The Antimicrobial Potential of the Hop (Humulus lupulus L.) Extract against Staphylococcus aureus and Oral Streptococci
Abstract
:1. Introduction
2. Results
2.1. Metabolite Profile of Hop Extracts
2.2. Antimicrobial Effect of an Extract from H. lupulus
2.3. Potentiation of Antimicrobial Agents by the H. lupulus Extract
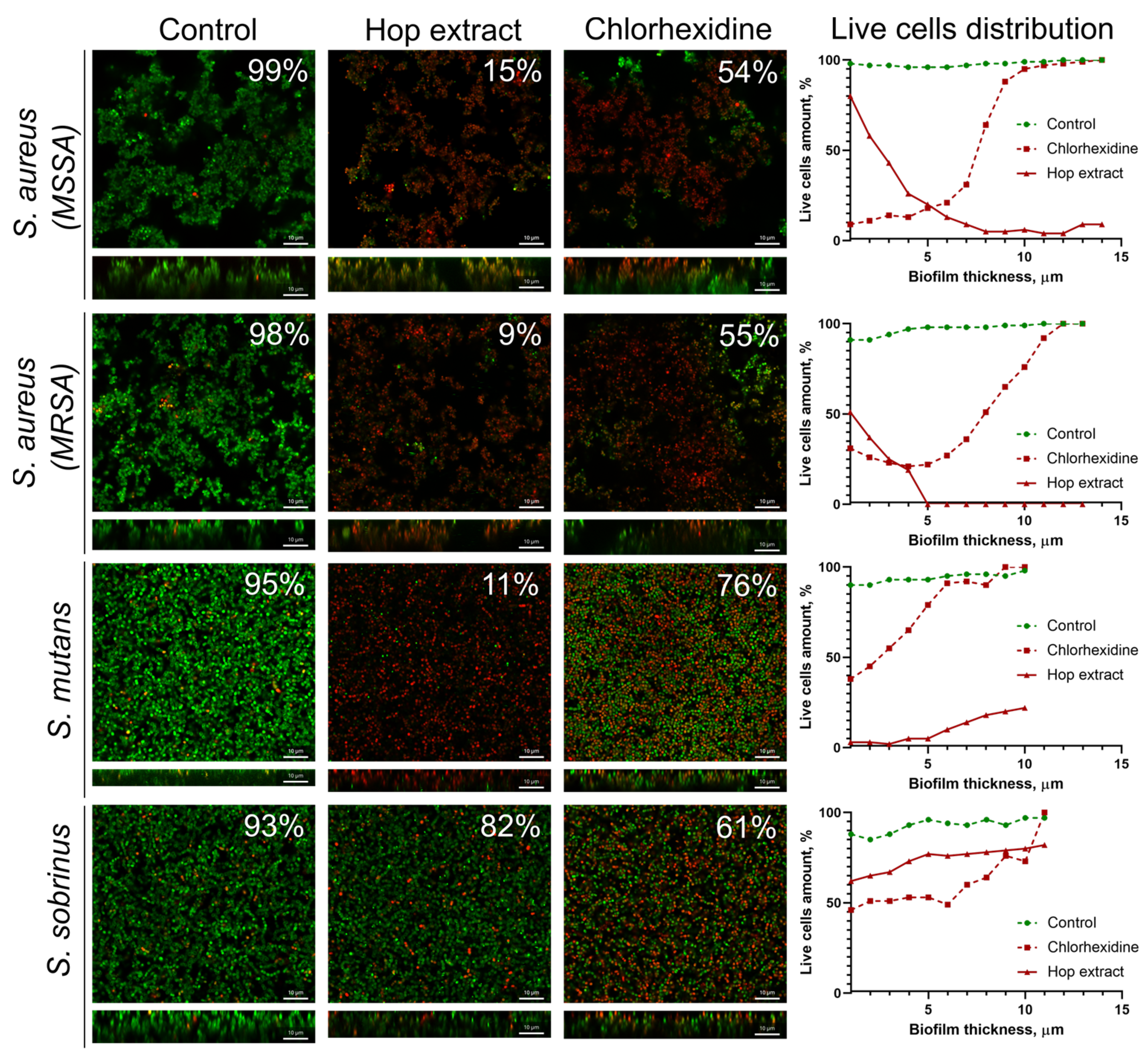
2.4. Extract from H. lupulus Kills Bacterial Cells in Biofilm
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sample Preparation
4.2. Solvents, Chemicals, and Apparatus
4.3. Extraction, Isolation, and Purification of Bioactive Compounds from Hop
4.4. Thin-Layer Chromatography
4.5. LC-MS Analysis
4.6. Bacterial Strains and Growth Conditions
4.7. Determination of Minimal Inhibitory Concentration (MIC)
4.8. Assessment of Synergy between Hop Extract and Antimicrobials
4.9. Biofilm Formation
4.10. Confocal Laser Scanning Microscopy
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, B.; González-Febles, J.; Garnier-Rodríguez, J.L.; Dadlani, S.; Bustabad-Reyes, S.; Sanz, M.; Sánchez-Alonso, F.; Sánchez-Piedra, C.; González-Dávila, E.; Díaz-González, F. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: A case-control study. Arthritis Res. Ther. 2019, 21, 27. [Google Scholar] [CrossRef]
- Moore, W.E.; Moore, L.V. The bacteria of periodontal diseases. Periodontology 2000 1994, 5, 66–77. [Google Scholar] [CrossRef]
- Diaz, P.I.; Chalmers, N.I.; Rickard, A.H.; Kong, C.; Milburn, C.L.; Palmer, R.J.; Kolenbrander, P.E. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 2006, 72, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J.; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial interactions and successions during plaque development. Periodontology 2000 2006, 42, 47–79. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, S.S.; Sweeney, E.L.; Cowley, D.M.; Liley, H.G.; Ranasinghe, P.D.; Charles, B.G.; Shaw, P.N.; Vagenas, D.; Duley, J.A.; Knox, C.L. Deep sequencing of the 16S ribosomal RNA of the neonatal oral microbiome: A comparison of breast-fed and formula-fed infants. Sci. Rep. 2016, 6, 38309. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; He, X.; Shi, W. Precision Reengineering of the Oral Microbiome for Caries Management. Adv. Dent. Res. 2019, 30, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Grier, A.; Faustoferri, R.; Alzoubi, S.; Gill, A.L.; Feng, C.; Liu, Y.; Quivey, R.; Kopycka-Kedzierawski, D.; Koo, H. Association between oral candida and bacteriome in children with severe ECC. J. Dent. Res. 2018, 97, 1468–1476. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, F.; Qiu, L.; Gao, M.; Xu, P.; Zhang, L.; Liao, X.; Wang, M.; Hu, X.; Sun, Y. Ecological influence by colonization of fluoride-resistant Streptococcus mutans in oral biofilm. Front. Cell. Infect. Microbiol. 2023, 12, 1942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Chu, C.-H.; Yu, O.Y. Oral microbiome and dental caries development. Dent. J. 2022, 10, 184. [Google Scholar] [CrossRef]
