Synthesis, Characterization, and Toxicity Assessment of Zinc Oxide-Doped Manganese Oxide Nanoparticles in a Macrophage Model
Abstract
1. Introduction
2. Results and Discussion
2.1. Nanoparticle Characterization
2.2. Nanoparticle Preparation for In Vitro Studies and Characterization in Vehicle and Cell Culture Media (DMEM)
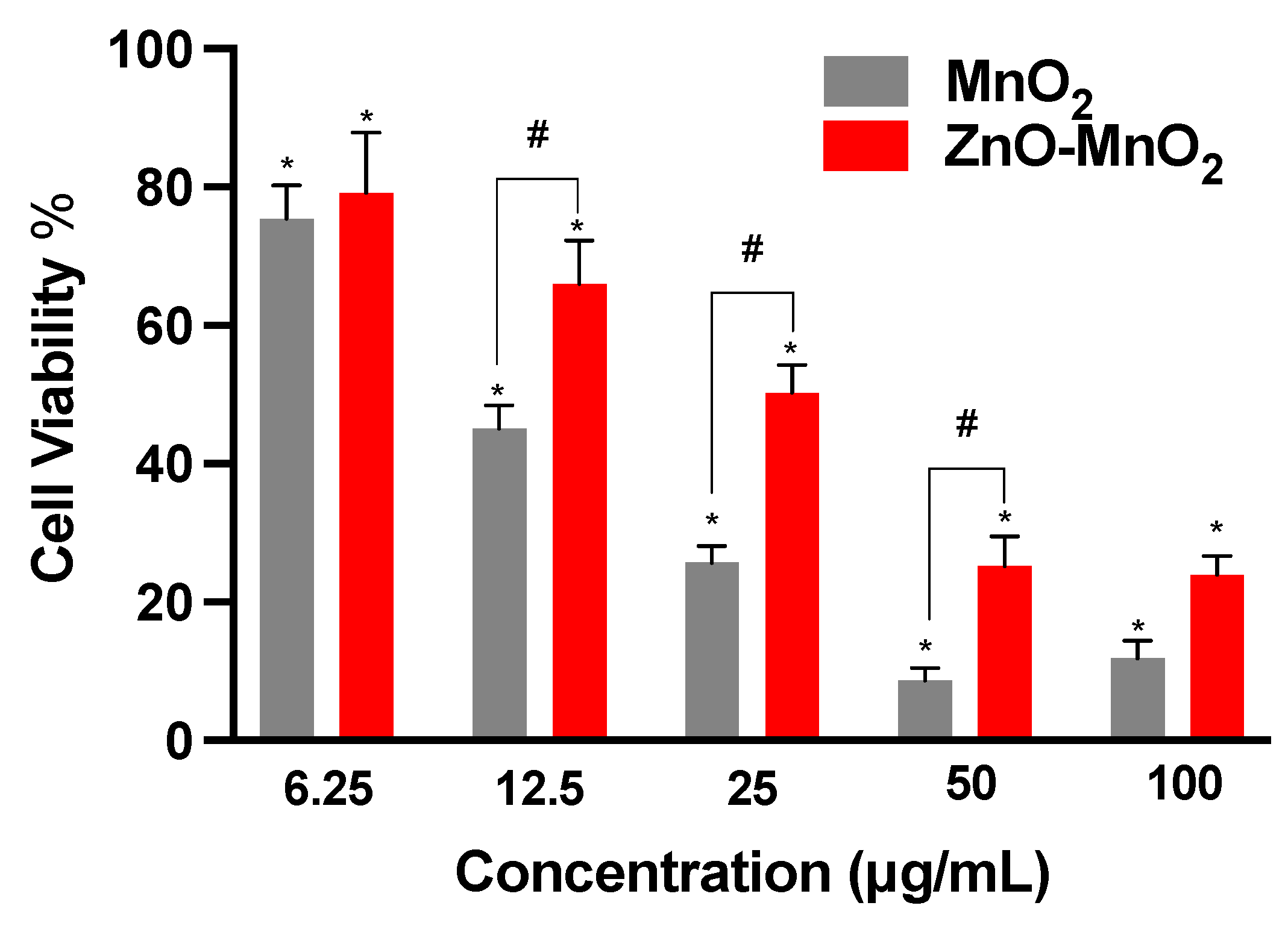
2.3. Cell Viability following Nanomaterial Treatment
2.4. Cell Functional Competence following Nanomaterial Treatment
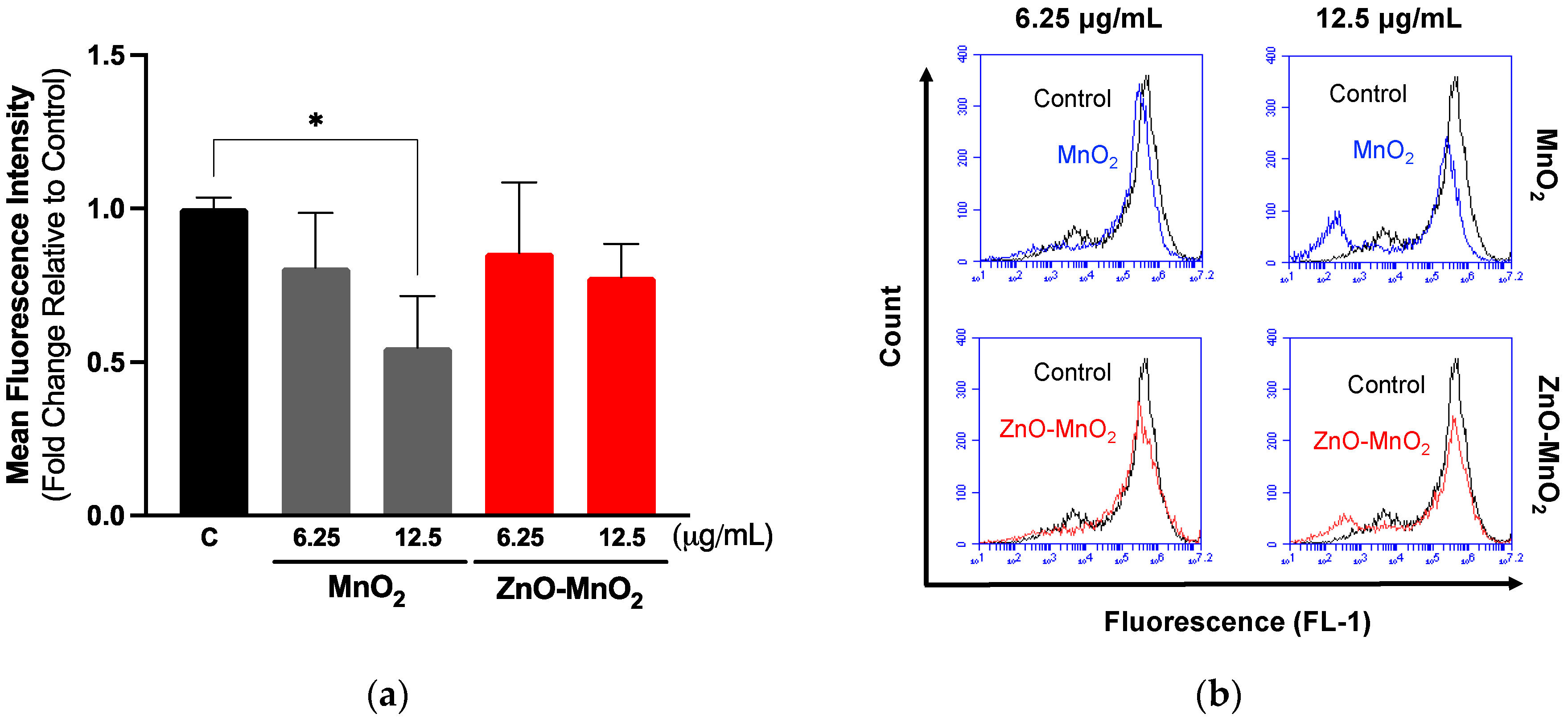
2.5. Inflammatory Response and Reactive Oxygen Species Generation following Nanomaterial Treatment
2.6. Antioxidant Response following Nanomaterial Treatment
2.7. Cellular Internalization following Nanomaterial Treatment
3. Materials and Methods
3.1. Nanoparticle Synthesis and Characterization
3.2. Nanoparticle Preparation for Biological Studies
3.3. Cell Culture and Viability Studies
3.4. Cell Phagocytosis
3.5. Gene Expression
3.6. ROS Generation
3.7. Western Blot Analysis
3.8. Nanoparticle Internalization
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Singh, A.V.; Laux, P.; Luch, A.; Sudrik, C.; Wiehr, S.; Wild, A.M.; Santomauro, G.; Bill, J.; Sitti, M. Review of emerging concepts in nanotoxicology: Opportunities and challenges for safer nanomaterial design. Toxicol. Mech. Methods 2019, 29, 378–387. [Google Scholar] [CrossRef]
- Liu, S.; Xia, T. Continued Efforts on Nanomaterial-Environmental Health and Safety Is Critical to Maintain Sustainable Growth of Nanoindustry. Small 2020, 16, e2000603. [Google Scholar] [CrossRef] [PubMed]
- Oksel Karakus, C.; Bilgi, E.; Winkler, D.A. Biomedical nanomaterials: Applications, toxicological concerns, and regulatory needs. Nanotoxicology 2021, 15, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ.-Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal-Organic Frameworks for Biomedical Applications. Small 2020, 16, e1906846. [Google Scholar] [CrossRef]
