Immunotherapy in the Battle Against Bone Metastases: Mechanisms and Emerging Treatments
Abstract
:1. Introduction
2. Bone Metastases: An Overview
2.1. The Metastatic Cascades
2.2. The Bone Microenvironment
3. The Immune System Interplay in Bone Metastases
3.1. Overview of the Immune System in Cancer
3.2. Innate and Adaptive Immunity
3.3. Immune Influence on Bone Metabolism
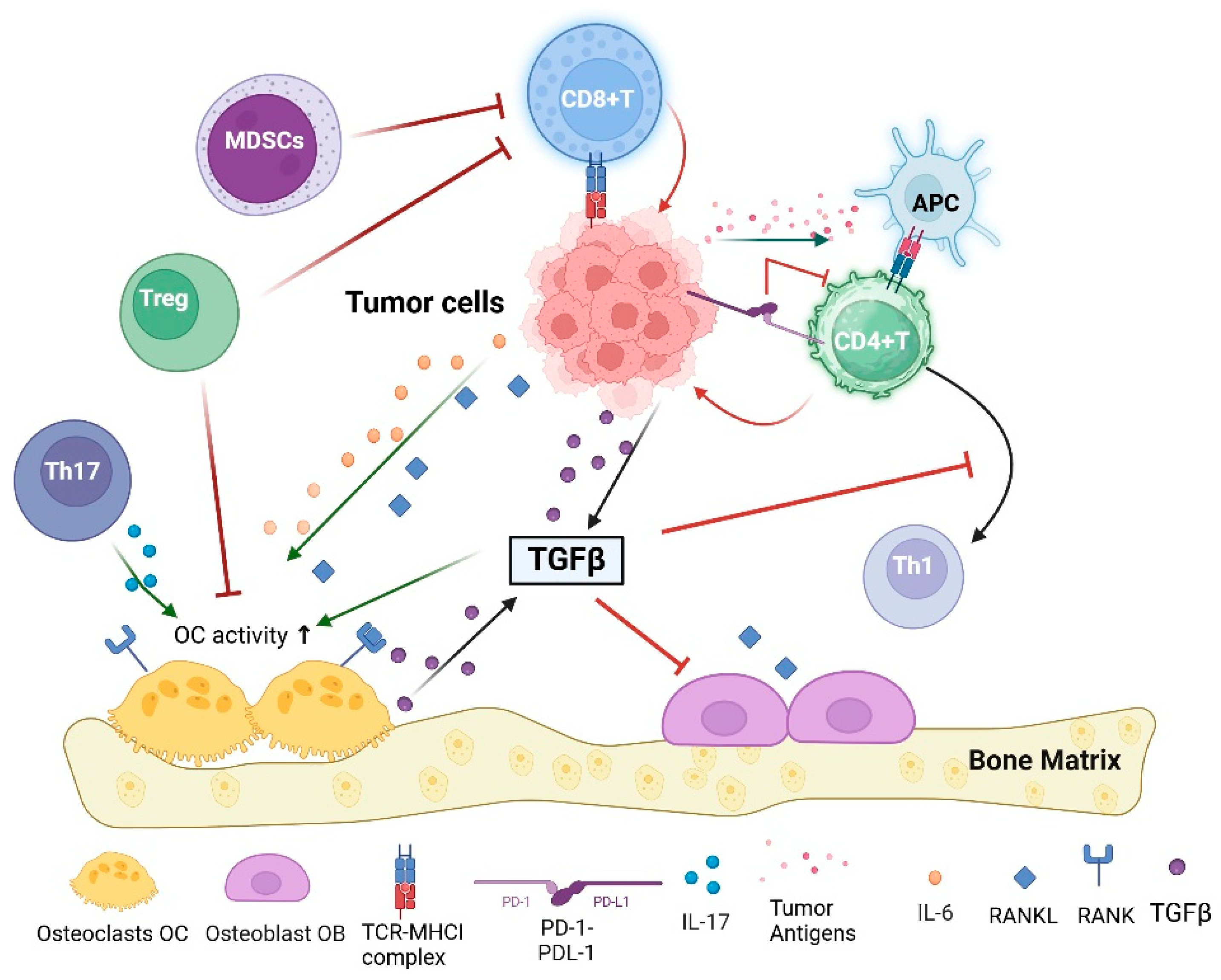
3.4. Immune System and Bone Metastases
4. Concept of Cancer Immunoediting
5. Introduction to Immunotherapy
5.1. Types of Immunotherapy
5.1.1. Checkpoints Inhibitors (ICIs)
5.1.2. CAR T-Cell Therapy
5.1.3. Cytokines and Other Immunomodulators
5.1.4. Cancer Vaccines
5.1.5. Newly Approved Drugs
6. Tumor Immunogenicity
7. Immunotherapy and Bone Metastases
7.1. Rationale for Immunotherapy in Bone Metastases
7.2. Preclinical Studies
7.2.1. Mouse Model to Study Bone Metastases
7.2.2. Innovative Modeling Approaches
8. Checkpoint Inhibitors in Bone Metastases
Challenges in Treating Bone Metastases with ICIs
9. Combination Strategies
9.1. Combining Immunotherapy with Bone Targeted Agents (Bisphosphonates or Denosumab)
9.2. Combining with Chemotherapy: Potential Synergies
9.3. Radiation and Immunotherapy: Abscopal Effect
9.4. Combining Checkpoint Inhibitors with Other Immunotherapies
10. Challenges and Limitations
10.1. Immune Escape Mechanisms Employed by Tumor Cells in Bone Metastases
10.2. Immune-Related Adverse Effects
11. Future Directions
Funding
Conflicts of Interest
References
- Rachner, T.D.; Coleman, R.; Hadji, P.; Hofbauer, L.C. Individualized Bone-Protective Management in Long-Term Cancer Survivors with Bone Metastases. J. Bone Miner. Res. 2021, 36, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Bado, I.; Zhang, X.H.-F. Bone Tropism in Cancer Metastases. Cold Spring Harb. Perspect. Med. 2020, 10, a036848. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Shane, E. Hypercalcemia: A Review. JAMA 2022, 328, 1624–1636. [Google Scholar] [CrossRef]
- Aielli, F.; Ponzetti, M.; Rucci, N. Bone Metastasis Pain, from the Bench to the Bedside. Int. J. Mol. Sci. 2019, 20, 280. [Google Scholar] [CrossRef]
- Elaasser, B.; Arakil, N.; Mohammad, K.S. Bridging the Gap in Understanding Bone Metastasis: A Multifaceted Perspective. Int. J. Mol. Sci. 2024, 25, 2846. [Google Scholar] [CrossRef]
- Patel, L.R.; Camacho, D.F.; Shiozawa, Y.; Pienta, K.J.; Taichman, R.S. Mechanisms of cancer cell metastasis to the bone: A multistep process. Futur. Oncol. 2011, 7, 1285–1297. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Li, C.-J.; Yiang, G.-T.; Cheng, Y.-L.; Tsai, A.P.; Hou, Y.-T.; Ho, Y.-C.; Hou, M.-F.; Chu, P.-Y. Molecular Regulation of Bone Metastasis Pathogenesis. Cell. Physiol. Biochem. 2018, 46, 1423–1438. [Google Scholar] [CrossRef]
- Krzeszinski, J.Y.; Wan, Y. New therapeutic targets for cancer bone metastasis. Trends Pharmacol. Sci. 2015, 36, 360–373. [Google Scholar] [CrossRef]
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol. Rev. 2021, 101, 797–855. [Google Scholar] [CrossRef]
- Araujo, A.; Cook, L.M.; Lynch, C.C.; Basanta, D. Size Matters: Metastatic Cluster Size and Stromal Recruitment in the Establishment of Successful Prostate Cancer to Bone Metastases. Bull. Math. Biol. 2018, 80, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Yasmin-Karim, S.; King, M.R.; Messing, E.M.; Lee, Y.-F. E-selectin ligand-1 controls circulating prostate cancer cell rolling/adhesion and metastasis. Oncotarget 2014, 5, 12097–12110. [Google Scholar] [CrossRef] [PubMed]
- Klusa, D.; Lohaus, F.; Franken, A.; Baumbach, M.; Cojoc, M.; Dowling, P.; Linge, A.; Offermann, A.; Löck, S.; Hušman, D.; et al. Dynamics of CXCR4 positive circulating tumor cells in prostate cancer patients during radiotherapy. Int. J. Cancer 2023, 152, 2639–2654. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Liang, Y.; Zhao, Q.; Wang, H.; Dong, J. CX3CL1 Induces Vertebral Microvascular Barrier Dysfunction via the Src/P115-RhoGEF/ROCK Signaling Pathway. Front. Cell. Neurosci. 2020, 14, 96. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Pedersen, E.A.; Havens, A.M.; Jung, Y.; Mishra, A.; Joseph, J.; Kim, J.K.; Patel, L.R.; Ying, C.; Ziegler, A.M.; et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Investig. 2011, 121, 1298–1312. [Google Scholar] [CrossRef]
- Ban, J.; Fock, V.; Aryee, D.N.T.; Kovar, H. Mechanisms, Diagnosis and Treatment of Bone Metastases. Cells 2021, 10, 2944. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Salerno, M.; Granchi, D.; Cenni, E.; Facchini, G.; Baldini, N. Change in FGF-2 circulating levels after arterial embolization in patients with bone metastases. Neoplasma 2018, 65, 262–268. [Google Scholar] [CrossRef]
- Ölken, E.A.; Aszodi, A.; Taipaleenmäki, H.; Saito, H.; Schönitzer, V.; Chaloupka, M.; Apfelbeck, M.; Böcker, W.; Saller, M.M. SFRP2 Overexpression Induces an Osteoblast-like Phenotype in Prostate Cancer Cells. Cells 2022, 11, 4081. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Xia, T.; Qiao, F.; Wang, N.; Jiang, Y.; Xin, H. Role and Regulation of Transcription Factors in Osteoclastogenesis. Int. J. Mol. Sci. 2023, 24, 16175. [Google Scholar] [CrossRef]
- Yahara, Y.; Nguyen, T.; Ishikawa, K.; Kamei, K.; Alman, B.A.; Ginhoux, F.; Martin, P. The origins and roles of osteoclasts in bone development, homeostasis and repair. Development 2022, 149, 199908. [Google Scholar] [CrossRef]
- Anwar, A.; Sapra, L.; Gupta, N.; Ojha, R.P.; Verma, B.; Srivastava, R.K. Fine-tuning osteoclastogenesis: An insight into the cellular and molecular regulation of osteoclastogenesis. J. Cell. Physiol. 2023, 238, 1431–1464. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Munoz, M.; Izadmehr, S.; Arumugam, D.; Wong, B.; Kirschenbaum, A.; Levine, A.C. Mechanisms of Osteoblastic Bone Metastasis in Prostate Cancer: Role of Prostatic Acid Phosphatase. J. Endocr. Soc. 2019, 3, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, H.; Yuan, S.; Liang, C.; Li, Y.; Zhu, S.; Chen, K. IL-17A deficiency inhibits lung cancer-induced osteoclastogenesis by promoting apoptosis of osteoclast precursor cells. PLoS ONE 2024, 19, e0299028. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, K.; Oshiro, H.; Katsuki, R.; Tsuha, Y.; Aoki, Y.; Tome, Y.; Nishida, K. Denosumab administration for bone metastases from solid tumors: A retrospective cross-sectional study. BMC Cancer 2023, 23, 999. [Google Scholar] [CrossRef]