- Vasconcelos, L.C.; Sampaio, F.C.; Sampaio, M.C.; Pereira, M.O.S.; Higino, J.S.; Peixoto, M.H. Minimum inhibitory concentration of adherence of Punica granatum Linn (pomegranate) gel against S. mutans, S. mitis and C. albicans. Braz. Dent. J. 2006, 17, 223–227. [Google Scholar] [CrossRef]
- Zeng, L.; Burne, R.A. Sucrose- and Fructose-Specific Effects on the Transcriptome of Streptococcus mutans, as Determined by RNA Sequencing. Appl. Environ. Microbiol. 2016, 82, 146–156. [Google Scholar] [CrossRef]
- Guo, L.; Hu, W.; He, X.; Lux, R.; McLean, J.; Shi, W. Investigating acid production by Streptococcus mutans with a surface-displayed pH-sensitive green fluorescent protein. PLoS ONE 2013, 8, e57182. [Google Scholar] [CrossRef]
- Hwang, G.; Liu, Y.; Kim, D.; Sun, V.; Aviles-Reyes, A.; Kajfasz, J.K.; Lemos, J.A.; Koo, H. Simultaneous spatiotemporal mapping of in situ pH and bacterial activity within an intact 3D microcolony structure. Sci. Rep. 2016, 6, 32841. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Kressirer, C.A.; Rothmiller, S.; Johansson, I.; Chalmers, N.I. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef]
- Daep, C.A.; Novak, E.A.; Lamont, R.J.; Demuth, D.R. Structural dissection and in vivo effectiveness of a peptide inhibitor of Porphyromonas gingivalis adherence to Streptococcus gordonii. Infect. Immun. 2011, 79, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Nagata, H.; Yamamoto, Y.; Tanaka, M.; Tanaka, J.; Minamino, N.; Shizukuishi, S. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus oralis functions as a coadhesin for Porphyromonas gingivalis major fimbriae. Infect. Immun. 2004, 72, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.Y.; Furtado Araujo, M.V.; Strausbaugh, L.D.; Terzi, E.; Ioannidou, E.; Diaz, P.I. Microbiome profiles in periodontitis in relation to host and disease characteristics. PLoS ONE 2015, 10, e0127077. [Google Scholar] [CrossRef] [PubMed]
- Tajkarimi, M.; Ibrahim, S.A.; Cliver, D. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, S. Perspective on plant products as antimicrobials agents: A review. Pharmacologia 2013, 4, 469–480. [Google Scholar] [CrossRef]
- Mehta, S.R.; Yusuf, S.; Peters, R.J.; Bertrand, M.E.; Lewis, B.S.; Natarajan, M.K.; Malmberg, K.; Rupprecht, H.-J.; Zhao, F.; Chrolavicius, S. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001, 358, 527–533. [Google Scholar] [CrossRef]