- Peng, X.; Ai, F.; Yan, L.; Ha, E.; Hu, X.; He, S.; Hu, J. Synthesis strategies and biomedical applications for doped inorganic semiconductor nanocrystals. Cell Rep. Phys. Sci. 2021, 2, 100436. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.A.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Role of Zn doping in oxidative stress mediated cytotoxicity of TiO2 nanoparticles in human breast cancer MCF-7 cells. Sci. Rep. 2016, 6, 30196. [Google Scholar] [CrossRef]
- Naskar, A.; Lee, S.; Kim, K.S. Antibacterial potential of Ni-doped zinc oxide nanostructure: Comparatively more effective against Gram-negative bacteria including multi-drug resistant strains. RSC Adv. 2020, 10, 1232–1242. [Google Scholar] [CrossRef]
- Saha, P.; Rakshit, R.; Mandal, K. Enhanced magnetic properties of Zn doped Fe3O4 nano hollow spheres for better bio-medical applications. J. Magn. Magn. Mater. 2019, 475, 130–136. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, G.; Qiu, L.; Ye, X. Facile synthesis of Mg-doped calcium silicate porous nanoparticles for targeted drug delivery and ovarian cancer treatment. Ceram. Int. 2021, 47, 24942–24948. [Google Scholar] [CrossRef]
- Saleh, N.B.; Milliron, D.J.; Aich, N.; Katz, L.E.; Liljestrand, H.M.; Kirisits, M.J. Importance of doping, dopant distribution, and defects on electronic band structure alteration of metal oxide nanoparticles: Implications for reactive oxygen species. Sci. Total Environ. 2016, 568, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Scimeca, J.C.; Ivanovski, S.; Verron, E. Double-edged sword: Therapeutic efficacy versus toxicity evaluations of doped titanium implants. Drug Discov. Today 2021, 26, 2734–2742. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, R.U.K.; Rama, A.; Govindan, I.; Naha, A. The Purview of Doped Nanoparticles: Insights into Their Biomedical Applications. In OpenNano; Elsevier: Amsterdam, The Netherlands, 2022; p. 100070. [Google Scholar]
- Avila, D.S.; Puntel, R.L.; Aschner, M. Manganese in health and disease. Interrelat. Between Essent. Met. Ions Hum. Dis. 2013, 13, 199–227. [Google Scholar] [CrossRef]
