Aerosol Inhalation of Luteolin-7-O-Glucuronide Exerts Anti-Inflammatory Effects by Inhibiting NLRP3 Inflammasome Activation
Abstract
1. Introduction
2. Results
2.1. Determination of the Real-Time Particle Size Distribution
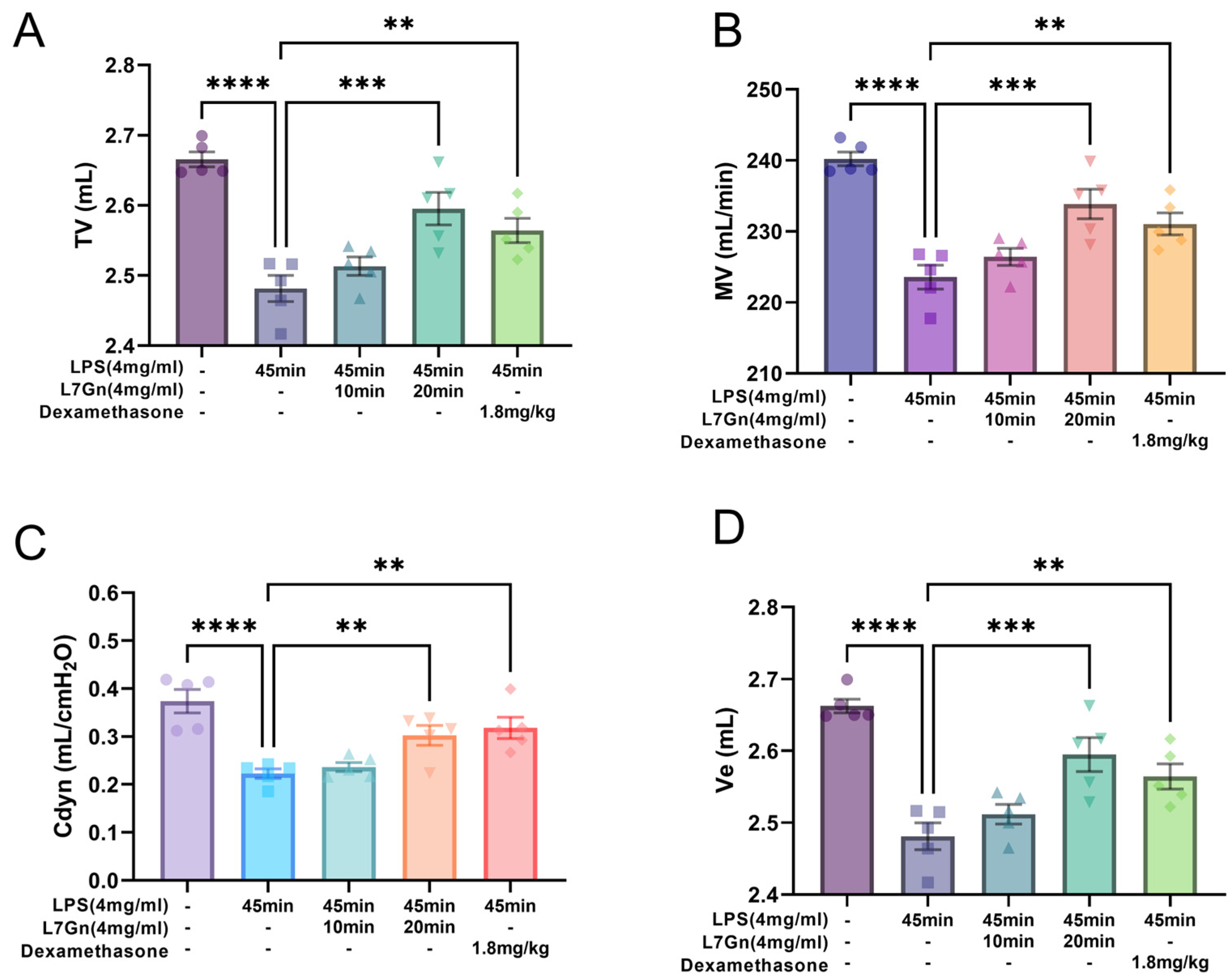
2.2. L7Gn Protects Against Pulmonary Function Damage
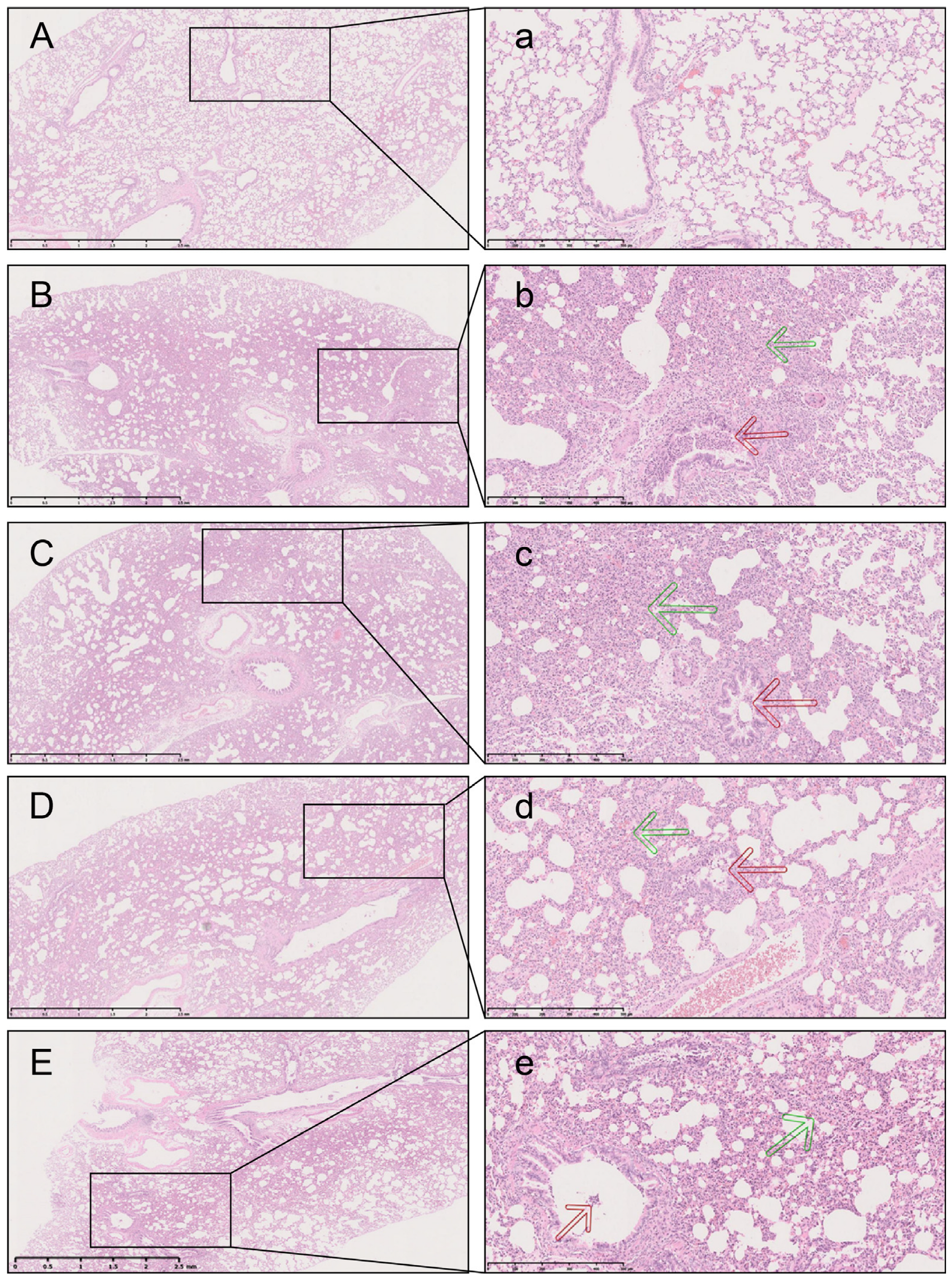
2.3. L7Gn Inhibits LPS-Induced Pulmonary Injury
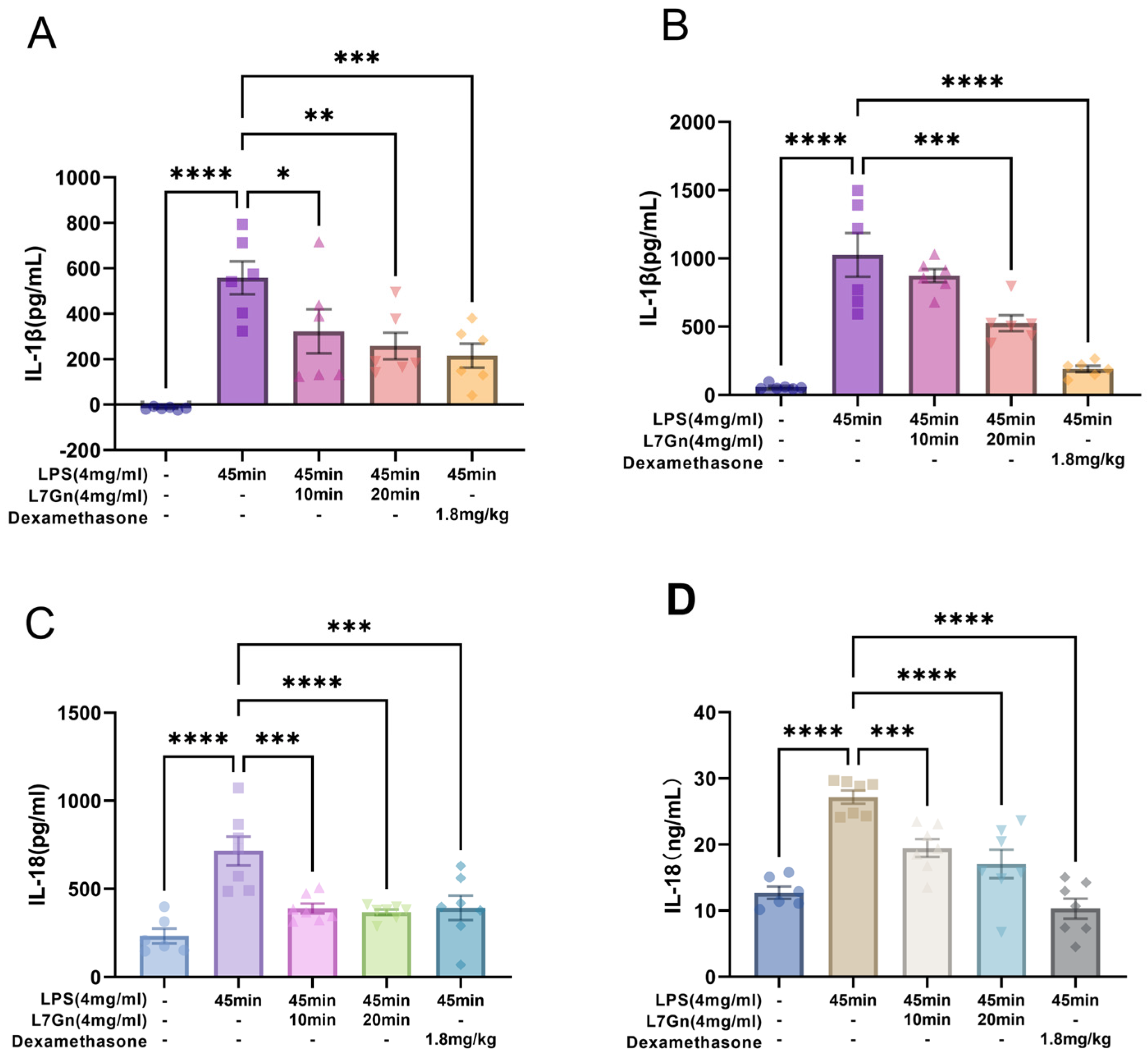
2.4. L7Gn Inhibits NLRP3 Inflammasome Activation in LPS-Induced ALI Rats
2.5. L7Gn Inhibits NLRP3 Inflammasome Activation in MH-S Cells Induced by LPS
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
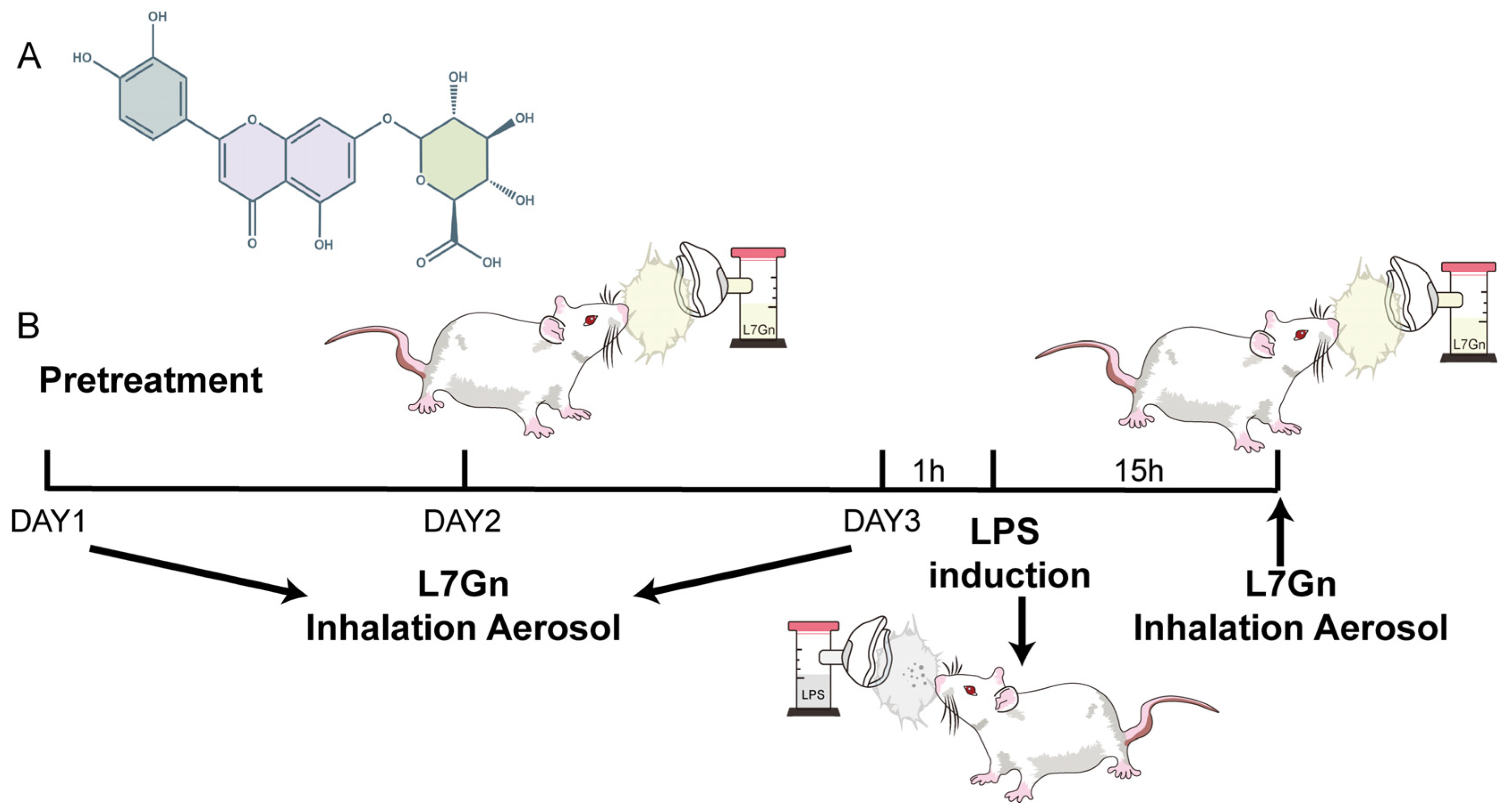
4.2. Dose and Method of Administration
4.3. Determination of Real-Time Particle Size for Aerosol Inhalation
4.4. Animal Experimental Design
4.5. Determination of the Lung Function
4.6. BALF Collection
4.7. ELISA of BALF and Lung Homogenates
4.8. Histological Analysis and Immunofluorescence
4.9. Inflammasome Activation Assays in MH-S Cells
4.10. RNA Isolation and Quantitative RT-PCR
4.11. Western Blotting
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome. JAMA 2018, 319, 698. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute Respiratory Distress Syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.; Liu, Y.; Rundberg Nilsson, A.; Gatchalian, R.; Crother, T.R.; Tourtellotte, W.G.; Zhang, Y.; Aleman-Muench, G.R.; Lewis, G.; Chen, W.; et al. Metformin Inhibition of Mitochondrial ATP and DNA Synthesis Abrogates NLRP3 Inflammasome Activation and Pulmonary Inflammation. Immunity 2021, 54, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bai, C.; Wang, X. The Value of the Lipopolysaccharide-Induced Acute Lung Injury Model in Respiratory Medicine. Expert Rev. Respir. Med. 2010, 4, 773–783. [Google Scholar] [CrossRef]
