Predicting Pharmacokinetics of Active Constituents in Spatholobi caulis by Using Physiologically Based Pharmacokinetic Models
Abstract
:1. Introduction
2. Results
2.1. Establishing the PBPK Models of Active Constituents
2.1.1. Parameterization
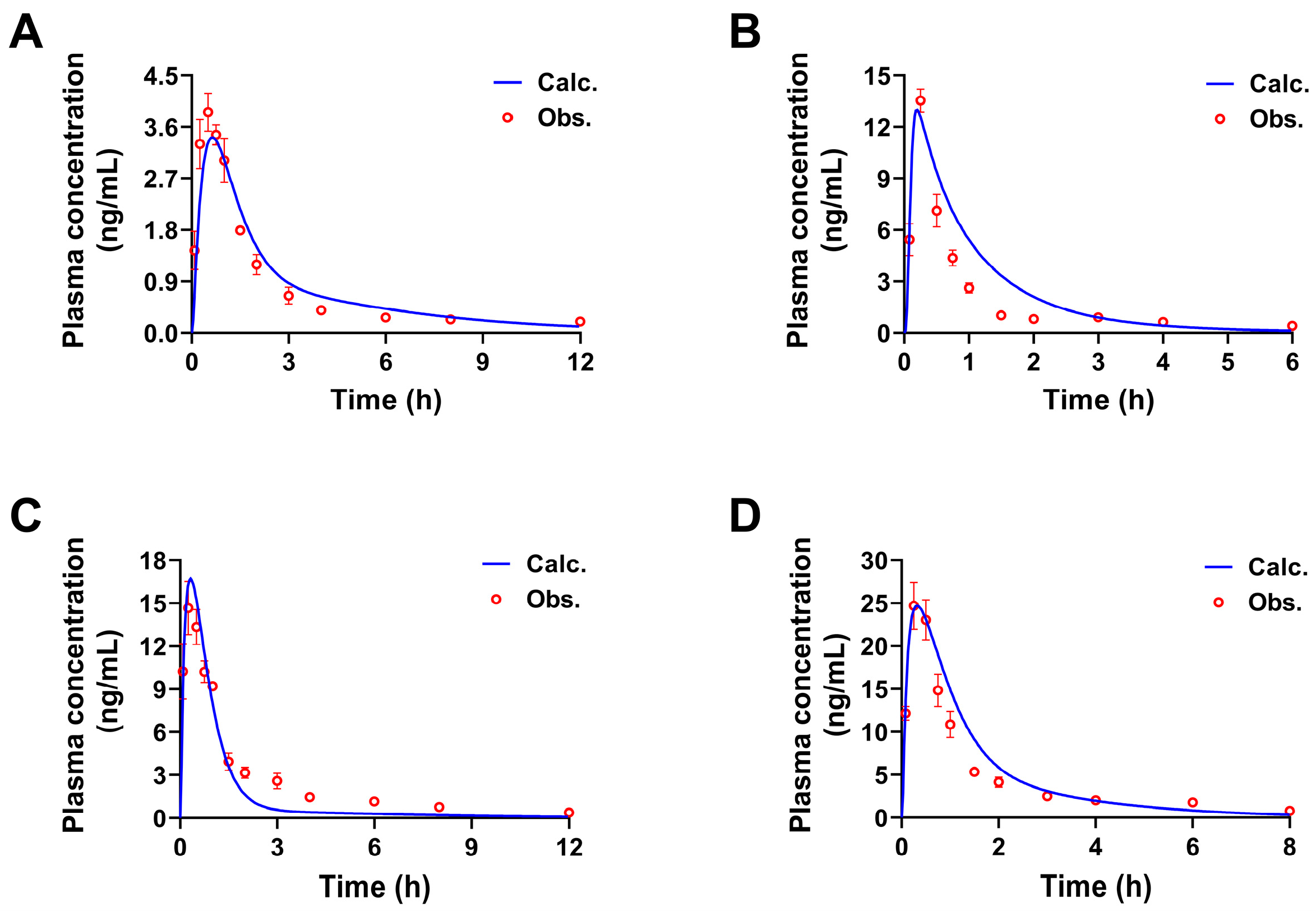
2.1.2. Construction and Validation of PBPK Models
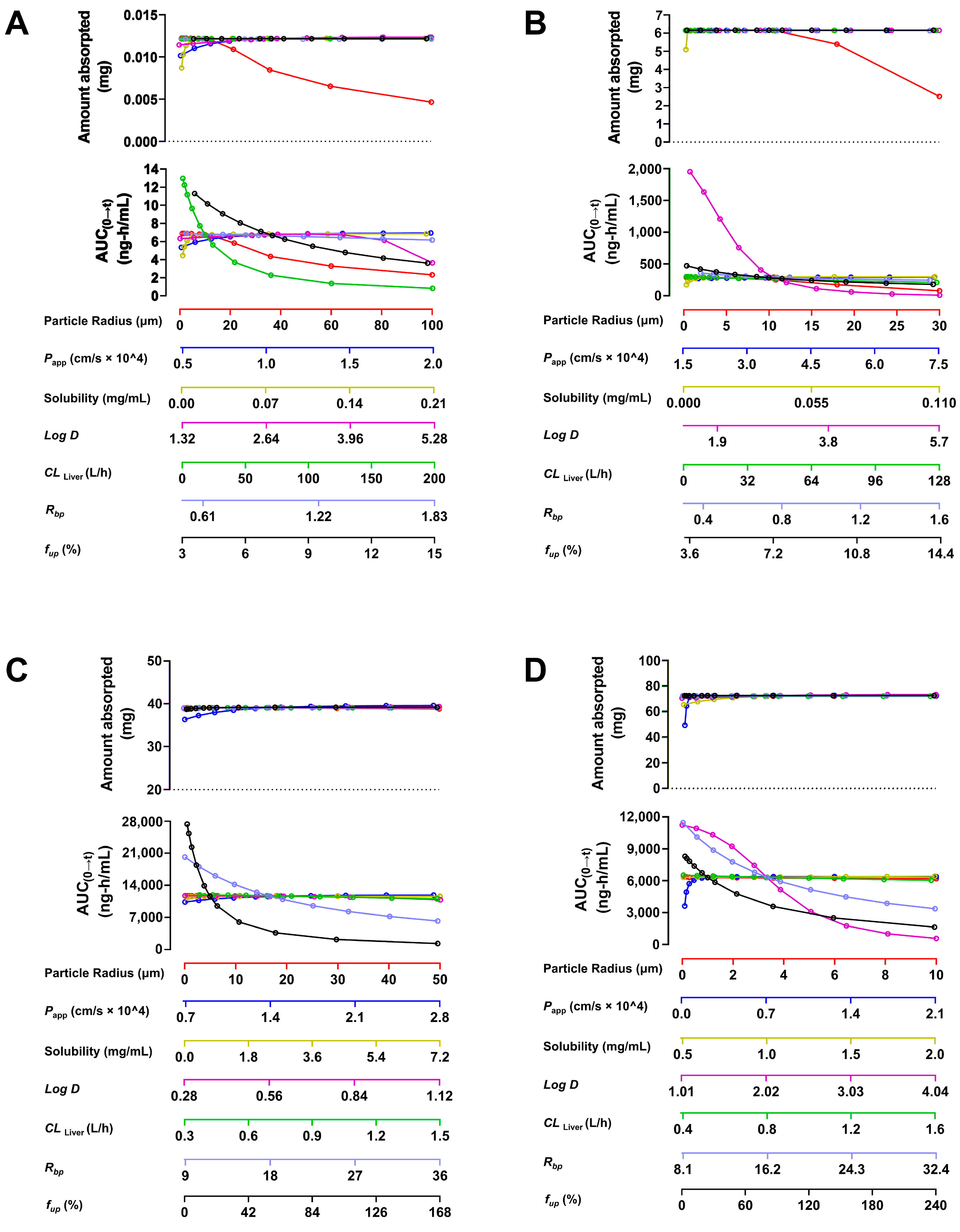
2.1.3. Sensitivity Analysis of Parameters
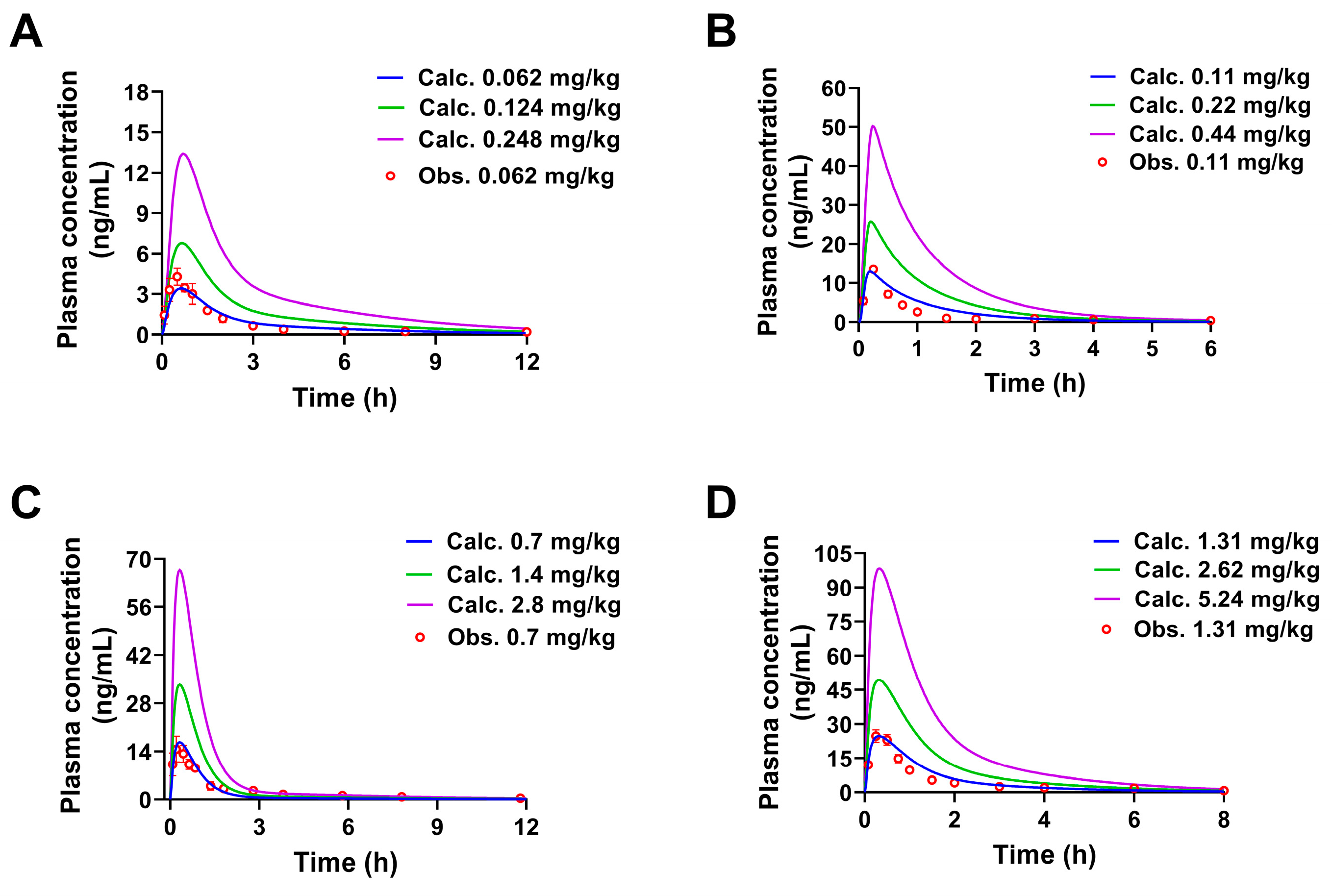
2.2. Pharmacokinetic Predictions of the Four Constituents in Rats at Different Doses
2.3. Pharmacokinetic Predictions of the Four Constituents in Humans
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Parameters for the PBPK Model
4.1.2. Experimental Verification Materials
4.2. Methods
4.2.1. Model Parameters and Assumptions
4.2.2. The Construction of the PBPK Model
4.2.3. Evaluation of the PBPK Model
4.2.4. Sensitivity Analysis
4.2.5. Prediction of the Pharmacokinetics Among Different Doses and Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, Volume I; China Medical Science and Technology Press: Beijing, China, 2020; pp. 202–203. [Google Scholar]
- Wang, N.; Wang, J.; Meng, X.; Li, T.; Wang, S.; Bao, Y. The pharmacological effects of Spatholobi Caulis tannin in cervical cancer and its precise therapeutic effect on related circRNA. Mol. Ther. Oncolytics 2019, 14, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ji, H. Experience and application of Simiaosan combine with Rattan drugs in the treatment of acute gouty arthritis. Rheum. Arthritis 2020, 9, 57–59, 75. [Google Scholar]
- Tan, J.; Lin, H.Q.; Wang, H.; Wu, F.L.; Dong, Q.H.; Liu, J.P.; Li, P.Y. Research progress on the pharmacological activities and clinical application of Jixueteng. Pharm. Clin. Chin. Mat. Med. 2018, 9, 61–65. [Google Scholar]