- Hoseinpour, V.; Ghaemi, N. Green synthesis of manganese nanoparticles: Applications and future perspective—A review. J. Photochem. Photobiol. B Biol. 2018, 189, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhu, Q.; Zeng, Y.; Zeng, Q.; Chen, X.; Zhan, Y. Manganese Oxide Nanoparticles As MRI Contrast Agents In Tumor Multimodal Imaging And Therapy. Int. J. Nanomed. 2019, 14, 8321–8344. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Zheng, P.; Ma, P.; Lin, J. Manganese Oxide Nanomaterials: Synthesis, Properties, and Theranostic Applications. Adv. Mater. 2020, 32, e1905823. [Google Scholar] [CrossRef]
- Qian, X.; Han, X.; Yu, L.; Xu, T.; Chen, Y. Manganese-based functional nanoplatforms: Nanosynthetic construction, physiochemical property, and theranostic applicability. Adv. Funct. Mater. 2020, 30, 1907066. [Google Scholar] [CrossRef]
- Lin, L.S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew. Chem. 2018, 130, 4996–5000. [Google Scholar] [CrossRef]
- Xu, J.; Han, W.; Yang, P.; Jia, T.; Dong, S.; Bi, H.; Gulzar, A.; Yang, D.; Gai, S.; He, F. Tumor microenvironment-responsive mesoporous MnO2-coated upconversion nanoplatform for self-enhanced tumor theranostics. Adv. Funct. Mater. 2018, 28, 1803804. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, W.; Pan, C.; Feng, L.; Ou, M.; Zeng, X.; Liang, X.; Wu, M.; Ji, X.; Mei, L. SnTe@ MnO2-SP nanosheet–based intelligent nanoplatform for second near-infrared light–mediated cancer theranostics. Adv. Funct. Mater. 2019, 29, 1903791. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, M.; Kumari, S.I.; Rahman, M.F.; Mahboob, M.; Grover, P. Toxicity assessment of manganese oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral exposure. J. Appl. Toxicol. 2013, 33, 1165–1179. [Google Scholar] [CrossRef]
- Katsnelson, B.A.; Minigaliyeva, I.A.; Panov, V.G.; Privalova, L.I.; Varaksin, A.N.; Gurvich, V.B.; Sutunkova, M.P.; Shur, V.Y.; Shishkina, E.V.; Valamina, I.E.; et al. Some patterns of metallic nanoparticles’ combined subchronic toxicity as exemplified by a combination of nickel and manganese oxide nanoparticles. Food Chem. Toxicol. 2015, 86, 351–364. [Google Scholar] [CrossRef]