- de Souza Xavier Costa, N.; Ribeiro Júnior, G.; dos Santos Alemany, A.A.; Belotti, L.; Zati, D.H.; Frota Cavalcante, M.; Matera Veras, M.; Ribeiro, S.; Kallás, E.G.; Nascimento Saldiva, P.H.; et al. Early and Late Pulmonary Effects of Nebulized LPS in Mice: An Acute Lung Injury Model. PLoS ONE 2017, 12, e0185474. [Google Scholar] [CrossRef] [PubMed]
- Poroyko, V.; Meng, F.; Meliton, A.; Afonyushkin, T.; Ulanov, A.; Semenyuk, E.; Latif, O.; Tesic, V.; Birukova, A.A.; Birukov, K.G. Alterations of Lung Microbiota in a Mouse Model of LPS-Induced Lung Injury. Am. J. Physiol.-Lung Cell Mol. Physiol. 2015, 309, L76–L83. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, X.; Wang, W.; Yang, Q.; Dong, Y.; Xu, N.; Zhang, C.; Song, X.; Ren, Z.; Zhao, F.; et al. Characteristic Anti-Inflammatory and Antioxidative Effects of Enzymatic- and Acidic- Hydrolysed Mycelium Polysaccharides by Oudemansiella Radicata on LPS-Induced Lung Injury. Carbohydr. Polym. 2019, 204, 142–151. [Google Scholar] [CrossRef]
- Johnson, D.B.; Lopez, M.J.; Kelley, B. Dexamethasone. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Levy, M.; Thaiss, C.A.; Elinav, E. Taming the Inflammasome. Nat. Med. 2015, 21, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Place, D.E.; Kanneganti, T.-D. Toward Targeting Inflammasomes: Insights into Their Regulation and Activation. Cell Res. 2020, 30, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; de Alba, E. Structure, Activation and Regulation of NLRP3 and AIM2 Inflammasomes. IJMS 2021, 22, 872. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and Regulation of the Inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Lupfer, C.R.; Kanneganti, T.-D. The Role of Inflammasome Modulation in Virulence. Virulence 2012, 3, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-J.; Jo, E.-K. NLRP3 Inflammasome and Host Protection against Bacterial Infection. J. Korean Med. Sci. 2013, 28, 1415. [Google Scholar] [CrossRef]