- Zheng, G.; Chang, B.; Lin, F.; Xie, D.; Hu, Q.; Yu, G.; Du, S.; Li, X. Meta-analysis comparing denosumab and zoledronic acid for treatment of bone metastases in patients with advanced solid tumours. Eur. J. Cancer Care 2017, 26, e12541. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Bozec, A.; Rauner, M.; Jakob, F.; Perner, S.; Pantel, K. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 2021, 18, 488–505. [Google Scholar] [CrossRef]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Muscarella, A.M.; Aguirre, S.; Hao, X.; Waldvogel, S.M.; Zhang, X.H. Exploiting bone niches: Progression of disseminated tumor cells to metastasis. J. Clin. Investig. 2021, 131, e143764. [Google Scholar] [CrossRef]
- Guise, T.A.; Mohammad, K.S.; Clines, G.; Stebbins, E.G.; Wong, D.H.; Higgins, L.S.; Vessella, R.; Corey, E.; Padalecki, S.; Suva, L.; et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 2006, 12 Pt 2, 6213s–6216s. [Google Scholar] [CrossRef]
- Davila, D.; Antoniou, A.; Chaudhry, M.A. Evaluation of osseous metastasis in bone scintigraphy. Semin. Nucl. Med. 2015, 45, 3–15. [Google Scholar] [CrossRef]
- Kuchuk, I.; Hutton, B.; Moretto, P.; Ng, T.; Addison, C.; Clemons, M. Incidence, consequences and treatment of bone metastases in breast cancer patients—Experience from a single cancer centre. J. Bone Oncol. 2013, 2, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhang, J.; Ji, P.; Ling, H.; Hu, X.; Shao, Z. Incidence proportions and prognosis of breast cancer patients with bone metastases at initial diagnosis. Cancer Med. 2018, 7, 4156–4169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, D.; Hong, S. Prevalence and prognosis of bone metastases in common solid cancers at initial diagnosis: A population-based study. BMJ Open 2023, 13, e069908. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-F.; Shen, J.; Li, X.; Rengan, R.; Silvestris, N.; Wang, M.; Derosa, L.; Zheng, X.; Belli, A.; Zhang, X.-L.; et al. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: A large population-based study. Ann. Transl. Med. 2020, 8, 482. [Google Scholar] [CrossRef]
- Xiaohong, L.; Qiuxia, F.; Ruie, L.; Daijie, W.; Muluh, T.A.; Zhang, Y. An Epic Advancement in Targeting Macrophages for Cancer Therapy Approach. Curr. Drug Deliv. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Chang, R.B.; Beatty, G.L. The interplay between innate and adaptive immunity in cancer shapes the productivity of cancer immunosurveillance. J. Leukoc. Biol. 2020, 108, 363–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, W.; Xu, C.; Nan, Y.; Mei, S.; Ju, D.; Wang, S.; Zhang, X. Innate Immunity in Cancer Biology and Therapy. Int. J. Mol. Sci. 2023, 24, 11233. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Mei, Q.; Zhao, B.; Chu, Q.; Dai, Z.; Wu, K. Exploiting innate immunity for cancer immunotherapy. Mol. Cancer 2023, 22, 187. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Maiorino, L.; Daßler-Plenker, J.; Sun, L.; Egeblad, M. Innate Immunity and Cancer Pathophysiology. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 425–457. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Marinelli Busilacchi, E.; Morsia, E.; Poloni, A. Bone Marrow Adipose Tissue. Cells 2024, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yao, Z.; Xue, L.; Wang, D.; Tan, Z. The role of immune cells in modulating chronic inflammation and osteonecrosis. Front. Immunol. 2022, 13, 1064245. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Gruber, R. Osteoimmunology: Inflammatory osteolysis and regeneration of the alveolar bone. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 52–69. [Google Scholar] [CrossRef]
- Kang, I.-H.; Baliga, U.K.; Chatterjee, S.; Chakraborty, P.; Choi, S.; Buchweitz, N.; Li, H.; Wu, Y.; Yao, H.; Mehrotra, S.; et al. Quantitative increase in T regulatory cells enhances bone remodeling in osteogenesis imperfecta. iScience 2022, 25, 104818. [Google Scholar] [CrossRef]
- Buchwald, Z.S.; Kiesel, J.R.; Yang, C.; DiPaolo, R.; Novack, D.V.; Aurora, R. Osteoclast-induced Foxp3+ CD8 T-cells limit bone loss in mice. Bone 2013, 56, 163–173. [Google Scholar] [CrossRef]
- Fischer, L.; Herkner, C.; Kitte, R.; Dohnke, S.; Riewaldt, J.; Kretschmer, K.; Garbe, A.I. Foxp3+ Regulatory T Cells in Bone and Hematopoietic Homeostasis. Front. Endocrinol. 2019, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol. Rev. 2017, 97, 1295–1349. [Google Scholar] [CrossRef]
- Onal, M.; Xiong, J.; Chen, X.; Thostenson, J.D.; Almeida, M.; Manolagas, S.C.; O’Brien, C.A. Receptor activator of nuclear factor κB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J. Biol. Chem. 2012, 287, 29851–29860. [Google Scholar] [CrossRef]
- Ren, Y.; Bäcker, H.; Müller, M.; Kienzle, A. The role of myeloid derived suppressor cells in musculoskeletal disorders. Front. Immunol. 2023, 14, 1139683. [Google Scholar] [CrossRef] [PubMed]
- Saxena, Y.; Routh, S.; Mukhopadhaya, A. Immunoporosis: Role of Innate Immune Cells in Osteoporosis. Front. Immunol. 2021, 12, 687037. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef]
- Cheng, A.; Vantucci, C.E.; Krishnan, L.; Ruehle, M.A.; Kotanchek, T.; Wood, L.B.; Roy, K.; Guldberg, R.E. Early systemic immune biomarkers predict bone regeneration after trauma. Proc. Natl. Acad. Sci. USA 2021, 118, 889118. [Google Scholar] [CrossRef]
- Bozec, A.; Zaiss, M.M.; Kagwiria, R.; Voll, R.; Rauh, M.; Chen, Z.; Mueller-Schmucker, S.; Kroczek, R.A.; Heinzerling, L.; Moser, M.; et al. T cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci. Transl. Med. 2014, 6, 235ra60. [Google Scholar] [CrossRef] [PubMed]
- El Khassawna, T.; Serra, A.; Bucher, C.H.; Petersen, A.; Schlundt, C.; Könnecke, I.; Malhan, D.; Wendler, S.; Schell, H.; Volk, H.-D.; et al. T Lymphocytes Influence the Mineralization Process of Bone. Front. Immunol. 2017, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-C.; Zhang, S.-W.; Yuan, Q. Deubiquitinating Enzymes and Bone Remodeling. Stem Cells Int. 2018, 2018, 3712083. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, a031245. [Google Scholar] [CrossRef]
- Walsh, M.C.; Takegahara, N.; Kim, H.; Choi, Y. Updating osteoimmunology: Regulation of bone cells by innate and adaptive immunity. Nat. Rev. Rheumatol. 2018, 14, 146–156. [Google Scholar] [CrossRef]
- Mori, G.; D’Amelio, P.; Faccio, R.; Brunetti, G. The Interplay between the bone and the immune system. Clin. Dev. Immunol. 2013, 2013, 720504. [Google Scholar] [CrossRef]
- Novince, C.M.; Whittow, C.R.; Aartun, J.D.; Hathaway, J.D.; Poulides, N.; Chavez, M.B.; Steinkamp, H.M.; Kirkwood, K.A.; Huang, E.; Westwater, C.; et al. Commensal Gut Microbiota Immunomodulatory Actions in Bone Marrow and Liver have Catabolic Effects on Skeletal Homeostasis in Health. Sci. Rep. 2017, 7, 5747. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.J.; Ahn, J.; Gao, B.; Gee, H.; Nagrial, A.; Hau, E.; da Silva, I.P. Site-Specific Response and Resistance Patterns in Patients with Advanced Non-Small-Cell Lung Cancer Treated with First-Line Systemic Therapy. Cancers 2024, 16, 2136. [Google Scholar] [CrossRef] [PubMed]