- Tang, P.; Liu, H.; Lin, B.; Wang, W.; Chen, W.; Lu, Z.; Li, P.; Gui, S.; Zhan, Y.; Lin, B. Spatholobi Caulis dispensing granule reduces deep vein thrombus burden through antiinflammation via SIRT1 and Nrf2. Phytomedicine 2020, 77, 153285. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; He, X.; Sheng, Y.; Yang, C.; Li, H.; Xu, J.; Xu, W.; Huang, K. Caulis Spatholobi ameliorates obesity through activating brown adipose tissue and modulating the composition of gut microbiota. Int. J. Mol. Sci. 2019, 20, 5150. [Google Scholar] [CrossRef]
- Sun, L.; Yang, L.; Du, X.; Liu, L.; Ran, Q.; Yang, Q.; Chen, Y.; Zhu, X.; Li, Q. Ethyl-acetate extract of Spatholobi Caulis blocked the pro-metastatic support from the hemato-microenvironment of colon cancer by specific disruption of tumor-platelet adhesion. Phytomedicine 2024, 128, 155420. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, W.; Li, Q.; Wang, K.; Wang, Y.; Lv, F.; Chen, X.; Peng, X.; Wang, Y.; Li, J.; et al. Effects of Caulis spatholobi polysaccharide on immunity, intestinal mucosal barrier function, and intestinal microbiota in cyclophosphamide-induced immunosuppressive chickens. Front. Vet. Sci. 2022, 18, 833842. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, X.; Zhang, Z.; Han, J.; Qu, S.; Liu, T.; Wang, G. Ononin: A candidate anti-parasitic drug isolated from Spatholobi Caulis against infections of dactylogyrus intermedius (Monogenea). Parasitol. Int. 2022, 88, 102535. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Hur, H.J.; Kim, S.H.; Park, S.J.; Hong, M.J.; Sung, M.J.; Kwon, D.Y.; Kim, M.S. Biochanin A improves hepatic steatosis and insulin resistance by regulating the hepatic lipid and glucose metabolic pathways in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 1944–1955. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, H.; Lin, X.; Qiu, F. Simultaneous quantification and rat pharmacokinetics of formononetin-7-O-β-D-glucoside and its metabolite formononetin by high-performance liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2020, 43, 2996–3005. [Google Scholar] [CrossRef]