- Grailer, J.J.; Canning, B.A.; Kalbitz, M.; Haggadone, M.D.; Dhond, R.M.; Andjelkovic, A.V.; Zetoune, F.S.; Ward, P.A. Critical Role for the NLRP3 Inflammasome during Acute Lung Injury. J. Immunol. 2014, 192, 5974–5983. [Google Scholar] [CrossRef]
- de Zoete, M.R.; Palm, N.W.; Zhu, S.; Flavell, R.A. Inflammasomes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016287. [Google Scholar] [CrossRef]
- He, X.; Mekasha, S.; Mavrogiorgos, N.; Fitzgerald, K.A.; Lien, E.; Ingalls, R.R. Inflammation and Fibrosis during Chlamydia pneumoniae Infection Is Regulated by IL-1 and the NLRP3/ASC Inflammasome. J. Immunol. 2010, 184, 5743–5754. [Google Scholar] [CrossRef]
- Fang, R.; Tsuchiya, K.; Kawamura, I.; Shen, Y.; Hara, H.; Sakai, S.; Yamamoto, T.; Fernandes-Alnemri, T.; Yang, R.; Hernandez-Cuellar, E.; et al. Critical Roles of ASC Inflammasomes in Caspase-1 Activation and Host Innate Resistance to Streptococcus pneumoniae Infection. J. Immunol. 2011, 187, 4890–4899. [Google Scholar] [CrossRef] [PubMed]
- McNeela, E.A.; Burke, Á.; Neill, D.R.; Baxter, C.; Fernandes, V.E.; Ferreira, D.; Smeaton, S.; El-Rachkidy, R.; McLoughlin, R.M.; Mori, A.; et al. Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4. PLoS Pathog. 2010, 6, e1001191. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C.; Scull, M.A.; Moore, C.B.; Holl, E.K.; McElvania-TeKippe, E.; Taxman, D.J.; Guthrie, E.H.; Pickles, R.J.; Ting, J.P.-Y. The NLRP3 Inflammasome Mediates In Vivo Innate Immunity to Influenza A Virus through Recognition of Viral RNA. Immunity 2009, 30, 556–565. [Google Scholar] [CrossRef]
- Momota, M.; Lelliott, P.; Kubo, A.; Kusakabe, T.; Kobiyama, K.; Kuroda, E.; Imai, Y.; Akira, S.; Coban, C.; Ishii, K.J. ZBP1 Governs the Inflammasome-Independent IL-1α and Neutrophil Inflammation That Play a Dual Role in Anti-Influenza Virus Immunity. Int. Immunol. 2020, 32, 203–212. [Google Scholar] [CrossRef]