- Hurtuková, K.; Fajstavrová, K.; Rimpelová, S.; Vokatá, B.; Fajstavr, D.; Kasálková, N.S.; Siegel, J.; Švorčík, V.; Slepička, P. Antibacterial Properties of a Honeycomb-like Pattern with Cellulose Acetate and Silver Nanoparticles. Materials 2021, 14, 4051. [Google Scholar] [CrossRef] [PubMed]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants 2021, 10, 569. [Google Scholar] [CrossRef]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Ody, P. The Complete Medicinal Herbal: A Practical Guide to the Healing Properties of Herbs; Simon and Schuster: New York, NY, USA, 2017. [Google Scholar]
- Ruddaraju, L.K.; Pammi, S.V.N.; Guntuku, G.S.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Joshi, R.K. A perspective on the phytopharmaceuticals responsible for the therapeutic applications. In Recent Advances in Drug Delivery Technology; IGI Global: Hershey, PA, USA, 2017; pp. 229–262. [Google Scholar]
- Wangchuk, P.; Keller, P.A.; Pyne, S.G.; Taweechotipatr, M.; Tonsomboon, A.; Rattanajak, R.; Kamchonwongpaisan, S. Evaluation of an ethnopharmacologically selected Bhutanese medicinal plants for their major classes of phytochemicals and biological activities. J. Ethnopharmacol. 2011, 137, 730–742. [Google Scholar] [CrossRef]
- Aleksic Sabo, V.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Ghirga, F.; Quaglio, D.; Mori, M.; Cammarone, S.; Iazzetti, A.; Goggiamani, A.; Ingallina, C.; Botta, B.; Calcaterra, A. A unique high-diversity natural product collection as a reservoir of new therapeutic leads. Org. Chem. Front. 2021, 8, 996–1025. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Shen, X.; Cao, X.; Zhan, Q.; Guo, Y.; Yu, F. Resveratrol enhances the antimicrobial effect of polymyxin B on Klebsiella pneumoniae and Escherichia coli isolates with polymyxin B resistance. BMC Microbiol. 2020, 20, 306. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Pai, B.M.; Rajesh, G.; Shenoy, R.; Rao, A. Anti-microbial efficacy of soursop leaf extract (Annona muricata) on oral pathogens: An in-vitro study. J. Clin. Diagn. Res. 2016, 10, ZC01–ZC04. [Google Scholar] [CrossRef] [PubMed]
- Hoglund, K.B.; Barnett, B.K.; Watson, S.A.; Melgarejo, M.B.; Kang, Y. Activity of bioactive garlic compounds on the oral microbiome: A literature review. Gen. Dent. 2020, 68, 27–33. [Google Scholar] [PubMed]
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A. Plant-based proteins and their multifaceted industrial applications. LWT 2022, 154, 112620. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, U.; Sanyal, R.; Jha, N.K.; Behl, T.; Mundhra, A.; Ghosh, A.; Kumar, M.; Proćków, J.; Dey, A. Neoechinulins: Molecular, cellular, and functional attributes as promising therapeutics against cancer and other human diseases. Biomed. Pharmacother. 2022, 145, 112378. [Google Scholar] [CrossRef]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A. Garlic (Allium sativum L.) bioactives and its role in alleviating oral pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Phitaktim, S.; Chomnawang, M.; Sirichaiwetchakoon, K.; Dunkhunthod, B.; Hobbs, G.; Eumkeb, G. Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus. BMC Microbiol. 2016, 16, 195. [Google Scholar] [CrossRef]