- D’amico, L.; Roato, I. The Impact of Immune System in Regulating Bone Metastasis Formation by Osteotropic Tumors. J. Immunol. Res. 2015, 2015, 143526. [Google Scholar] [CrossRef]
- Abbott, A.G.; Meyers, D.E.; Elmi-Assadzadeh, G.; Stukalin, I.; Marro, A.; Puloski, S.K.T.; Morris, D.G.; Cheung, W.Y.; Monument, M.J. Effectiveness of immune checkpoint inhibitor therapy on bone metastases in non-small-cell lung cancer. Front. Immunol. 2024, 15, 1379056. [Google Scholar] [CrossRef]
- Cheng, M.L.; Fong, L. Effects of RANKL-Targeted Therapy in Immunity and Cancer. Front. Oncol. 2014, 3, 329. [Google Scholar] [CrossRef]
- Wang, H.; Ashton, R.; Hensel, J.A.; Lee, J.H.; Khattar, V.; Wang, Y.; Deshane, J.S.; Ponnazhagan, S. RANKL-Targeted Combination Therapy with Osteoprotegerin Variant Devoid of TRAIL Binding Exerts Biphasic Effects on Skeletal Remodeling and Antitumor Immunity. Mol. Cancer Ther. 2020, 19, 2585–2597. [Google Scholar] [CrossRef]
- Li, B.; Wang, P.; Jiao, J.; Wei, H.; Xu, W.; Zhou, P. Roles of the RANKL–RANK Axis in Immunity—Implications for Pathogenesis and Treatment of Bone Metastasis. Front. Immunol. 2022, 13, 824117. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Ricci, M.; Scarpi, E.; Bongiovanni, A.; Ricci, R.; Riva, N.; Liverani, C.; De Vita, A.; La Manna, F.; Oboldi, D.; et al. RANKL: A promising circulating marker for bone metastasis response. Oncol. Lett. 2016, 12, 2970–2975. [Google Scholar] [CrossRef]
- Capietto, A.-H.; Faccio, R. Immune regulation of bone metastasis. BoneKEy Rep. 2014, 3, 600. [Google Scholar] [CrossRef]
- Monteran, L.; Ershaid, N.; Sabah, I.; Fahoum, I.; Zait, Y.; Shani, O.; Cohen, N.; Eldar-Boock, A.; Satchi-Fainaro, R.; Erez, N. Bone metastasis is associated with acquisition of mesenchymal phenotype and immune suppression in a model of spontaneous breast cancer metastasis. Sci. Rep. 2020, 10, 13838. [Google Scholar] [CrossRef]
- Kähkönen, T.E.; Halleen, J.M.; Bernoulli, J. Osteoimmuno-Oncology: Therapeutic Opportunities for Targeting Immune Cells in Bone Metastasis. Cells 2021, 10, 1529. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.; Xu, C.; Li, B.; Chen, J.; Chen, J.; Wang, Z. Immune Checkpoint Inhibitor Therapy for Bone Metastases: Specific Microenvironment and Current Situation. J. Immunol. Res. 2021, 2021, 8970173. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Zhao, S.; Miah, A.; Wei, L.; Patel, S.; Johns, A.; Grogan, M.; Bertino, E.M.; He, K.; Shields, P.G.; et al. Bone Metastases, Skeletal-Related Events, and Survival in Patients with Metastatic Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. J. Natl. Compr Cancer Netw. 2021, 19, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Furubayashi, N.; Nakagawa, T.; Nishiyama, N.; Kitamura, H.; Hori, Y.; Kuroiwa, K.; Son, Y.; Seki, N.; Tomoda, T.; et al. Site-specific Response to Nivolumab in Renal Cell Carcinoma. Anticancer. Res. 2021, 41, 1539–1545. [Google Scholar] [CrossRef]
- Asano, Y.; Yamamoto, N.; Demura, S.; Hayashi, K.; Takeuchi, A.; Kato, S.; Miwa, S.; Igarashi, K.; Higuchi, T.; Yonezawa, H.; et al. The Therapeutic Effect and Clinical Outcome of Immune Checkpoint Inhibitors on Bone Metastasis in Advanced Non-Small-Cell Lung Cancer. Front. Oncol. 2022, 12, 871675. [Google Scholar] [CrossRef]
- Trivedi, T.; Pagnotti, G.M.; Guise, T.A.; Mohammad, K.S. The Role of TGF-β in Bone Metastases. Biomolecules 2021, 11, 1643. [Google Scholar] [CrossRef]
- Pagnotti, G.M.; Trivedi, T.; Mohammad, K.S. Translational Strategies to Target Metastatic Bone Disease. Cells 2022, 11, 1309. [Google Scholar] [CrossRef] [PubMed]
- Akhund, S.A. Mechanism of TGFβ in Bone Metastases and its Potential Therapeutic Uses. J. Orthop. Res. Ther. 2023, 8, 1316. [Google Scholar]
- Chiechi, A.; Waning, D.L.; Stayrook, K.R.; Buijs, J.T.; Guise, T.A.; Mohammad, K.S. Role of TGF-β in breast cancer bone metastases. Adv. Biosci. Biotechnol. 2013, 4, 15–30. [Google Scholar] [CrossRef]
- Brylka, L.J.; Schinke, T. Chemokines in Physiological and Pathological Bone Remodeling. Front. Immunol. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Sousa, S.; Määttä, J. The role of tumour-associated macrophages in bone metastasis. J. Bone Oncol. 2016, 5, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Batoon, L.; McCauley, L.K. Cross Talk Between Macrophages and Cancer Cells in the Bone Metastatic Environment. Front. Endocrinol. 2021, 12, 763846. [Google Scholar] [CrossRef] [PubMed]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Antonangeli, F.; Sozzani, S.; Santoni, A.; Cippitelli, M.; Soriani, A. The senescence journey in cancer immunoediting. Mol. Cancer 2024, 23, 68. [Google Scholar] [CrossRef]
- Vesely, M.D.; Schreiber, R.D. Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy. Ann. N. Y. Acad. Sci. 2013, 1284, 1–5. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Q.; Ren, X. Novel strategies for cancer immunotherapy: Counter-immunoediting therapy. J. Hematol. Oncol. 2023, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- O’sullivan, T.; Saddawi-Konefka, R.; Vermi, W.; Koebel, C.M.; Arthur, C.; White, J.M.; Uppaluri, R.; Andrews, D.M.; Ngiow, S.F.; Teng, M.W.; et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J. Exp. Med. 2012, 209, 1869–1882. [Google Scholar] [CrossRef]
- Gubin, M.M.; Vesely, M.D. Cancer Immunoediting in the Era of Immuno-oncology. Clin. Cancer Res. 2022, 28, 3917–3928. [Google Scholar] [CrossRef]
- Hu, M.; Li, Y.; Lu, Y.; Wang, M.; Li, Y.; Wang, C.; Li, Q.; Zhao, H. The regulation of immune checkpoints by the hypoxic tumor microenvironment. PeerJ 2021, 9, e11306. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Ishino, T.; Ueda, Y.; Nagasaki, J.; Sadahira, T.; Dansako, H.; Araki, M.; Togashi, Y. Activated CTLA-4-independent immunosuppression of Treg cells disturbs CTLA-4 blockade-mediated antitumor immunity. Cancer Sci. 2023, 114, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Lanzar, Z.; Clark, J.T.; Hart, A.P.; Douglas, B.B.; Shallberg, L.; O’dea, K.; Christian, D.A.; Hunter, C.A. PD-1 and CTLA-4 exert additive control of effector regulatory T cells at homeostasis. Front. Immunol. 2023, 14, 997376. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Fritz, J.M.; Lenardo, M.J. Development of immune checkpoint therapy for cancer. J. Exp. Med. 2019, 216, 1244–1254. [Google Scholar] [CrossRef]
- He, R.; Zhao, X.; Liu, J.; Zhou, Y.; Zhang, X.; Cheng, F. PD-1 and CTLA-4 inhibitors in combination vs. alone for the treatment of advanced melanoma: A systematic review and meta-analysis. Medicine 2022, 101, e30561. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.; Vassiliou, G.S. T-Cell Cancer after CAR T-Cell Therapy. N. Engl. J. Med. 2024, 390, 2120–2121. [Google Scholar] [CrossRef]