- Mate, Z.; Horvath, E.; Kozma, G.; Simon, T.; Konya, Z.; Paulik, E.; Papp, A.; Szabo, A. Size-Dependent Toxicity Differences of Intratracheally Instilled Manganese Oxide Nanoparticles: Conclusions of a Subacute Animal Experiment. Biol. Trace Elem. Res. 2016, 171, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, J.; Milosevic, A.; Hauser, D.; Lehner, R.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Biodistribution, Clearance, and Long-Term Fate of Clinically Relevant Nanomaterials. Adv. Mater. 2018, 30, e1704307. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Yu, Y.; Qiu, Q.; Sun, X.; Meng, F.; Bi, Y.; Gu, J.; Wang, Y.; Zhang, F.; Huo, H. Mechanisms of BPA Degradation and Toxicity Resistance in Rhodococcus equi. Microorganisms 2022, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Jangid, A.K.; Pooja, D.; Kulhari, H. Design of manganese-based nanomaterials for pharmaceutical and biomedical applications. J. Mater. Chem. B 2024, 12, 577–608. [Google Scholar] [CrossRef] [PubMed]
- Olivito, F.; Jagdale, P.; Oza, G. Synthesis and Biodegradation Test of a New Polyether Polyurethane Foam Produced from PEG 400, (L)-Lysine Ethyl Ester Diisocyanate (L-LDI) and Bis-hydroxymethyl Furan (BHMF). Toxics 2023, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Di Bernardo, I.; Kreider, P.; Tran-Phu, T.; Cai, X.; Wang, N.; Zhu, Y.; Venkataraman, M.B.; Lipton-Duffin, J.; Bayon, A. Lattice expansion in optimally doped manganese oxide: An effective structural parameter for enhanced thermochemical water splitting. ACS Catal. 2019, 9, 9880–9890. [Google Scholar] [CrossRef]
- Huang, K.Z.; Zhang, H. Direct electron-transfer-based peroxymonosulfate activation by iron-doped manganese oxide (δ-MnO2) and the development of galvanic oxidation processes (GOPs). Environ. Sci. Technol. 2019, 53, 12610–12620. [Google Scholar] [CrossRef] [PubMed]
- Balaghi, S.E.; Triana, C.A.; Patzke, G.R. Molybdenum-doped manganese oxide as a highly efficient and economical water oxidation catalyst. ACS Catalysis 2020, 10, 2074–2087. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Kuniyil, M.; Khan, M.; Shaik, M.R.; Alwarthan, A.; Labis, J.P.; Siddiqui, M.R.H. Synthesis and comparative catalytic study of zinc oxide (ZnO x) nanoparticles promoted MnCO3, MnO2 and Mn2O3 for selective oxidation of benzylic alcohols using molecular oxygen. Mater. Express 2017, 7, 79–92. [Google Scholar] [CrossRef]
