Effects of Ketamine vs. Midazolam in Adolescent Treatment Resistant Depression
Abstract
1. Introduction
2. Results
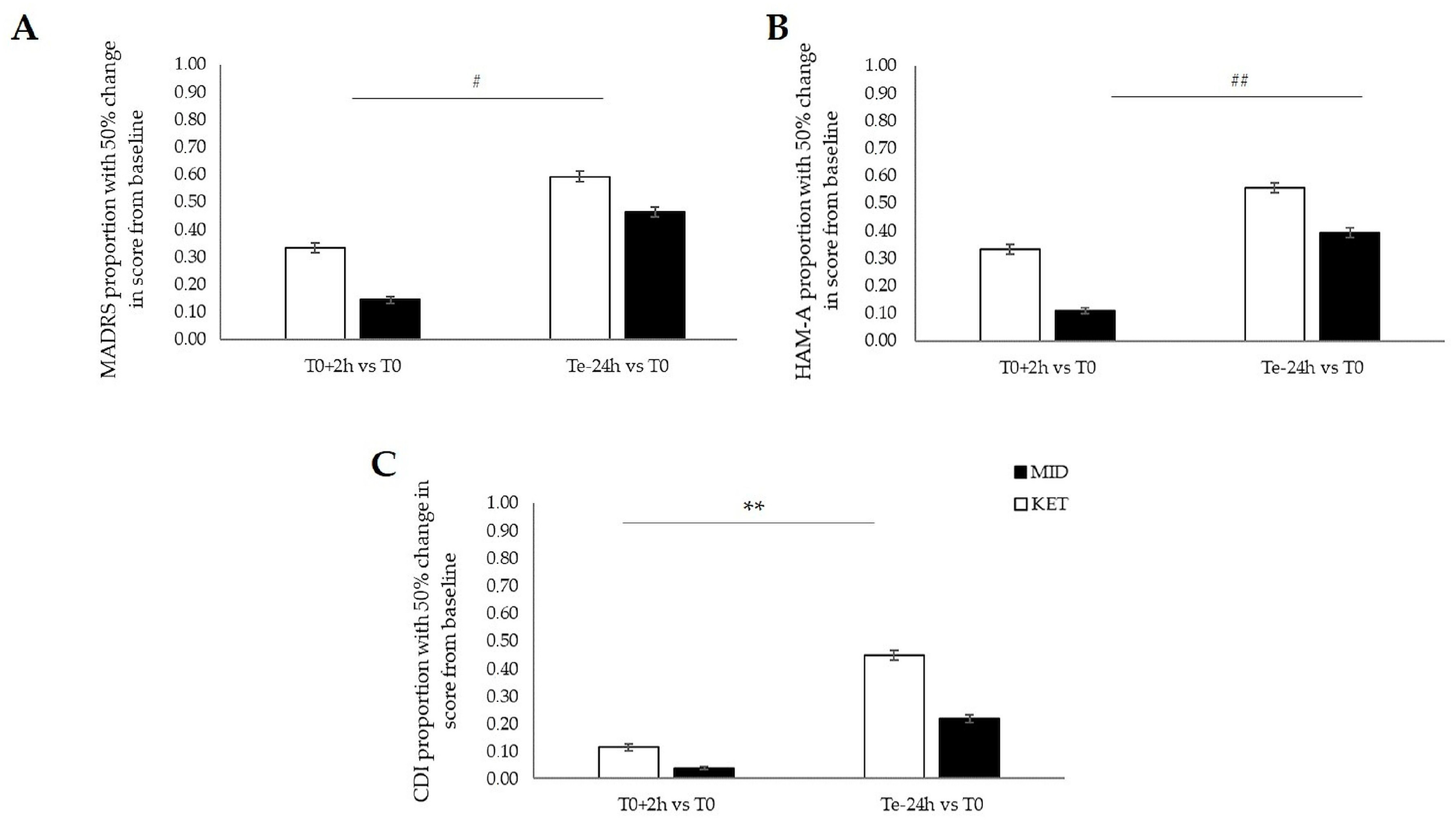
2.1. Primary Outcomes: Between-Group Comparisons
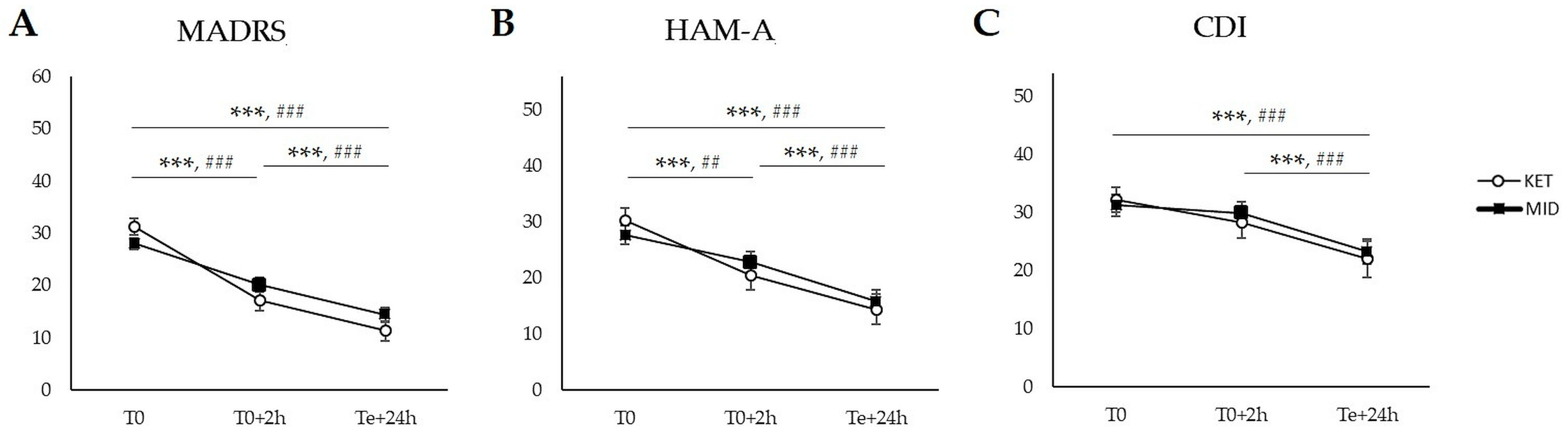
2.2. Secondary Outcomes: Within-Group Comparisons
3. Discussion
Limitations
4. Materials and Methods
4.1. Ethics Approval
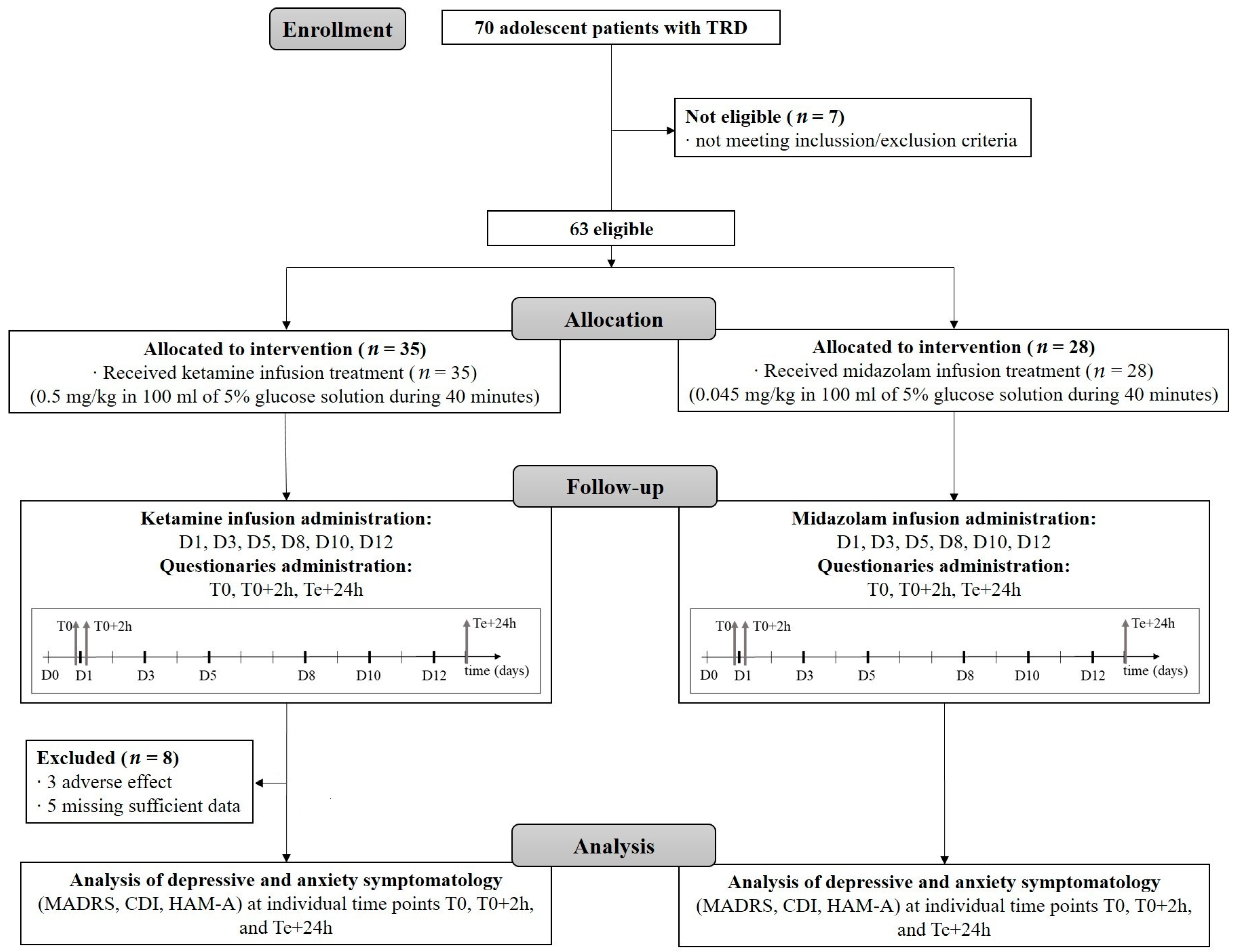
4.2. Study Design and Patients
4.3. Study Procedures
4.4. Psychometric Measures
4.5. Outcomes
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benton, T.D.; Boyd, R.C.; Njoroge, W.F.M. Addressing the Global Crisis of Child and Adolescent Mental Health. JAMA Pediatr. 2021, 175, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Shorey, S.; Ng, E.D.; Wong, C.H.J. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br. J. Clin. Psychol. 2022, 61, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, T.M.; Gillham, J.E.; Seligman, M.E.P. Gender, Anxiety, and Depressive Symptoms: A Longitudinal Study of Early Adolescents. J. Early Adolesc. 2009, 29, 307. [Google Scholar] [CrossRef] [PubMed]
- Bridge, J.A.; Goldstein, T.R.; Brent, D.A. Adolescent suicide and suicidal behavior. J. Child Psychol. Psychiatry 2006, 47, 372–394. [Google Scholar] [CrossRef] [PubMed]
- Verboom, C.E.; Sijtsema, J.J.; Verhulst, F.C.; Penninx, B.W.J.H.; Ormel, J. Longitudinal associations between depressive problems, academic performance, and social functioning in adolescent boys and girls. Dev. Psychol. 2014, 50, 247–257. [Google Scholar] [CrossRef]
- Dwyer, J.B.; Stringaris, A.; Brent, D.A.; Bloch, M.H. Annual Research Review: Defining and treating pediatric treatment-resistant depression. J. Child Psychol. Psychiatry 2020, 61, 312–332. [Google Scholar] [CrossRef]
- Fergusson, D.M.; Woodward, L.J. Mental health, educational, and social role outcomes of adolescents with depression. Arch. Gen. Psychiatry 2002, 59, 225–231. [Google Scholar] [CrossRef]
- Bernaras, E.; Jaureguizar, J.; Garaigordobil, M. Child and Adolescent Depression: A Review of Theories, Evaluation Instruments, Prevention Programs, and Treatments. Front. Psychol. 2019, 10, 543. [Google Scholar] [CrossRef]