- Jacobson, C.; Emmert, A.; Rosenthal, M.B. CAR T-Cell Therapy: A Microcosm for the Challenges Ahead in Medicare. JAMA 2019, 322, 923–924. [Google Scholar] [CrossRef]
- Han, D.; Xu, Z.; Zhuang, Y.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Hematological Malignancies. J. Cancer 2021, 12, 326–334. [Google Scholar] [CrossRef]
- Chen, P.; Xia, Y.; Lei, W.; Zhong, S.; Jiang, H.; Ren, L.; Qian, W.; Liu, H. Case report: Hashimoto’s thyroiditis after CD19 chimeric antigen receptor T-cell therapy. Front. Immunol. 2022, 13, 995496. [Google Scholar] [CrossRef]
- Feins, S.; Kong, W.; Williams, E.F.; Milone, M.C.; Fraietta, J.A. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am. J. Hematol. 2019, 94, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Beer, T.M.; Gerritsen, W.; Oudard, S.; Wiechno, P.; Kukielka-Budny, B.; Samal, V.; Hajek, J.; Feyerabend, S.; Khoo, V.; et al. Efficacy and Safety of Autologous Dendritic Cell-Based Immunotherapy, Docetaxel, and Prednisone vs Placebo in Patients with Metastatic Castration-Resistant Prostate Cancer: The VIABLE Phase 3 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Biery, D.N.; Turicek, D.P.; Diorio, C.; Schroeder, B.A.; Shah, N.N. Need for standardization of cytokine profiling in CAR T cell therapy. Mol. Ther. 2024, 32, 2979–2983. [Google Scholar] [CrossRef]
- Schroeder, T.; Martens, T.; Fransecky, L.; Valerius, T.; Schub, N.; Pott, C.; Baldus, C.; Stölzel, F. Management of chimeric antigen receptor T (CAR-T) cell-associated toxicities. Intensiv. Care Med. 2024, 50, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Mashima, H.; Zhang, R.; Kobayashi, T.; Hagiya, Y.; Tsukamoto, H.; Liu, T.; Iwama, T.; Yamamoto, M.; Lin, C.; Nakatsuka, R.; et al. Generation of GM-CSF-producing antigen-presenting cells that induce a cytotoxic T cell-mediated antitumor response. OncoImmunology 2020, 9, 1814620. [Google Scholar] [CrossRef] [PubMed]
- Palucka, A.K.; Coussens, L.M. The basis of oncoimmunology. Cell 2016, 164, 1233–1247. [Google Scholar] [CrossRef]
- Hong, D.S.; Van Tine, B.A.; Biswas, S.; McAlpine, C.; Johnson, M.L.; Olszanski, A.J.; Clarke, J.M.; Araujo, D.; Blumenschein, G.R.; Kebriaei, P.; et al. Autologous T cell therapy for MAGE-A4+ solid cancers in HLA-A*02+ patients: A phase 1 trial. Nat. Med. 2023, 29, 104–114. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Araujo, D.M.; Razak, A.R.A.; Agulnik, M.; Attia, S.; Blay, J.-Y.; Garcia, I.C.; Charlson, J.A.; Choy, E.; Demetri, G.D.; et al. Afamitresgene autoleucel for advanced synovial sarcoma and myxoid round cell liposarcoma (SPEARHEAD-1): An international, open-label, phase 2 trial. Lancet 2024, 403, 1460–1471. [Google Scholar] [CrossRef]
- Keam, S.J. Afamitresgene Autoleucel: First Approval. Mol. Diagn. Ther. 2024, 28, 861–866. [Google Scholar] [CrossRef]
- Wang, L.; Geng, H.; Liu, Y.; Liu, L.; Chen, Y.; Wu, F.; Liu, Z.; Ling, S.; Wang, Y.; Zhou, L. Hot and cold tumors: Immunological features and the therapeutic strategies. Medcomm 2023, 4, e343. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N.; Suarez-Carmona, M.; Charoentong, P.; Weis, C.-A.; Hirsch, D.; Bankhead, P.; Horning, M.; Ferber, D.; Kel, I.; Herpel, E.; et al. Topography of cancer-associated immune cells in human solid tumors. eLife 2018, 7, e36967. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.Y.; Lou, Y.; Sexton-Bonacci, R.E. Editorial: Novel immunological characteristics and immunotherapeutic targets in pancreatic cancer. Front. Immunol. 2024, 15, 1428740. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fang, Y.; Zhang, Y.; Wang, H.; Yang, Z.; Ding, D. Supramolecular Self-Assembly-Facilitated Aggregation of Tumor-Specific Transmembrane Receptors for Signaling Activation and Converting Immunologically Cold to Hot Tumors. Adv. Mater. 2021, 33, 2008518. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Sun, Z.-J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef]
- Horii, T.; Orikawa, Y.; Ohira, Y.; Eta, R.; Kobayashi, N.; Sato, T.; Watanabe, T.; Tanaka, T. Potential of Z-100, extracted from Mycobacterium tuberculosis strain Aoyama B, as a hot tumor inducer. Cancer Cell Int. 2022, 22, 392. [Google Scholar] [CrossRef]
- Sivagnanalingam, U.; Beatty, P.L.; Finn, O.J. Myeloid derived suppressor cells in cancer, premalignancy and inflammation: A roadmap to cancer immunoprevention. Mol. Carcinog. 2020, 59, 852–861. [Google Scholar] [CrossRef]
- Xu, J.; Shi, Q.; Wang, B.; Ji, T.; Guo, W.; Ren, T.; Tang, X. The role of tumor immune microenvironment in chordoma: Promising immunotherapy strategies. Front. Immunol. 2023, 14, 1257254. [Google Scholar] [CrossRef]
- Wang, B.; Bai, J.; Tian, B.; Chen, H.; Yang, Q.; Chen, Y.; Xu, J.; Zhang, Y.; Dai, H.; Ma, Q.; et al. Genetically Engineered Hematopoietic Stem Cells Deliver TGF-β Inhibitor to Enhance Bone Metastases Immunotherapy. Adv. Sci. 2022, 9, e2201451. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Yamazaki, T.; McCarty, K.; Fox, N.; Phillips, M.; Alice, A.; Blair, T.; Whiteford, M.; O’brien, D.; Ahmad, R.; et al. TGFβ suppresses CD8+ T cell expression of CXCR3 and tumor trafficking. Nat. Commun. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Murphy, D.A.; Osteicochea, D.; Atkins, A.; Sannes, C.; McClarnon, Z.; Adjei, I.M. Optimizing Oxygen-Production Kinetics of Manganese Dioxide Nanoparticles Improves Hypoxia Reversal and Survival in Mice with Bone Metastases. Mol. Pharm. 2024, 21, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; D’incà, F.; Gelibter, A.; Chiari, R.; Grossi, F.; Delmonte, A.; Passaro, A.; Signorelli, D.; Gelsomino, F.; Galetta, D.; et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J. Immunother. Cancer 2019, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, T.E.; Halleen, J.M.; MacRitchie, G.; Andersson, R.M.; Bernoulli, J. Insights into immuno-oncology drug development landscape with focus on bone metastasis. Front. Immunol. 2023, 14, 1121878. [Google Scholar] [CrossRef] [PubMed]
- Qiang, H.; Lei, Y.; Shen, Y.; Li, J.; Zhong, H.; Zhong, R.; Zhang, X.; Chang, Q.; Lu, J.; Feng, H.; et al. Pembrolizumab monotherapy or combination therapy for bone metastases in advanced non-small cell lung cancer: A real-world retrospective study. Transl. Lung Cancer Res. 2022, 11, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, K.; Peng, Y.; Wu, W.; Chen, F.; Shao, Z.; Zhang, Z. Animal models of cancer metastasis to the bone. Front. Oncol. 2023, 13, 1165380. [Google Scholar] [CrossRef]