- Farooqui, A.; Khan, A.; Borghetto, I.; Kazmi, S.U.; Rubino, S.; Paglietti, B. Synergistic antimicrobial activity of Camellia sinensis and Juglans regia against multidrug-resistant bacteria. PLoS ONE 2015, 10, e0118431. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; Neuville, L.; Moreau, N.J.; Genet, J.-P.; Dos Santos, A.F.; De Andrade, M.C.C.; Sant’Ana, A.E.G. Multidrug resistance reversal agent from Jatropha elliptica. Phytochemistry 2005, 66, 1804–1811. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.Y.; Trizna, E.Y.; Sulaiman, R.K.; Pavelyev, R.S.; Gilfanov, I.R.; Lisovskaya, S.A.; Ostolopovskaya, O.V.; Frolova, L.L.; Kutchin, A.V.; Guseva, G.B. Increasing the Efficacy of Treatment of Staphylococcus aureus–Candida albicans Mixed Infections with Myrtenol. Antibiotics 2022, 11, 1743. [Google Scholar] [CrossRef]
- Moir, M. Hops—A millennium review. J. Am. Soc. Brew. Chem. 2000, 58, 131–146. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G. Empirical prediction and validation of antibacterial inhibitory effects of various plant essential oils on common pathogenic bacteria. Int. J. Food Microbiol. 2015, 202, 35–41. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Sato, Y. Antifungal activities of hop bitter resins and related compounds. Agric. Biol. Chem. 1985, 49, 399–403. [Google Scholar]
- Alonso-Esteban, J.I.; Pinela, J.; Barros, L.; Ćirić, A.; Soković, M.; Calhelha, R.C.; Torija-Isasa, E.; de Cortes Sánchez-Mata, M.; Ferreira, I.C. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) Seeds. Ind. Crops Prod. 2019, 134, 154–159. [Google Scholar] [CrossRef]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Xin, G.; Wei, Z.; Ji, C.; Zheng, H.; Gu, J.; Ma, L.; Huang, W.; Morris-Natschke, S.L.; Yeh, J.L.; Zhang, R.; et al. Xanthohumol isolated from Humulus lupulus prevents thrombosis without increased bleeding risk by inhibiting platelet activation and mtDNA release. Free Radic. Biol. Med. 2017, 108, 247–257. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Satoh-Yamaguchi, K.; Ono, M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine 2009, 16, 369–376. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Miyajima, F.; Roberts, P.; Ellison, L.; Pickard, D.J.; Martin, M.J.; Connor, T.R.; Harris, S.R.; Fairley, D.; Bamford, K.B.; et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat. Genet. 