A New Intervention for Implementation of Pharmacogenetics in Psychiatry: A Description of the PSY-PGx Clinical Study
Abstract
:1. Introduction
Objectives
2. Experimental Design
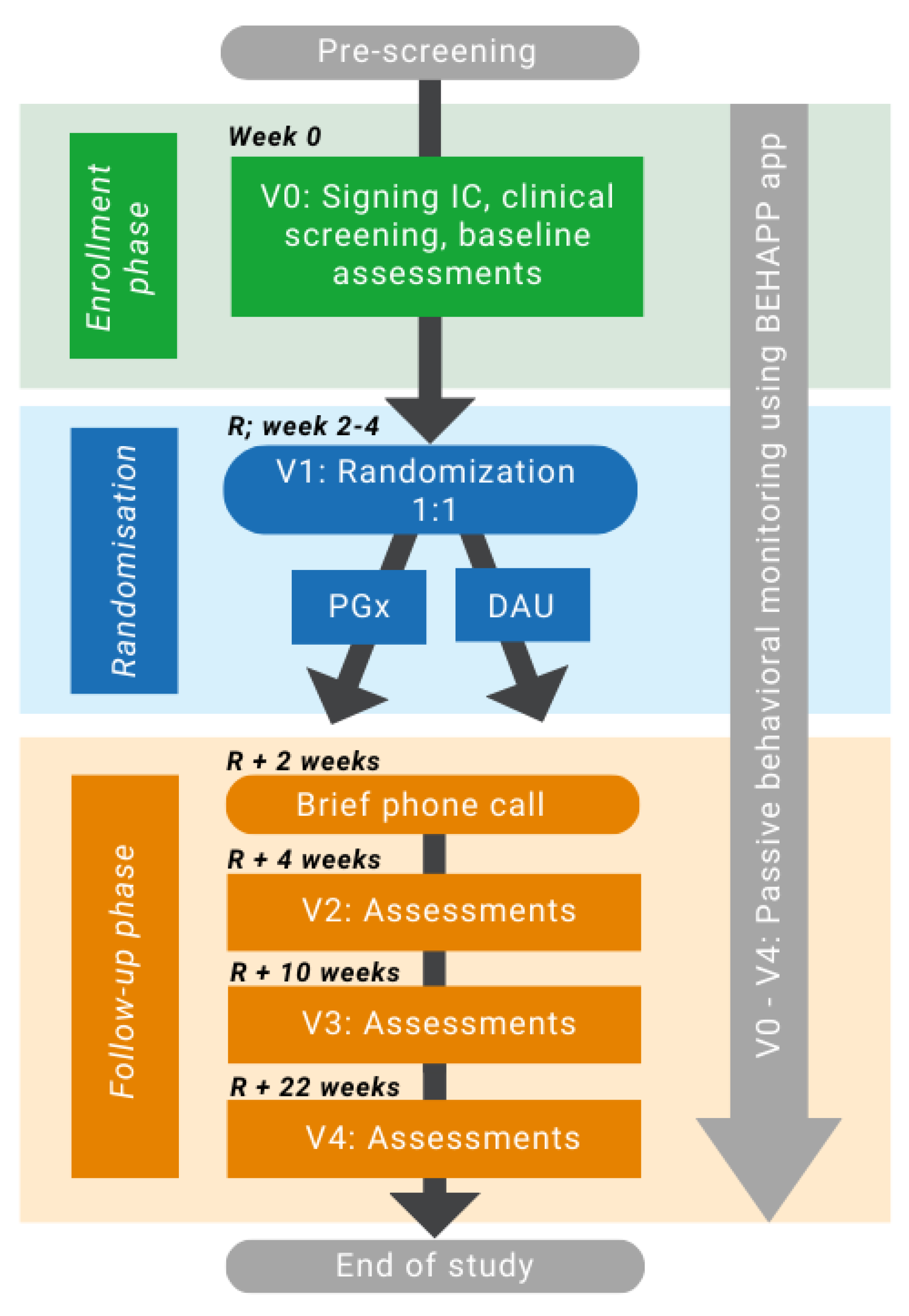
2.1. Study Design
2.2. Recruitment and Participants
2.3. Inclusion and Exclusion Criteria
3. Procedures
3.1. Study Procedure
3.2. Study Readouts (Table S1 in Supplementary Materials)
3.2.1. Psychiatric Evaluation
3.2.2. Patient Characteristics
3.2.3. Primary and Secondary Outcomes
3.2.4. Anthropometric Readouts
3.2.5. Clinical Chemistry and Therapeutic Drug Monitoring (TDM)
3.2.6. Passive Behavioral Monitoring
3.3. Genotyping
3.4. Dosing Recommendations
3.5. Sample Size and Power Calculation
3.6. Statistical Analysis
3.7. Overarching Project
4. Expected Results
5. Strengths and Limitations
6. Ethics and Dissemination
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wittchen, H.U.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The Size and Burden of Mental Disorders and Other Disorders of the Brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S. Epidemiology of Anxiety Disorders in the 21st Century. Dialogues Clin. Neurosci. 2022, 17, 327–335. [Google Scholar] [CrossRef] [PubMed]
- WHO. Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 2 February 2023).