- Lewis, C.P.; Nakonezny, P.A.; Sonmez, A.I.; Ozger, C.; Garzon, J.F.; Camsari, D.D.; Yuruk, D.; Romanowicz, M.; Shekunov, J.; Zaccariello, M.J.; et al. A Dose-Finding, Biomarker Validation, and Effectiveness Study of Transcranial Magnetic Stimulation for Adolescents with Depression. J. Am. Acad. Child Adolesc. Psychiatry 2024. [Google Scholar] [CrossRef]
- Mullen, S. Major depressive disorder in children and adolescents. Ment. Health Clin. 2018, 8, 275–283. [Google Scholar] [CrossRef]
- Ayvaci, E.R.; Croarkin, P.E. Special Populations: Treatment-Resistant Depression in Children and Adolescents. Psychiatr. Clin. N. Am. 2023, 46, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Faries, E.; Mabe, L.A.; Franzen, R.L.; Murtaza, S.; Nathani, K.; Ahmed, B.; Prokop, L.; Mohamed, K.; Ahmed, A.T. Interventional approaches to treatment resistant depression (DTR) in children and adolescents: A systematic review and meta-analysis. J. Affect. Disord. 2024, 367, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Vlisides, P.E. Ketamine: 50 Years of Modulating the Mind. Front. Hum. Neurosci. 2016, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Sanacora, G.; Duman, R.S.; Krystal, J.H. Ketamine and Rapid-Acting Antidepressants: A Window into a New Neurobiology for Mood Disorder Therapeutics. Annu. Rev. Med. 2015, 66, 509–523. [Google Scholar] [CrossRef]
- Phillips, J.L.; Norris, S.; Talbot, J.; Birmingham, M.; Hatchard, T.; Ortiz, A.; Owoeye, O.; Batten, L.A.; Blier, P. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. Am. J. Psychiatry 2019, 176, 401–409. [Google Scholar] [CrossRef]
- Strasburger, S.E.; Bhimani, P.M.; Kaabe, J.H.; Krysiak, J.T.; Nanchanatt, D.L.; Nguyen, T.N.; Pough, K.A.; Prince, T.A.; Ramsey, N.S.; Savsani, K.H.; et al. What is the mechanism of Ketamine’s rapid-onset antidepressant effect? A concise overview of the surprisingly large number of possibilities. J. Clin. Pharm. Ther. 2017, 42, 147–154. [Google Scholar] [CrossRef]
- Duman, R.S. Ketamine and rapid-acting antidepressants: A new era in the battle against depression and suicide. F1000Research 2018, 7, F1000 Faculty Rev-659. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Perez, A.M.; Pillemer, S.; Stern, J.; Parides, M.K.; aan het Rot, M.; Collins, K.A.; Mathew, S.J.; Charney, D.S.; Iosifescu, D.V. Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression. Biol. Psychiatry 2013, 74, 250–256. [Google Scholar] [CrossRef]