- Gac, W. The influence of silver on the structural, redox and catalytic properties of the cryptomelane-type manganese oxides in the low-temperature CO oxidation reaction. Appl. Catal. B Environ. 2007, 75, 107–117. [Google Scholar] [CrossRef]
- Dubal, D.; Dhawale, D.; Salunkhe, R.; Pawar, S.; Lokhande, C. A novel chemical synthesis and characterization of Mn3O4 thin films for supercapacitor application. Appl. Surf. Sci. 2010, 256, 4411–4416. [Google Scholar] [CrossRef]
- Maslen, E.; Streltsov, V.; Streltsova, N.; Ishizawa, N. Electron density and optical anisotropy in rhombohedral carbonates. III. Synchrotron X-ray studies of CaCO3, MgCO3 and MnCO3. Acta Crystallogr. Sect. B Struct. Sci. 1995, 51, 929–939. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef]
- Eom, H.J.; Choi, J. Oxidative stress of CeO2 nanoparticles via p38-Nrf-2 signaling pathway in human bronchial epithelial cell, Beas-2B. Toxicol. Lett. 2009, 187, 77–83. [Google Scholar] [CrossRef]
- Zhang, T.; Gaffrey, M.J.; Thomas, D.G.; Weber, T.J.; Hess, B.M.; Weitz, K.K.; Piehowski, P.D.; Petyuk, V.A.; Moore, R.J.; Qian, W.J.; et al. A proteome-wide assessment of the oxidative stress paradigm for metal and metal-oxide nanomaterials in human macrophages. NanoImpact 2020, 17, 100194. [Google Scholar] [CrossRef]
- DeLoid, G.; Casella, B.; Pirela, S.; Filoramo, R.; Pyrgiotakis, G.; Demokritou, P.; Kobzik, L. Effects of engineered nanomaterial exposure on macrophage innate immune function. NanoImpact 2016, 2, 70–81. [Google Scholar] [CrossRef]
- Alsaleh, N.B.; Assiri, M.A.; Aljarbou, A.M.; Almutairi, M.M.; As Sobeai, H.M.; Alshamrani, A.A.; Almudimeegh, S. Adverse Responses following Exposure to Subtoxic Concentrations of Zinc Oxide and Nickle Oxide Nanoparticles in the Raw 264.7 Cells. Toxics 2023, 11, 674. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Figueiredo Borgognoni, C.; Kim, J.H.; Zucolotto, V.; Fuchs, H.; Riehemann, K. Human macrophage responses to metal-oxide nanoparticles: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 694–703. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, T.; Gaffrey, M.J.; Weitz, K.K.; Moore, R.J.; Li, X.; Xian, M.; Thrall, B.D.; Qian, W.J. Stochiometric quantification of the thiol redox proteome of macrophages reveals subcellular compartmentalization and susceptibility to oxidative perturbations. Redox Biol. 2020, 36, 101649. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, N.; Zhu, M.; Lu, J.; Zhong, H.; Xue, X.; Guo, S.; Li, M.; Wei, X.; Tao, Y.; et al. TiO2 nanoparticles cause mitochondrial dysfunction, activate inflammatory responses, and attenuate phagocytosis in macrophages: A proteomic and metabolomic insight. Redox Biol. 2018, 15, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Alsaleh, N.B.; Minarchick, V.C.; Mendoza, R.P.; Sharma, B.; Podila, R.; Brown, J.M. Silver nanoparticle immunomodulatory potential in absence of direct cytotoxicity in RAW 264.7 macrophages and MPRO 2.1 neutrophils. J. Immunotoxicol. 2019, 16, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Nawrot, T.S.; Baccarelli, A.A. Hallmarks of environmental insults. Cell 2021, 184, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Pokhrel, S.; Ji, Z.; Henderson, B.L.; Xia, T.; Li, L.; Zink, J.I.; Nel, A.E.; Mädler, L. Role of Fe doping in tuning the band gap of TiO2 for the photo-oxidation-induced cytotoxicity paradigm. J. Am. Chem. Soc. 2011, 133, 11270–11278. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.P.; et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef] [PubMed]
- Persaud, I.; Raghavendra, A.J.; Paruthi, A.; Alsaleh, N.B.; Minarchick, V.C.; Roede, J.R.; Podila, R.; Brown, J.M. Defect-induced electronic states amplify the cellular toxicity of ZnO nanoparticles. Nanotoxicology 2020, 14, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; et al. Decreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryos. ACS Nano 2011, 5, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Naatz, H.; Lin, S.; Li, R.; Jiang, W.; Ji, Z.; Chang, C.H.; Koser, J.; Thoming, J.; Xia, T.; Nel, A.E.; et al. Safe-by-Design CuO Nanoparticles via Fe-Doping, Cu-O Bond Length Variation, and