2013, 45, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, C.; Menten, J.F.M.; Pereira, R.; Fagundes, N.S.; Napty, G.; Pedroso, A.; Bigaton, A.D.; Andreote, F.D. Hops β-acids and zinc bacitracin affect the performance and intestinal microbiota of broilers challenged with Eimeria acervulina and Eimeria tenella. Anim. Feed Sci. Technol. 2015, 207, 181–189. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Virani, S.; Zavro, M.; Haas, G.J. Inhibition of Streptococcus mutans and Other Oral streptococci by hop (Humulus lupulus L.) constituents. Econ. Bot. 2003, 57, 118–125. [Google Scholar] [CrossRef]
- Mani, A.; Mahalingam, G. Effect of anti-biofilm potential of different medicinal plants: Review. Asian J. Pharm. Clin. Res. 2017, 10, 24–32. [Google Scholar]
- Brookes, Z.L.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Verspecht, T.; Rodriguez Herrero, E.; Khodaparast, L.; Khodaparast, L.; Boon, N.; Bernaerts, K.; Quirynen, M.; Teughels, W. Development of antiseptic adaptation and cross-adapatation in selected oral pathogens in vitro. Sci. Rep. 2019, 9, 8326. [Google Scholar] [CrossRef]
- Deschepper, M.; Waegeman, W.; Eeckloo, K.; Vogelaers, D.; Blot, S. Effects of chlorhexidine gluconate oral care on hospital mortality: A hospital-wide, observational cohort study. Intensive Care Med. 2018, 44, 1017–1026. [Google Scholar] [CrossRef]
- Čermák, P.; Palečková, V.; Houška, M.; Strohalm, J.; Novotna, P.; Mikyška, A.; Jurkova, M.; Sikorova, M. Inhibitory effects of fresh hops on Helicobacter pylori strains. Czech J. Food Sci. 2015, 33, 302–307. [Google Scholar] [CrossRef]
- Stompor, M.; Żarowska, B. Antimicrobial Activity of Xanthohumol and Its Selected Structural Analogues. Molecules 2016, 21, 608. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Menghini, L.; Ferrante, C.; Recchia, E.; Castro-Amorim, J.; Gameiro, P.; Cellini, L.; Bessa, L.J. Hop Extract: An Efficacious Antimicrobial and Anti-biofilm Agent against Multidrug-Resistant Staphylococci Strains and. Front. Microbiol. 2020, 11, 1852. [Google Scholar] [CrossRef]
- Chin, Y.C.; Chang, N.C.; Anderson, H.H. Factors Influencing the Antibiotic Activity of Lupulon. J. Clin. Investig. 1949, 28 Pt 1, 909–915. [Google Scholar] [CrossRef]
- Roehrer, S.; Behr, J.; Stork, V.; Ramires, M.; Médard, G.; Frank, O.; Kleigrewe, K.; Hofmann, T.; Minceva, M. Xanthohumol C, a minor bioactive hop compound: Production, purification strategies and antimicrobial test. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1095, 39–49. [Google Scholar] [CrossRef]