- Strong, C.E.; Kabbaj, M. On the safety of repeated ketamine infusions for the treatment of depression: Effects of sex and developmental periods. Neurobiol. Stress 2018, 9, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.B.; Landeros-Weisenberger, A.; Johnson, J.A.; Londono Tobon, A.; Flores, J.M.; Nasir, M.; Couloures, K.; Sanacora, G.; Bloch, M.H. Efficacy of Intravenous Ketamine in Adolescent Treatment-Resistant Depression: A Randomized Midazolam-Controlled Trial. Am. J. Psychiatry 2021, 178, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant Efficacy of Ketamine in Treatment-Resistant Major Depression: A Two-Site Randomized Controlled Trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.R.; Amatya, P.; Roback, M.G.; Albott, C.S.; Westlund Schreiner, M.; Ren, Y.; Eberly, L.E.; Carstedt, P.; Samikoglu, A.; Gunlicks-Stoessel, M.; et al. Intravenous Ketamine for Adolescents with Treatment-Resistant Depression: An Open-Label Study. J. Child Adolesc. Psychopharmacol. 2018, 28, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Matveychuk, D.; Thomas, R.K.; Swainson, J.; Khullar, A.; MacKay, M.-A.; Baker, G.B.; Dursun, S.M. Ketamine as an antidepressant: Overview of its mechanisms of action and potential predictive biomarkers. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320916657. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, P.; Liang, P. Neuropsychopharmacological effects of midazolam on the human brain. Brain Inform. 2020, 7, 15. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Farmer, C.; Ballard, E.D.; Mathew, S.J.; Grunebaum, M.F.; Murrough, J.W.; Sos, P.; Wang, G.; Gueorguieva, R.; Carlos A Zarate, J. Impact of midazolam vs. saline on effect size estimates in controlled trials of ketamine as a rapid-acting antidepressant. Neuropsychopharmacology 2019, 44, 1233. [Google Scholar] [CrossRef]
- Dubovsky, S.L.; Marshall, D. Benzodiazepines Remain Important Therapeutic Options in Psychiatric Practice. Psychother. Psychosom. 2022, 91, 307–334. [Google Scholar] [CrossRef]