- Miller, L.S.; Pietras, E.M.; Uricchio, L.H.; Hirano, K.; Rao, S.; Lin, H.; O’Connell, R.M.; Iwakura, Y.; Cheung, A.L.; Cheng, G.; et al. Inflammasome-Mediated Production of IL-1β Is Required for Neutrophil Recruitment against Staphylococcus aureus In Vivo. J. Immunol. 2007, 179, 6933–6942. [Google Scholar] [CrossRef]
- Mishra, B.B.; Moura-Alves, P.; Sonawane, A.; Hacohen, N.; Griffiths, G.; Moita, L.F.; Anes, E. Mycobacterium Tuberculosis Protein ESAT-6 Is a Potent Activator of the NLRP3/ASC Inflammasome. Cell Microbiol. 2010, 12, 1046–1063. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Knoten, C.A.; Morris, A.R.; Hauser, A.R. ASC Acts in a Caspase-1-Independent Manner to Worsen Acute Pneumonia Caused by Pseudomonas Aeruginosa. J. Med. Microbiol. 2018, 67, 1168–1180. [Google Scholar] [CrossRef]
- Cohen, T.S.; Prince, A.S. Activation of Inflammasome Signaling Mediates Pathology of Acute P. Aeruginosa Pneumonia. J. Clin. Investig. 2013, 123, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.D.; Crother, T.R.; Gonazalez, R.; Jupelli, M.; Chen, S.; Dagvadorj, J.; Arditi, M.; Shimada, K. The NLRP3 Inflammasome Is Required for the Development of Hypoxemia in LPS/Mechanical Ventilation Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2014, 50, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Rahte, S.; Evans, R.; Eugster, P.; Marcourt, L.; Wolfender, J.-L.; Kortenkamp, A.; Tasdemir, D. Salvia Officinalis for Hot Flushes: Towards Determination of Mechanism of Activity and Active Principles. Planta. Med. 2013, 79, 753–760. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, M.; Li, C.; Huang, W.; Gu, K.; Yu, H.; Yuan, Y.; Wang, Y.; Yang, B.; et al. Stepwise Rapid Tracking Strategy to Identify Active Molecules from Ixeris Sonchifolia Hance Based on “‘Affinity Mass Spectrometry-Atomic Force Microscopy Imaging’” Technology. Talanta 2020, 217, 121031. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, Y.; Duan, S.; Sun, D. The Antialgal Mechanism of Luteolin-7-O-Glucuronide on Phaeocystis Globosa by Metabolomics Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3222. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, H.; Chen, Q.; Zhao, M.; Sun, D.; Duan, S. Growth Inhibition of Phaeocystis Globosa Induced by Luteolin-7-O-Glucuronide from Seagrass Enhalus Acoroides. Int. J. Environ. Res. Public Health 2019, 16, 2615. [Google Scholar] [CrossRef]
- Ferlemi, A.-V.; Katsikoudi, A.; Kontogianni, V.G.; Kellici, T.F.; Iatrou, G.; Lamari, F.N.; Tzakos, A.G.; Margarity, M. Rosemary Tea Consumption Results to Anxiolytic-and Anti-Depressant-like Behavior of Adult Male Mice and Inhibits All Cerebral Area and Liver Cholinesterase Activity; Phytochemical Investigation and in Silico Studies. Chem.-Biol. Interact. 2015, 237, 47–57. [Google Scholar] [CrossRef]
- Guvenalp, Z.; Ozbek, H.; Karadayi, M.; Gulluce, M.; Kuruuzum-Uz, A.; Salih, B.; Demirezer, O. Two Antigenotoxic Chalcone Glycosides from Mentha longifolia Subsp. longifolia. Pharm. Biol. 2015, 53, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cha, K.H.; Kim, C.Y.; Nho, C.W.; Pan, C.-H. Bioavailability of Hydroxycinnamic Acids from Crepidiastrum Denticulatum Using Simulated Digestion and Caco-2 Intestinal Cells. J. Agric. Food Chem. 2014, 62, 5290–5295. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-C.; Park, J.; Cho, S. Anti-Inflammatory and Anti-Oxidative Effects of Luteolin-7-O-Glucuronide in LPS-Stimulated Murine Macrophages through TAK1 Inhibition and Nrf2 Activation. Int. J. Mol. Sci. 2020, 21, 2007. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Jiang, J.-G.; Zhang, X.-M.; Zhu, W. Identification of Luteolin 7-O-β-D-Glucuronide from Cirsium Japonicum and Its Anti-Inflammatory Mechanism. J. Funct. Foods 2018, 46, 521–528. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, M.; Qian, L.; Wen, D.; Wu, G. Luteolin Attenuates High Glucose-Induced Podocyte Injury via Suppressing NLRP3 Inflammasome Pathway. Life Sci. 2019, 225, 1–7. [Google Scholar] [CrossRef]
