Fluoroquinolones and Biofilm: A Narrative Review
Abstract
:1. Introduction
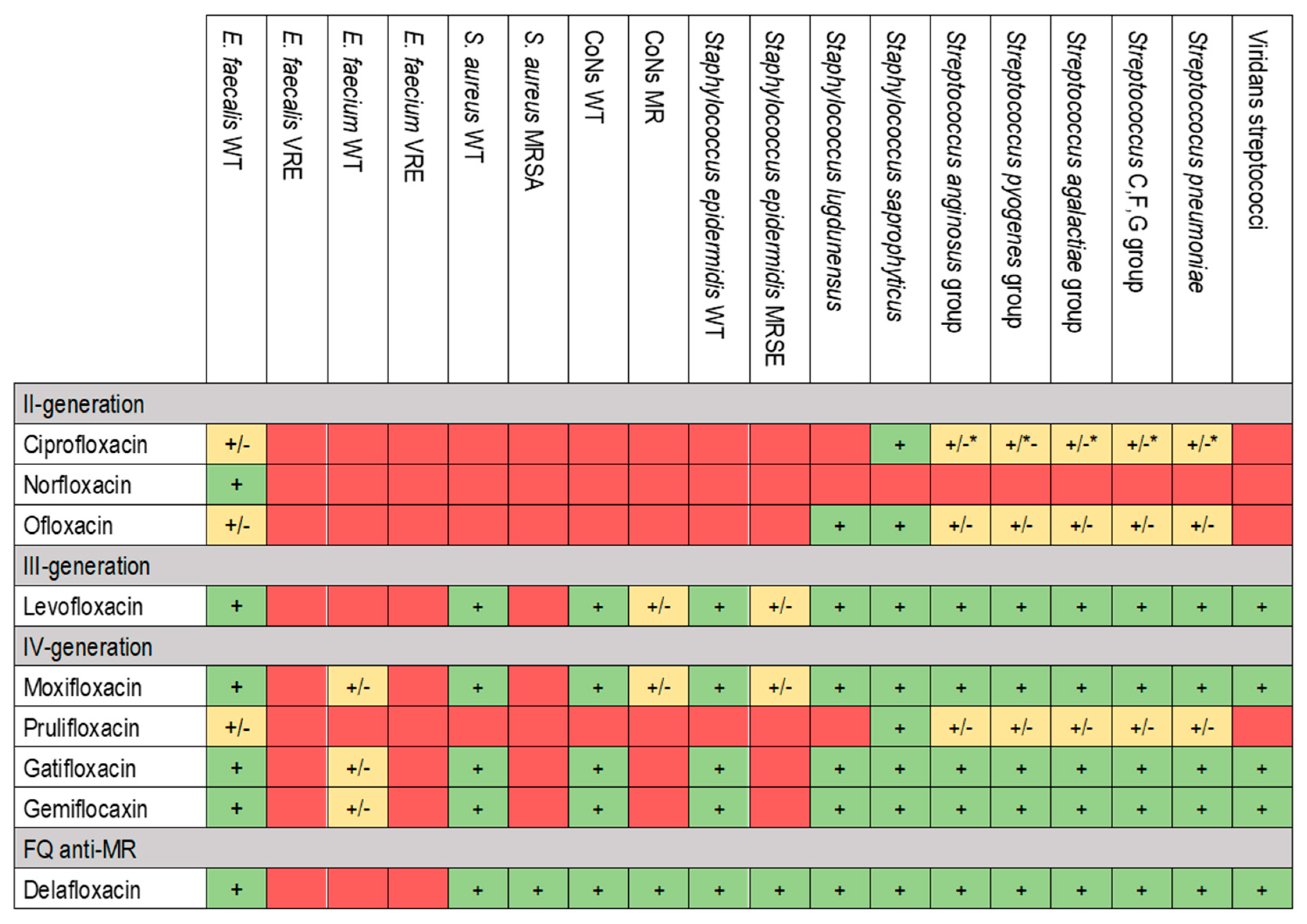
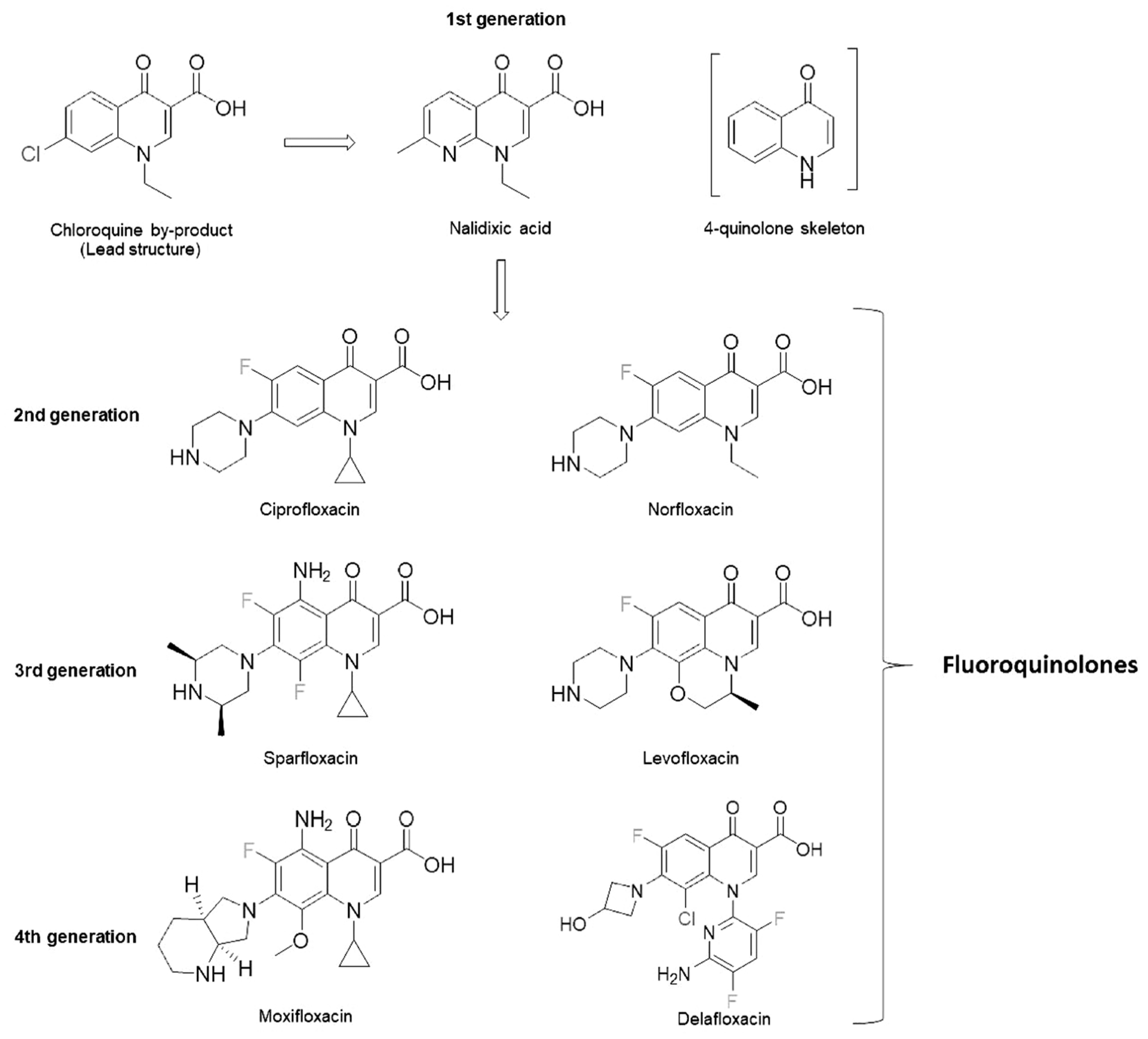
2. Pharmacology and Characteristics of Fluoroquinolones
3. Activity Against Biofilm and Microbiology
4. Central Nervous System, Eyes and Ear Infections
5. Osteoarticular and Prosthetic Joint Infections
6. Vascular Prosthetic Infections and Endocarditis
7. Pulmonary Infections
8. Reproductive and Urinary Tract Infections
9. Skin and Soft Tissue Infections
10. Digestive Infections
11. Discussion
12. Materials and Methods
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tdm, P.; Zm, Z.; Mat, B. Quinolone Antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Adjei, M.D.; Deck, J.; Heinze, T.M.; Freeman, J.P.; Williams, A.J.; Sutherland, J.B. Identification of Metabolites Produced from N-Phenylpiperazine by Mycobacterium Spp. J. Ind. Microbiol. Biotechnol. 2007, 34, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.I.; MacGowan, A.P. Development of the Quinolones. J. Antimicrob. Chemother. 2003, 51 (Suppl. 1), 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J.; Nelligan, J.; Costerton, J.W. A Scanning and Transmission Electron Microscopic Study of an Infected Endocardial Pacemaker Lead. Circulation 1982, 66, 1339–1341. [Google Scholar] [CrossRef]
- Del Pozo, J.L. Biofilm-Related Disease. Expert Rev. Anti Infect. Ther. 2018, 16, 51–65. [Google Scholar] [CrossRef]
- Rottier, W.; Seidelman, J.; Wouthuyzen-Bakker, M. Antimicrobial Treatment of Patients with a Periprosthetic Joint Infection: Basic Principles. Arthroplasty 2023, 5, 10. [Google Scholar] [CrossRef]
- FDA Updates Warnings for Fluoroquinolone Antibiotics on Risks of Mental Health and Low Blood Sugar Adverse Reactions. Available online: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse (accessed on 18 October 2024).