- Parellada, M.; Moreno, C.; Moreno, M.; Espliego, A.; de Portugal, E.; Arango, C. Placebo effect in child and adolescent psychiatric trials. Eur. Neuropsychopharmacol. 2012, 22, 787–799. [Google Scholar] [CrossRef]
- Benasi, G.; Guidi, J.; Offidani, E.; Balon, R.; Rickels, K.; Fava, G.A. Benzodiazepines as a Monotherapy in Depressive Disorders: A Systematic Review. Psychother. Psychosom. 2018, 87, 65–74. [Google Scholar] [CrossRef]
- Shi, D.C.; Chen, L.; Kosik-Gonzalez, C.; Bangerter, A.; Fu, D.J. Measuring Remission and Response using CDRS-R and MADRS: An Analysis in Adolescents with MDD and Imminent Risk for Suicide. In Proceedings of the 20th Annual Scientific Meeting of International Society for CNS Clinical Trials and Methodology (ISCTM), Washington, DC, USA, 21–23 February 2024. [Google Scholar]
- Domany, Y.; Shelton, R.C.; McCullumsmith, C.B. Ketamine for acute suicidal ideation. An emergency department intervention: A randomized, double-blind, placebo-controlled, proof-of-concept trial. Depress. Anxiety 2020, 37, 224–233. [Google Scholar] [CrossRef]
- Lehmann, M.; Seifritz, E.; Henning, A.; Walter, M.; Böker, H.; Scheidegger, M.; Grimm, S. Differential effects of rumination and distraction on ketamine induced modulation of resting state functional connectivity and reactivity of regions within the default-mode network. Soc. Cogn. Affect. Neurosci. 2016, 11, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, L.R.; Phillips, A.G. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol. Sci. 2021, 42, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.H.; Kaye, A.P.; Jefferson, S.; Girgenti, M.J.; Wilkinson, S.T.; Sanacora, G.; Esterlis, I. Ketamine and the neurobiology of depression: Toward next-generation rapid-acting antidepressant treatments. Proc. Natl. Acad. Sci. USA 2023, 120, e2305772120. [Google Scholar] [CrossRef] [PubMed]
- Glue, P.; Neehoff, S.; Sabadel, A.; Broughton, L.; Le Nedelec, M.; Shadli, S.; McNaughton, N.; Medlicott, N.J. Effects of ketamine in patients with treatment-refractory generalized anxiety and social anxiety disorders: Exploratory double-blind psychoactive-controlled replication study. J. Psychopharmacol. 2020, 34, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Marchi, M.; Magarini, F.M.; Galli, G.; Mordenti, F.; Travascio, A.; Uberti, D.; Micheli, E.D.; Pingani, L.; Ferrari, S.; Galeazzi, G.M. The effect of ketamine on cognition, anxiety, and social functioning in adults with psychiatric disorders: A systematic review and meta-analysis. Front. Neurosci. 2022, 16, 1011103. [Google Scholar] [CrossRef]