- Greenberg, P.E.; Fournier, A.A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics 2021, 39, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Van Westrhenen, R.; Aitchison, K.J.; Ingelman-Sundberg, M.; Jukić, M.M. Pharmacogenomics of Antidepressant and Antipsychotic Treatment: How Far Have We Got and Where Are We Going? Front. Psychiatry 2020, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Van Westrhenen, R.; Ingelman-Sundberg, M. Editorial: From Trial and Error to Individualised Pharmacogenomics-Based Pharmacotherapy in Psychiatry. Front. Pharmacol. 2021, 12, 725565. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, S.; Drago, A.; Fabbri, C.; Gibiino, S.; Calati, R.; Serretti, A. Pharmacogenetics of Antidepressant Response. J. Psychiatry Neurosci. 2011, 36, 87. [Google Scholar] [CrossRef]
- Vanderkooy, J.D.; Kennedy, S.H.; Bagby, R.M. Antidepressant Side Effects in Depression Patients Treated in a Naturalistic Setting: A Study of Bupropion, Moclobemide, Paroxetine, Sertraline, and Venlafaxine. Can. J. Psychiatry 2002, 47, 174–180. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M. Genetic Polymorphisms of Cytochrome P450 2D6 (CYP2D6): Clinical Consequences, Evolutionary Aspects and Functional Diversity. Pharmacogenom. J. 2005, 5, 6–13. [Google Scholar] [CrossRef]
- Altar, C.A.; Carhart, J.; Allen, J.D.; Hall-Flavin, D.; Winner, J.; Dechairo, B. Clinical Utility of Combinatorial Pharmacogenomics-Guided Antidepressant Therapy: Evidence from Three Clinical Studies. Complex Psychiatry 2015, 1, 145–155. [Google Scholar] [CrossRef]
- Wong, W.L.E.; Fabbri, C.; Laplace, B.; Li, D.; van Westrhenen, R.; Lewis, C.M.; Dawe, G.S.; Young, A.H. The Effects of CYP2C19 Genotype on Proxies of SSRI Antidepressant Response in the UK Biobank. Pharmaceuticals 2023, 16, 1277. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T. Clinical Use of Pharmacogenomics in Psychiatry: The Future Has Not yet Arrived. Eur. Neuropsychopharmacol. 2022, 58, 4–6. [Google Scholar] [CrossRef] [PubMed]
- van Westrhenen, R.; van Schaik, R.H.N.; van Gelder, T.; Birkenhager, T.K.; Bakker, P.R.; Houwink, E.J.F.; Bet, P.M.; Hoogendijk, W.J.G.; van Weelden-Hulshof, M.J.M. Policy and Practice Review: A First Guideline on the Use of Pharmacogenetics in Clinical Psychiatric Practice. Front. Pharmacol. 2021, 12, 640032. [Google Scholar] [CrossRef]
- Swen, J.J.; Nijenhuis, M.; De Boer, A.; Grandia, L.; Maitland-Van Der Zee, A.H.; Mulder, H.; Rongen, G.A.P.J.M.; Van Schaik, R.H.N.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From Bench to Byte—An Update of Guidelines. Clin. Pharmacol. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Muller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC®) for CYP2D6 and CYP2C19 Genotypes and Dosing of Tricyclic Antidepressants: 2016 Update. Clin. Pharmacol. Ther. 2017, 102, 37. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.C.; Stanton, J.D.; Bharthi, K.; Al Maruf, A.; Müller, D.J.; Bousman, C.A. Pharmacogenomic Testing and Depressive Symptom Remission: A Systematic Review and Meta-Analysis of Prospective, Controlled Clinical Trials. Clin. Pharmacol. Ther. 2022, 112, 1303–1317. [Google Scholar] [CrossRef]
- Bousman, C.A.; Arandjelovic, K.; Mancuso, S.G.; Eyre, H.A.; Dunlop, B.W. Pharmacogenetic Tests and Depressive Symptom Remission: A Meta-Analysis of Randomized Controlled Trials. Pharmacogenomics 2019, 20, 37–47. [Google Scholar] [CrossRef]