- Willmott, C.J.; Maxwell, A. A Single Point Mutation in the DNA Gyrase A Protein Greatly Reduces Binding of Fluoroquinolones to the Gyrase-DNA Complex. Antimicrob. Agents Chemother. 1993, 37, 126–127. [Google Scholar] [CrossRef]
- Brar, R.K.; Jyoti, U.; Patil, R.K.; Patil, H.C. Fluoroquinolone Antibiotics: An Overview. Adesh Univ. J. Med. Sci. Res. 2020, 2, 26–30. [Google Scholar] [CrossRef]
- Bhatt, S.; Chatterjee, S. Fluoroquinolone Antibiotics: Occurrence, Mode of Action, Resistance, Environmental Detection, and Remediation—A Comprehensive Review. Environ. Pollut. 2022, 315, 120440. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, L. The Antibacterial Activity of Fluoroquinolone Derivatives: An Update (2018-2021). Eur. J. Med. Chem. 2021, 224, 113741. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone Antibiotics: An Emerging Class of Environmental Micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, S.; Hersh, A.L.; Shapiro, D.J.; Fleming-Dutra, K.E.; Pavia, A.T.; Hicks, L.A. Opportunities to Improve Fluoroquinolone Prescribing in the United States for Adult Ambulatory Care Visits. Clin. Infect. Dis. 2018, 67, 134–136. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.A.; Gelone, S.P. Fluoroquinolones. Infect. Dis. Clin. N. Am. 2000, 14, 489–513. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.L.; Hecker, M.T.; Sethi, A.K.; Donskey, C.J. Unnecessary Use of Fluoroquinolone Antibiotics in Hospitalized Patients. BMC Infect. Dis. 2011, 11, 187. [Google Scholar] [CrossRef]
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of Quinolone Action and Resistance: Where Do We Stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How Antibiotics Kill Bacteria: From Targets to Networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Blondeau, J.M. Fluoroquinolones: Mechanism of Action, Classification, and Development of Resistance. Surv. Ophthalmol. 2004, 49, S73–S78. [Google Scholar] [CrossRef]
- Qu, C.; Wu, Z.; Pan, D.; Cai, Z.; Liu, X. Characterization of Lactobacillus Reuteri WQ-Y1 with the Ciprofloxacin Degradation Ability. Biotechnol. Lett. 2021, 43, 855–864. [Google Scholar] [CrossRef]
- Dube, P.S.; Legoabe, L.J.; Beteck, R.M. Quinolone: A Versatile Therapeutic Compound Class. Mol. Divers. 2023, 27, 1501–1526. [Google Scholar] [CrossRef]
- Zahari, N.I.N.; Engku Abd Rahman, E.N.S.; Irekeola, A.A.; Ahmed, N.; Rabaan, A.A.; Alotaibi, J.; Alqahtani, S.A.; Halawi, M.Y.; Alamri, I.A.; Almogbel, M.S.; et al. A Review of the Resistance Mechanisms for β-Lactams, Macrolides and Fluoroquinolones among Streptococcus Pneumoniae. Med. Kaunas Lith. 2023, 59, 1927. [Google Scholar] [CrossRef]
- Xu, P.; Chen, H.; Xu, J.; Wu, M.; Zhu, X.; Wang, F.; Chen, S.; Xu, J. Moxifloxacin Is an Effective and Safe Candidate Agent for Tuberculosis Treatment: A Meta-Analysis. Int. J. Infect. Dis. 2017, 60, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, S.H. The Role of Moxifloxacin in Tuberculosis Therapy. Eur. Respir. Rev. 2016, 25, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Stein, G.E. Delafloxacin: A New Anti-Methicillin-Resistant Staphylococcus Aureus Fluoroquinolone. Clin. Infect. Dis. 2019, 68, 1058–1062. [Google Scholar] [CrossRef]
- Lee, A.; Lamb, Y.N.; Shirley, M. Delafloxacin: A Review in Community-Acquired Pneumonia. Drugs 2022, 82, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.R.; Slattery, J.; Pinheiro, L.; Kurz, X.; Hedenmalm, K. Indications for Systemic Fluoroquinolone Therapy in Europe and Prevalence of Primary-Care Prescribing in France, Germany and the UK: Descriptive Population-Based Study. Clin. Drug Investig. 2018, 38, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.; Schreiber, Y.S.; Hopko, J.; Kirkwood, T.; Kattini, R.; Poirier, D.; Madden, S. Fluoroquinolone Use in a Rural Practice. Can. J. Rural. Med. 2021, 26, 14–18. [Google Scholar] [CrossRef]
- Aminimanizani, A.; Beringer, P.; Jelliffe, R. Comparative Pharmacokinetics and Pharmacodynamics of the Newer Fluoroquinolone Antibacterials. Clin. Pharmacokinet. 2001, 40, 169–187. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Jain, S. Fluoroquinolone Antibacterials: A Review on Chemistry, Microbiology and Therapeutic Prospects. Acta Pol. Pharm. 2009, 66, 587–604. [Google Scholar]
- Van Bambeke, F.; Tulkens, P.M. Safety Profile of the Respiratory Fluoroquinolone Moxifloxacin: Comparison with Other Fluoroquinolones and Other Antibacterial Classes. Drug Saf. 2009, 32, 359–378. [Google Scholar] [CrossRef]
- Tam, P.K.; Ho, C.T.K. Fluoroquinolone-Induced Achilles Tendinitis. Hong Kong Med. J. Xianggang Yi Xue Za Zhi 2014, 20, 545–547. [Google Scholar] [CrossRef]
- Annisa, N.; Barliana, M.I.; Santoso, P.; Ruslami, R. Transporter and Metabolizer Gene Polymorphisms Affect Fluoroquinolone Pharmacokinetic Parameters. Front. Pharmacol. 2022, 13, 1063413. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Biophysics of Biofilm Infection. Pathog. Dis. 2014, 70, 212–218. [Google Scholar] [CrossRef]
- Tolker-Nielsen, T. Biofilm Development. Microbiol. Spectr. 2015, 3, 51–66. [Google Scholar] [CrossRef]
- Høiby, N. Understanding Bacterial Biofilms in Patients with Cystic Fibrosis: Current and Innovative Approaches to Potential Therapies. J. Cyst. Fibros. 2002, 1, 249–254. [Google Scholar] [CrossRef]
- Niveditha, S.; Pramodhini, S.; Umadevi, S.; Kumar, S.; Stephen, S. The Isolation and the Biofilm Formation of Uropathogens in the Patients with Catheter Associated Urinary Tract Infections (UTIs). J. Clin. Diagn. Res. JCDR 2012, 6, 1478–1482. [Google Scholar] [CrossRef]
- Hemmati, F.; Rezaee, M.A.; Ebrahimzadeh, S.; Yousefi, L.; Nouri, R.; Kafil, H.S.; Gholizadeh, P. Novel Strategies to Combat Bacterial Biofilms. Mol. Biotechnol. 2021, 63, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Meneses, L.; Brandão, A.C.; Coenye, T.; Braga, A.C.; Pires, D.P.; Azeredo, J. A Systematic Review of the Use of Bacteriophages for in Vitro Biofilm Control. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 919–928. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Conen, A.; Raabe, A.; Schaller, K.; Fux, C.A.; Vajkoczy, P.; Trampuz, A. Management of Neurosurgical Implant-Associated Infections. Swiss Med. Wkly. 2020, 150, w20208. [Google Scholar] [CrossRef]
- Olson, M.E.; Ceri, H.; Morck, D.W.; Buret, A.G.; Read, R.R. Biofilm Bacteria: Formation and Comparative Susceptibility to Antibiotics. Can. J. Vet. Res. Rev. Can. Rech. Veterinaire 2002, 66, 86–92. [Google Scholar]
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter Pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. MMBR 2018, 82, e00001-18. [Google Scholar] [CrossRef] [PubMed]
- Przekwas, J.; Gębalski, J.; Kwiecińska-Piróg, J.; Wiktorczyk-Kapischke, N.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E.; Rutkowska, D.; Skowron, K. The Effect of Fluoroquinolones and Antioxidans on Biofilm Formation by Proteus Mirabilis Strains. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 22. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Alzoubi, K.H.; Ahmed, W.S.; Magaji, A.S. In Vitro Comparison of Antibacterial and Antibiofilm Activities of Selected Fluoroquinolones against Pseudomonas Aeruginosa and Methicillin-Resistant Staphylococcus Aureus. Pathogens 2019, 8, 12. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm Infections, Their Resilience to Therapy and Innovative Treatment Strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; van de Beek, D.; Bleck, T.P.; Garton, H.J.L.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Waheed, A.; Panese, S.; Scarparo, C.; Solinas, M.; Parisi, S.G.; Geremia, N. Treatment with Cefiderocol in K. Pneumoniae KPC Nosocomial External Ventricular Drainage Meningitis: A Brief Report. Infez. Med. 2022, 30, 454–458. [Google Scholar] [CrossRef]
- Motta, F.; Antonello, C.E. Analysis of Complications in 430 Consecutive Pediatric Patients Treated with Intrathecal Baclofen Therapy: 14-Year Experience. J. Neurosurg. Pediatr. 2014, 13, 301–306. [Google Scholar] [CrossRef]
- Ramírez, P.; Gordón, M.; Soriano, A.; Gil-Perotin, S.; Marti, V.; Gonzalez-Barbera, E.M.; Sanchez-Aguilar, M.T.; Simal, J.A.; Bonastre, J. Assessment of the in Vivo Formation of Biofilm on External Ventricular Drainages. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1437–1443. [Google Scholar] [CrossRef]
- Destache, C.J.; Pakiz, C.B.; Larsen, C.; Owens, H.; Dash, A.K. Cerebrospinal Fluid Penetration and Pharmacokinetics of Levofloxacin in an Experimental Rabbit Meningitis Model. J. Antimicrob. Chemother. 2001, 47, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Lutsar, I.; McCracken, G.H.; Friedland, I.R. Antibiotic Pharmacodynamics in Cerebrospinal Fluid. Clin. Infect. Dis. 1998, 27, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Nau, R.; Sörgel, F.; Eiffert, H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef] [PubMed]