- Whittaker, E.; Dadabayev, A.R.; Joshi, S.A.; Glue, P. Systematic review and meta-analysis of randomized controlled trials of ketamine in the treatment of refractory anxiety spectrum disorders. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211056743. [Google Scholar] [CrossRef]
- Tully, J.L.; Dahlén, A.D.; Haggarty, C.J.; Schiöth, H.B.; Brooks, S. Ketamine treatment for refractory anxiety: A systematic review. Br. J. Clin. Pharmacol. 2022, 88, 4412–4426. [Google Scholar] [CrossRef] [PubMed]
- Hartland, H.; Mahdavi, K.; Jelen, L.A.; Strawbridge, R.; Young, A.H.; Alexander, L. A transdiagnostic systematic review and meta-analysis of ketamine’s anxiolytic effects. J. Psychopharmacol. 2023, 37, 764–774. [Google Scholar] [CrossRef]
- Brent, D.A.; Greenhill, L.L.; Compton, S.; Emslie, G.; Wells, K.; Walkup, J.T.; Vitiello, B.; Bukstein, O.; Stanley, B.; Posner, K.; et al. The Treatment of Adolescent Suicide Attempters Study (TASA): Predictors of Suicidal Events in an Open Treatment Trial. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 987–996. [Google Scholar] [CrossRef]
- Vitiello, B.; Silva, S.; Rohde, P.; Kratochvil, C.; Kennard, B.; Reinecke, M.; Mayes, T.; Posner, K.; May, D.E.; March, J.S. Suicidal Events in the Treatment for Adolescents with Depression Study (TADS). J. Clin. Psychiatry 2009, 70, 741–747. [Google Scholar] [CrossRef]
- Murrough, J.W.; Soleimani, L.; DeWilde, K.E.; Collins, K.A.; Lapidus, K.A.; Iacoviello, B.M.; Lener, M.; Kautz, M.; Kim, J.; Stern, J.B.; et al. Ketamine for rapid reduction of suicidal ideation: A randomized controlled trial. Psychol. Med. 2015, 45, 3571–3580. [Google Scholar] [CrossRef] [PubMed]
- Grunebaum, M.F.; Galfalvy, H.C.; Choo, T.-H.; Keilp, J.G.; Moitra, V.K.; Parris, M.S.; Marver, J.E.; Burke, A.K.; Milak, M.S.; Sublette, M.E.; et al. Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial. Am. J. Psychiatry 2018, 175, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.T.; Ballard, E.D.; Bloch, M.H.; Mathew, S.J.; Murrough, J.W.; Feder, A.; Sos, P.; Wang, G.; Zarate, C.A.; Sanacora, G. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am. J. Psychiatry 2018, 175, 150–158. [Google Scholar] [CrossRef]