- Jelgersma, C.; Vajkoczy, P. How to Target Spinal Metastasis in Experimental Research: An Overview of Currently Used Experimental Mouse Models and Future Prospects. Int. J. Mol. Sci. 2021, 22, 5420. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Y.; Qiao, X.; Yu, Y.; Song, Z.; Liu, Z.; Tian, Z.; Chen, S.; Zhang, X.; Wang, X. Experimental research on spinal metastasis with mouse models. Chin. Med. J. 2023, 136, 3008–3009. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Zhang, D.; Chen, Q.; Xu, J.; Tang, L.; Luo, J.; Mai, Q.; Lu, X.; Tan, L.; et al. Development of a precision tumor bone metastasis model by a magnetic micro-living-motor system. Colloids Surf. B Biointerfaces 2024, 238, 113877. [Google Scholar] [CrossRef]
- Azar, H.K.; Gharibshahian, M.; Rostami, M.; Mansouri, V.; Sabouri, L.; Beheshtizadeh, N.; Rezaei, N. The progressive trend of modeling and drug screening systems of breast cancer bone metastasis. J. Biol. Eng. 2024, 18, 14. [Google Scholar] [CrossRef]
- Brom, V.C.; Strauss, A.C.; Sieberath, A.; Salber, J.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Agonistic and antagonistic targeting of immune checkpoint molecules differentially regulate osteoclastogenesis. Front. Immunol. 2023, 14, 988365. [Google Scholar] [CrossRef]
- Moseley, K.F.; Naidoo, J.; Bingham, C.O.; Carducci, M.A.; Forde, P.M.; Gibney, G.T.; Lipson, E.J.; Shah, A.A.; Sharfman, W.H.; Cappelli, L.C. Immune-related adverse events with immune checkpoint inhibitors affecting the skeleton: A seminal case series. J. Immunother. Cancer 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Chen, S.; Zhou, F.; Zhao, J.; Zhao, W.; Su, C. Adverse impact of bone metastases on clinical outcomes of patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac. Cancer 2020, 11, 2812–2819. [Google Scholar] [CrossRef]
- Rosner, S.; Sen, F.; Postow, M. Response after treatment with pembrolizumab in a patient with myelophthisis due to melanoma: The role of checkpoint inhibition in the bone. J. Immunother. Cancer 2017, 5, 34. [Google Scholar] [CrossRef]
- Ihle, C.L.; Provera, M.D.; Straign, D.M.; Smith, E.E.; Edgerton, S.M.; Van Bokhoven, A.; Lucia, M.S.; Owens, P. Distinct tumor microenvironments of lytic and blastic bone metastases in prostate cancer patients. J. Immunother. Cancer 2019, 7, 293. [Google Scholar] [CrossRef]
- Conway, J.W.; Braden, J.; Wilmott, J.S.; Scolyer, R.A.; Long, G.V.; da Silva, I.P. The effect of organ-specific tumor microenvironments on response patterns to immunotherapy. Front. Immunol. 2022, 13, 1030147. [Google Scholar] [CrossRef]
- Yu, G.; Corn, P.G.; Mak, C.S.L.; Liang, X.; Zhang, M.; Troncoso, P.; Song, J.H.; Lin, S.-C.; Song, X.; Liu, J.; et al. Prostate cancer–induced endothelial-cell-to-osteoblast transition drives immunosuppression in the bone–tumor microenvironment through Wnt pathway–induced M2 macrophage polarization. Proc. Natl. Acad. Sci. USA 2024, 121, 2903121. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhao, W.; Ye, B.; Chen, D. Combination of Immune Checkpoint Inhibitors and Anti-Angiogenic Agents in Brain Metastases from Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 670313. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Yu, Y. Screening responsive or resistant biomarkers of immune checkpoint inhibitors based on online databases. Front. Med. 2019, 13, 24–31. [Google Scholar] [CrossRef]
- Joseph, G.J.; Johnson, D.B.; Johnson, R.W. Immune checkpoint inhibitors in bone metastasis: Clinical challenges, toxicities, and mechanisms. J. Bone Oncol. 2023, 43, 100505. [Google Scholar] [CrossRef]
- Angela, Y.; Haferkamp, S.; Weishaupt, C.; Ugurel, S.; Becker, J.C.; Oberndörfer, F.; Alar, V.; Satzger, I.; Gutzmer, R. Combination of denosumab and immune checkpoint inhibition: Experience in 29 patients with metastatic melanoma and bone metastases. Cancer Immunol. Immunother. 2019, 68, 1187–1194. [Google Scholar] [CrossRef]
- Cao, Y.; Afzal, M.Z.; Shirai, K. Does denosumab offer survival benefits?—Our experience with denosumab in metastatic non-small cell lung cancer patients treated with immune-checkpoint inhibitors. J. Thorac. Dis. 2021, 13, 4668–4677. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-S.; Lei, S.-Y.; Li, J.-L.; Xing, P.-Y.; Hao, X.-Z.; Xu, F.; Xu, H.-Y.; Wang, Y. Efficacy and safety of concomitant immunotherapy and denosumab in patients with advanced non-small cell lung cancer carrying bone metastases: A retrospective chart review. Front. Immunol. 2022, 13, 908436. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-P.; Wu, C.-C.; Shyr, C.-R. Combination of novel intravesical xenogeneic urothelial cell immunotherapy and chemotherapy enhances anti-tumor efficacy in preclinical murine bladder tumor models. Cancer Immunol. Immunother. 2020, 70, 1419–1433. [Google Scholar] [CrossRef]
- Xing, W.; Zhao, L.; Zheng, Y.; Liu, B.; Liu, X.; Li, T.; Zhang, Y.; Ma, B.; Yang, Y.; Shang, Y.; et al. The Sequence of Chemotherapy and Toripalimab Might Influence the Efficacy of Neoadjuvant Chemoimmunotherapy in Locally Advanced Esophageal Squamous Cell Cancer—A Phase II Study. Front. Immunol. 2021, 12, 772450. [Google Scholar] [CrossRef]
- Nabrinsky, E.; Macklis, J.; Bitran, J. A Review of the Abscopal Effect in the Era of Immunotherapy. Cureus 2022, 14, 29620. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Masui, K.; Yamazaki, H.; Takenaka, T.; Asai, S.; Taniguchi, H.; Nakamura, T.; Ukimura, O.; Yamada, K. Abscopal effect of high-dose-rate brachytherapy on pelvic bone metastases from renal cell carcinoma: A case report. J. Contemp. Brachytherapy 2019, 11, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Le, T.Q.; Massarelli, E.; Hendifar, A.E.; Tuli, R. Radiation therapy and PD-1/PD-L1 blockade: The clinical development of an evolving anticancer combination. J. Immunother. Cancer 2018, 6, 46. [Google Scholar] [CrossRef]
- Mannavola, F.; Mandala, M.; Todisco, A.; Sileni, V.C.; Palla, M.; Minisini, A.M.; Pala, L.; Morgese, F.; Di Guardo, L.; Stucci, L.S.; et al. An Italian Retrospective Survey on Bone Metastasis in Melanoma: Impact of Immunotherapy and Radiotherapy on Survival. Front. Oncol. 2020, 10, 1652. [Google Scholar] [CrossRef]
- Galluzzo, S.; Santini, D.; Vincenzi, B.; Caccamo, N.; Meraviglia, F.; Salerno, A.; Dieli, F.; Tonini, G. Immunomodulating role of bisphosphonates on human gamma delta T cells: An intriguing and promising aspect of their antitumour activity. Expert Opin. Ther. Targets 2007, 11, 941–954. [Google Scholar] [CrossRef]