- Tunkel, A.R.; Hartman, B.J.; Kaplan, S.L.; Kaufman, B.A.; Roos, K.L.; Scheld, W.M.; Whitley, R.J. Practice Guidelines for the Management of Bacterial Meningitis. Clin. Infect. Dis. 2004, 39, 1267–1284. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The Resistance Mechanisms of Bacteria against Ciprofloxacin and New Approaches for Enhancing the Efficacy of This Antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef]
- Karvouniaris, M.; Brotis, A.; Tsiakos, K.; Palli, E.; Koulenti, D. Current Perspectives on the Diagnosis and Management of Healthcare-Associated Ventriculitis and Meningitis. Infect. Drug Resist. 2022, 15, 697–721. [Google Scholar] [CrossRef]
- Verderosa, A.D.; de la Fuente-Núñez, C.; Mansour, S.C.; Cao, J.; Lu, T.K.; Hancock, R.E.W.; Fairfull-Smith, K.E. Ciprofloxacin-Nitroxide Hybrids with Potential for Biofilm Control. Eur. J. Med. Chem. 2017, 138, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Voinescu, A.; Licker, M.; Muntean, D.; Musuroi, C.; Musuroi, S.I.; Izmendi, O.; Vulpie, S.; Jumanca, R.; Munteanu, M.; Cosnita, A. A Comprehensive Review of Microbial Biofilms on Contact Lenses: Challenges and Solutions. Infect. Drug Resist. 2024, 17, 2659–2671. [Google Scholar] [CrossRef]
- Borroni, D.; Paytuví-Gallart, A.; Sanseverino, W.; Gómez-Huertas, C.; Bonci, P.; Romano, V.; Giannaccare, G.; Rechichi, M.; Meduri, A.; Oliverio, G.W.; et al. Exploring the Healthy Eye Microbiota Niche in a Multicenter Study. Int. J. Mol. Sci. 2022, 23, 10229. [Google Scholar] [CrossRef]
- Borroni, D.; Rocha de Lossada, C.; Mazzotta, C.; Sánchez-González, J.-M.; Papa, F.; Gabrielli, F. Ocular Microbiome Evaluation in Dry Eye Disease and Meibomian Gland Dysfunction: Values of Variables. Exp. Eye Res. 2023, 236, 109656. [Google Scholar] [CrossRef]
- Ballesteros-Sánchez, A.; Sánchez-González, J.-M.; Borrone, M.A.; Borroni, D.; Rocha-de-Lossada, C. The Influence of Lid-Parallel Conjunctival Folds and Conjunctivochalasis on Dry Eye Symptoms with and Without Contact Lens Wear: A Review of the Literature. Ophthalmol. Ther. 2024, 13, 651–670. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Kunimoto, D.Y.; Kazi, L.; Regillo, C.D.; Ho, A.C.; Belmont, J.; Maguire, J.; Vander, J.; Brown, G.C. Endophthalmitis after Open Globe Injury: Microbiologic Spectrum and Susceptibilities of Isolates. Am. J. Ophthalmol. 2006, 142, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Pachigolla, G.; Blomquist, P.; Cavanagh, H.D. Microbial Keratitis Pathogens and Antibiotic Susceptibilities: A 5-Year Review of Cases at an Urban County Hospital in North Texas. Eye Contact Lens 2007, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.P.; Bahatheg, G.; Carnt, N.; Kalaiselvan, P.; Kumar, N.; Kuppusamy, R.; Rayamajhee, B.; Sara, M.; Stapleton, F.; Vijay, A.K.; et al. Biofilms and Contact Lenses: Problems and Solutions. Microbiol. Aust. 2023, 44, 96–99. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and Device-Associated Infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Lodha, D.; Karolia, R.; Sharma, S.; Joseph, J.; Das, T.; Dave, V.P. Biofilm Formation and Its Effect on the Management of Culture-Positive Bacterial Endophthalmitis. Indian J. Ophthalmol. 2022, 70, 472–476. [Google Scholar] [CrossRef]
- Wozniak, R.A.F.; Aquavella, J.V. Antibiotics in Ophthalmology Practice. Expert Rev. Ophthalmol. 2017, 12, 243–250. [Google Scholar] [CrossRef]
- Marone, P.; Perversi, L.; Monzillo, V.; Maserati, R.; Antoniazzi, E. Ocular Infections: Antibiotics and Bacterial Adhesion on Biomaterials Used in Ocular Surgery. Ophthalmologica 2010, 209, 315–318. [Google Scholar] [CrossRef]
- Gentili, V.; Strazzabosco, G.; Spena, R.; Rizzo, S.; Beltrami, S.; Schiuma, G.; Alogna, A.; Rizzo, R. Comparison Between Moxifloxacin and Chloramphenicol for the Treatment of Bacterial Eye Infections. Curr. Ther. Res. 2024, 100, 100740. [Google Scholar] [CrossRef]
- Diriba, K.; Kassa, T.; Alemu, Y.; Bekele, S. In Vitro Biofilm Formation and Antibiotic Susceptibility Patterns of Bacteria from Suspected External Eye Infected Patients Attending Ophthalmology Clinic, Southwest Ethiopia. Int. J. Microbiol. 2020, 2020, 8472395. [Google Scholar] [CrossRef]
- Thirumalmuthu, K.; Devarajan, B.; Prajna, L.; Mohankumar, V. Mechanisms of Fluoroquinolone and Aminoglycoside Resistance in Keratitis-Associated Pseudomonas Aeruginosa. Microb. Drug Resist. Larchmt. N 2019, 25, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Garty, S.; Shirakawa, R.; Warsen, A.; Anderson, E.M.; Noble, M.L.; Bryers, J.D.; Ratner, B.D.; Shen, T.T. Sustained Antibiotic Release from an Intraocular Lens-Hydrogel Assembly for Cataract Surgery. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6109–6116. [Google Scholar] [CrossRef]
- Schilder, A.G.M.; Chonmaitree, T.; Cripps, A.W.; Rosenfeld, R.M.; Casselbrant, M.L.; Haggard, M.P.; Venekamp, R.P. Otitis Media. Nat. Rev. Dis. Primer 2016, 2, 16063. [Google Scholar] [CrossRef]
- CDC Ear Infection Basics. Available online: https://www.cdc.gov/ear-infection/about/index.html (accessed on 29 October 2024).
- Harmes, K.M.; Blackwood, R.A.; Burrows, H.L.; Cooke, J.M.; Harrison, R.V.; Passamani, P.P. Otitis Media: Diagnosis and Treatment. Am. Fam. Physician 2013, 88, 435–440. [Google Scholar]
- Coker, T.R.; Chan, L.S.; Newberry, S.J.; Limbos, M.A.; Suttorp, M.J.; Shekelle, P.G.; Takata, G.S. Diagnosis, Microbial Epidemiology, and Antibiotic Treatment of Acute Otitis Media in Children: A Systematic Review. JAMA 2010, 304, 2161–2169. [Google Scholar] [CrossRef]
- Jacobs, M.R.; Dagan, R.; Appelbaum, P.C.; Burch, D.J. Prevalence of Antimicrobial-Resistant Pathogens in Middle Ear Fluid: Multinational Study of 917 Children with Acute Otitis Media. Antimicrob. Agents Chemother. 1998, 42, 589. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.C.; Massa, H.M.; Thornton, R.B.; Cripps, A.W. Predominant Bacteria Detected from the Middle Ear Fluid of Children Experiencing Otitis Media: A Systematic Review. PLoS ONE 2016, 11, e0150949. [Google Scholar] [CrossRef]
- Lampikoski, H.; Aarnisalo, A.A.; Jero, J.; Kinnari, T.J. Mastoid Biofilm in Chronic Otitis Media. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2012, 33, 785–788. [Google Scholar] [CrossRef]
- Gu, X.; Keyoumu, Y.; Long, L.; Zhang, H. Detection of Bacterial Biofilms in Different Types of Chronic Otitis Media. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 2877–2883. [Google Scholar] [CrossRef]
- Van Hoecke, H.; De Paepe, A.-S.; Lambert, E.; Van Belleghem, J.D.; Cools, P.; Van Simaey, L.; Deschaght, P.; Vaneechoutte, M.; Dhooge, I. Haemophilus Influenzae Biofilm Formation in Chronic Otitis Media with Effusion. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3553–3560. [Google Scholar] [CrossRef]
- Mittal, R.; Lisi, C.V.; Gerring, R.; Mittal, J.; Mathee, K.; Narasimhan, G.; Azad, R.K.; Yao, Q.; Grati, M.; Yan, D.; et al. Current Concepts in the Pathogenesis and Treatment of Chronic Suppurative Otitis Media. J. Med. Microbiol. 2015, 64, 1103–1116. [Google Scholar] [CrossRef]
- Grubb, M.S.; Spaugh, D.C. Treatment Failure, Recurrence, and Antibiotic Prescription Rates for Different Acute Otitis Media Treatment Methods. Clin. Pediatr. (Phila.) 2010, 49, 970–975. [Google Scholar] [CrossRef]
- Moriyama, S.; Hotomi, M.; Shimada, J.; Billal, D.S.; Fujihara, K.; Yamanaka, N. Formation of Biofilm by Haemophilus Influenzae Isolated from Pediatric Intractable Otitis Media. Auris. Nasus. Larynx 2009, 36, 525–531. [Google Scholar] [CrossRef]
- Takei, S.; Hotomi, M.; Yamanaka, N. Minimal Biofilm Eradication Concentration of Antimicrobial Agents against Nontypeable Haemophilus Influenzae Isolated from Middle Ear Fluids of Intractable Acute Otitis Media. J. Infect. Chemother. 2013, 19, 504–509. [Google Scholar] [CrossRef]
- Kaji, C.; Watanabe, K.; Apicella, M.A.; Watanabe, H. Antimicrobial Effect of Fluoroquinolones for the Eradication of Nontypeable Haemophilus Influenzae Isolates within Biofilms. Tohoku J. Exp. Med. 2008, 214, 121–128. [Google Scholar] [CrossRef]
- Novotny, L.A.; Chiang, T.; Goodman, S.D.; Elmaraghy, C.A.; Bakaletz, L.O. Humanized Anti-DNABII Fab Fragments Plus Ofloxacin Eradicated Biofilms in Experimental Otitis Media. Laryngoscope 2021, 131, E2698–E2704. [Google Scholar] [CrossRef]
- Khomtchouk, K.M.; Joseph, L.I.; Khomtchouk, B.B.; Kouhi, A.; Massa, S.; Xia, A.; Koliesnik, I.; Pletzer, D.; Bollyky, P.L.; Santa Maria, P.L. Treatment with a Neutrophil Elastase Inhibitor and Ofloxacin Reduces P. Aeruginosa Burden in a Mouse Model of Chronic Suppurative Otitis Media. NPJ Biofilms Microbiomes 2021, 7, 31. [Google Scholar] [CrossRef]