- Zou, Y.; Luo, X.; Feng, Y.; Fang, S.; Tian, J.; Yu, B.; Li, J. Luteolin Prevents THP-1 Macrophage Pyroptosis by Suppressing ROS Production via Nrf2 Activation. Chem.-Biol. Interact. 2021, 345, 109573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-C.; Li, Z.; Xu, W.; Xiang, C.-H.; Ma, Y.-F. Luteolin Alleviates NLRP3 Inflammasome Activation and Directs Macrophage Polarization in Lipopolysaccharide-Stimulated RAW264.7 Cells. Am. J. Transl. Res. 2018, 10, 265–273. [Google Scholar]
- Lee, M.N.; Lee, Y.; Wu, D.; Pae, M. Luteolin Inhibits NLRP3 Inflammasome Activation via Blocking ASC Oligomerization. J. Nutr. Biochem. 2021, 92, 108614. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.G.; Ryckman, D.M. Assessment and Development of the Antifungal Agent Caspofungin for Aerosolized Pulmonary Delivery. Pharmaceutics 2021, 13, 504. [Google Scholar] [CrossRef]
- Jen, H.-I.; Lin, Z.-Y.; Guo, J.-X.; Lee, C.-I. The Effects of Divalent Cation-Chelated Prion Fibrils on the Immune Response of EOC 13.31 Microglia Cells. Cells 2020, 9, 2285. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Jo, S.-G.; Lee, H.-N.; Choi, J.-H.; Lee, Y.-J.; Kim, Y.-M.; Cho, J.-Y.; Lee, S.K.; Park, J.-H. Tendril Extract of Cucurbita Moschata Suppresses NLRP3 Inflammasome Activation in Murine Macrophages and Human Trophoblast Cells. Int. J. Med. Sci. 2020, 17, 1006–1014. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Peng, D.; Liu, X.; Wu, C.; Guo, P.; Wei, J. Agarwood Essential Oil Displays Sedative-Hypnotic Effects through the GABAergic System. Molecules 2017, 22, 2190. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, E.; Hamblin, N.; Edwards, C.; Baker-Glenn, C.; Hurley, C. Developing Inhaled Drugs for Respiratory Diseases: A Medicinal Chemistry Perspective. Drug Discov. Today 2022, 27, 134–150. [Google Scholar] [CrossRef]