- Kunzmann, V.; Bauer, E.; Feurle, J.; Weissinger, F.; Tony, H.P.; Wilhelm, M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000, 96, 384–392. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, M.; Wang, L.; Wang, F.; Deng, H.; Yang, Y.; Sun, N.; Li, R.; Chen, Y.; Lin, X.; et al. Effects of combining immune checkpoint inhibitors and anti-angiogenic agents on bone metastasis in non-small cell lung cancer patients. Hum. Vaccines Immunother. 2023, 19, 2241310. [Google Scholar] [CrossRef] [PubMed]
- Del Conte, A.; De Carlo, E.; Bertoli, E.; Stanzione, B.; Revelant, A.; Bertola, M.; Spina, M.; Bearz, A. Bone Metastasis and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer (NSCLC): Microenvironment and Possible Clinical Implications. Int. J. Mol. Sci. 2022, 23, 6832. [Google Scholar] [CrossRef] [PubMed]
- Blazar, B.R.; Murphy, W.J. Bone marrow transplantation and approaches to avoid graft-versus-host disease (GVHD). Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1747–1767. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Tian, Z.; Lv, G.; Zhang, L.; Jiang, G.; Sun, K.; Wang, C.; Bu, X.; Li, R.; Shi, Y.; et al. Immunosuppressive effect of bone marrow-derived mesenchymal stem cells in inflammatory microenvironment favours the growth of B16 melanoma cells. J. Cell. Mol. Med. 2010, 15, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Chou, M.-H.; Meng, E.; Kao, C.-C. A Rare Case Report of Metastatic Urothelial Carcinoma to Skull with Significant Reossification after Pembrolizumab. Medicina 2021, 57, 987. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Lin, C.-S. Regression of advanced maxillary sinus cancer with orbital invasion by combined chemotherapy and immunotherapy: A one-year follow-up case report. Mol. Clin. Oncol. 2022, 16, 94. [Google Scholar] [CrossRef]
- Kumar, S.; Mulia, G.E.; Figueiredo, M.L. Cabozantinib and IL-27 combinatorial therapy for bone-metastatic prostate cancer. Front. Mol. Biosci. 2023, 10, 1259336. [Google Scholar] [CrossRef]
- Yu, D.-P.; Cheng, X.; Liu, Z.-D.; Xu, S.-F. Comparative beneficiary effects of immunotherapy against chemotherapy in patients with advanced NSCLC: Meta-analysis and systematic review. Oncol. Lett. 2017, 14, 1568–1580. [Google Scholar] [CrossRef]
- Vivier, E.; Rebuffet, L.; Narni-Mancinelli, E.; Cornen, S.; Igarashi, R.Y.; Fantin, V.R. Natural killer cell therapies. Nature 2024, 626, 727–736. [Google Scholar] [CrossRef]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nano-Micro Lett. 2020, 12, 142. [Google Scholar] [CrossRef]
- Buchwald, Z.S.; Wynne, J.; Nasti, T.H.; Zhu, S.; Mourad, W.F.; Yan, W.; Gupta, S.; Khleif, S.N.; Khan, M.K. Radiation, Immune Checkpoint Blockade and the Abscopal Effect: A Critical Review on Timing, Dose and Fractionation. Front. Oncol. 2018, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Brooker, R.; Brada, M. Combining immunotherapy and radiotherapy in lung cancer. J. Thorac. Dis. 2018, 10 (Suppl. 13), S1447–S1460. [Google Scholar] [CrossRef] [PubMed]
- Kalina, J.L.; Neilson, D.S.; Comber, A.P.; Rauw, J.M.; Alexander, A.S.; Vergidis, J.; Lum, J.J. Immune Modulation by Androgen Deprivation and Radiation Therapy: Implications for Prostate Cancer Immunotherapy. Cancers 2017, 9, 13. [Google Scholar] [CrossRef]
- Jagodinsky, J.C.; Harari, P.M.; Morris, Z.S. The Promise of Combining Radiation Therapy with Immunotherapy. Int. J. Radiat. Oncol. 2020, 108, 6–16. [Google Scholar] [CrossRef]
- Amin, S.; Baine, M.J.; Meza, J.L.; Lin, C. Association of Immunotherapy with Survival Among Patients with Brain Metastases Whose Cancer Was Managed with Definitive Surgery of the Primary Tumor. JAMA Netw. Open 2020, 3, e2015444. [Google Scholar] [CrossRef]
- Dagoglu, N.; Karaman, S.; Caglar, H.B.; Oral, E.N. Abscopal Effect of Radiotherapy in the Immunotherapy Era: Systematic Review of Reported Cases. Cureus 2019, 11, e4103. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, M.E.; Vanpouille-Box, C.; Melero, I.; Formenti, S.C.; Demaria, S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. 2018, 39, 644–655. [Google Scholar] [CrossRef]
- Sridharan, V.; Margalit, D.N.; Lynch, S.A.; Severgnini, M.; Zhou, J.; Chau, N.G.; Rabinowits, G.; Lorch, J.H.; Hammerman, P.S.; Hodi, F.S.; et al. Definitive chemoradiation alters the immunologic landscape and immune checkpoints in head and neck cancer. Br. J. Cancer 2016, 115, 252–260. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Wilkins, A.; McDonald, F.; Harrington, K.; Melcher, A. Radiotherapy enhances responses of lung cancer to CTLA-4 blockade. J. Immunother. Cancer 2019, 7, 64. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.Y.; Deftos, M.; Wang, C.C. Pneumonitis in combined Anti-programmed Death-1 Immunotherapy and Radiation Therapy for Renal Cell Carcinoma. Cureus 2018, 10, e3748. [Google Scholar] [CrossRef] [PubMed]
- Chicas-Sett, R.; Morales-Orue, I.; Rodriguez-Abreu, D.; Lara-Jimenez, P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: A systematic review. Clin. Transl. Radiat. Oncol. 2018, 9, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Garelli, E.; Rittmeyer, A.; Putora, P.M.; Glatzer, M.; Dressel, R.; Andreas, S. Abscopal effect in lung cancer: Three case reports and a concise review. Immunotherapy 2019, 11, 1445–1461. [Google Scholar] [CrossRef]
- Protopapa, M.; Kouloulias, V.; Nikoloudi, S.; Papadimitriou, C.; Gogalis, G.; Zygogianni, A. From Whole-Brain Radiotherapy to Immunotherapy: A Multidisciplinary Approach for Patients with Brain Metastases from NSCLC. J. Oncol. 2019, 2019, 3267409. [Google Scholar] [CrossRef]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newell, P.; Bahjat, K.S.; et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef]
- Cushman, T.R.; Gomez, D.; Kumar, R.; Likacheva, A.; Chang, J.Y.; Cadena, A.P.; Paris, S.; Welsh, J.W. Combining radiation plus immunotherapy to improve systemic immune response. J. Thorac. Dis. 2018, 10, S468–S479. [Google Scholar] [CrossRef]
- Vatner, R.E.; Cooper, B.T.; Vanpouille-Box, C.; Demaria, S.; Formenti, S.C. Combinations of immunotherapy and radiation in cancer therapy. Front. Oncol. 2014, 4, 325. [Google Scholar] [CrossRef]
- Chandra, R.A.; Wilhite, T.J.; Balboni, T.A.; Alexander, B.M.; Spektor, A.; Ott, P.A.; Ng, A.K.; Hodi, F.S.; Schoenfeld, J.D. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. OncoImmunology 2015, 4, e1046028. [Google Scholar] [CrossRef]
- Lin, A.J.; Roach, M.; Bradley, J.; Robinson, C. Combining stereotactic body radiation therapy with immunotherapy: Current data and future directions. Transl. Lung Cancer Res. 2019, 8, 107–115. [Google Scholar] [CrossRef]
- Du, D.; Song, T.; Dai, H.; Jing, Z.; Chen, P.; Wu, S. Stereotactic body radiation therapy and thymosin alpha-1-induced anti-tumor effects in heavily pretreated, metastatic esophageal squamous cell carcinoma patients. OncoImmunology 2018, 7, e1450128. [Google Scholar] [CrossRef]