- Senneville, E.; Gachet, B.; Blondiaux, N.; Robineau, O. Do Anti-Biofilm Antibiotics Have a Place in the Treatment of Diabetic Foot Osteomyelitis? Antibiotics 2023, 12, 317. [Google Scholar] [CrossRef]
- Almasri, D.; Dahman, Y. Prosthetic Joint Infections: Biofilm Formation, Management, and the Potential of Mesoporous Bioactive Glass as a New Treatment Option. Pharmaceutics 2023, 15, 1401. [Google Scholar] [CrossRef]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of Removed Hip and Knee Prostheses for Diagnosis of Infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef]
- Ramage, G.; Tunney, M.M.; Patrick, S.; Gorman, S.P.; Nixon, J.R. Formation of Propionibacterium Acnes Biofilms on Orthopaedic Biomaterials and Their Susceptibility to Antimicrobials. Biomaterials 2003, 24, 3221–3227. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID Guideline for the Diagnosis and Treatment of Biofilm Infections 2014. Clin. Microbiol. Infect. 2015, 21 (Suppl. 1), S1–S25. [Google Scholar] [CrossRef]
- Høiby, N.; Moser, C.; Oliver, A.; Williams, C.; Ramage, G.; Borghi, E.; Azeredo, J.; Macia, M.D.; European Society for Clinical Microbiology and Infectious Disease Study Group for Biofilms (ESGB). To Update or Not to Update the ESCMID Guidelines for the Diagnosis and Treatment of Biofilm Infections—That Is the Question! The Opinion of the ESGB Board. Biofilm 2023, 6, 100135. [Google Scholar] [CrossRef]
- Wang, L.; Di Luca, M.; Tkhilaishvili, T.; Trampuz, A.; Gonzalez Moreno, M. Synergistic Activity of Fosfomycin, Ciprofloxacin, and Gentamicin Against Escherichia Coli and Pseudomonas Aeruginosa Biofilms. Front. Microbiol. 2019, 10, 2522. [Google Scholar] [CrossRef]
- Widmer, A.F.; Wiestner, A.; Frei, R.; Zimmerli, W. Killing of Nongrowing and Adherent Escherichia Coli Determines Drug Efficacy in Device-Related Infections. Antimicrob. Agents Chemother. 1991, 35, 741–746. [Google Scholar] [CrossRef]
- Holmberg, A.; Mörgelin, M.; Rasmussen, M. Effectiveness of Ciprofloxacin or Linezolid in Combination with Rifampicin against Enterococcus Faecalis in Biofilms. J. Antimicrob. Chemother. 2012, 67, 433–439. [Google Scholar] [CrossRef]
- Fily, F.; Jolivet-Gougeon, A.; Polard, E.; Gicquel, T.; Dupont, M.; Verdier, M.C.; Arvieux, C. Moxifloxacin-Rifampicin Combination for the Treatment of Non-Staphylococcal Gram-Positive Orthopedic Implant-Related Infections. Med. Mal. Infect. 2019, 49, 540–544. [Google Scholar] [CrossRef]
- Perez-Alba, E.; Flores-Treviño, S.; Villarreal-Salazar, V.; Bocanegra-Ibarias, P.; Vilchez-Cavazos, F.; Camacho-Ortiz, A. Planktonic and Biofilm States of Staphylococcus Aureus Isolated from Bone and Joint Infections and the in Vitro Effect of Orally Available Antibiotics. J. Appl. Microbiol. 2023, 134, lxad258. [Google Scholar] [CrossRef]
- Greimel, F.; Scheuerer, C.; Gessner, A.; Simon, M.; Kalteis, T.; Grifka, J.; Benditz, A.; Springorum, H.-R.; Schaumburger, J. Efficacy of Antibiotic Treatment of Implant-Associated Staphylococcus Aureus Infections with Moxifloxacin, Flucloxacillin, Rifampin, and Combination Therapy: An Animal Study. Drug Des. Devel. Ther. 2017, 11, 1729–1736. [Google Scholar] [CrossRef]
- Kalteis, T.; Beckmann, J.; Schröder, H.-J.; Schaumburger, J.; Linde, H.-J.; Lerch, K.; Lehn, N. Treatment of Implant-Associated Infections with Moxifloxacin: An Animal Study. Int. J. Antimicrob. Agents 2006, 27, 444–448. [Google Scholar] [CrossRef]
- Munaweera, I.; Shaikh, S.; Maples, D.; Nigatu, A.S.; Sethuraman, S.N.; Ranjan, A.; Greenberg, D.E.; Chopra, R. Temperature-Sensitive Liposomal Ciprofloxacin for the Treatment of Biofilm on Infected Metal Implants Using Alternating Magnetic Fields. Int. J. Hyperth. 2018, 34, 189. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Onyilofor, S.; Ebube, N.K.; Owusu-Ababio, G. Preparation and Characterization of Ofloxacin Microspheres for the Eradication of Bone Associated Bacterial Biofilm. J. Microencapsul. 1999, 16, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Rzhepishevska, O.; Grenho, L.; Malheiros, D.; Gonçalves, L.; Almeida, A.J.; Jordão, L.; Ribeiro, I.A.; Ramstedt, M.; Gomes, P.; et al. Levofloxacin-Loaded Bone Cement Delivery System: Highly Effective against Intracellular Bacteria and Staphylococcus Aureus Biofilms. Int. J. Pharm. 2017, 532, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.C.; Ribeiro, I.A.C.; Guedes, R.C.; Pinto, R.; Vaz, M.A.; Gonçalves, L.M.; Almeida, A.J.; Bettencourt, A.F. Key-Properties Outlook of a Levofloxacin-Loaded Acrylic Bone Cement with Improved Antibiotic Delivery. Int. J. Pharm. 2015, 485, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Sedghizadeh, P.P.; Cherian, P.; Roshandel, S.; Tjokro, N.; Chen, C.; Junka, A.F.; Hu, E.; Neighbors, J.; Pawlak, J.; Russell, R.G.G.; et al. Real-Time Impedance-Based Monitoring of the Growth and Inhibition of Osteomyelitis Biofilm Pathogen Staphylococcus Aureus Treated with Novel Bisphosphonate-Fluoroquinolone Antimicrobial Conjugates. Int. J. Mol. Sci. 2023, 24, 1985. [Google Scholar] [CrossRef]
- Sedghizadeh, P.P.; Sun, S.; Junka, A.F.; Richard, E.; Sadrerafi, K.; Mahabady, S.; Bakhshalian, N.; Tjokro, N.; Bartoszewicz, M.; Oleksy, M.; et al. Design, Synthesis, and Antimicrobial Evaluation of a Novel Bone-Targeting Bisphosphonate-Ciprofloxacin Conjugate for the Treatment of Osteomyelitis Biofilms. J. Med. Chem. 2017, 60, 2326–2343. [Google Scholar] [CrossRef]
- Memar, M.Y.; Ahangarzadeh Rezaee, M.; Barzegar-Jalali, M.; Gholikhani, T.; Adibkia, K. The Antibacterial Effect of Ciprofloxacin Loaded Calcium Carbonate (CaCO3) Nanoparticles Against the Common Bacterial Agents of Osteomyelitis. Curr. Microbiol. 2023, 80, 173. [Google Scholar] [CrossRef]
- Wilson, W.R.; Bower, T.C.; Creager, M.A.; Amin-Hanjani, S.; O’Gara, P.T.; Lockhart, P.B.; Darouiche, R.O.; Ramlawi, B.; Derdeyn, C.P.; Bolger, A.F.; et al. Vascular Graft Infections, Mycotic Aneurysms, and Endovascular Infections: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e412–e460. [Google Scholar] [CrossRef]
- Chiesa, R.; Astore, D.; Frigerio, S.; Garriboli, L.; Piccolo, G.; Castellano, R.; Scalamogna, M.; Odero, A.; Pirrelli, S.; Biasi, G.; et al. Vascular Prosthetic Graft Infection: Epidemiology, Bacteriology, Pathogenesis and Treatment. Acta Chir. Belg. 2002, 102, 238–247. [Google Scholar] [CrossRef]
- Antonios, V.S.; Noel, A.A.; Steckelberg, J.M.; Wilson, W.R.; Mandrekar, J.N.; Harmsen, W.S.; Baddour, L.M. Prosthetic Vascular Graft Infection: A Risk Factor Analysis Using a Case-Control Study. J. Infect. 2006, 53, 49–55. [Google Scholar] [CrossRef]
- Bandyk, D.F.; Bergamini, T.M.; Kinney, E.V.; Seabrook, G.R.; Towne, J.B. In Situ Replacement of Vascular Prostheses Infected by Bacterial Biofilms. J. Vasc. Surg. 1991, 13, 575–583. [Google Scholar] [CrossRef]
- Sohail, M.R.; Uslan, D.Z.; Khan, A.H.; Friedman, P.A.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Stoner, S.; Baddour, L.M. Management and Outcome of Permanent Pacemaker and Implantable Cardioverter-Defibrillator Infections. J. Am. Coll. Cardiol. 2007, 49, 1851–1859. [Google Scholar] [CrossRef]
- Moore, W.S.; Rosson, C.T.; Hall, A.D.; Thomas, A.N. Transient Bacteremia. A Cause of Infection in Prosthetic Vascular Grafts. Am. J. Surg. 1969, 117, 342–343. [Google Scholar] [CrossRef]
- Gharamti, A.; Kanafani, Z.A. Vascular Graft Infections: An Update. Infect. Dis. Clin. North Am. 2018, 32, 789–809. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Voyich, J.M.; Fischer, E.R.; Braughton, K.R.; Whitney, A.R.; DeLeo, F.R.; Otto, M. Polysaccharide Intercellular Adhesin (PIA) Protects Staphylococcus Epidermidis against Major Components of the Human Innate Immune System. Cell. Microbiol. 2004, 6, 269–275. [Google Scholar] [CrossRef]
- Leroy, O.; Meybeck, A.; Sarraz-Bournet, B.; d’Elia, P.; Legout, L. Vascular Graft Infections. Curr. Opin. Infect. Dis. 2012, 25, 154–158. [Google Scholar] [CrossRef]
- Tello-Díaz, C.; Muñoz, E.; Palau, M.; Gomis, X.; Gavaldà, J.; Gil-Sala, D.; Fernández-Hidalgo, N.; Bellmunt-Montoya, S. Antibiotic Efficacy against Methicillin-Susceptible Staphylococcus Aureus Biofilms on Synthetic and Biological Vascular Grafts. Ann. Vasc. Surg. 2024, 108, 475–483. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lee, M.-T.G.; Chen, Y.-S.; Lee, S.-H.; Chen, Y.-S.; Chen, S.-C.; Chang, S.-C. Risk of Aortic Dissection and Aortic Aneurysm in Patients Taking Oral Fluoroquinolone. JAMA Intern. Med. 2015, 175, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Sixt, T.; Aho, S.; Chavanet, P.; Moretto, F.; Denes, E.; Mahy, S.; Blot, M.; Catherine, F.-X.; Steinmetz, E.; Piroth, L. Long-Term Prognosis Following Vascular Graft Infection: A 10-Year Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac054. [Google Scholar] [CrossRef]