- Fang, L.; Tong, Y.; Li, M.; Wang, C.; Li, Y.; Yuan, M.; Zhang, X.; Wang, G.; Wang, J.; Su, P. Anxiety in adolescents and subsequent risk of suicidal behavior: A systematic review and meta-analysis. J. Affect. Disord. 2024, 358, 97–104. [Google Scholar] [CrossRef]
- Hochschild, A.; Keilp, J.G.; Madden, S.P.; Burke, A.K.; Mann, J.J.; Grunebaum, M.F. Ketamine vs midazolam: Mood improvement reduces suicidal ideation in depression. J. Affect. Disord. 2022, 300, 10–16. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5TM, 5th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013; 947p, ISBN 978-0-89042-554-1. [Google Scholar]
- Shiroma, P.R.; Thuras, P.; Wels, J.; Albott, C.S.; Erbes, C.; Tye, S.; Lim, K.O. A randomized, double-blind, active placebo-controlled study of efficacy, safety, and durability of repeated vs single subanesthetic ketamine for treatment-resistant depression. Transl. Psychiatry 2020, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Freeman, M.P.; Flynn, M.; Judge, H.; Hoeppner, B.B.; Cusin, C.; Ionescu, D.F.; Mathew, S.J.; Chang, L.C.; Iosifescu, D.V.; et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol. Psychiatry 2020, 25, 1592–1603. [Google Scholar] [CrossRef]
- Aldrete, J.A. The post-anesthesia recovery score revisited. J. Clin. Anesth. 1995, 7, 89–91. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry J. Ment. Sci. 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. The Assessment of Anxiety States by Rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M. The Children’s Depression, Inventory (CDI). Psychopharmacol. Bull. 1985, 21, 995–998. [Google Scholar] [PubMed]
- Quilty, L.C.; Robinson, J.J.; Rolland, J.; Fruyt, F.D.; Rouillon, F.; Bagby, R.M. The structure of the Montgomery–Åsberg depression rating scale over the course of treatment for depression. Int. J. Methods Psychiatr. Res. 2013, 22, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Geijer, J.; Baigi, A.; Aiff, H. Inter-rater reliability among psychiatrists when assessing depression according to the Montgomery-Åsberg Depression Rating Scale. Nord. J. Psychiatry 2021, 75, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Szegedi, A.; Wetzel, H.; Benkert, O. Moderate and severe depression: Gradations for the Montgomery–Åsberg Depression Rating Scale. J. Affect. Disord. 