- van Schaik, R.H.N.; Müller, D.J.; Serretti, A.; Ingelman-Sundberg, M. Pharmacogenetics in Psychiatry: An Update on Clinical Usability. Front. Pharmacol. 2020, 11, 1443. [Google Scholar] [CrossRef]
- Arranz, M.J.; Salazar, J.; Hernández, M.H. Pharmacogenetics of Antipsychotics: Clinical Utility and Implementation. Behav. Brain Res. 2021, 401, 113058. [Google Scholar] [CrossRef]
- Solomon, H.V.; Cates, K.W.; Li, K.J. Does Obtaining CYP2D6 and CYP2C19 Pharmacogenetic Testing Predict Antidepressant Response or Adverse Drug Reactions? Psychiatry Res. 2019, 271, 604–613. [Google Scholar] [CrossRef]
- Vos, C.F.; Ter Hark, S.E.; Schellekens, A.F.A.; Spijker, J.; van der Meij, A.; Grotenhuis, A.J.; Mihaescu, R.; Kievit, W.; Donders, R.; Aarnoutse, R.E.; et al. Effectiveness of Genotype-Specific Tricyclic Antidepressant Dosing in Patients With Major Depressive Disorder: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2312443. [Google Scholar] [CrossRef] [PubMed]
- Swen, J.J.; van der Wouden, C.H.; Manson, L.E.; Abdullah-Koolmees, H.; Blagec, K.; Blagus, T.; Böhringer, S.; Cambon-Thomsen, A.; Cecchin, E.; Cheung, K.C.; et al. A 12-Gene Pharmacogenetic Panel to Prevent Adverse Drug Reactions: An Open-Label, Multicentre, Controlled, Cluster-Randomised Crossover Implementation Study. Lancet 2023, 401, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarian, S.; Wong, G.W.K.; Bunka, M.; Edwards, L.; Cressman, S.; Conte, T.; Price, M.; Schuetz, C.; Riches, L.; Landry, G.; et al. Cost-Effectiveness of Pharmacogenomic-Guided Treatment for Major Depression. CMAJ 2023, 195, E1499–E1508. [Google Scholar] [CrossRef] [PubMed]
- Slomp, C.; Morris, E.; Edwards, L.; Hoens, A.M.; Landry, G.; Riches, L.; Ridgway, L.; Bryan, S.; Austin, J. Pharmacogenomic Testing for Major Depression: A Qualitative Study of the Perceptions of People with Lived Experience and Professional Stakeholders. Can. J. Psychiatry 2023, 68, 436–452. [Google Scholar] [CrossRef]
- Hall-Flavin, D.K.; Winner, J.G.; Allen, J.D.; Jordan, J.J.; Nesheim, R.S.; Snyder, K.A.; Drews, M.S.; Eisterhold, L.L.; Biernacka, J.M.; Mrazek, D.A. Using a Pharmacogenomic Algorithm to Guide the Treatment of Depression. Transl. Psychiatry 2012, 2, e172. [Google Scholar] [CrossRef] [PubMed]
- Hall-Flavin, D.K.; Winner, J.G.; Allen, J.D.; Carhart, J.M.; Proctor, B.; Snyder, K.A.; Drews, M.S.; Eisterhold, L.L.; Geske, J.; Mrazek, D.A. Utility of Integrated Pharmacogenomic Testing to Support the Treatment of Major Depressive Disorder in a Psychiatric Outpatient Setting. Pharmacogenet. Genom. 2013, 23, 535–548. [Google Scholar] [CrossRef]
- Bradley, P.; Shiekh, M.; Mehra, V.; Vrbicky, K.; Layle, S.; Olson, M.C.; Maciel, A.; Cullors, A.; Garces, J.A.; Lukowiak, A.A. Improved Efficacy with Targeted Pharmacogenetic-Guided Treatment of Patients with Depression and Anxiety: A Randomized Clinical Trial Demonstrating Clinical Utility. J. Psychiatr. Res. 2018, 96, 100–107. [Google Scholar] [CrossRef]
- Pérez, V.; Salavert, A.; Espadaler, J.; Tuson, M.; Saiz-Ruiz, J.; Sáez-Navarro, C.; Bobes, J.; Baca-García, E.; Vieta, E.; Olivares, J.M.; et al. Efficacy of Prospective Pharmacogenetic Testing in the Treatment of Major Depressive Disorder: Results of a Randomized, Double-Blind Clinical Trial. BMC Psychiatry 2017, 17, 250. [Google Scholar] [CrossRef]