- Bauer, J.; Robineau, O.; Sobocinski, J.; D’Elia, P.; Boucher, A.; Lafon-Desmurs, B.; Tetart, M.; Meybeck, A.; Patoz, P.; Senneville, E. Enterococcus-Related Vascular Graft Infection: A Case Series. Infect. Dis. Now 2024, 54, 104940. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Yamamura, K.; Fujimoto, K.; Mizuno, K.; Sakurai, T.; Ohta, M.; Nabeshima, T. Prophylaxis of Local Vascular Graft Infection with Levofloxacin Incorporated into Albumin-Sealed Dacron Graft (LVFX-ALB Graft). Microbiol. Immunol. 1999, 43, 317–321. [Google Scholar] [CrossRef]
- Bin Abdulhak, A.A.; Baddour, L.M.; Erwin, P.J.; Hoen, B.; Chu, V.H.; Mensah, G.A.; Tleyjeh, I.M. Global and Regional Burden of Infective Endocarditis, 1990-2010: A Systematic Review of the Literature. Glob. Heart 2014, 9, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Lauten, A.; Martinović, M.; Kursawe, L.; Kikhney, J.; Affeld, K.; Kertzscher, U.; Falk, V.; Moter, A. Bacterial Biofilms in Infective Endocarditis: An in Vitro Model to Investigate Emerging Technologies of Antimicrobial Cardiovascular Device Coatings. Clin. Res. Cardiol. 2021, 110, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Kouijzer, J.J.P.; Noordermeer, D.J.; van Leeuwen, W.J.; Verkaik, N.J.; Lattwein, K.R. Native Valve, Prosthetic Valve, and Cardiac Device-Related Infective Endocarditis: A Review and Update on Current Innovative Diagnostic and Therapeutic Strategies. Front. Cell Dev. Biol. 2022, 10, 995508. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-J.; Yeh, C.-Y.; Shun, C.-T.; Hsu, R.-B.; Cheng, H.-W.; Lin, C.-S.; Chia, J.-S. Platelets Enhance Biofilm Formation and Resistance of Endocarditis-Inducing Streptococci on the Injured Heart Valve. J. Infect. Dis. 2012, 205, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the Management of Endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Iversen, K.; Ihlemann, N.; Gill, S.U.; Madsen, T.; Elming, H.; Jensen, K.T.; Bruun, N.E.; Høfsten, D.E.; Fursted, K.; Christensen, J.J.; et al. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N. Engl. J. Med. 2019, 380, 415–424. [Google Scholar] [CrossRef]
- Bundgaard, H.; Ihlemann, N.; Gill, S.U.; Bruun, N.E.; Elming, H.; Madsen, T.; Jensen, K.T.; Fursted, K.; Christensen, J.J.; Schultz, M.; et al. Long-Term Outcomes of Partial Oral Treatment of Endocarditis. N. Engl. J. Med. 2019, 380, 1373–1374. [Google Scholar] [CrossRef]
- Pries-Heje, M.M.; Wiingaard, C.; Ihlemann, N.; Gill, S.U.; Bruun, N.E.; Elming, H.; Povlsen, J.A.; Madsen, T.; Jensen, K.T.; Fursted, K.; et al. Five-Year Outcomes of the Partial Oral Treatment of Endocarditis (POET) Trial. N. Engl. J. Med. 2022, 386, 601–602. [Google Scholar] [CrossRef]
- Cremieux, A.C.; Maziere, B.; Vallois, J.M.; Ottaviani, M.; Azancot, A.; Raffoul, H.; Bouvet, A.; Pocidalo, J.J.; Carbon, C. Evaluation of Antibiotic Diffusion into Cardiac Vegetations by Quantitative Autoradiography. J. Infect. Dis. 1989, 159, 938–944. [Google Scholar] [CrossRef]
- McAllister, D.A.; Liu, L.; Shi, T.; Chu, Y.; Reed, C.; Burrows, J.; Adeloye, D.; Rudan, I.; Black, R.E.; Campbell, H.; et al. Global, Regional, and National Estimates of Pneumonia Morbidity and Mortality in Children Younger than 5 Years between 2000 and 2015: A Systematic Analysis. Lancet Glob. Health 2019, 7, e47–e57. [Google Scholar] [CrossRef]
- Ding, L.; Wang, J.; Cai, S.; Smyth, H.; Cui, Z. Pulmonary Biofilm-Based Chronic Infections and Inhaled Treatment Strategies. Int. J. Pharm. 2021, 604, 120768. [Google Scholar] [CrossRef] [PubMed]
- Kolpen, M.; Kragh, K.N.; Enciso, J.B.; Faurholt-Jepsen, D.; Lindegaard, B.; Egelund, G.B.; Jensen, A.V.; Ravn, P.; Mathiesen, I.H.M.; Gheorge, A.G.; et al. Bacterial Biofilms Predominate in Both Acute and Chronic Human Lung Infections. Thorax 2022, 77, 1015–1022. [Google Scholar] [CrossRef]
- Short, B.; Williams, C.; Litherland, G.; Lundy, F.; Mackay, W.; Ramage, G. Development and Characterisation of a Multi-Species COPD Biofilm. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Short, B.; Delaney, C.; Johnston, W.; Litherland, G.J.; Lockhart, J.C.; Williams, C.; Mackay, W.G.; Ramage, G. Informed Development of a Multi-Species Biofilm in Chronic Obstructive Pulmonary Disease. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2024, 132, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas Aeruginosa Biofilms in Cystic Fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef]
- Diaconu, O.; Siriopol, I.; Poloșanu, L.I.; Grigoraș, I. Endotracheal Tube Biofilm and Its Impact on the Pathogenesis of Ventilator-Associated Pneumonia. J. Crit. Care Med. Univ. Med. Si Farm. Din Targu-Mures 2018, 4, 50–55. [Google Scholar] [CrossRef]
- Pirrone, M.; Pinciroli, R.; Berra, L. Microbiome, Biofilms, and Pneumonia in the ICU. Curr. Opin. Infect. Dis. 2016, 29, 160–166. [Google Scholar] [CrossRef]
- Inglis, T.J.; Millar, M.R.; Jones, J.G.; Robinson, D.A. Tracheal Tube Biofilm as a Source of Bacterial Colonization of the Lung. J. Clin. Microbiol. 1989, 27, 2014–2018. [Google Scholar] [CrossRef]
- Adair, C.G.; Gorman, S.P.; Feron, B.M.; Byers, L.M.; Jones, D.S.; Goldsmith, C.E.; Moore, J.E.; Kerr, J.R.; Curran, M.D.; Hogg, G.; et al. Implications of Endotracheal Tube Biofilm for Ventilator-Associated Pneumonia. Intensive Care Med. 1999, 25, 1072–1076. [Google Scholar] [CrossRef]
- Vandecandelaere, I.; Matthijs, N.; Van Nieuwerburgh, F.; Deforce, D.; Vosters, P.; De Bus, L.; Nelis, H.J.; Depuydt, P.; Coenye, T. Assessment of Microbial Diversity in Biofilms Recovered from Endotracheal Tubes Using Culture Dependent and Independent Approaches. PLoS ONE 2012, 7, e38401. [Google Scholar] [CrossRef] [PubMed]
- Vandecandelaere, I.; Matthijs, N.; Nelis, H.J.; Depuydt, P.; Coenye, T. The Presence of Antibiotic-Resistant Nosocomial Pathogens in Endotracheal Tube Biofilms and Corresponding Surveillance Cultures. Pathog. Dis. 2013, 69, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Pneumatikos, I.A.; Dragoumanis, C.K.; Bouros, D.E. Ventilator-Associated Pneumonia or Endotracheal Tube-Associated Pneumonia? An Approach to the Pathogenesis and Preventive Strategies Emphasizing the Importance of Endotracheal Tube. Anesthesiology 2009, 110, 673–680. [Google Scholar] [CrossRef]
- Vandecandelaere, I.; Coenye, T. Microbial Composition and Antibiotic Resistance of Biofilms Recovered from Endotracheal Tubes of Mechanically Ventilated Patients. Adv. Exp. Med. Biol. 2015, 830, 137–155. [Google Scholar] [CrossRef]
- Restrepo, M.; Peterson, J.; Fernandez, J.; Qin, Z.; Fisher, A.; Nicholson, S. Comparison of the Bacterial Etiology of Early-Onset and Late-Onset Ventilator-Associated Pneumonia in Subjects Enrolled in 2 Large Clinical Studies. Respir. Care 2013, 58, 1220–1225. [Google Scholar] [CrossRef]
- Boltey, E.; Yakusheva, O.; Costa, D.K. 5 Nursing Strategies to Prevent Ventilator-Associated Pneumonia. Am. Nurse Today 2017, 12, 42. [Google Scholar] [PubMed]
- Klompas, M.; Branson, R.; Cawcutt, K.; Crist, M.; Eichenwald, E.C.; Greene, L.R.; Lee, G.; Maragakis, L.L.; Powell, K.; Priebe, G.P.; et al. Strategies to Prevent Ventilator-Associated Pneumonia, Ventilator-Associated Events, and Nonventilator Hospital-Acquired Pneumonia in Acute-Care Hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2022, 43, 687–713. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, D.; Chommeloux, J.; Pineton de Chambrun, M.; Hékimian, G.; Luyt, C.-E. Antibiotic Stewardship in the ICU: Time to Shift into Overdrive. Ann. Intensive Care 2023, 13, 39. [Google Scholar] [CrossRef]
- Ture, Z.; Güner, R.; Alp, E. Antimicrobial Stewardship in the Intensive Care Unit. J. Intensive Med. 2023, 3, 244–253. [Google Scholar] [CrossRef]
- Geremia, N.; Zuglian, G.; Salvador, A.; Solinas, M.; Boraso, S.; Tini, L.; Fullin, G.; Vanin, M.; Lazzari, F.; Panese, S. Impact of Infection Control Measures and Antibiotic Stewardship Programs on Multidrug-Resistant Klebsiella Pneumoniae Prevalence in Intensive Care Unit. Infect. Dis. Trop. Med. 2024, 10, e1413. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-Acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Shams, W.E.; Evans, M.E. Guide to Selection of Fluoroquinolones in Patients with Lower Respiratory Tract Infections. Drugs 2005, 65, 949–991. [Google Scholar] [CrossRef]
- Wise, R. Comparative Penetration of Selected Fluoroquinolones into Respiratory Tract Fluids and Tissues. Am. J. Med. 1991, 91, 67S–70S. [Google Scholar] [CrossRef]