- Mamagkaki, A.; Bouris, I.; Parsonidis, P.; Vlachou, I.; Gougousi, M.; Papasotiriou, I. Genistein as a dietary supplement; formulation, analysis and pharmacokinetics study. PLoS ONE 2021, 16, e0250599. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anticancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef] [PubMed]
- Raju, K.S.R.; Rashid, M.; Gundeti, M.; Taneia, I.; Malik, M.Y.; Singh, S.K.; Chaturvedi, S.; Challagundla, M.; Singh, S.P.; Gayen, J.R.; et al. LC-ESI-MS/MS method for the simultaneous determination of isoformononetin, daidzein, and equol in rat plasma: Application to a preclinical pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1129, 121776. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, T.; Jin, X. UPLC-MS/MS assay for simultaneous determination of four compounds in rat plasma: Application to pharmacokinetic study after oral administration of Caulis Spatholobi extract. Biomed. Chromatogr. 2016, 30, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Zhang, Y.B.; Yang, X.W.; Xu, W.; Liu, L.; Zhang, P.; Gong, Y.; Liu, N.F.; Peng, K.F. Simultaneous determination of twenty-five compounds with anti-inflammatory activity in Spatholobi Caulis by using an optimized UFLC-MS/MS method: An application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2021, 204, 114267. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, F.; Wang, X.; Liang, H.; Yao, X.; Liu, D. Dose prediction and pharmacokinetic simulation of XZP-5610, a small molecule for NASH therapy, using allometric scaling and physiologically based pharmacokinetic models. Pharmaceuticals 2024, 17, 369. [Google Scholar] [CrossRef]
- Gao, J.; Feng, F.; Wang, L.X.; Chong, X.M.; Wang, C.; Yin, L.H. Evaluation on the efficacy of moxifloxacin hydrochloride based on physiological pharmacokinetic models. Acta Pharma Sin. 2022, 57, 2153–2157. [Google Scholar]