2000, 60, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Maier, W.; Buller, R.; Philipp, M.; Heuser, I. The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 1988, 14, 61–68. [Google Scholar] [CrossRef]
- Rabinowitz, J.; Williams, J.B.W.; Hefting, N.; Anderson, A.; Brown, B.; Fu, D.J.; Kadriu, B.; Kott, A.; Mahableshwarkar, A.; Sedway, J.; et al. Consistency checks to improve measurement with the Hamilton Rating Scale for Anxiety (HAM-A). J. Affect. Disord. 2023, 325, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Smucker, M.R.; Craighead, W.E.; Craighead, L.W.; Green, B.J. Normative and reliability data for the Children’s Depression Inventory. J. Abnorm. Child Psychol. 1986, 14, 25–39. [Google Scholar] [CrossRef]
- Figueras Masip, A.; Amador-Campos, J.A.; Gómez-Benito, J.; del Barrio Gándara, V. Psychometric properties of the Children’s Depression Inventory in community and clinical sample. Span. J. Psychol. 2010, 13, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.R.; Park, J.H.; Kim, S.H. Cut-Off Scores of the Children’s Depression Inventory for Screening and Rating Severity in Korean Adolescents. Psychiatry Investig. 2015, 12, 23–28. [Google Scholar] [CrossRef]
- Stein, D.J.; Khoo, J.-P.; Picarel-Blanchot, F.; Olivier, V.; Van Ameringen, M. Efficacy of Agomelatine 25–50 mg for the Treatment of Anxious Symptoms and Functional Impairment in Generalized Anxiety Disorder: A Meta-Analysis of Three Placebo-Controlled Studies. Adv. Ther. 2021, 38, 1567–1583. [Google Scholar] [CrossRef]
- Cuijpers, P.; Karyotaki, E.; Ciharova, M.; Miguel, C.; Noma, H.; Stikkelbroek, Y.; Weisz, J.R.; Furukawa, T.A. The effects of psychological treatments of depression in children and adolescents on response, reliable change, and deterioration: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2023, 32, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Kadam, P.; Bhalerao, S. Sample size calculation. Int. J. Ayurveda Res. 2010, 1, 55–57. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Ketamine Group (n = 27) | Midazolam Group (n = 28) | p-Value |