- Mitani, A.; Ito, K.; Vuppusetty, C.; Barnes, P.J.; Mercado, N. Restoration of Corticosteroid Sensitivity in Chronic Obstructive Pulmonary Disease by Inhibition of Mammalian Target of Rapamycin. Am. J. Respir. Crit. Care Med. 2016, 193, 143–153. [Google Scholar] [CrossRef]
- Thomas, R.J. Particle Size and Pathogenicity in the Respiratory Tract. Virulence 2013, 4, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Jeon, E.; Kim, J.-C.; Kang, S.G.; Yoon, S.; Ko, H.-J.; Kim, P.-H.; Lee, G.-S. Lentinan from Shiitake Selectively Attenuates AIM2 and Non-Canonical Inflammasome Activation While Inducing pro-Inflammatory Cytokine Production. Sci. Rep. 2017, 7, 1314. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J.; Collins, C.J.; Coombs, D.W.; Gilkison, I.S.; Hardy, C.J.; Healey, G.; Karantabias, G.; Johnson, N.; Karlsson, A.; Kilgour, J.D.; et al. Association of Inhalation Toxicologists (AIT) Working Party Recommendation for Standard Delivered Dose Calculation and Expression in Non-Clinical Aerosol Inhalation Toxicology Studies with Pharmaceuticals. Inhal. Toxicol. 2008, 20, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-C.; Zheng, X.-J.; Du, L.-J.; Sun, J.-Y.; Shen, Z.-X.; Shi, C.; Sun, S.; Zhang, Z.; Chen, X.; Qin, M.; et al. High Salt Primes a Specific Activation State of Macrophages, M(Na). Cell Res. 2015, 25, 893–910. [Google Scholar] [CrossRef]
- van den Brand, J.M.A.; Stittelaar, K.J.; van Amerongen, G.; Rimmelzwaan, G.F.; Simon, J.; de Wit, E.; Munster, V.; Bestebroer, T.; Fouchier, R.A.M.; Kuiken, T.; et al. Severity of Pneumonia Due to New H1N1 Influenza Virus in Ferrets Is Intermediate between That Due to Seasonal H1N1 Virus and Highly Pathogenic Avian Influenza H5N1 Virus. J. Infect. Dis. 2010, 201, 993–999. [Google Scholar] [CrossRef] [PubMed][Green Version]
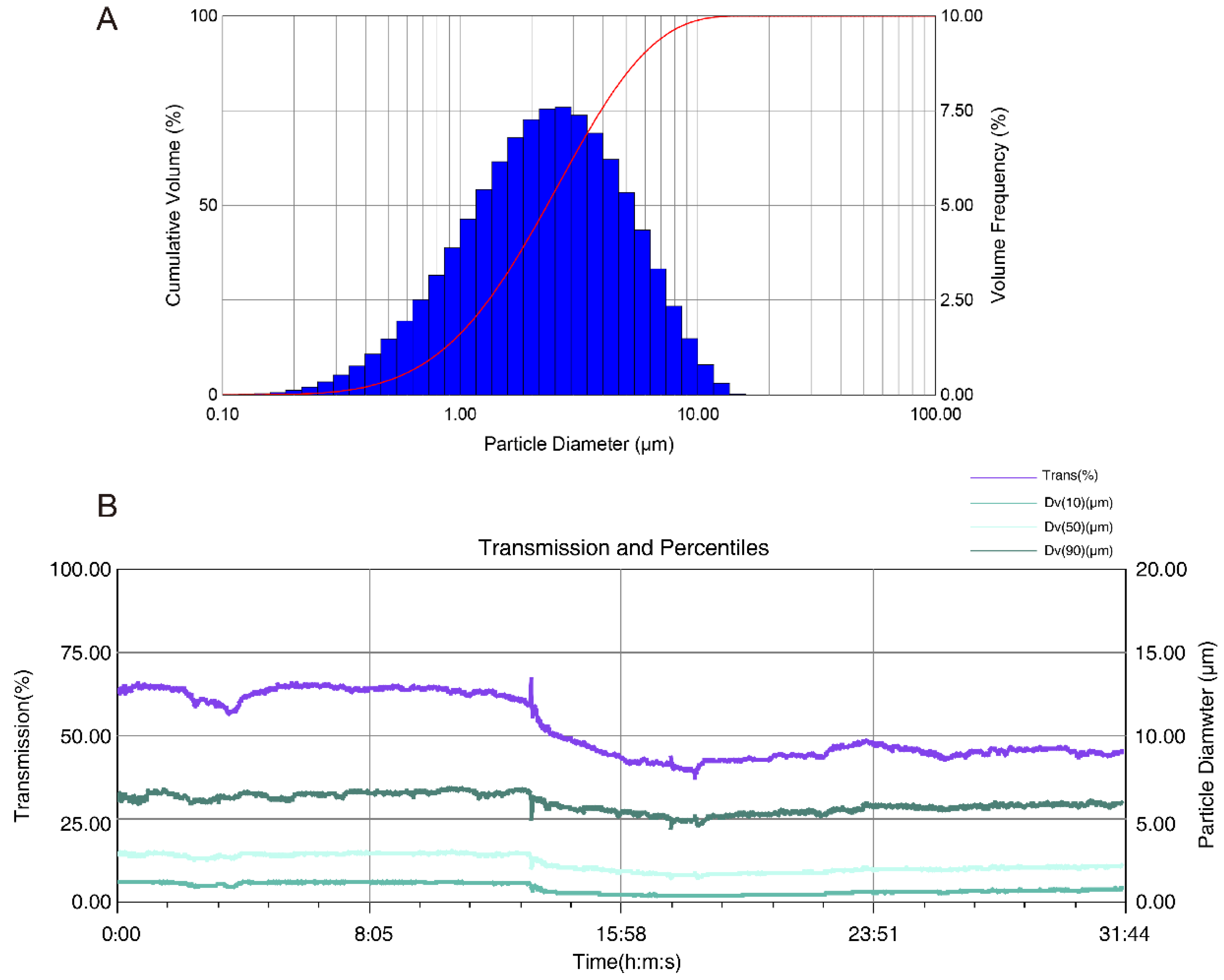


| Title | Average | б | Min | Max |
|---|---|---|---|---|
| Trans (%) | 51.70 | 9.06 | 37.50 | 66.80 |