- Singh, A.B. Improved Antidepressant Remission in Major Depression via a Pharmacokinetic Pathway Polygene Pharmacogenetic Report. Clin. Psychopharmacol. Neurosci. 2015, 13, 150. [Google Scholar] [CrossRef]
- Winner, J.G.; Carhart, J.M.; Altar, A.; Allen, J.D.; Dechairo, B.M. A Prospective, Randomized, Double-Blind Study Assessing the Clinical Impact of Integrated Pharmacogenomic Testing for Major Depressive Disorder. Discov. Med. 2013, 16, 219–227. [Google Scholar]
- Greden, J.F.; Parikh, S.V.; Rothschild, A.J.; Thase, M.E.; Dunlop, B.W.; DeBattista, C.; Conway, C.R.; Forester, B.P.; Mondimore, F.M.; Shelton, R.C.; et al. Impact of Pharmacogenomics on Clinical Outcomes in Major Depressive Disorder in the GUIDED Trial: A Large, Patient- and Rater-Blinded, Randomized, Controlled Study. J. Psychiatr. Res. 2019, 111, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Oslin, D.W.; Lynch, K.G.; Shih, M.C.; Ingram, E.P.; Wray, L.O.; Chapman, S.R.; Kranzler, H.R.; Gelernter, J.; Pyne, J.M.; Stone, A.; et al. Effect of Pharmacogenomic Testing for Drug-Gene Interactions on Medication Selection and Remission of Symptoms in Major Depressive Disorder: The PRIME Care Randomized Clinical Trial. JAMA 2022, 328, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wang, S.-M.; Bahk, W.-M.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Mandelli, L.; Pae, C.-U.; Serretti, A. A Pharmacogenomic-Based Antidepressant Treatment for Patients with Major Depressive Disorder: Results from an 8-Week, Randomized, Single-Blinded Clinical Trial. Clin. Psychopharmacol. Neurosci. 2018, 16, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Zhao, W.; Qiu, Y.; Wu, H.; Chen, J.; Fang, Y.; Guo, W.; Li, L. Preliminary Clinical Investigation of Combinatorial Pharmacogenomic Testing for the Optimized Treatment of Depression: A Randomized Single-Blind Study. Front. Neurosci. 2019, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Dowd, D.; Fava, M.; Lencz, T.; Krause, D.S. Randomized, Controlled, Participant- and Rater-Blind Trial of Pharmacogenomic Test-Guided Treatment versus Treatment as Usual for Major Depressive Disorder. Depress. Anxiety 2020, 37, 834–841. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Chen, Y.; Demodena, A.; Leckband, S.G.; Fischer, E.; Golshan, S.; Suppes, T.; Kelsoe, J.R. A Prospective Study to Determine the Clinical Utility of Pharmacogenetic Testing of Veterans with Treatment-Resistant Depression. J. Psychopharmacol. 2021, 35, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Zai, C.C.; Altar, C.A.; Tanner, J.A.; Davies, P.E.; Traxler, P.; Li, J.; Cogan, E.S.; Kucera, M.T.; Gugila, A.; et al. Clinical Utility of Combinatorial Pharmacogenomic Testing in Depression: A Canadian Patient- and Rater-Blinded, Randomized, Controlled Trial. Transl. Psychiatry 2022, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- de Leon, J. Precision Psychiatry: The Complexity of Personalizing Antipsychotic Dosing. Eur. Neuropsychopharmacol. 2022, 58, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, J.M.J.L.; Nijenhuis, M.; Soree, B.; Guchelaar, H.J.; Swen, J.J.; van Schaik, R.H.N.; van der Weide, J.; Rongen, G.A.P.J.M.; Buunk, A.M.; de Boer-Veger, N.J.; et al. Dutch Pharmacogenetics Working Group (DPWG) Guideline for the Gene-Drug Interaction between CYP2C19 and CYP2D6 and SSRIs. Eur. J. Hum. Genet. 2021, 30, 1114–1120. [Google Scholar] [CrossRef]