- Meng, X.; Feng, R.; Yang, L.; Xing, L.; Yu, J. The Role of Radiation Oncology in Immuno-Oncology. Oncologist 2019, 24, S42–S52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, W.; Li, N.; Neri, S.; Sharma, A.; Jiang, W.; Lin, S.H. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Front. Pharmacol. 2018, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, M.; Kohrt, H.; Levy, R.; Jones, J.; Camphausen, K.; Dicker, A.; Demaria, S.; Formenti, S. Current clinical trials testing combinations of immunotherapy and radiation. Semin. Radiat. Oncol. 2014, 25, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Shi, M.; Xu, H.; Bai, S.; Xiong, X.; Wei, Q.; Yang, L. Combined Treatment of Radiotherapy and Immunotherapy for Urological Malignancies: Current Evidence and Clinical Considerations. Cancer Manag. Res. 2021, 13, 1719–1731. [Google Scholar] [CrossRef]
- Salama, A.K.S.; Postow, M.A.; Salama, J.K. Irradiation and immunotherapy: From concept to the clinic. Cancer 2016, 122, 1659–1671. [Google Scholar] [CrossRef]
- Kumar, R.; Kim, J.; Deek, M.P.; Eskander, M.F.; Gulhati, P.; In, H.; Kennedy, T.; Shah, M.M.; Grandhi, M.S.; Berim, L.; et al. Combination of Immunotherapy and Radiation Therapy in Gastrointestinal Cancers: An Appraisal of the Current Literature and Ongoing Research. Curr. Oncol. 2023, 30, 6432–6446. [Google Scholar] [CrossRef]
- Arellano, D.L.; Juárez, P.; Verdugo-Meza, A.; Almeida-Luna, P.S.; Corral-Avila, J.A.; Drescher, F.; Olvera, F.; Jiménez, S.; Elzey, B.D.; Guise, T.A.; et al. Bone Microenvironment-Suppressed T Cells Increase Osteoclast Formation and Osteolytic Bone Metastases in Mice. J. Bone Miner. Res. 2022, 37, 1446–1463. [Google Scholar] [CrossRef]
- Ratti, C.; Botti, L.; Cancila, V.; Galvan, S.; Torselli, I.; Garofalo, C.; Manara, M.C.; Bongiovanni, L.; Valenti, C.F.; Burocchi, A.; et al. Trabectedin Overrides Osteosarcoma Differentiative Block and Reprograms the Tumor Immune Environment Enabling Effective Combination with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2017, 23, 5149–5161. [Google Scholar] [CrossRef]
- Rieunier, G.; Wu, X.; Macaulay, V.M.; Lee, A.V.; Weyer-Czernilofsky, U.; Bogenrieder, T. Bad to the Bone: The Role of the Insulin-Like Growth Factor Axis in Osseous Metastasis. Clin. Cancer Res. 2019, 25, 3479–3485. [Google Scholar] [CrossRef]
- Hillerdal, V.; Essand, M. Chimeric antigen receptor-engineered T cells for the treatment of metastatic prostate cancer. BioDrugs 2015, 29, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Tamada, S.; Ikarashi, D.; Tsuyukubo, T.; Iwasaki, K.; Isurugi, K.; Ono, S.; Takata, R.; Fujisawa, H.; Obara, W. Efficacy of combination therapy with pembrolizumab and axitinib for metastatic renal collecting duct cell carcinoma: A report on two cases. IJU Case Rep. 2022, 5, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Vilinovszki, O.; Andratschke, N.; Huellner, M.; Curioni-Fontecedro, A.; Kroeze, S.G.C. True abscopal effect in a patient with metastatic non-small cell lung cancer. Radiat. Oncol. 2021, 16, 194. [Google Scholar] [CrossRef]
- Xiang, L.; Gilkes, D.M. The Contribution of the Immune System in Bone Metastasis Pathogenesis. Int. J. Mol. Sci. 2019, 20, 999. [Google Scholar] [CrossRef]
- Baschuk, N.; Rautela, J.; Parker, B.S. Bone specific immunity and its impact on metastasis. BoneKEy Rep. 2015, 4, 665. [Google Scholar] [CrossRef]
- Grimm, M.; Gasser, M.; Bueter, M.; Strehl, J.; Wang, J.; Nichiporuk, E.; Meyer, D.; Germer, C.T.; Waaga-Gasser, A.M.; Thalheimer, A. Evaluation of immunological escape mechanisms in a mouse model of colorectal liver metastases. BMC Cancer 2010, 10, 82. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Sosnoski, D.M.; Mastro, A.M. Breast cancer metastasis to the bone: Mechanisms of bone loss. Breast Cancer Res. 2010, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Di Maio, M.; Valsecchi, A.A.; Santini, D.; Tucci, M.; De Giorgi, U.; Bossi, P.; Ibrahim, T.; Cavanna, L.; Lanzetta, G.; et al. Treatment of bone metastases from solid tumors with bone-modifying agents: A web survey of Italian oncologists investigating patterns of practice drug prescription and prevention of side effects. Support. Care Cancer 2024, 32, 202. [Google Scholar] [CrossRef]
- Drudge-Coates, L.; van Muilekom, E.; de la Torre-Montero, J.C.; Leonard, K.; van Oostwaard, M.; Niepel, D.; Jensen, B.T. Management of bone health in patients with cancer: A survey of specialist nurses. Support Care Cancer 2020, 28, 1151–1162. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Rinnerthaler, G.; Greil, R. Bone-targeted therapy in metastatic breast cancer—All well-established knowledge? Breast Care 2014, 9, 323–330. [Google Scholar] [CrossRef]
- Liede, A.; Hernandez, R.K.; Wade, S.W.; Bo, R.; Nussbaum, N.C.; Ahern, E.; Dougall, W.C.; Smyth, M.J. An observational study of concomitant immunotherapies and denosumab in patients with advanced melanoma or lung cancer. OncoImmunology 2018, 7, e1480301. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.M.; Swee, D.S.M. Severe and refractory hypocalcaemia secondary to osteoblastic bone metastases in bladder signet ring carcinoma: A case report and literature review. Medicine 2022, 101, e29731. [Google Scholar] [CrossRef]
- Rief, H.; Bruckner, T.; Schlampp, I.; Bostel, T.; Welzel, T.; Debus, J.; Förster, R. Resistance training concomitant to radiotherapy of spinal bone metastases—Survival and prognostic factors of a randomized trial. Radiat. Oncol. 2016, 11, 97. [Google Scholar] [CrossRef]
- Rüttinger, D.; Li, R.; Urba, W.J.; Fox, B.A.; Hu, H.-M. Regression of bone metastases following adoptive transfer of anti-CD3-activated and IL-2-expanded tumor vaccine draining lymph node cells. Clin. Exp. Metastasis 2004, 21, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Iuliani, M.; Simonetti, S.; Ribelli, G.; Napolitano, A.; Pantano, F.; Vincenzi, B.; Tonini, G.; Santini, D. Current and Emerging Biomarkers Predicting Bone Metastasis Development. Front. Oncol. 2020, 10, 789. [Google Scholar] [CrossRef]
- Wood, S.L.; Brown, J.E. Personal Medicine and Bone Metastases: Biomarkers, Micro-RNAs and Bone Metastases. Cancers 2020, 12, 2109. [Google Scholar] [CrossRef] [PubMed]
- Thysell, E.; Vidman, L.; Ylitalo, E.B.; Jernberg, E.; Crnalic, S.; Iglesias-Gato, D.; Flores-Morales, A.; Stattin, P.; Egevad, L.; Widmark, A.; et al. Gene expression profiles define molecular subtypes of prostate cancer bone metastases with different outcomes and morphology traceable back to the primary tumor. Mol. Oncol. 2019, 13, 1763–1777. [Google Scholar] [CrossRef]