- Choi, G.W.; Kang, D.W.; Kim, J.H.; Cho, S.J.; Lee, Y.B.; Kwon, I.H.; Cho, H.Y. Sex, age, and species differences of perfluorooctanoic acid modeled by flow- versus permeability-limited physiologically-based pharmacokinetic models. Toxicology 2024, 505, 153806. [Google Scholar] [CrossRef]
- Ruiz, P.; Emond, C.; McLanahan, E.D.; Joshi-Barr, S.; Mumtaz, M. Exploring mechanistic toxicity of mixtures using PBPK modeling and computational systems biology. Toxicol. Sci. 2020, 174, 38–50. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Mukherjee, D.; Salem, A.H.; Miles, D.; Menon, R.M.; Gibbs, J.P. Dose adjustment of venetoclax when co-administered with posaconazole: Clinical drug-drug interaction predictions using a PBPK approach. Cancer Chemother. Pharmacol. 2021, 87, 465–474. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration, CDER. The Use of Physiologically Based Pharmacokinetic Analyses-Biopharmaceutics Applications for Oral Drug Product Development, Manufacturing Changes, and Controls (Guidance for Industry); U.S. Department of Health and Human Services, Food and Drug Administration, CDER: Rockville, MD, USA, 2020; pp. 1–3. [Google Scholar]
- Li, X.M.; Su, B.D.; Huang, Z.W.; Ning, L.; Li, Y.B. Predicting pharmacokinetic behavior of aconitine in humans based on physiological pharmacokinetic model. Chin. Tradit. Herb. Drugs 2023, 54, 425–433. [Google Scholar]
- Liu, X.Y.; Zhang, Y.B.; Yang, X.W.; Wu, X.W.; Yang, Y.F.; Xu, W.; Wan, M.Q.; Gong, Y.; Liu, N.F.; Zhang, P. Biological analysis of constituents in Spatholobi Caulis by UFLC-MS/MS: Enhanced quantification and application to permeability properties study in Caco-2 cell monolayer model. J. Pharm. Biomed. Anal. 2023, 226, 115235. [Google Scholar] [CrossRef]
- Wu, S.; Xu, W.; Wang, F.R.; Yang, X.W. Study of the biotransformation of Tongmai formula by human intestinal flora and its intestinal permeability across the Caco-2 cell monolayer. Molecules 2015, 20, 18704–18716. [Google Scholar] [CrossRef]