- Beunk, L.; Nijenhuis, M.; Soree, B.; de Boer-Veger, N.J.; Buunk, A.M.; Guchelaar, H.J.; Houwink, E.J.F.; Risselada, A.; Rongen, G.A.P.J.M.; van Schaik, R.H.N.; et al. Dutch Pharmacogenetics Working Group (DPWG) Guideline for the Gene-Drug Interaction between CYP2D6, CYP3A4 and CYP1A2 and Antipsychotics. Eur. J. Hum. Genet. 2023, 1–8. [Google Scholar] [CrossRef]
- Yoshida, K.; Müller, D.J. Pharmacogenetics of Antipsychotic Drug Treatment: Update and Clinical Implications. Rev. Artic. Mol Neuropsychiatry 2019, 5, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Jukic, M.M.; Smith, R.L.; Haslemo, T.; Molden, E.; Ingelman-Sundberg, M. Effect of CYP2D6 Genotype on Exposure and Efficacy of Risperidone and Aripiprazole: A Retrospective, Cohort Study. Lancet Psychiatry 2019, 6, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Minelli, A.; Barlati, S.; Baune, B.T. Evaluating Study Designs and Treatment Outcomes of Antidepressant Pharmacogenetic Clinical Trials—Challenges and Future Perspectives. A Critical Review. Eur. Neuropsychopharmacol. 2022, 59, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, K.; Tansey, K.; Dernovšek, M.Z.; Hauser, J.; Henigsberg, N.; Maier, W.; Mors, O.; Placentino, A.; Rietschel, M.; Souery, D.; et al. Genetic Differences in Cytochrome P450 Enzymes and Antidepressant Treatment Response. J. Psychopharmacol. 2014, 28, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Stingl, J.C.; Brockmöller, J.; Viviani, R. Genetic Variability of Drug-Metabolizing Enzymes: The Dual Impact on Psychiatric Therapy and Regulation of Brain Function. Mol. Psychiatry 2012, 18, 273–287. [Google Scholar] [CrossRef]
- Walden, L.M.; Brandl, E.J.; Tiwari, A.K.; Cheema, S.; Freeman, N.; Braganza, N.; Kennedy, J.L.; Müller, D.J. Genetic Testing for CYP2D6 and CYP2C19 Suggests Improved Outcome for Antidepressant and Antipsychotic Medication. Psychiatry Res. 2019, 279, 111–115. [Google Scholar] [CrossRef]
- Islam, F.; Marshe, V.S.; Magarbeh, L.; Frey, B.N.; Milev, R.V.; Soares, C.N.; Parikh, S.V.; Placenza, F.; Strother, S.C.; Hassel, S.; et al. Effects of CYP2C19 and CYP2D6 Gene Variants on Escitalopram and Aripiprazole Treatment Outcome and Serum Levels: Results from the CAN-BIND 1 Study. Transl. Psychiatry 2022, 12, 366. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M.; Persson, A.; Jukic, M.M. Polymorphic Expression of CYP2C19 and CYP2D6 in the Developing and Adult Human Brain Causing Variability in Cognition, Risk for Depression and Suicide: The Search for the Endogenous Substrates. Pharmacogenomics 2014, 15, 1841–1844. [Google Scholar] [CrossRef]
- Zhou, Y.; Ingelman-Sundberg, M.; Lauschke, V.M. Worldwide Distribution of Cytochrome P450 Alleles: A Meta-Analysis of Population-Scale Sequencing Projects. Clin. Pharmacol. Ther. 2017, 102, 688–700. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar]
- WHO. Medication Safety in Polypharmacy: Technical Report. Available online: https://apps.who.int/iris/handle/10665/325454 (accessed on 3 February 2023).
- Castor. EDC Castor Electronic Data Capture [Online]. Available online: https://castoredc.com (accessed on 7 January 2023).
- Behapp. Digital Phenotyping. Available online: https://www.behapp.com/ (accessed on 10 January 2023).