- Usman, M.; Marcus, A.; Fatima, A.; Aslam, B.; Zaid, M.; Khattak, M.; Bashir, S.; Masood, S.; Rafaque, Z.; Dasti, J.I. Synergistic Effects of Gentamicin, Cefepime, and Ciprofloxacin on Biofilm of Pseudomonas Aeruginosa. Infect. Drug Resist. 2023, 16, 5887–5898. [Google Scholar] [CrossRef]
- Han, J.; Luo, J.; Du, Z.; Chen, Y.; Liu, T. Synergistic Effects of Baicalin and Levofloxacin Against Hypervirulent Klebsiella Pneumoniae Biofilm In Vitro. Curr. Microbiol. 2023, 80, 126. [Google Scholar] [CrossRef]
- Vandevelde, N.M.; Tulkens, P.M.; Van Bambeke, F. Antibiotic Activity against Naive and Induced Streptococcus Pneumoniae Biofilms in an in Vitro Pharmacodynamic Model. Antimicrob. Agents Chemother. 2014, 58, 1348–1358. [Google Scholar] [CrossRef]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, ciae403. [Google Scholar] [CrossRef]
- Craddock, V.D.; Steere, E.L.; Harman, H.; Britt, N.S. Activity of Delafloxacin and Comparator Fluoroquinolones against Multidrug-Resistant Pseudomonas Aeruginosa in an In Vitro Cystic Fibrosis Sputum Model. Antibiotics 2023, 12, 1078. [Google Scholar] [CrossRef]
- Millar, B.C.; McCaughan, J.; Rendall, J.C.; Moore, J.E. Delafloxacin--A Novel Fluoroquinolone for the Treatment of Ciprofloxacin-Resistant Pseudomonas Aeruginosa in Patients with Cystic Fibrosis. Clin. Respir. J. 2021, 15, 116–120. [Google Scholar] [CrossRef]
- Kocsis, B.; Szabo, D. New Treatment Options for Lower Respiratory Tract Infections. Expert Opin. Pharmacother. 2017, 18, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Siala, W.; Tulkens, P.M.; Van Bambeke, F. A Combined Pharmacodynamic Quantitative and Qualitative Model Reveals the Potent Activity of Daptomycin and Delafloxacin against Staphylococcus Aureus Biofilms. Antimicrob. Agents Chemother. 2013, 57, 2726–2737. [Google Scholar] [CrossRef]
- Siala, W.; Mingeot-Leclercq, M.-P.; Tulkens, P.M.; Hallin, M.; Denis, O.; Van Bambeke, F. Comparison of the Antibiotic Activities of Daptomycin, Vancomycin, and the Investigational Fluoroquinolone Delafloxacin against Biofilms from Staphylococcus Aureus Clinical Isolates. Antimicrob. Agents Chemother. 2014, 58, 6385–6397. [Google Scholar] [CrossRef]
- Kara, A.; Massaro, C.; Giammanco, G.M.; Alduina, R.; Boussoualim, N. Phylogenetic Diversity, Antibiotic Resistance, and Virulence of Escherichia Coli Strains from Urinary Tract Infections in Algeria. Antibiotics 2024, 13, 773. [Google Scholar] [CrossRef]
- David, A.; Tahrioui, A.; Tareau, A.-S.; Forge, A.; Gonzalez, M.; Bouffartigues, E.; Lesouhaitier, O.; Chevalier, S. Pseudomonas Aeruginosa Biofilm Lifecycle: Involvement of Mechanical Constraints and Timeline of Matrix Production. Antibiotics 2024, 13, 688. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Mishra, M.P.; Bhattacharyay, D. Targeting Microbial Bio-Film: An Update on MDR Gram-Negative Bio-Film Producers Causing Catheter-Associated Urinary Tract Infections. Appl. Biochem. Biotechnol. 2022, 194, 2796–2830. [Google Scholar] [CrossRef]
- Ronald, A. The Etiology of Urinary Tract Infection: Traditional and Emerging Pathogens. Am. J. Med. 2002, 113, 14–19. [Google Scholar] [CrossRef]
- Nielsen, D.W.; Klimavicz, J.S.; Cavender, T.; Wannemuehler, Y.; Barbieri, N.L.; Nolan, L.K.; Logue, C.M. The Impact of Media, Phylogenetic Classification, and E. Coli Pathotypes on Biofilm Formation in Extraintestinal and Commensal E. Coli From Humans and Animals. Front. Microbiol. 2018, 9, 902. [Google Scholar] [CrossRef]
- Mirzahosseini, H.K.; Najmeddin, F.; Najafi, A.; Ahmadi, A.; Sharifnia, H.; Khaledi, A.; Mojtahedzadeh, M. Correlation of Biofilm Formation, Virulence Factors, and Phylogenetic Groups among Escherichia Coli Strains Causing Urinary Tract Infection: A Global Systematic Review and Meta-Analysis. J. Res. Med. Sci. 2023, 28, 66. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-C.; Tseng, C.-C.; Wu, A.-B.; Lin, W.-H.; Teng, C.-H.; Yan, J.-J.; Wu, J.-J. Bacterial Characteristics and Glycemic Control in Diabetic Patients with Escherichia Coli Urinary Tract Infection. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sharma, M.; Chaudhary, U. Biofilm and Multidrug Resistance in Uropathogenic Escherichia Coli. Pathog. Glob. Health 2015, 109, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Egbule, O.S.; Konye, O.P.; Iweriebor, B.C. Assessment of Biofilm Forming Capability and Antibiotic Resistance in Proteus Mirabilis Colonizing Indwelling Catheter. Pak. J. Biol. Sci. PJBS 2024, 27, 268–275. [Google Scholar] [CrossRef]
- Jacobsen, S.M.; Shirtliff, M.E. Proteus Mirabilis Biofilms and Catheter-Associated Urinary Tract Infections. Virulence 2011, 2, 460–465. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.; Li, M.; Liu, Y.; Ma, J.; Wang, X.; Yu, Z.; Cheng, W.; Zhang, W.; Sun, H.; et al. Relationship between Biofilm Formation and Antibiotic Resistance of Klebsiella Pneumoniae and Updates on Antibiofilm Therapeutic Strategies. Front. Cell. Infect. Microbiol. 2024, 14, 1324895. [Google Scholar] [CrossRef]
- Rastegar, E.; Malekzadegan, Y.; Khashei, R.; Hadi, N. Quinolone Resistance and Biofilm Formation Capability of Uropathogenic Escherichia Coli Isolates from an Iranian Inpatients’ Population. Mol. Biol. Rep. 2023, 50, 8073–8079. [Google Scholar] [CrossRef]
- WHO Updates List of Drug-Resistant Bacteria Most Threatening to Human Health. Available online: https://www.who.int/news/item/17-05-2024-who-updates-list-of-drug-resistant-bacteria-most-threatening-to-human-health (accessed on 18 October 2024).
- Giovagnorio, F.; De Vito, A.; Madeddu, G.; Parisi, S.G.; Geremia, N. Resistance in Pseudomonas Aeruginosa: A Narrative Review of Antibiogram Interpretation and Emerging Treatments. Antibiotics 2023, 12, 1621. [Google Scholar] [CrossRef]
- Aniba, R.; Dihmane, A.; Raqraq, H.; Ressmi, A.; Nayme, K.; Timinouni, M.; Barguigua, A. Molecular and Phenotypic Characterization of Biofilm Formation and Antimicrobial Resistance Patterns of Uropathogenic Staphylococcus Haemolyticus Isolates in Casablanca, Morocco. Diagn. Microbiol. Infect. Dis. 2024, 110, 116483. [Google Scholar] [CrossRef]
- Raya, S.; Belbase, A.; Dhakal, L.; Govinda Prajapati, K.; Baidya, R.; Kishor Bimali, N. In-Vitro Biofilm Formation and Antimicrobial Resistance of Escherichia Coli in Diabetic and Nondiabetic Patients. BioMed Res. Int. 2019, 2019, 1474578. [Google Scholar] [CrossRef]
- Colpan, A.; Johnston, B.; Porter, S.; Clabots, C.; Anway, R.; Thao, L.; Kuskowski, M.A.; Tchesnokova, V.; Sokurenko, E.V.; Johnson, J.R.; et al. Escherichia Coli Sequence Type 131 (ST131) Subclone H30 as an Emergent Multidrug-Resistant Pathogen among US Veterans. Clin. Infect. Dis. 2013, 57, 1256–1265. [Google Scholar] [CrossRef]
- Elhosseini, M.A.; El-Banna, T.E.; Sonbol, F.I.; El-Bouseary, M.M. Potential Antivirulence Activity of Sub-Inhibitory Concentrations of Ciprofloxacin against Proteus Mirabilis Isolates: An in-Vitro and in-Vivo Study. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, R.; Abd El-Rahman, O.A.; Mansour, L.E.; Hanora, A.S.; Hashem, A.M.; Ashour, M.S. Antimicrobial Activities against Biofilm Formed by Proteus Mirabilis Isolates from Wound and Urinary Tract Infections. Indian J. Med. Microbiol. 2012, 30, 76–80. [Google Scholar] [CrossRef]
- Whelan, S.; O’Grady, M.C.; Corcoran, G.D.; Finn, K.; Lucey, B. Effect of Sub-Inhibitory Concentrations of Nitrofurantoin, Ciprofloxacin, and Trimethoprim on In Vitro Biofilm Formation in Uropathogenic Escherichia Coli (UPEC). Med. Sci. 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- González, M.J.; Robino, L.; Iribarnegaray, V.; Zunino, P.; Scavone, P. Effect of Different Antibiotics on Biofilm Produced by Uropathogenic Escherichia Coli Isolated from Children with Urinary Tract Infection. Pathog. Dis. 2017, 75, ftx053. [Google Scholar] [CrossRef]
- Rafaque, Z.; Abid, N.; Liaqat, N.; Afridi, P.; Siddique, S.; Masood, S.; Kanwal, S.; Dasti, J.I. In-Vitro Investigation of Antibiotics Efficacy Against Uropathogenic Escherichia Coli Biofilms and Antibiotic Induced Biofilm Formation at Sub-Minimum Inhibitory Concentration of Ciprofloxacin. Infect. Drug Resist. 2020, 13, 2801–2810. [Google Scholar] [CrossRef]
- Slade-Vitković, M.; Batarilo, I.; Bielen, L.; Maravić-Vlahoviček, G.; Bedenić, B. In Vitro Antibiofilm Activity of Fosfomycin Alone and in Combination with Other Antibiotics against Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas Aeruginosa. Pharmaceuticals 2024, 17, 769. [Google Scholar] [CrossRef]
- Saini, H.; Chhibber, S.; Harjai, K. Azithromycin and Ciprofloxacin: A Possible Synergistic Combination against Pseudomonas Aeruginosa Biofilm-Associated Urinary Tract Infections. Int. J. Antimicrob. Agents 2015, 45, 359–367. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhang, H.; Chi, Y.; Cai, Y. The Potentiation Activity of Azithromycin in Combination with Colistin or Levofloxacin Against Pseudomonas Aeruginosa Biofilm Infection. Infect. Drug Resist. 2024, 17, 1259–1266. [Google Scholar] [CrossRef]