- Yuan, Y.; He, Q.; Zhang, S.; Li, M.; Tang, Z.; Zhu, X.; Jiao, Z.; Cai, W.; Xiang, X. Application of physiologically based pharmacokinetic modeling in preclinical studies: A feasible strategy to Practice the Principles of 3Rs. Front. Pharmacol. 2022, 13, 895556. [Google Scholar] [CrossRef] [PubMed]
- Jogiraju, V.K.; Avvari, S.; Gollen, R.; Taft, D.R. Application of physiologically based pharmacokinetic modeling to predict drug disposition in pregnant populations. Biopharm. Drug Dispos. 2017, 38, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.; Nagar, S.; Korzekwa, K. Methods to Predict Volume of Distribution. Curr. Pharmacol. Rep. 2019, 5, 391–399. [Google Scholar] [CrossRef]
- Fan, C.; Basharat, Z.; Mah, K.; Wei, C.R. Computerional approach for drug discovery against Gardnerella vaginalis in quest for safer and effective treatments for bacterial vaginosis. Sci. Rep. 2024, 14, 17437. [Google Scholar]
- Cho, C.K.; Kang, P.; Jang, G.G.; Lee, Y.J.; Bae, J.W.; Choi, C.I. PBPK modeling to predict the pharmacokinetics of venlafaxine and its active metabolite in different CYP2D6 genotypes and drug–drug interactions with clarithromycin and paroxetine. Arch. Pharm. Res. 2024, 47, 481–504. [Google Scholar] [CrossRef]
- Kolli, A.R.; Kuczaj, A.K.; Martin, F.; Hayes, A.W.; Peitsch, M.C.; Hoeng, J. Bridging inhaled aerosol dosimetry to physiologically based pharmacokinetic modeling for toxicological assessment: Nicotine delivery systems and beyond. Crit. Rev. Toxicol. 2019, 49, 725–741. [Google Scholar] [CrossRef]
- Zhao, J.J.; Zheng, F.Z.; Ma, J.F.; Ma, M.Y.; Guo, J. Study on the mechanism of the drug pair-Tripterygium Wilfordii and Caulis Spatholobi in the treatment of rheumatoid arthritis based on network pharmacology. Arthritis Rheumatol. 2020, 9, 11–15, 23. [Google Scholar]
- Li, C.; Cheng, C.; Jia, W.W.; Yang, J.L.; Yu, X.; Olaleye, O.E. Multi-compound pharmacokinetic research on Chinese herbal medicines: Identifying potentially therapeutic compounds and characterizing their disposition and pharmacokinetics. Acta Pharm. Sin. 2021, 56, 2426–2446. [Google Scholar]