- Abbott, C.H.; Zisk, A.; Bounoua, N.; Diamond, G.S.; Kobak, R. Predicting Patterns of Treatment Response and Outcome for Adolescents Who Are Suicidal and Depressed. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, C.; Lenze, E.J.; Dew, M.A.; Begley, A.E.; Mulsant, B.H.; Dombrovski, A.Y.; Pollock, B.G.; Stack, J.; Miller, M.D.; Reynolds, C.F. Effect of Comorbid Anxiety on Treatment Response and Relapse Risk in Late-Life Depression: Controlled Study. Br. J. Psychiatry 2007, 190, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Altamura, A.C.; Buoli, M.; Serati, M. Duration of Illness and Duration of Untreated Illness in Relation to Drug Response in Psychiatric Disorders. Neuropsychiatry 2011, 1, 81–90. [Google Scholar] [CrossRef]
- Hancock, N.; Scanlan, J.N.; Honey, A.; Bundy, A.C.; O’Shea, K. Recovery Assessment Scale—Domains and Stages (RAS-DS): Its Feasibility and Outcome Measurement Capacity. Aust. N. Z. J. Psychiatry 2015, 49, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, P.W.; Salzer, M.; Ralph, R.O.; Sangster, Y.; Keck, L. Examining the Factor Structure of the Recovery Assessment Scale. Schizophr. Bull. 2004, 30, 1035–1041. [Google Scholar] [CrossRef]
- Salzer, M.S.; Brusilovskiy, E. Advancing Recovery Science: Reliability and Validity Properties of the Recovery Assessment Scale. Psychiatr. Serv. 2014, 65, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.B.W. A Structured Interview Guide for the Hamilton Depression Rating Scale. Arch. Gen. Psychiatry 1988, 45, 742–747. [Google Scholar] [CrossRef]
- Hamilton, M. The Assessment of Anxiety States by Rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Wisniewski, S.R.; Rush, A.J.; Balasubramani, G.K.; Trivedi, M.H.; Nierenberg, A.A. Self-Rated Global Measure of the Frequency, Intensity, and Burden of Side Effects. J. Psychiatry Pract. 2006, 12, 71–79. [Google Scholar] [CrossRef]
- Lingjærde, O.; Ahlfors, U.G.; Bech, P.; Dencker, S.J.; Elgen, K. The UKU Side Effect Rating Scale. A New Comprehensive Rating Scale for Psychotropic Drugs and a Cross-Sectional Study of Side Effects in Neuroleptic-Treated Patients. Acta Psychiatr. Scand. 1987, 76 (Suppl. S334), 1–100. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and Preliminary Testing of the New Five-Level Version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.R.; Sánchez-Moreno, J.; Martínez-Aran, A.; Salamero, M.; Torrent, C.; Reinares, M.; Comes, M.; Colom, F.; Van Riel, W.; Ayuso-Mateos, J.; et al. Validity and Reliability of the Functioning Assessment Short Test (FAST) in Bipolar Disorder. Clin. Pract. Epidemiol. Ment. Health 2007, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Jeremić, A.; Milosavljević, F.; Vladimirov, S.; Batinić, B.; Marković, B.; Jukić, M. Validation of a Quick and Simple Chromatographic Method for Simultaneous Quantification of Sertraline, Escitalopram, Risperidone and Paliperidone Levels in the Human Plasma. Arch. Pharm. 2021, 71, 365–377. [Google Scholar] [CrossRef]
- Jongs, N.; Jagesar, R.; van Haren, N.E.M.; Penninx, B.W.J.H.; Reus, L.; Visser, P.J.; van der Wee, N.J.A.; Koning, I.M.; Arango, C.; Sommer, I.E.C.; et al. A Framework for Assessing Neuropsychiatric Phenotypes by Using Smartphone-Based Location Data. Transl. Psychiatry 2020, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Jagesar, R.R.; Vorstman, J.A.; Kas, M.J. Requirements and Operational Guidelines for Secure and Sustainable Digital Phenotyping: Design and Development Study. J. Med. Internet Res. 2021, 23, e20996. [Google Scholar] [CrossRef]
- Milosavljević, F.; Bukvić, N.; Pavlović, Z.; Miljević, Č.; Pešić, V.; Molden, E.; Ingelman-Sundberg, M.; Leucht, S.; Jukić, M.M. Association of CYP2C19 and CYP2D6 Poor and Intermediate Metabolizer Status With Antidepressant and Antipsychotic Exposure: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2021, 78, 270–280. [Google Scholar] [CrossRef]
- Jukić, M.M.; Haslemo, T.; Molden, E.; Ingelman-Sundberg, M. Impact of CYP2C19 Genotype on Escitalopram Exposure and Therapeutic Failure: A Retrospective Study Based on 2,087 Patients. Am. J. Psychiatry 2018, 175, 463–470. [Google Scholar] [CrossRef]
- UK Biobank. Available online: https://www.ukbiobank.ac.uk/ (accessed on 1 June 2023).
