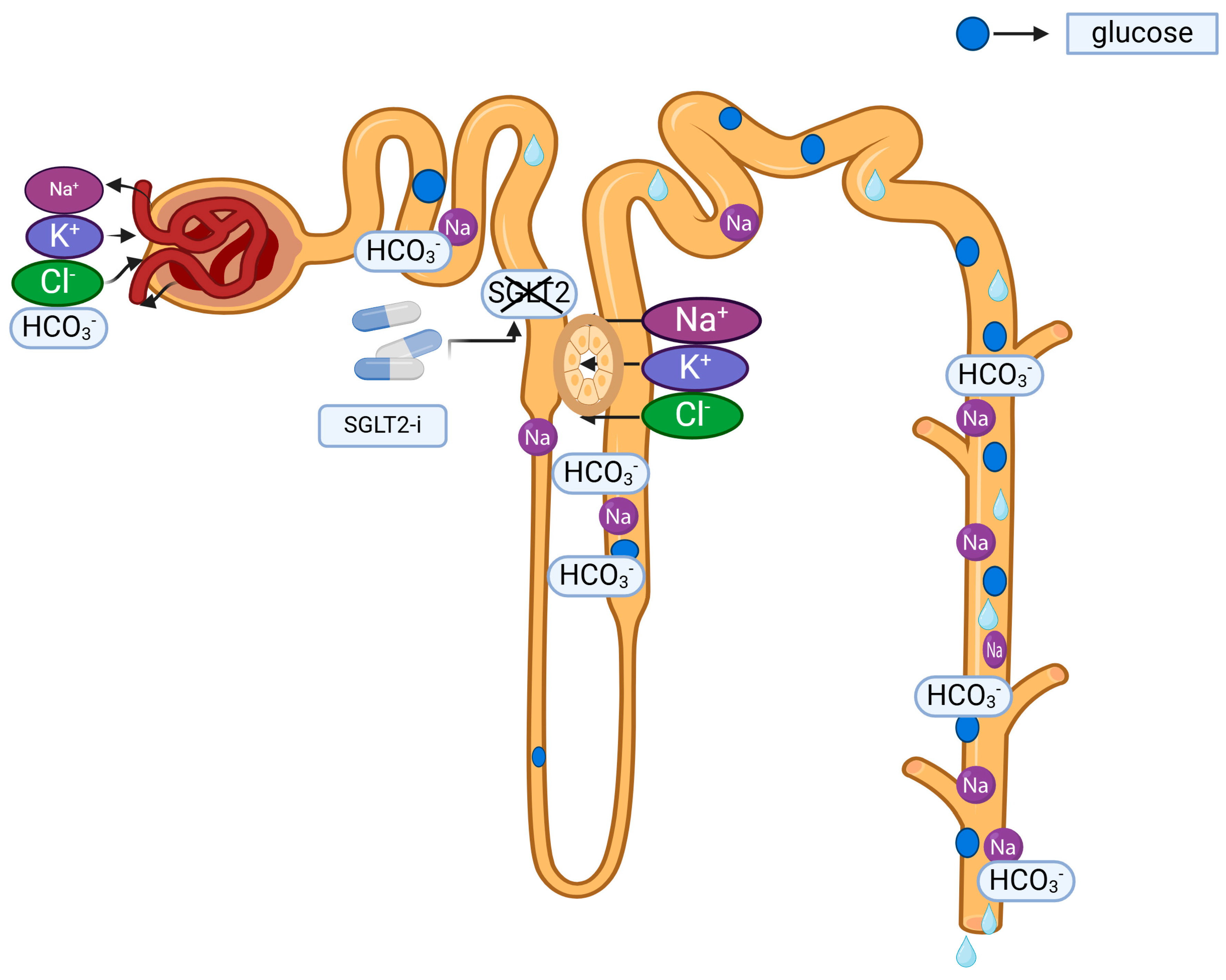

| Variables | Categorization | n (n = 51) | Percentage (%) |
|---|---|---|---|
| Gender | Male | 18 | 35.3 |
| Female | 33 | 64.7 | |
| Smoking | Yes | 14 | 27.5 |
| No | 37 | 72.5 | |
| DM Type | T1DM | 25 | 49 |
| T2DM | 22 | 43.1 | |
| LADA | 4 | 7.8 | |
| SGLT2-is | Yes | 19 | 37.3 |
| No | 32 | 62.7 | |
| EuDKA | Yes | 5 | 9.8 |
| No | 46 | 90.2 | |
| UTI | Yes | 13 | 25.5 |
| No | 38 | 74.5 | |
| Vulvovaginitis | Yes | 6 | 11.8 |
| No | 45 | 88.2 | |
| HT | Yes | 16 | 31.4 |
| No | 35 | 68.6 | |
| Hyperlipidemia | Yes | 3 | 5.9 |
| No | 48 | 94.1 | |
| Mortality | Yes | 4 | 7.8 |
| No | 47 | 92.2 | |
| Intubation | Yes | 5 | 9.8 |
| No | 46 | 90.2 | |
| DM Treatment | No Treatment | 1 | 2 |
| Insulin | 28 | 54.9 | |
| OAD | 13 | 25.5 | |
| Insulin + OAD | 7 | 13.7 | |
| Insulin Pump | 2 | 3.9 |
| Variables | SGLT2-is (+) | SGLT2-is (−) | Total | p–Value | |
|---|---|---|---|---|---|
| Sex | Male | 7 | 11 | 18 | 0.859 |
| Female | 12 | 21 | 33 | ||
| Age (year) | 59 ± 18 | 37 ± 17 | <0.001 | ||
| Height (cm) | 164 ± 7 | 164 ± 9 | 0.795 | ||
| Weight (kg) | 70 ± 16 | 67 ± 14 | 0.525 | ||
| BMI (kg/m2) | 26 ± 6 | 25 ± 5 | 0.429 | ||
| Smoking (+) | 5 (26.3) | 9 (28.1) | 14 | 0.889 | |
| DM Type | T1DM | 0 | 25 | 25 | <0.001 |
| T2DM | 7 | 15 | 22 | <0.001 | |
| LADA | 4 | 0 | 4 | <0.001 | |
| EuDKA (+) | 5 | 0 | 5 | 0.005 * | |
| HT (+) | 6 (31.6) | 10 (31.3) | 16 | >0.05 | |
| UTI | 8 (42.1) | 5 (15.6) | 13 | 0.036 * | |
| Vulvovaginitis | 6 (31.6) | 0 (0) | 6 | 0.001 * | |
| GUS infection—female | 10 (52.6) | 5 (15.6) | 15 | 0.005 * | |
| GUS infection—total | 12 (63.2) | 7 (21.9) | 19 | 0.003 * | |
| Variables | SGLT2-is (+) | SGLT2-is (−) | p–Value |
|---|---|---|---|
| HbA1c (%) | 11.2 ± 2.5 | 10.9 ± 2.2 | 0.623 |
| Glucose (mg/dL) | 398 ± 191 | 564 ± 206 | 0.006 * |
| BUN (mg/dL) | 25 ± 13 | 27 ± 23 | 0.649 |
| Creatinine (mg/dL) | 1.3 ± 0.5 | 1.6 ± 1.2 | 0.247 |
| Na+ (mmol/L) | 135 ± 5 | 130 ± 6 | 0.008 * |
| cNa+ (mmol/L) | 140 ± 4 | 137 ± 6 | 0.205 |
| K+ (mmol/L) | 4.5 ± 0.8 | 4.8 ± 0.9 | 0.176 |