Biological Assessment in Cells and Zebrafish Embryos. ACS Nano 2017, 11, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Huo, D.; Li, H.; Zhang, B.; Li, Y.; Liang, A.; Wang, H.; Yu, Q.; Li, M. ROS-independent toxicity of Fe3O4 nanoparticles to yeast cells: Involvement of mitochondrial dysfunction. Chem. Biol. Interact. 2018, 287, 20–26. [Google Scholar] [CrossRef]
- Yusuf, A.; Brophy, A.; Gorey, B.; Casey, A. Liposomal encapsulation of silver nanoparticles enhances cytotoxicity and causes induction of reactive oxygen species-independent apoptosis. J. Appl. Toxicol. 2018, 38, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.O.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Refsnes, M.; Skuland, T.; Lilleaas, E.; Ovrevik, J.; Lag, M. Concentration-dependent cytokine responses of silica nanoparticles and role of ROS in human lung epithelial cells. Basic Clin. Pharmacol. Toxicol. 2019, 125, 304–314. [Google Scholar] [CrossRef]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 2014, 6, 7052–7061. [Google Scholar] [CrossRef]
- Alsaleh, N.B.; Brown, J.M. Immune responses to engineered nanomaterials: Current understanding and challenges. Curr. Opin. Toxicol. 2018, 10, 8–14. [Google Scholar] [CrossRef]
- Cortez-Jugo, C.; Czuba-Wojnilowicz, E.; Tan, A.; Caruso, F. A Focus on “Bio” in Bio-Nanoscience: The Impact of Biological Factors on Nanomaterial Interactions. Adv. Healthc. Mater. 2021, 10, e2100574. [Google Scholar] [CrossRef]
- Assal, M.E.; Kuniyil, M.; Khan, M.; Shaik, M.R.; Al-Warthan, A.; Siddiqui, M.R.H.; Labis, J.P.; Adil, S.F. Comparative catalytic evaluation of nano-ZrOx promoted manganese catalysts: Kinetic study and the effect of dopant on the aerobic oxidation of secondary alcohols. Adv. Mater. Sci. Eng. 2017, 2017, 3958319. [Google Scholar] [CrossRef]
- Almomen, A.; Alsaleh, N.B.; El-Toni, A.M.; El-Mahrouky, M.A.; Alhowyan, A.A.; Alkholief, M.; Alshamsan, A.; Khurana, N.; Ghandehari, H. In Vitro Safety Assessment of In-House Synthesized Titanium Dioxide Nanoparticles: Impact of Washing and Temperature Conditions. Int. J. Mol. Sci. 2023, 24, 9966. [Google Scholar] [CrossRef]