| Dv(10) (μm) | 0.77 | 0.31 | 0.36 | 1.27 |
| Dv(50) (μm) | 2.32 | 0.48 | 1.48 | 3.04 |
| Dv(90) (μm) | 5.91 | 0.52 | 4.47 | 6.88 |
| Group | Bronchiolar Inflammation Grade | p-Value | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Control | 10 | 0 | 0 | 0 | 0 | |
| LPS | 0 | 2 | 7 | 1 | 0 | 0.0001 ## |
| LPS + L7Gn | 0 | 8 | 2 | 0 | 0 | 0.044 * |
| LPS + L7Gn | 0 | 9 | 1 | 0 | 0 | 0.020 * |
| LPS + Dex | 1 | 6 | 3 | 0 | 0 | 0.046 * |
| Gene | Primer Forward (5′ → 3′) | Primer Reverse (5′ → 3′) |
|---|---|---|
| IL-1β | TGTTCTTACAGGAGAGGGTAGAC | GTTCATCTCGGAGCCTGTAGTG |
| IL-18 | GACAGCCTGTGTTCGAGGATATG | TGTTCTTACAGGAGAGGGTAGAC |
| GADPH | GTTCATCTCGGAGCCTGTAGTG | ATGCCAGTGAGCTTCCCGTTCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Song, L.; Li, H.; Gao, Y.; Chen, T.; Zhang, Z.; Hon, H.; Ye, Z.; Zhang, G. Aerosol Inhalation of Luteolin-7-O-Glucuronide Exerts Anti-Inflammatory Effects by Inhibiting NLRP3 Inflammasome Activation. Pharmaceuticals 2024, 17, 1731. https://doi.org/10.3390/ph17121731
Li J, Song L, Li H, Gao Y, Chen T, Zhang Z, Hon H, Ye Z, Zhang G. Aerosol Inhalation of Luteolin-7-O-Glucuronide Exerts Anti-Inflammatory Effects by Inhibiting NLRP3 Inflammasome Activation. Pharmaceuticals. 2024; 17(12):1731. https://doi.org/10.3390/ph17121731
Chicago/Turabian StyleLi, Jianliang, Ling Song, Han Li, Yunhang Gao, Tengfei Chen, Zhongxiu Zhang, Hongping Hon, Zuguang Ye, and Guangping Zhang. 2024. "Aerosol Inhalation of Luteolin-7-O-Glucuronide Exerts Anti-Inflammatory Effects by Inhibiting NLRP3 Inflammasome Activation" Pharmaceuticals 17, no. 12: 1731. https://doi.org/10.3390/ph17121731
APA StyleLi, J., Song, L., Li, H., Gao, Y., Chen, T., Zhang, Z., Hon, H., Ye, Z., & Zhang, G. (2024). Aerosol Inhalation of Luteolin-7-O-Glucuronide Exerts Anti-Inflammatory Effects by Inhibiting NLRP3 Inflammasome Activation. Pharmaceuticals, 17(12), 1731. https://doi.org/10.3390/ph17121731