|---|---|---|---|
| Average age (years) | 14.9 ± 0.3 | 15.2 ± 0.2 | 0.494 |
| BMI (kg/m2) | 23.0 ± 1.2 | 23.3 ± 0.9 | 0.826 |
| Sex | Only female | Only female | - |
| SBP (mmHg) | 116.0 ± 2.3 | 112.0 ± 1.7 | 0.100 |
| DBP (mmHg) | 72.9 ± 1.8 | 71.2 ± 1.9 | 0.528 |
| HR (bpm) | 88.4 ± 2.7 | 87.9 ± 3.1 | 0.894 |
| Blood oxygen saturation (%) | 98.1 ± 0.2 | 98.0 ± 0.2 | 0.715 |
| Adequate AD Trials per patient | 2 (1, 2) | 2 (1, 3) | 0.458 |
| Current AD medication | none | none | - |
| Previous AD treatment | Fluoxetine (n = 21) | Fluoxetine (n = 20) | - |
| Sertraline (n = 12) | Sertraline (n = 14) | ||
| Fluvoxamine (n = 5) | Escitalopram (n = 7) | ||
| Trazodone (n = 5) | Trazodone (n = 6) | ||
| Vortioxetine (n = 4) | Vortioxetine (n = 4) | ||
| Escitalopram (n = 3) | Fluvoxamine (n = 2) | ||
| Sertraline (n = 12) | Citalopram (n = 1) | ||
| Citalopram (n = 2) | Sertraline (n = 14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macejova, A.; Kovacova, V.; Tonhajzerova, I.; Visnovcova, Z.; Ferencova, N.; Mlyncekova, Z.; Kukucka, T.; Ondrejka, I. Effects of Ketamine vs. Midazolam in Adolescent Treatment Resistant Depression. Pharmaceuticals 2024, 17, 1627. https://doi.org/10.3390/ph17121627
Macejova A, Kovacova V, Tonhajzerova I, Visnovcova Z, Ferencova N, Mlyncekova Z, Kukucka T, Ondrejka I. Effects of Ketamine vs. Midazolam in Adolescent Treatment Resistant Depression. Pharmaceuticals. 2024; 17(12):1627. https://doi.org/10.3390/ph17121627
Chicago/Turabian StyleMacejova, Andrea, Veronika Kovacova, Ingrid Tonhajzerova, Zuzana Visnovcova, Nikola Ferencova, Zuzana Mlyncekova, Tomas Kukucka, and Igor Ondrejka. 2024. "Effects of Ketamine vs. Midazolam in Adolescent Treatment Resistant Depression" Pharmaceuticals 17, no. 12: 1627. https://doi.org/10.3390/ph17121627
APA StyleMacejova, A., Kovacova, V., Tonhajzerova, I., Visnovcova, Z., Ferencova, N., Mlyncekova, Z., Kukucka, T., & Ondrejka, I. (2024). Effects of Ketamine vs. Midazolam in Adolescent Treatment Resistant Depression. Pharmaceuticals, 17(12), 1627. https://doi.org/10.3390/ph17121627