- THL Biobank. Available online: https://thl.fi/en/web/thl-biobank/for-researchers (accessed on 1 June 2023).
- Vieta, E. Personalised Medicine Applied to Mental Health: Precision Psychiatry. Rev. Psiquiatr. Salud. Ment. 2015, 8, 117–118. [Google Scholar] [CrossRef]
- Dean, O.M.; Walker, A.J. Current Approaches to Precision Medicine in Psychiatry: Are We Just Spinning Our Wheels? Eur. Neuropsychopharmacol. 2023, 66, 11–13. [Google Scholar] [CrossRef]
- Lundh, A.; Lexchin, J.; Mintzes, B.; Schroll, J.B.; Bero, L. Industry Sponsorship and Research Outcome. Cochrane Database Syst. Rev. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
| Genotype | Enzyme Function | Alleles |
|---|---|---|
| CYP2C19Null | Loss of function | CYP2C19*2, *3, *4, *5, *6, *8 |
| CYP2C19Inc | Increase | CYP2C19*17 |
| CYP2D6Null | Loss of function | CYP2D6*3, *4, *5, *6, *7, *8, *11, *12, *14, *18, *19, *20, *29, *38, *42 |
| CYP2D6Red | Reduction | CYP2D6 *9, *10, *17, and *41 |
| CYP2D6xN | Increase | CYP2D6 multiplication |
| CYP2C19 | Genotype | Escitalopram Daily Dose | Sertraline Daily Dose | CYP2D6 | Genotype | Risperidone Daily Dose | Aripiprazole Daily Dose |
|---|---|---|---|---|---|---|---|
| Poor (PM) | Null/Null | 5 mg | 50 mg | Poor (PM) | Null/Null | 3–4 mg 4 mg | 15–20 mg >10 mg Max 10 mg |
| Intermediate (IM) | Wt/Null Inc/Null | 5–10 mg | 100 mg | Intermediate (IM) | Null/Red Red/Red | 4–5 mg | 20–25 mg |
| Intermediate (IM+) | Wt/Null | 5–6 mg | 25–30 mg | ||||
| Normal (NM) | Wt/Wt | 15–20 mg | 100–150 mg | Normal (NM) | Wt/Wt | 6 mg | 30 mg |
| Ultrarapid (UM) | Wt/Inc Inc/Inc | 15–20 mg | 100–150 mg | Ultrarapid (UM) | Wt/Wt × 2 | 6 mg Avoid or 12 mg | 30 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelgrim, T.A.D.; Philipsen, A.; Young, A.H.; Juruena, M.; Jimenez, E.; Vieta, E.; Jukić, M.; Van der Eycken, E.; Heilbronner, U.; Moldovan, R.; et al. A New Intervention for Implementation of Pharmacogenetics in Psychiatry: A Description of the PSY-PGx Clinical Study. Pharmaceuticals 2024, 17, 151. https://doi.org/10.3390/ph17020151
Pelgrim TAD, Philipsen A, Young AH, Juruena M, Jimenez E, Vieta E, Jukić M, Van der Eycken E, Heilbronner U, Moldovan R, et al. A New Intervention for Implementation of Pharmacogenetics in Psychiatry: A Description of the PSY-PGx Clinical Study. Pharmaceuticals. 2024; 17(2):151. https://doi.org/10.3390/ph17020151
Chicago/Turabian StylePelgrim, Teuntje A. D., Alexandra Philipsen, Allan H. Young, Mario Juruena, Ester Jimenez, Eduard Vieta, Marin Jukić, Erik Van der Eycken, Urs Heilbronner, Ramona Moldovan, and et al. 2024. "A New Intervention for Implementation of Pharmacogenetics in Psychiatry: A Description of the PSY-PGx Clinical Study" Pharmaceuticals 17, no. 2: 151. https://doi.org/10.3390/ph17020151