- Simpson, W.J.; Smith, A.R. Factors affecting antibacterial activity of hop compounds and their derivatives. J. Appl. Bacteriol. 1992, 72, 327–334. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Bogdanova, K.; Röderova, M.; Kolar, M.; Langova, K.; Dusek, M.; Jost, P.; Kubelkova, K.; Bostik, P.; Olsovska, J. Antibiofilm activity of bioactive hop compounds humulone, lupulone and xanthohumol toward susceptible and resistant staphylococci. Res. Microbiol. 2018, 169, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mironova, A.V.; Karimova, A.V.; Bogachev, M.I.; Kayumov, A.R.; Trizna, E.Y. Alterations in Antibiotic Susceptibility of Staphylococcus aureus and Klebsiella pneumoniae in Dual Species Biofilms. Int. J. Mol. Sci. 2023, 24, 8475. [Google Scholar] [CrossRef] [PubMed]
- Cendra, M.D.M.; Blanco-Cabra, N.; Pedraz, L.; Torrents, E. Optimal environmental and culture conditions allow the in vitro coexistence of Pseudomonas aeruginosa and Staphylococcus aureus in stable biofilms. Sci. Rep. 2019, 9, 16284. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.C.; Rice, S.A. Influence of interspecies interactions on the spatial organization of dual species bacterial communities. Biofilm 2020, 2, 100035. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.; Oluwatuyi, M.; Veitch, N.C.; Gray, A.I. Bacterial resistance modifying agents from Lycopus europaeus. Phytochemistry 2003, 62, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, P.; Katta, S.; Andrei, I.; Babu Rao Ambati, V.; Leonida, M.; Haas, G.J. Positive antibacterial co-action between hop (Humulus lupulus) constituents and selected antibiotics. Phytomedicine 2008, 15, 194–201. [Google Scholar] [CrossRef]
- Gagos, M. Process for Preparation of Xanthohumol. Patent US9556097B2, 31 January 2017. [Google Scholar]
- Zhang, X.; Liang, X.; Xiao, H.; Xu, Q. Direct characterization of bitter acids in a crude hop extract by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 180–187. [Google Scholar] [CrossRef]
- Santos, S.A.; Freire, C.S.; Domingues, M.R.M.; Silvestre, A.J.; Neto, C.P. Characterization of phenolic components in polar extracts of Eucalyptus globulus Labill. bark by high-performance liquid chromatography–mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Negri, G.; Di Santi, D.; Tabach, R. Bitter acids from hydroethanolic extracts of Humulus lupulus L., Cannabaceae, used as anxiolytic. Rev. Bras. Farmacogn. 2010, 20, 850–859. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus–a story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar]
- Kayumov, A.R.; Khakimullina, E.N.; Sharafutdinov, I.S.; Trizna, E.Y.; Latypova, L.Z.; Lien, H.T.; Margulis, A.B.; Bogachev, M.I.; Kurbangalieva, A.R. Inhibition of biofilm formation in Bacillus subtilis by new halogenated furanones. J. Antibiot. 