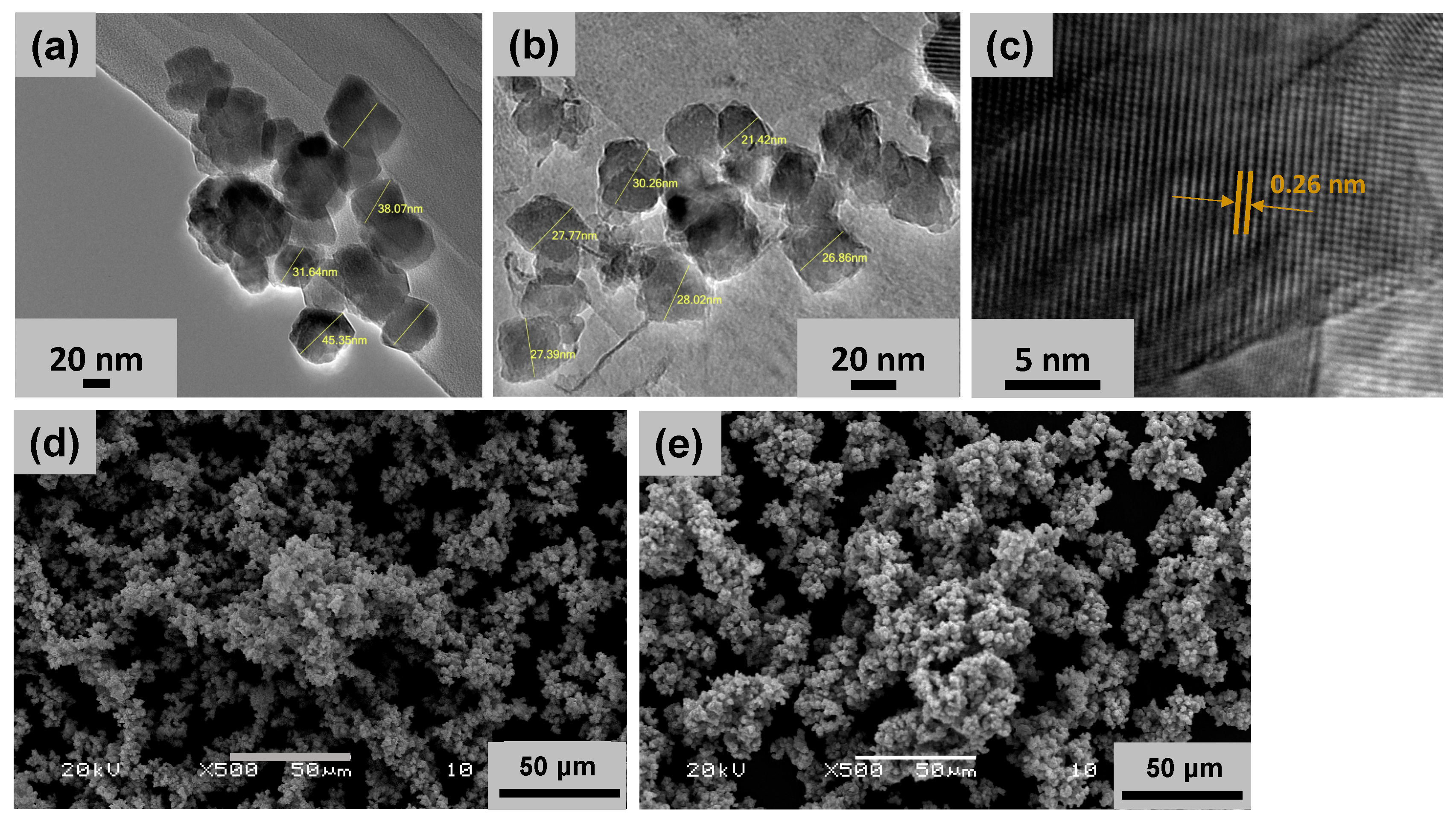




| Nanoparticle—Media | Hydrodynamic Size (dh) (nm) | Zeta Potential (ζ) (mV) | PDI |
|---|---|---|---|
| MnO2 NPs—UPW | 197 ± 35 | −16.2 ± 3.7 | 0.20 |
| MnO2 NPs—DMEM | 483 ± 15 | −4.8 ± 1 | 0.25 |
| ZnO-MnO2 NPs—UPW | 245 ± 7 | −20 ± 5.2 | 0.25 |
| ZnO-MnO2 NPs—DMEM | 546 ± 30 | −5.5 ± 1.8 | 0.29 |
| Gene | Primer Sequence |
|---|---|
| TNF-α | Forward 5′-ACTGAACTTCGGGGTGATTG-3′ Reverse 5′- GCTTGGTGGTTTGCTACGAC-3′ |
| IL-1β | Forward 5′-CTATGGCAACTGTCCCTGAA-3′ Reverse 5′-GGCTTGGAAGCAATCCTTAATC-3′ |
| GAPDH | Forward 5′-GGAAAGCTGTGGCGTGAT-3′ Reverse 5′-AAGGTGGAAGAATGGGAGTT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaleh, N.B.; Aljarbou, A.M.; Assal, M.E.; Assiri, M.A.; Almutairi, M.M.; As Sobeai, H.M.; Alshamrani, A.A.; Almudimeegh, S.; Hatshan, M.R.; Adil, S.F. Synthesis, Characterization, and Toxicity Assessment of Zinc Oxide-Doped Manganese Oxide Nanoparticles in a Macrophage Model. Pharmaceuticals 2024, 17, 168. https://doi.org/10.3390/ph17020168
Alsaleh NB, Aljarbou AM, Assal ME, Assiri MA, Almutairi MM, As Sobeai HM, Alshamrani AA, Almudimeegh S, Hatshan MR, Adil SF. Synthesis, Characterization, and Toxicity Assessment of Zinc Oxide-Doped Manganese Oxide Nanoparticles in a Macrophage Model. Pharmaceuticals. 2024; 17(2):168. https://doi.org/10.3390/ph17020168
Chicago/Turabian StyleAlsaleh, Nasser B., Anas M. Aljarbou, Mohamed E. Assal, Mohammed A. Assiri, Mohammed M. Almutairi, Homood M. As Sobeai, Ali A. Alshamrani, Sultan Almudimeegh, Mohammad R. Hatshan, and Syed F. Adil. 2024. "Synthesis, Characterization, and Toxicity Assessment of Zinc Oxide-Doped Manganese Oxide Nanoparticles in a Macrophage Model" Pharmaceuticals 17, no. 2: 168. https://doi.org/10.3390/ph17020168
APA StyleAlsaleh, N. B., Aljarbou, A. M., Assal, M. E., Assiri, M. A., Almutairi, M. M., As Sobeai, H. M., Alshamrani, A. A., Almudimeegh, S., Hatshan, M. R., & Adil, S. F. (2024). Synthesis, Characterization, and Toxicity Assessment of Zinc Oxide-Doped Manganese Oxide Nanoparticles in a Macrophage Model. Pharmaceuticals, 17(2), 168. https://doi.org/10.3390/ph17020168