- Zeng, B.; Li, Y.; Khan, N.; Su, A.; Yang, Y.; Mi, P.; Jiang, B.; Liang, Y.; Duan, L. Yin-Yang: Two Sides of Extracellular Vesicles in Inflammatory Diseases. J. Nanobiotechnol. 2024, 22, 514. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, A.; Uzair, B.; Sajjad, S.; Samin, G.; Ali Khan, B.; Khan Leghari, S.A.; Khan Niazi, M.B.; Abbas, S. Synthesis and Characterization of Carbon Dots Coated CaCO3 Nanocarrier for Levofloxacin Against Multidrug Resistance Extended-Spectrum Beta-Lactamase Escherichia Coli of Urinary Tract Infection Origin. Microb. Drug Resist. Larchmt. N 2022, 28, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Pekmezovic, M.; Mogavero, S.; Naglik, J.R.; Hube, B. Host–Pathogen Interactions during Female Genital Tract Infections. Trends Microbiol. 2019, 27, 982–996. [Google Scholar] [CrossRef]
- Pan, J.; Singh, A.; Hanning, K.; Hicks, J.; Williamson, A. A Role for the ATP-Dependent DNA Ligase Lig E of Neisseria Gonorrhoeae in Biofilm Formation. BMC Microbiol. 2024, 24, 29. [Google Scholar] [CrossRef]
- Goldstein, E.; Moss, E.; Bennett-Slater, S.; Ferguson, L.; McInally, C.; McHugh, M.; Maxwell, A.; Winter, A.; Gunson, R.N. Impact of Molecular Ciprofloxacin Resistance Testing in Management of Gonorrhoea in a Large Urban Clinic. Sex. Transm. Infect. 2024, 100, 226–230. [Google Scholar] [CrossRef]
- Collins, J.A.; Oviatt, A.A.; Chan, P.F.; Osheroff, N. Target-Mediated Fluoroquinolone Resistance in Neisseria Gonorrhoeae: Actions of Ciprofloxacin against Gyrase and Topoisomerase IV. ACS Infect. Dis. 2024, 10, 1351–1360. [Google Scholar] [CrossRef]
- Kagawa, N.; Aoki, K.; Komori, K.; Ishii, Y.; Shimuta, K.; Ohnishi, M.; Tateda, K. Molecular Epidemiological and Antimicrobial-Resistant Mechanisms Analysis of Prolonged Neisseria Gonorrhoeae Collection between 1971 and 2005 in Japan. JAC-Antimicrob. Resist. 2024, 6, dlae040. [Google Scholar] [CrossRef] [PubMed]
- Yueyue, W.; Feichen, X.; Yixuan, X.; Lu, L.; Yiwen, C.; Xiaoxing, Y. Pathogenicity and Virulence of Mycoplasma Genitalium: Unraveling Ariadne’s Thread. Virulence 2022, 13, 1161–1183. [Google Scholar] [CrossRef] [PubMed]
- Evsyutina, D.V.; Semashko, T.A.; Galyamina, M.A.; Kovalchuk, S.I.; Ziganshin, R.H.; Ladygina, V.G.; Fisunov, G.Y.; Pobeguts, O.V. Molecular Basis of the Slow Growth of Mycoplasma Hominis on Different Energy Sources. Front. Cell. Infect. Microbiol. 2022, 12, 918557. [Google Scholar] [CrossRef] [PubMed]
- Hackett, A.; Yossepowitch, O.; Goor, Y.; Sheffer, R.; Schwartz, O.; Sheftel, Y.; Weiss, Y.; Maor, Y. Prevalence and Risk Factors for Antimicrobial Resistance of Mycoplasma Genitalium Infections in a High-Risk Population. J. Clin. Med. 2024, 13, 4924. [Google Scholar] [CrossRef]
- Ertl, N.G.; Anderson, T.K.; Pardo, C.J.; Maidment, T.I.; Murray, G.L.; Bradshaw, C.S.; Whiley, D.M.; Sweeney, E.L. Concurrent parC and gyrA Fluoroquinolone Resistance Mutations and Associated Strains in Mycoplasma Genitalium in Queensland, Australia. J. Antimicrob. Chemother. 2024, 79, 467–469. [Google Scholar] [CrossRef]
- Rowlands, R.S.; Kragh, K.; Sahu, S.; Maddocks, S.E.; Bolhuis, A.; Spiller, O.B.; Beeton, M.L. A Requirement for Flow to Enable the Development of Ureaplasma Parvum Biofilms in Vitro. J. Appl. Microbiol. 2021, 131, 2579–2585. [Google Scholar] [CrossRef]
- Wu, Y.; Majidzadeh, N.; Li, Y.; Zafar Shakourzadeh, M.; Hajilari, S.; Kouhsari, E.; Azizian, K. Trends of Fluoroquinolones Resistance in Mycoplasma and Ureaplasma Urogenital Isolates: Systematic Review and Meta-Analysis. J. Glob. Antimicrob. Resist. 2024, 36, 13–25. [Google Scholar] [CrossRef]
- Garcia, E.M.; Klimowicz, A.K.; Edupuganti, L.; Topf, M.A.; Bhide, S.R.; Slusser, D.J.; Leib, S.M.; Coddington, C.L.; Matveyev, A.; Buck, G.A.; et al. Phase Variable Colony Variants Are Conserved across Gardnerella Spp. and Exhibit Different Virulence-Associated Phenotypes. mSphere 2024, 9, e0045024. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Schilling, J.; Mendling, W. Response of Gardnerella Vaginalis Biofilm to 5 Days of Moxifloxacin Treatment. FEMS Immunol. Med. Microbiol. 2011, 61, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Bassetti, M.; Concia, E.; De Simone, G.; De Rosa, F.G.; Grossi, P.; Novelli, A.; Menichetti, F.; Petrosillo, N.; Tinelli, M.; et al. Diagnosis and Management of Skin and Soft-Tissue Infections (SSTI). A Literature Review and Consensus Statement: An Update. J. Chemother. Florence Italy 2017, 29, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Viaggi, B.; Cangialosi, A.; Langer, M.; Olivieri, C.; Gori, A.; Corona, A.; Finazzi, S.; Di Paolo, A. Tissue Penetration of Antimicrobials in Intensive Care Unit Patients: A Systematic Review-Part II. Antibiotics 2022, 11, 1193. [Google Scholar] [CrossRef]
- Martin, S.J.; Zeigler, D.G. The Use of Fluoroquinolones in the Treatment of Skin Infections. Expert Opin. Pharmacother. 2004, 5, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Malin, D.; Nicolau, D.P.; Wiskirchen, D.E. Antibiotic Tissue Penetration in Diabetic Foot Infections A Review of the Microdialysis Literature and Needs for Future Research. J. Am. Podiatr. Med. Assoc. 2015, 105, 520–531. [Google Scholar] [CrossRef]
- Wagner, C.; Sauermann, R.; Joukhadar, C. Principles of Antibiotic Penetration into Abscess Fluid. Pharmacology 2006, 78, 1–10. [Google Scholar] [CrossRef]
- Eckmann, C.; Nathwani, D.; Lawson, W.; Corman, S.; Solem, C.; Stephens, J.; Macahilig, C.; Li, J.; Charbonneau, C.; Baillon-Plot, N.; et al. Comparison of Vancomycin and Linezolid in Patients with Peripheral Vascular Disease and/or Diabetes in an Observational European Study of Complicated Skin and Soft-Tissue Infections Due to Methicillin-Resistant Staphylococcus Aureus. Clin. Microbiol. Infect. 2015, 21 (Suppl. 2), S33–S39. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, 147–159. [Google Scholar] [CrossRef]
- Patenall, B.L.; Ridgley, J.D.; Jenkins, A.T.A.; Young, A.E. Evidence of Bacterial Biofilms within Acute Wounds: A Systematic Review. J. Wound Care 2023, 32, 273–278. [Google Scholar] [CrossRef]
- Gomes, F.; Furtado, G.E.; Henriques, M.; Sousa, L.B.; Santos-Costa, P.; Bernardes, R.; Apóstolo, J.; Parreira, P.; Salgueiro-Oliveira, A. The Skin Microbiome of Infected Pressure Ulcers: A Review and Implications for Health Professionals. Eur. J. Clin. Investig. 2022, 52, e13688. [Google Scholar] [CrossRef]
- Buch, P.J.; Chai, Y.; Goluch, E.D. Treating Polymicrobial Infections in Chronic Diabetic Wounds. Clin. Microbiol. Rev. 2019, 32, e00091-18. [Google Scholar] [CrossRef] [PubMed]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Farulla, I.; Prignano, G.; Gallo, M.T.; Vespaziani, M.; Cavallo, I.; Sperduti, I.; Pontone, M.; Bordignon, V.; Cilli, L.; et al. Biofilm Is a Major Virulence Determinant in Bacterial Colonization of Chronic Skin Ulcers Independently from the Multidrug Resistant Phenotype. Int. J. Mol. Sci. 2017, 18, 1077. [Google Scholar] [CrossRef]
- Versey, Z.; da Cruz Nizer, W.S.; Russell, E.; Zigic, S.; DeZeeuw, K.G.; Marek, J.E.; Overhage, J.; Cassol, E. Biofilm-Innate Immune Interface: Contribution to Chronic Wound Formation. Front. Immunol. 2021, 12, 648554. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Rao, P.; Hm, S.; Gs, S.; Rai, R.V. Biofilm-Associated Infections in Chronic Wounds and Their Management. Adv. Exp. Med. Biol. 2023, 1370, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Senneville, É.; Albalawi, Z.; van Asten, S.A.; Abbas, Z.G.; Allison, G.; Aragón-Sánchez, J.; Embil, J.M.; Lavery, L.A.; Alhasan, M.; Oz, O.; et al. IWGDF/IDSA Guidelines on the Diagnosis and Treatment of Diabetes-Related Foot Infections (IWGDF/IDSA 2023). Clin. Infect. Dis. 2024, 79, 286. [Google Scholar] [CrossRef]
- Sartelli, M.; Guirao, X.; Hardcastle, T.C.; Kluger, Y.; Boermeester, M.A.; Raşa, K.; Ansaloni, L.; Coccolini, F.; Montravers, P.; Abu-Zidan, F.M.; et al. 2018 WSES/SIS-E Consensus Conference: Recommendations for the Management of Skin and Soft-Tissue Infections. World J. Emerg. Surg. WJES 2018, 13, 58. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Al-Askar, A.A.; AbdElgawad, H.; Hagras, F.A.; Ramadan, A.A.; Kamel, M.R.; Ahmed, M.A.; Atia, K.H.; Hashem, A.H. Wound Dressing Scaffold with High Anti-Biofilm Performance Based on Ciprofloxacin-Loaded Chitosan-Hydrolyzed Starch Nanocomposite: In Vitro and In Vivo Study. Appl. Biochem. Biotechnol. 2023, 195, 6421–6439. [Google Scholar] [CrossRef]