2015, 68, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Trizna, E.Y.; Khakimullina, E.N.; Latypova, L.Z.; Kurbangalieva, A.R.; Sharafutdinov, I.S.; Evtyugin, V.G.; Babynin, E.V.; Bogachev, M.I.; Kayumov, A.R. Thio Derivatives of 2(5H)-Furanone As Inhibitors against Bacillus subtilis Biofilms. Acta Naturae 2015, 7, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R.; Canton, R.; Brown, D.F.J.; Giske, C.G.; Heisig, P.; MacGowan, A.P.; Mouton, J.W.; Nordmann, P.; Rodloff, A.C.; Rossolini, G.M.; et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2013, 19, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Sharafutdinov, I.S.; Trizna, E.Y.; Baidamshina, D.R.; Ryzhikova, M.N.; Sibgatullina, R.R.; Khabibrakhmanova, A.M.; Latypova, L.Z.; Kurbangalieva, A.R.; Rozhina, E.V.; Klinger-Strobel, M.; et al. Antimicrobial Effects of Sulfonyl Derivative of 2(5H)-Furanone against Planktonic and Biofilm Associated Methicillin-Resistant and -Susceptible Staphylococcus aureus. Front. Microbiol. 2017, 8, 2246. [Google Scholar] [CrossRef]
- Sulaiman, R.; Trizna, E.; Kolesnikova, A.; Khabibrakhmanova, A.; Kurbangalieva, A.; Bogachev, M.; Kayumov, A. Antimicrobial and Biofilm-Preventing Activity of l-Borneol Possessing 2 (5 H)-Furanone Derivative F131 against S. aureus—C. albicans Mixed Cultures. Pathogens 2022, 12, 26. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Bogachev, M.I.; Volkov, V.Y.; Markelov, O.A.; Trizna, E.Y.; Baydamshina, D.R.; Melnikov, V.; Murtazina, R.R.; Zelenikhin, P.V.; Sharafutdinov, I.S.; Kayumov, A.R. Fast and simple tool for the quantification of biofilm-embedded cells sub-populations from fluorescent microscopic images. PLoS ONE 2018, 13, e0193267. [Google Scholar] [CrossRef] [PubMed]
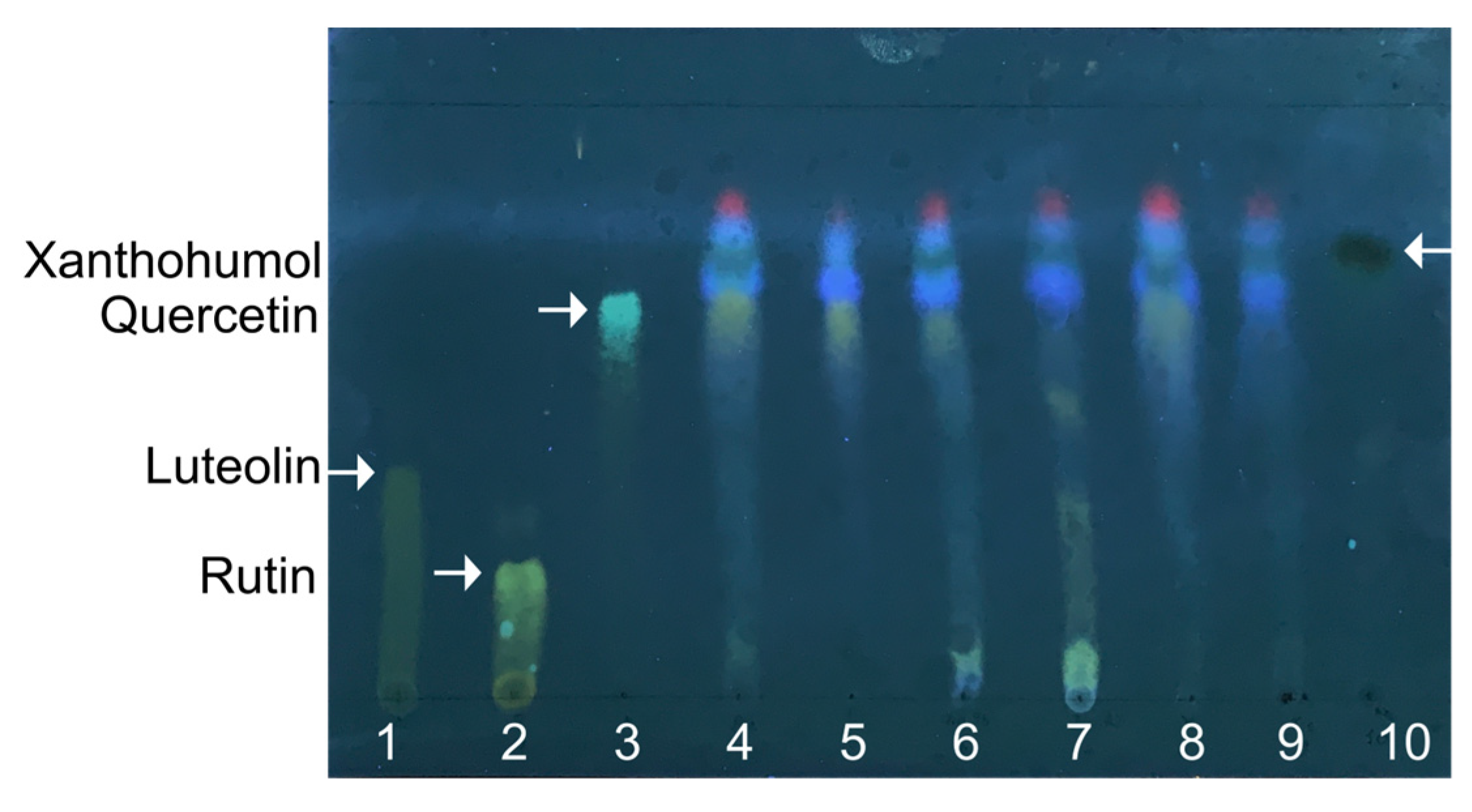
| Number | Retention Time | Molecular Weight (m/z) | Molecular Formula | Identified Compound |
|---|---|---|---|---|
| 1 | 4.16 | 407.1 | C25H28O5 | 2′,4′,6′,4-tetrahydroxy-3′-geranylchalcone |
| 2 | 7.83 | 333.0 | C19H26O5 | Posthumulone |
| 3 | 10.2 | 347.4 | C20H28O5 | Cohumulone |
| 4 | 10.87 | 408.7 | C25H36O4 | Colupulone |
| 5 | 13.71 | 361.5 | C21H30O5 | Humulone |
| 6 | 14.79 | 423.1 | C26H38O4 | Lupulone |
| 7 | 18.09 | 360.9 | C22H32O5 | Adhumulone |
| 8 | 23.94 | 385.3 | C22H32O5 | Prehumulone |
| 9 | 31.85 | 386.2 | C22H32O5 | Adprehumulene |
| 10 | 33.96 | 446.8 | C27H40O4 | Adprelupulone |
| Bacterial Strains and Isolates | MIC, µg/mL | |||
|---|---|---|---|---|
| Extract from H. lupulus | Amikacin | Ciprofloxacin | Ceftriaxone | |
| S. aureus ATCC (MSSA) | 10 | 32 | 16 | 4 |
| S. aureus 18 (MSSA clinical isolate) | 10 | 16 | 8 | 2 |
| S. aureus 25 (MSSA clinical isolate) | 40 | 16 | 2048 | 0.5 |
| S. aureus 26 (MSSA clinical isolate) | 10 | 64 | 8 | 2048 |
| S. aureus 67 (MRSA clinical isolate) | 40 | 2048 | 8 | 2048 |
| S. aureus 68 (MRSA clinical isolate) | 10 | 4 | 1 | 1 |
| S. aureus 73 (MRSA clinical isolate) | 40 | 128 | 2048 | 1 |