- Ma, B.L.; Ma, Y.M. Pharmacokinetic herb-drug interactions with traditional Chinese medicine: Progress, causes of conflicting results and suggestions for future research. Drug Metab. Rev. 2016, 48, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.Y.; Fan, M.X.; Zhao, H.Y.; Li, M.X.; Wu, X.; Gao, W.Y. Pharmacokinetics and bioavailability of the isoflavones formononetin and ononin and their in vitro absorption in using chamber and Caco-2 cell models. J. Agric. Food Chem. 2018, 66, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.S.; Lian, F.M.; Yu, T.Y.; Zhao, Y.R. Clinical application and dosage of suberect spatholobus stem. J. Changchun Univ. Chin. Med. 2022, 38, 374–377. [Google Scholar]
- Yang, Y.; Wang, Y.; Zeng, W.; Zhou, J.; Xu, M.; Lan, Y.; Liu, L.; Shen, J.; Zhang, C.; He, Q. Physiologically-based pharmacokinetic/pharmacodynamic modeling of meropenem in critically ill patients. Sci. Rep. 2024, 14, 19269. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.A.; Wu, S. Application of structural and functional pharmacokinetic analogs for physiologically based pharmacokinetic model development and evaluation. Regul. Toxicol. Pharmacol. 2020, 114, 104667. [Google Scholar] [CrossRef]
- Patel, D.; Dierks, E. Single-species allometric scaling: A strategic approach to support drug discovery. J. Pharm. Res. Int. 2018, 22, 1–7. [Google Scholar] [CrossRef]
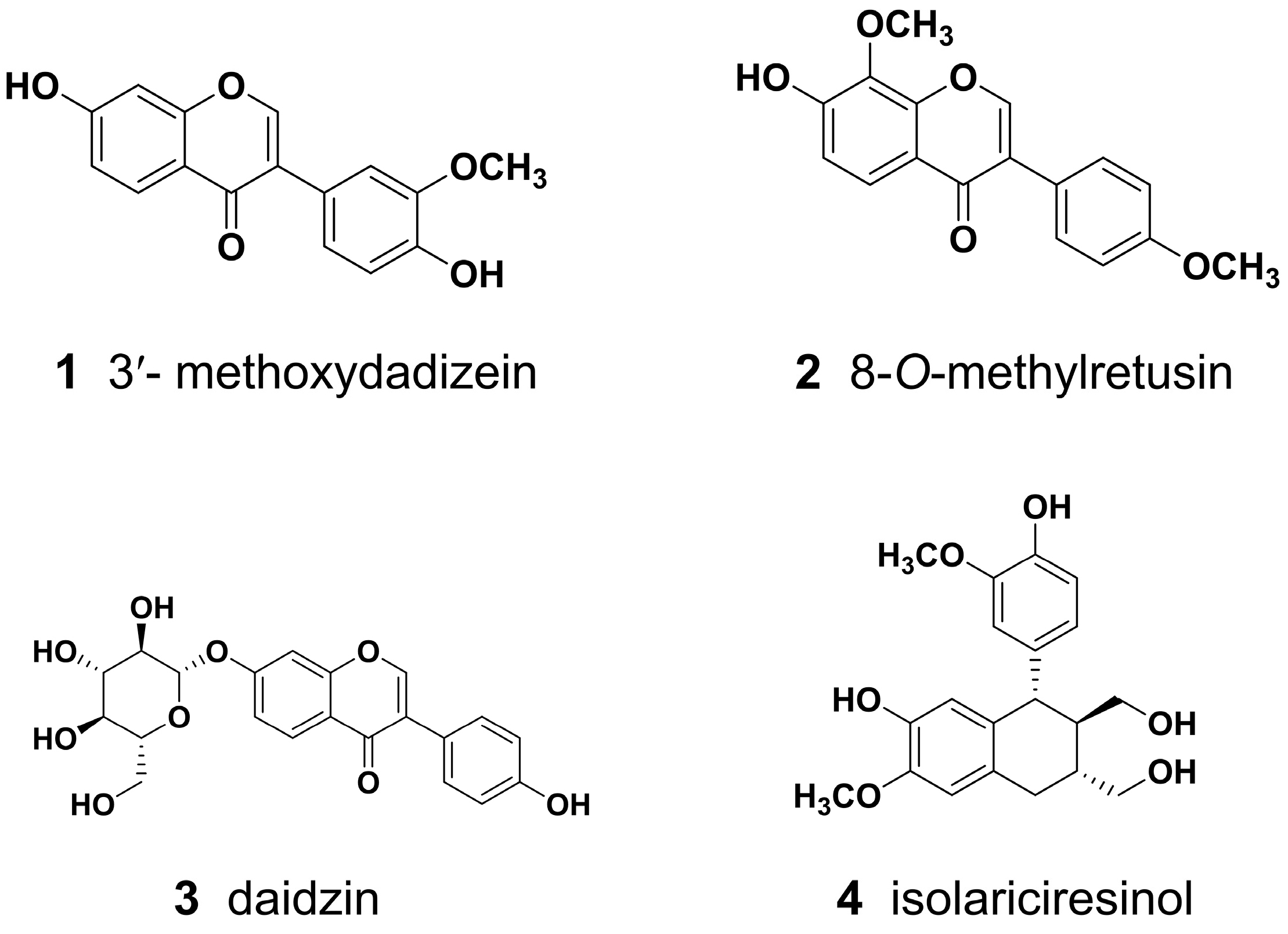

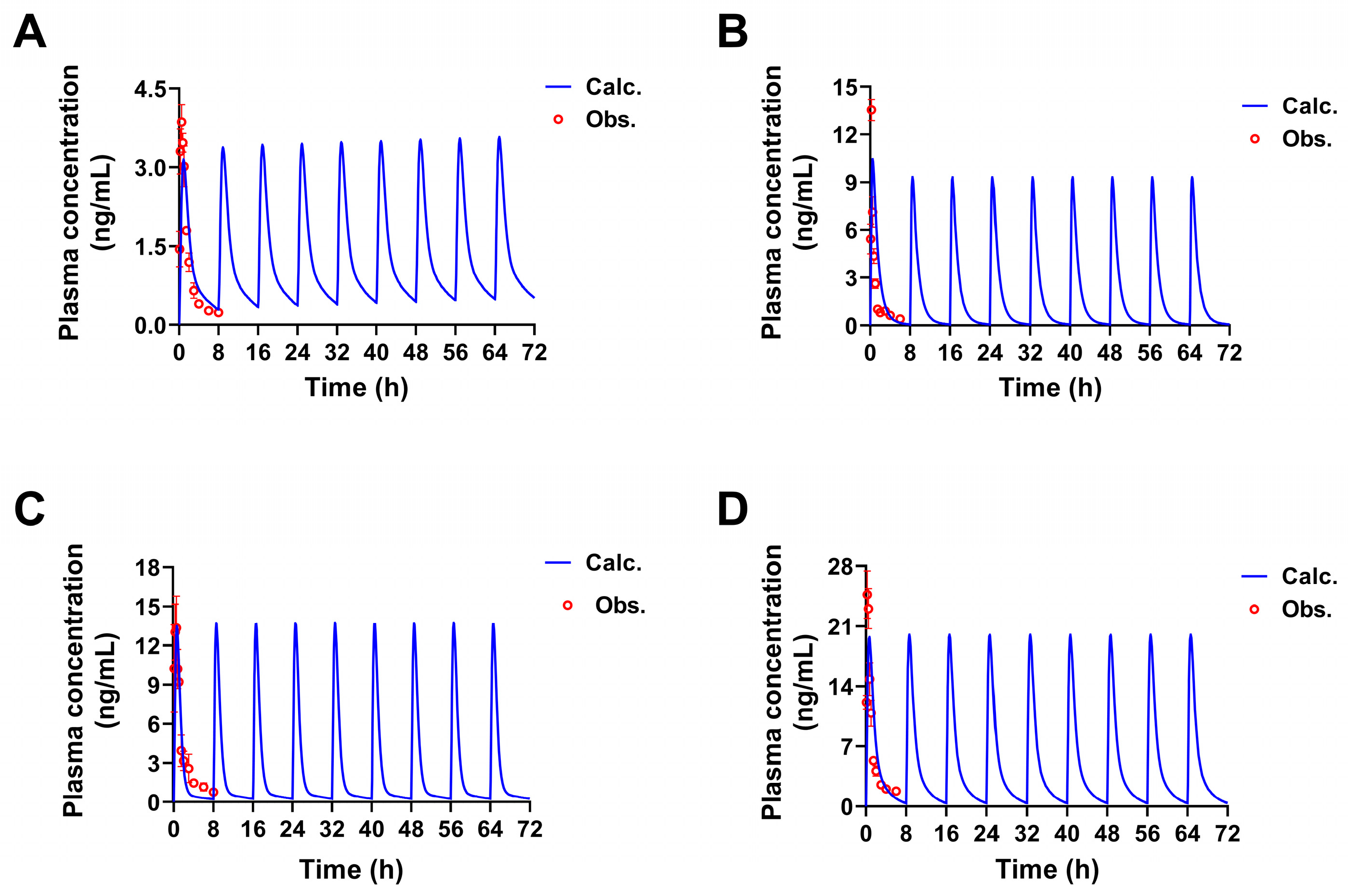


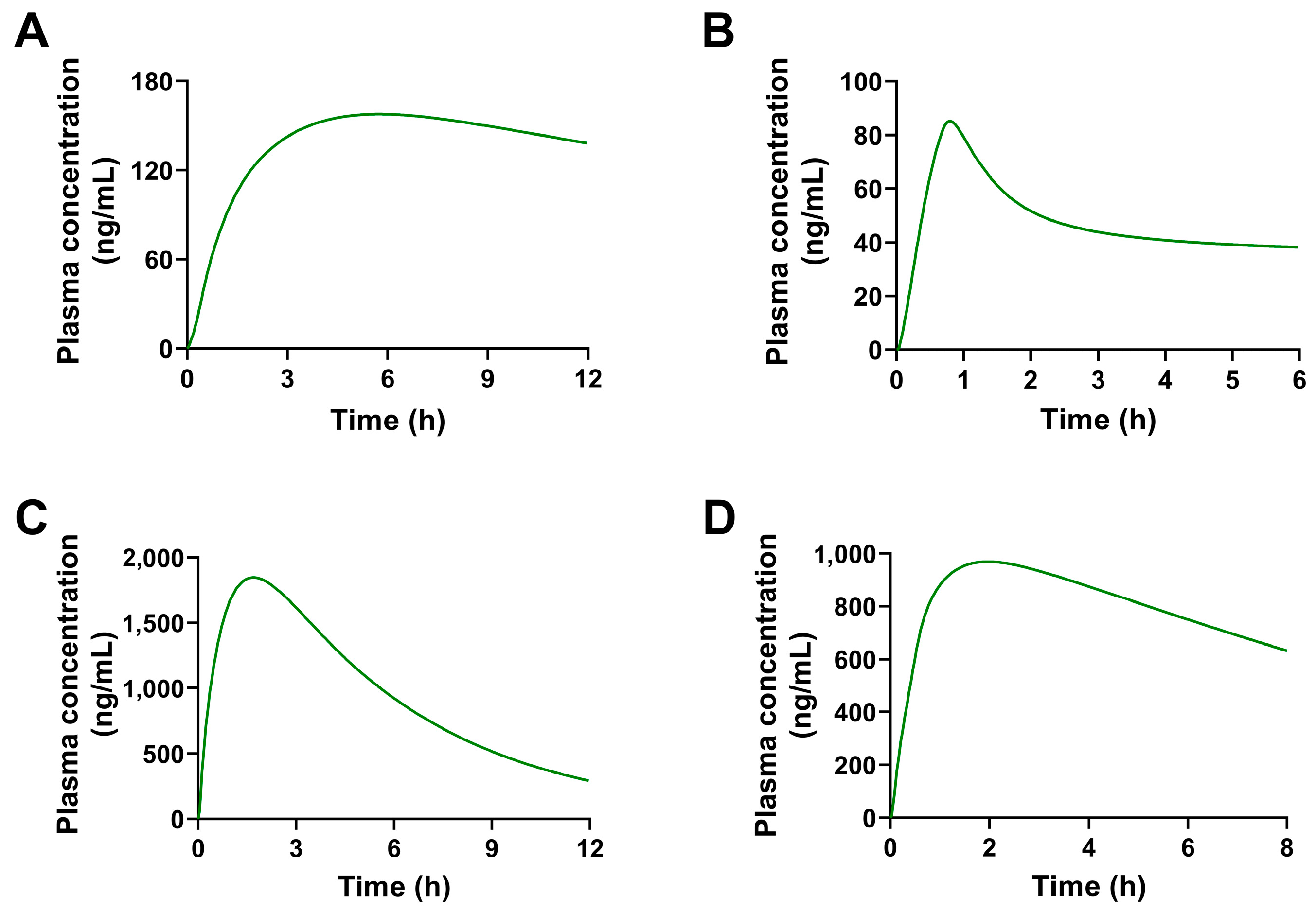
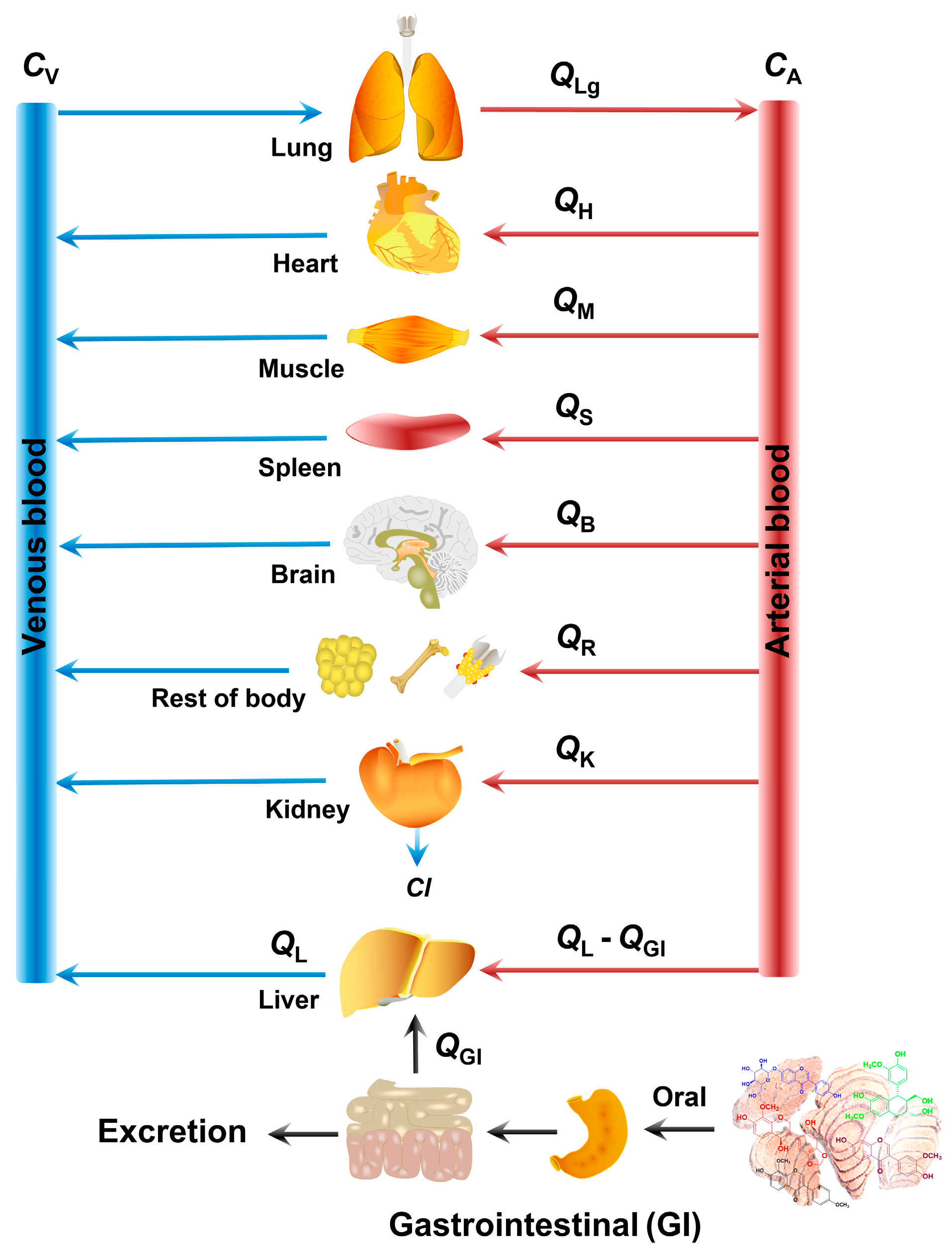
| Parameters | 1 | 2 | 3 | 4 | Source |
|---|---|---|---|---|---|
| MW | 284.27 | 298.30 | 416.38 | 360.41 | ChemDraw 21.0 |
| Log P | 2.676 | 2.805 | 0.541 | 1.591 | ADMET Predictor |
| pKa | 8.510 | 8.830 | 9.650 | 9.370 | ADMET Predictor |