| Cl− (mmol/L) | 103 (90−112) | 98 (41−113) | 0.036 * |
| Ca2+ (mmol/L) | 8.9 ± 0.7 | 8.9 ± 0.8 | 0.996 |
| Mg2+ (mEq/L) | 2.3 ± 0.9 | 2 ± 0.5 | 0.201 |
| P(mEq/L) | 3.9 ± 3.4 | 4 ± 2.1 | 0.898 |
| Albumin (mg/dL) | 3.6 ± 0.5 | 3.6 ± 0.9 | 0.885 |
| WBC (103 µL) | 13.5 ± 6.5 | 14.5 ± 12 | 0.734 |
| Neutrophil (103 µL) | 11.7 ± 6 | 11.5 ± 10.4 | 0.935 |
| Lymphocyte (103 µL) | 1.4 ± 0.8 | 2 ± 1.8 | 0.121 |
| Hemoglobin (gr/dL) | 14.2 ± 3.2 | 13 ± 2.6 | 0.147 |
| Platelet (103 µL) | 261 ± 149 | 303 ± 115 | 0.262 |
| CRP (mg/dL) | 4.8 (0.3–36) | 4.2 (0.3–30) | 0.822 |
| pH | 7.17 ± 0.17 | 7.16 ± 0.16 | 0.853 |
| HCO3 (mmol/L) | 12.5 ± 5.5 | 11.7 ± 4.5 | 0.796 |
| Lactate mmol/L | 3.3 ± 3.7 | 2.6 ± 1.9 | 0.344 |
| PO2 (mmHg) | 66 ± 42 | 69 ± 37 | 0.733 |
| O2 Saturation (%) | 97 ± 3 | 97 ± 2 | 0.232 |
| Urine Density (g/cm3) | 1019 ± 7 | 1019 ± 7 | 0.831 |
| Urine WBC (/HPF) | 16 (1−70) | 22 (0−617) | <0.001 * |
| Urine RBC (/HPF) | 12 (0−51) | 9 (0−75) | 0.178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genc, S.; Evren, B.; Yigit, O.S.; Sahin, I.; Dayanan, R.; Klisic, A.; Erturk, A.; Mercantepe, F. Evolving Clinical Features of Diabetic Ketoacidosis: The Impact of SGLT2 Inhibitors. Pharmaceuticals 2024, 17, 1553. https://doi.org/10.3390/ph17111553
Genc S, Evren B, Yigit OS, Sahin I, Dayanan R, Klisic A, Erturk A, Mercantepe F. Evolving Clinical Features of Diabetic Ketoacidosis: The Impact of SGLT2 Inhibitors. Pharmaceuticals. 2024; 17(11):1553. https://doi.org/10.3390/ph17111553
Chicago/Turabian StyleGenc, Selin, Bahri Evren, Onur Selcuk Yigit, Ibrahim Sahin, Ramazan Dayanan, Aleksandra Klisic, Ayse Erturk, and Filiz Mercantepe. 2024. "Evolving Clinical Features of Diabetic Ketoacidosis: The Impact of SGLT2 Inhibitors" Pharmaceuticals 17, no. 11: 1553. https://doi.org/10.3390/ph17111553
APA StyleGenc, S., Evren, B., Yigit, O. S., Sahin, I., Dayanan, R., Klisic, A., Erturk, A., & Mercantepe, F. (2024). Evolving Clinical Features of Diabetic Ketoacidosis: The Impact of SGLT2 Inhibitors. Pharmaceuticals, 17(11), 1553. https://doi.org/10.3390/ph17111553