| S. mutans (clinical isolate) | 625 | 8 | 0.06 | 0.5 |
| S. sobrinus (clinical isolate) | 625 | 64 | 16 | 16 |
| S. salivarius (clinical isolate) | 625 | 2048 | 16 | 16 |
| S. gordonii (clinical isolate) | 625 | 16 | 0.06 | 0.06 |
| Bacterial Strains and Isolates | Amikacin | Ciprofloxacin | Ceftriaxone | |||
|---|---|---|---|---|---|---|
| FICI | MIC Decrease, Fold | FICI | MIC Decrease, Fold | FICI | MIC Decrease, Fold | |
| S. aureus ATCC (MSSA) | 0.375 | 8 | 0.75 | 2 | 1.25 | 1 |
| S. aureus 18 (MSSA clinical isolate) | 2.25 | 0.5 | 0.27 | 64 | 0.75 | 2 |
| S. aureus 25 (MSSA clinical isolate) | 0.27 | 64 | 1.25 | 1 | 0.37 | 8 |
| S. aureus 26 (MSSA clinical isolate) | 0.375 | 8 | 0.27 | 64 | 1.25 | 1 |
| S. aureus 67 (MRSA clinical isolate) | 1.25 | 1 | 0.26 | 128 | 1.25 | 1 |
| S. aureus 68 (MRSA clinical isolate) | 1.25 | 1 | 1.25 | 1 | 0.75 | 2 |
| S. aureus 73 (MRSA clinical isolate) | 0.25 | 1024 | 1.25 | 1 | 0.31 | 16 |
| S. mutans (clinical isolate) | 0.27 | 64 | 1.25 | 1 | 0.375 | 8 |
| S. sobrinus (clinical isolate) | 0.25 | 512 | 0.25 | 256 | 0.25 | 256 |
| S. salivarius (clinical isolate) | 1.25 | 1 | 0.25 | 256 | 0.25 | 256 |
| S. gordonii (clinical isolate) | 0.26 | 128 | 8.6 | 0.25 | 1.25 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaliullina, A.; Kolesnikova, A.; Khairullina, L.; Morgatskaya, O.; Shakirova, D.; Patov, S.; Nekrasova, P.; Bogachev, M.; Kurkin, V.; Trizna, E.; et al. The Antimicrobial Potential of the Hop (Humulus lupulus L.) Extract against Staphylococcus aureus and Oral Streptococci. Pharmaceuticals 2024, 17, 162. https://doi.org/10.3390/ph17020162
Khaliullina A, Kolesnikova A, Khairullina L, Morgatskaya O, Shakirova D, Patov S, Nekrasova P, Bogachev M, Kurkin V, Trizna E, et al. The Antimicrobial Potential of the Hop (Humulus lupulus L.) Extract against Staphylococcus aureus and Oral Streptococci. Pharmaceuticals. 2024; 17(2):162. https://doi.org/10.3390/ph17020162
Chicago/Turabian StyleKhaliullina, Alyona, Alyona Kolesnikova, Leysan Khairullina, Olga Morgatskaya, Dilyara Shakirova, Sergey Patov, Polina Nekrasova, Mikhail Bogachev, Vladimir Kurkin, Elena Trizna, and et al. 2024. "The Antimicrobial Potential of the Hop (Humulus lupulus L.) Extract against Staphylococcus aureus and Oral Streptococci" Pharmaceuticals 17, no. 2: 162. https://doi.org/10.3390/ph17020162
APA StyleKhaliullina, A., Kolesnikova, A., Khairullina, L., Morgatskaya, O., Shakirova, D., Patov, S., Nekrasova, P., Bogachev, M., Kurkin, V., Trizna, E., & Kayumov, A. (2024). The Antimicrobial Potential of the Hop (Humulus lupulus L.) Extract against Staphylococcus aureus and Oral Streptococci. Pharmaceuticals, 17(2), 162. https://doi.org/10.3390/ph17020162