- Giordano, P.; Song, J.; Pertel, P.; Herrington, J.; Kowalsky, S. Sequential Intravenous/Oral Moxifloxacin versus Intravenous Piperacillin-Tazobactam Followed by Oral Amoxicillin-Clavulanate for the Treatment of Complicated Skin and Skin Structure Infection. Int. J. Antimicrob. Agents 2005, 26, 357–365. [Google Scholar] [CrossRef]
- Vick-Fragoso, R.; Hernández-Oliva, G.; Cruz-Alcázar, J.; Amábile-Cuevas, C.F.; Arvis, P.; Reimnitz, P.; Bogner, J.R.; STIC Study Group. Efficacy and Safety of Sequential Intravenous/Oral Moxifloxacin vs Intravenous/Oral Amoxicillin/Clavulanate for Complicated Skin and Skin Structure Infections. Infection 2009, 37, 407–417. [Google Scholar] [CrossRef]
- Gyssens, I.C.; Dryden, M.; Kujath, P.; Nathwani, D.; Schaper, N.; Hampel, B.; Reimnitz, P.; Alder, J.; Arvis, P. A Randomized Trial of the Efficacy and Safety of Sequential Intravenous/Oral Moxifloxacin Monotherapy versus Intravenous Piperacillin/Tazobactam Followed by Oral Amoxicillin/Clavulanate for Complicated Skin and Skin Structure Infections. J. Antimicrob. Chemother. 2011, 66, 2632–2642. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Giordano, P.; Choudhri, S.; Song, J. Treating Diabetic Foot Infections with Sequential Intravenous to Oral Moxifloxacin Compared with Piperacillin-Tazobactam/Amoxicillin-Clavulanate. J. Antimicrob. Chemother. 2007, 60, 370–376. [Google Scholar] [CrossRef]
- Ribeiro, Á.C.d.S.; Santos, F.F.; Valiatti, T.B.; Lenzi, M.H.; Santos, I.N.M.; Neves, R.F.B.; Moses, I.B.; de Meneses, J.P.; Di Sessa, R.G.D.G.; Salles, M.J.; et al. Comparative in Vitro Activity of Delafloxacin and Other Antimicrobials against Isolates from Patients with Acute Bacterial Skin, Skin-Structure Infection and Osteomyelitis. Braz. J. Infect. Dis. 2024, 28, 103867. [Google Scholar] [CrossRef] [PubMed]
- Jandl, B.; Dighe, S.; Gasche, C.; Makristathis, A.; Muttenthaler, M. Intestinal Biofilms: Pathophysiological Relevance, Host Defense, and Therapeutic Opportunities. Clin. Microbiol. Rev. 2024, 37, e0013323. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Furrie, E.; Cummings, J.H.; Macfarlane, G.T. Chemotaxonomic Analysis of Bacterial Populations Colonizing the Rectal Mucosa in Patients with Ulcerative Colitis. Clin. Infect. Dis. 2004, 38, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Tomkovich, S.; Dejea, C.M.; Winglee, K.; Drewes, J.L.; Chung, L.; Housseau, F.; Pope, J.L.; Gauthier, J.; Sun, X.; Mühlbauer, M.; et al. Human Colon Mucosal Biofilms from Healthy or Colon Cancer Hosts Are Carcinogenic. J. Clin. Investig. 2019, 129, 1699–1712. [Google Scholar] [CrossRef]
- Fidelle, M.; Yonekura, S.; Picard, M.; Cogdill, A.; Hollebecque, A.; Roberti, M.P.; Zitvogel, L. Resolving the Paradox of Colon Cancer Through the Integration of Genetics, Immunology, and the Microbiota. Front. Immunol. 2020, 11, 600886. [Google Scholar] [CrossRef]
- Prudent, V.; Demarre, G.; Vazeille, E.; Wery, M.; Quenech’Du, N.; Ravet, A.; Dauverd-Girault, J.; van Dijk, E.; Bringer, M.-A.; Descrimes, M.; et al. The Crohn’s Disease-Related Bacterial Strain LF82 Assembles Biofilm-like Communities to Protect Itself from Phagolysosomal Attack. Commun. Biol. 2021, 4, 627. [Google Scholar] [CrossRef]
- Ma, L.; Feng, J.; Zhang, J.; Lu, X. Campylobacter Biofilms. Microbiol. Res. 2022, 264, 127149. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrowicz, A.; Carolak, E.; Dutkiewicz, A.; Błachut, A.; Waszczuk, W.; Grzymajlo, K. Better Together-Salmonella Biofilm-Associated Antibiotic Resistance. Gut Microbes 2023, 15, 2229937. [Google Scholar] [CrossRef]
- Rubio-Mendoza, D.; Martínez-Meléndez, A.; Maldonado-Garza, H.J.; Córdova-Fletes, C.; Garza-González, E. Review of the Impact of Biofilm Formation on Recurrent Clostridioides Difficile Infection. Microorganisms 2023, 11, 2525. [Google Scholar] [CrossRef] [PubMed]
- Harrell, J.E.; Hahn, M.M.; D’Souza, S.J.; Vasicek, E.M.; Sandala, J.L.; Gunn, J.S.; McLachlan, J.B. Salmonella Biofilm Formation, Chronic Infection, and Immunity Within the Intestine and Hepatobiliary Tract. Front. Cell. Infect. Microbiol. 2020, 10, 624622. [Google Scholar] [CrossRef] [PubMed]
- de Lastours, V.; Fantin, B. Impact of Fluoroquinolones on Human Microbiota. Focus on the Emergence of Antibiotic Resistance. Future Microbiol. 2015, 10, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of Antibiotic Penetration Limitation in Klebsiella Pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef]
- Aditya, V.; Kotian, A.; Saikrishnan, S.; Rohit, A.; Mithoor, D.; Karunasagar, I.; Deekshit, V.K. Effect of Ciprofloxacin and in Vitro Gut Conditions on Biofilm of Escherichia Coli Isolated from Clinical and Environmental Sources. J. Appl. Microbiol. 2022, 132, 964–977. [Google Scholar] [CrossRef]
- Gastaldi Guerrieri, C.; Teixeira Gonçalves, M.; Ferreira da Silva, A.; Souza Dos Santos, A.L.; Dos Santos, K.V.; Cruz Spano, L. Remarkable Antibiofilm Activity of Ciprofloxacin, Cefoxitin, and Tobramycin, by Themselves or in Combination, against Enteroaggregative Escherichia Coli in Vitro. Diagn. Microbiol. Infect. Dis. 2023, 107, 116048. [Google Scholar] [CrossRef]
- Eberhardt, N.; Santamarina, B.G.; Enghardt, M.-L.; Rohland, O.; Hussain, I.; Tannert, A.; Thieme, L.; Rubio, I.; Rödel, J.; Löffler, B.; et al. The Effects of Photoactivated Ciprofloxacin and Bile Acids on Biofilms on Bile Duct Catheters. Int. J. Antimicrob. Agents 2024, 63, 107086. [Google Scholar] [CrossRef]




| Infection Area | Risk Factors | Pathogens | Biofilm Formation |
|---|---|---|---|
| CNS | Shunts/drains, Intrathecal infusion pumps Deep brain stimulation hardware | S. aureus, CoNS, Cutibacterium acnes, Enterobacterales spp., A. baumannii | High risk due to external/internal devices |
| Eye | Contact lenses Surgery Trauma Ocular devices | Staphylococcus spp., Corynebacterium spp., Bacillus spp., Pseudomonas spp. | Common in contact lenses and storage cases |
| Ear | Viral respiratory infections Eustachian tube dysfunction | S. pneumoniae, Haemophilus influenzae, Moraxella catharralis, S. aureus, P. aeruginosa | Biofilm formation in chronic/recurrent otitis media and “swimmer” ear |
| Infection | Microbiology | Management | Role of Fluoroquinolones |
|---|---|---|---|
| VGI | Mostly Gram-positive (S. aureus, CoNs, enterococci). Increasing MDR Gram-negatives, P. aeruginosa, and candida. | Excision, debridement, and antibiotics. Chronic suppression with antibiotics. | Effective in biofilm reduction. Useful in chronic treatment and certain infections. |
| IE | S. aureus, streptococci, and enterococci. | Medical and surgical therapy. | Used for oral step-down therapy in specific cases. Limited studies on efficacy. Some suggest quick biofilm penetration for treatment. |
| Infections | Microorganisms Implicated in Biofilm Formation | Role of Biofilm | Role of Fluoroquinolones |
|---|---|---|---|
| UTIs | E. coli, Klebsiella spp., P. aeruginosa, Staphylococcus spp. | Biofilm formation is common in 65–80% of cases. Contributes to recurrent and complicated urinary tract infections. | Effective against most uropathogens. Risk of enhanced biofilm formation at sub-inhibitory concentrations. |
| Genital Tract Infections | N. gonorrhoeae, Mycoplasma hominis, Mycoplasma genitalium, Gardnerella vaginalis | Biofilm protects pathogens from immune system and antibiotics. Facilitates resistance gene transfer. | Useful for N. gonorrhoeae treatment, but resistance is emerging. Limited evidence for Mycoplasma spp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geremia, N.; Giovagnorio, F.; Colpani, A.; De Vito, A.; Botan, A.; Stroffolini, G.; Toc, D.-A.; Zerbato, V.; Principe, L.; Madeddu, G.; et al. Fluoroquinolones and Biofilm: A Narrative Review. Pharmaceuticals 2024, 17, 1673. https://doi.org/10.3390/ph17121673
Geremia N, Giovagnorio F, Colpani A, De Vito A, Botan A, Stroffolini G, Toc D-A, Zerbato V, Principe L, Madeddu G, et al. Fluoroquinolones and Biofilm: A Narrative Review. Pharmaceuticals. 2024; 17(12):1673. https://doi.org/10.3390/ph17121673
Chicago/Turabian StyleGeremia, Nicholas, Federico Giovagnorio, Agnese Colpani, Andrea De Vito, Alexandru Botan, Giacomo Stroffolini, Dan-Alexandru Toc, Verena Zerbato, Luigi Principe, Giordano Madeddu, and et al. 2024. "Fluoroquinolones and Biofilm: A Narrative Review" Pharmaceuticals 17, no. 12: 1673. https://doi.org/10.3390/ph17121673
APA StyleGeremia, N., Giovagnorio, F., Colpani, A., De Vito, A., Botan, A., Stroffolini, G., Toc, D.-A., Zerbato, V., Principe, L., Madeddu, G., Luzzati, R., Parisi, S. G., & Di Bella, S. (2024). Fluoroquinolones and Biofilm: A Narrative Review. Pharmaceuticals, 17(12), 1673. https://doi.org/10.3390/ph17121673