- Zhao, C.; Lou, Y.; Wang, Y.; Wang, D.; Tang, L.; Gao, X.; Zhang, K.; Xu, W.; Liu, T.; Xiao, J. A gene expression signature-based nomogram model in prediction of breast cancer bone metastases. Cancer Med. 2019, 8, 200–208. [Google Scholar] [CrossRef]
- Yang, W.; Pan, Q.; Huang, F.; Hu, H.; Shao, Z. Research progress of bone metastases: From disease recognition to clinical practice. Front. Oncol. 2022, 12, 1105745. [Google Scholar] [CrossRef]
- Nadar, R.A.; Farbod, K.; der Schilden, K.C.-V.; Schlatt, L.; Crone, B.; Asokan, N.; Curci, A.; Brand, M.; Bornhaeuser, M.; Iafisco, M.; et al. Targeting of radioactive platinum-bisphosphonate anticancer drugs to bone of high metabolic activity. Sci. Rep. 2020, 10, 5889. [Google Scholar] [CrossRef]
- Handkiewicz-Junak, D.; Poeppel, T.D.; Bodei, L.; Aktolun, C.; Ezziddin, S.; Giammarile, F.; Delgado-Bolton, R.C.; Gabriel, M. EANM guidelines for radionuclide therapy of bone metastases with beta-emitting radionuclides. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 846–859. [Google Scholar] [CrossRef] [PubMed]

| Combination Therapy | Therapeutic Agents | Mechanisms of Action | Clinical Outcomes | Key Studies & Findings | Challenges and Limitations |
|---|---|---|---|---|---|
| ICIs + Bone-Targeted Agents | Denosumab, Zoledronic Acid | ICIs restore T-cell activity by blocking immune checkpoint pathways (PD-1/PD-L1 or CTLA-4); denosumab inhibits RANKL-mediated osteoclastogenesis [8,141,142]. | Prolonged progression-free survival in NSCLC patients; improved outcomes in melanoma with bone metastases [8,141]. | Combination of denosumab and nivolumab showed significant overall response rates in patients with metastatic melanoma [141]. | Immunosuppressive bone microenvironment; limited stratification of clinical trial patients by metastasis sites; inconsistent benefits in different cancers [8,142]. |
| ICIs + Chemotherapy | Pembrolizumab + Cytotoxic Agents | Chemotherapy induces immunogenic cell death and tumor antigen release, enhancing ICI efficacy [144,145]. | Tumor regression and reossification observed in bone metastases [144]. | Enhanced T-cell activation observed when chemotherapy precedes ICI therapy [145]. | Lack of optimized sequencing and dosing; potential overlapping toxicities [8,144]. |
| ICIs + Radiotherapy | Nivolumab + Local Radiotherapy | Radiation induces immunogenic cell death, releasing antigens that stimulate systemic immune responses and the abscopal effect [8,146,147,148]. | Improved survival rates and systemic responses in metastatic cancers, including melanoma [146,149]. | Timing and sequencing critical; abscopal effect observed in some cases but inconsistent outcomes in others [148,149]. | Heterogeneity in tumor microenvironment and immune suppression; variable effectiveness across tumor types [8,148]. |
| CAR T-cell Therapy + ICIs | Genetically Modified T-cells | CAR T-cells target tumor antigens directly; ICIs prevent immune exhaustion by blocking inhibitory checkpoints [8,98,101]. | Effective in hematologic malignancies; limited efficacy in solid tumors like bone metastases [101]. | Potential synergy with checkpoint inhibitors demonstrated in preclinical studies [101]. | Immunosuppressive bone microenvironment; high risk of cytokine release syndrome; challenges in solid tumors [98,104]. |
| Bone-Targeted Agents + Chemotherapy | Bisphosphonates + Cytotoxic Agents | Bisphosphonates reduce osteoclast activity and bone resorption; chemotherapy induces cytotoxicity and immune modulation [150,151]. | Stabilization of bone lesions noted; impact on overall survival remains unclear [8,151]. | Synergy in reducing bone resorption and enhancing tumor antigen presentation [150]. | Long-term outcomes unclear; risk of combined toxicities; limited data on efficacy in bone metastases [8,150]. |
| ICIs + Anti-Angiogenic Agents | Nivolumab + Bevacizumab | Anti-angiogenics normalize tumor vasculature, enhancing ICI delivery and immune cell infiltration into the tumor microenvironment [8,152]. | Improved progression-free survival in NSCLC patients with bone metastases [152]. | Evidence supports synergistic effects of ICIs and anti-angiogenic agents in reducing tumor progression [152]. | Risk of immune-related adverse events (irAEs); increased toxicity; need for robust predictive biomarkers [8,152]. |
| Combination Therapy | Common Side Effects | Severe Side Effects |
|---|---|---|
| ICIs + Bone-Targeted Agents | Fatigue, musculoskeletal pain, and mild skin rash associated with ICIs and bone agents like denosumab [141,142]. | Osteonecrosis of the jaw (rare, linked to bone-targeted agents); immune-related adverse events (irAEs) such as pneumonitis [142]. |
| ICIs + Chemotherapy | Nausea, diarrhea, and mild hematologic toxicity such as anemia [144,145]. | Severe neutropenia, febrile infections, and exacerbation of chemotherapy-induced toxicities [145] |
| ICIs + Radiotherapy | Fatigue and localized skin irritation from radiotherapy; flu-like symptoms from ICIs [8,148]. | Severe irAEs, including pneumonitis, colitis, or inflammatory changes misinterpreted as disease progression [8,149] |
| CAR T-cell Therapy + ICIs | Fever, fatigue, and mild cytokine release syndrome (CRS) symptoms like hypotension and tachycardia [98,104]. | Severe CRS, neurotoxicity, and life-threatening immune hyperactivation (e.g., macrophage activation syndrome) [104,105]. |
| Bone-Targeted Agents + Chemotherapy | Fatigue, mild nausea, and increased bone pain during initial treatment (flare reaction) [150,151]. | Long-term osteonecrosis of the jaw; severe myelosuppression or cumulative toxicities with chemotherapy [151] |
| ICIs + Anti-Angiogenic Agents | Hypertension, proteinuria, and fatigue due to anti-angiogenic agents like bevacizumab; mild irAEs from ICIs [152]. | Severe thromboembolic events, gastrointestinal perforation, or exacerbated irAEs (e.g., colitis or hepatitis) [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, F.N.; Mohammad, K.S. Immunotherapy in the Battle Against Bone Metastases: Mechanisms and Emerging Treatments. Pharmaceuticals 2024, 17, 1591. https://doi.org/10.3390/ph17121591
Hamza FN, Mohammad KS. Immunotherapy in the Battle Against Bone Metastases: Mechanisms and Emerging Treatments. Pharmaceuticals. 2024; 17(12):1591. https://doi.org/10.3390/ph17121591
Chicago/Turabian StyleHamza, Fatheia N., and Khalid Said Mohammad. 2024. "Immunotherapy in the Battle Against Bone Metastases: Mechanisms and Emerging Treatments" Pharmaceuticals 17, no. 12: 1591. https://doi.org/10.3390/ph17121591
APA StyleHamza, F. N., & Mohammad, K. S. (2024). Immunotherapy in the Battle Against Bone Metastases: Mechanisms and Emerging Treatments. Pharmaceuticals, 17(12), 1591. https://doi.org/10.3390/ph17121591