| Log D | 2.640 | 2.850 | 0.560 | 2.020 | GastroPlus |
| Solubility (mg/mL) | 0.0205 (pH 6.32) | 0.0108 (pH 6.60) | 0.720 (pH 6.40) | 0.201 (pH 6.17) | GastroPlus |
| Papp (×10−5 cm/s) | 0.220 | 2.480 | 0.400 | 0.220 | Our study [24,25] |
| Rbp,rat | 0.915 | 0.875 | 1.044 | 0.830 | ADMET Predictor |
| Rbp,human | 0.807 | 0.777 | 0.744 | 0.787 | ADMET Predictor |
| fup,rat | 8.403 | 11.816 | 39.933 | 31.535 | ADMET Predictor |
| fup,human | 7.394 | 7.07 | 17.891 | 16.139 | ADMET Predictor |
| CLrat (L/h) | 2.786 | 1.906 | 4.669 | 7.152 | GastroPlus |
| Vssrat (L) | 54.730 | 5.860 | 17.36 | 17.03 | GastroPlus |
| Kp | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Lung | 0.45 | 2.42 | 0.48 | 1.25 |
| Adipose | 0.07 | 6.84 | 0.15 | 2.23 |
| Muscle | 0.39 | 1.19 | 0.36 | 0.70 |
| Liver | 0.37 | 2.05 | 0.37 | 1.04 |
| Spleen | 0.39 | 1.14 | 0.39 | 0.69 |
| Heart | 0.40 | 1.67 | 0.43 | 0.92 |
| Brain | 1.60 | 4.73 | 0.44 | 2.11 |
| Kidney | 0.73 | 1.92 | 0.42 | 1.02 |
| Skin | 1.09 | 2.72 | 0.47 | 1.35 |
| ReproOrg | 0.41 | 1.93 | 0.43 | 1.02 |
| RedMarrow | 0.33 | 2.51 | 0.31 | 1.17 |
| YellowMarrow | 0.07 | 6.84 | 0.15 | 2.23 |
| Rest of body | 0.42 | 1.16 | 0.40 | 0.71 |
| Constituents | Parameters | Cmax (μg/mL) | Tmax (h) | AUC0→t (μg∙h/mL) | AUC0→∞ (μg∙h/mL) |
|---|---|---|---|---|---|
| 1 | Obs. | 0.0039 | 0.42 | 0.0084 | 0.0090 |
| Calc. | 0.0034 | 0.64 | 0.0092 | 0.0097 | |
| FE | 1.15 | 1.52 | 1.10 | 1.08 | |
| 2 | Obs. | 0.0136 | 0.25 | 0.0097 | 0.0115 |
| Calc. | 0.0130 | 0.20 | 0.0144 | 0.0146 | |
| FE | 1.05 | 1.25 | 1.48 | 1.27 | |
| 3 | Obs. | 0.0151 | 0.21 | 0.0284 | 0.0305 |
| Calc. | 0.0167 | 0.32 | 0.0196 | 0.0200 | |
| FE | 1.11 | 1.52 | 1.45 | 1.52 | |
| 4 | Obs. | 0.0262 | 0.31 | 0.0347 | 0.0383 |
| Calc. | 0.0247 | 0.32 | 0.0395 | 0.0403 | |
| FE | 1.06 | 1.03 | 1.14 | 1.05 |
| Constituents | Parameters | Cmax (μg/mL) | Tmax (h) | AUC0→t (μg∙h/mL) | AUC0→∞ (μg∙h/mL) |
|---|---|---|---|---|---|
| 1 | Calc. | 0.0068 | 0.64 | 0.0183 | 0.0193 |
| Val. | 0.0072 | 0.55 | 0.0191 | 0.0200 | |
| FE | 1.06 | 1.16 | 1.04 | 1.04 | |
| 2 | Calc. | 0.0257 | 0.20 | 0.0288 | 0.0292 |
| Val. | 0.0253 | 0.25 | 0.0153 | 0.0155 | |
| FE | 1.02 | 1.25 | 1.88 | 1.88 | |
| 3 | Calc. | 0.0334 | 0.32 | 0.0393 | 0.0400 |
| Val. | 0.0272 | 0.25 | 0.0355 | 0.0359 | |
| FE | 1.23 | 1.28 | 1.11 | 1.11 | |
| 4 | Calc. | 0.0494 | 0.32 | 0.0791 | 0.0805 |
| Val. | 0.0417 | 0.30 | 0.0557 | 0.0593 | |
| FE | 1.16 | 1.20 | 1.42 | 1.36 |
| Constituents | Cmax (μg/mL) | Tmax (h) | AUC0→t (μg∙h/mL) | AUC0→∞ (μg∙h/mL) |
|---|---|---|---|---|
| 1 | 0.1578 | 5.76 | 1.635 | 6.650 |
| 2 | 0.0852 | 0.800 | 0.286 | 2.145 |
| 3 | 1.849 | 1.68 | 11.639 | 13.130 |
| 4 | 0.9699 | 1.97 | 6.359 | 13.601 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Du, R.; Zhang, T.; Li, Y.; Li, L.; Yang, Z.; Zhang, Y.; Wang, Q. Predicting Pharmacokinetics of Active Constituents in Spatholobi caulis by Using Physiologically Based Pharmacokinetic Models. Pharmaceuticals 2024, 17, 1621. https://doi.org/10.3390/ph17121621
Liu X, Du R, Zhang T, Li Y, Li L, Yang Z, Zhang Y, Wang Q. Predicting Pharmacokinetics of Active Constituents in Spatholobi caulis by Using Physiologically Based Pharmacokinetic Models. Pharmaceuticals. 2024; 17(12):1621. https://doi.org/10.3390/ph17121621
Chicago/Turabian StyleLiu, Xiaoyan, Ruihu Du, Tao Zhang, Yingzi Li, Ludi Li, Zheng Yang, Youbo Zhang, and Qi Wang. 2024. "Predicting Pharmacokinetics of Active Constituents in Spatholobi caulis by Using Physiologically Based Pharmacokinetic Models" Pharmaceuticals 17, no. 12: 1621. https://doi.org/10.3390/ph17121621
APA StyleLiu, X., Du, R., Zhang, T., Li, Y., Li, L., Yang, Z., Zhang, Y., & Wang, Q. (2024). Predicting Pharmacokinetics of Active Constituents in Spatholobi caulis by Using Physiologically Based Pharmacokinetic Models. Pharmaceuticals, 17(12), 1621. https://doi.org/10.3390/ph17121621









