Potential of DPD ((S)-4,5-dihydroxy-2,3-pentanedione) Analogs in Microparticulate Formulation as Vaccine Adjuvants
Abstract
:1. Introduction
2. Results
2.1. Microparticle Formulation and Quatitation
2.2. Particle Size Analysis
2.3. Zeta Potential Measurement
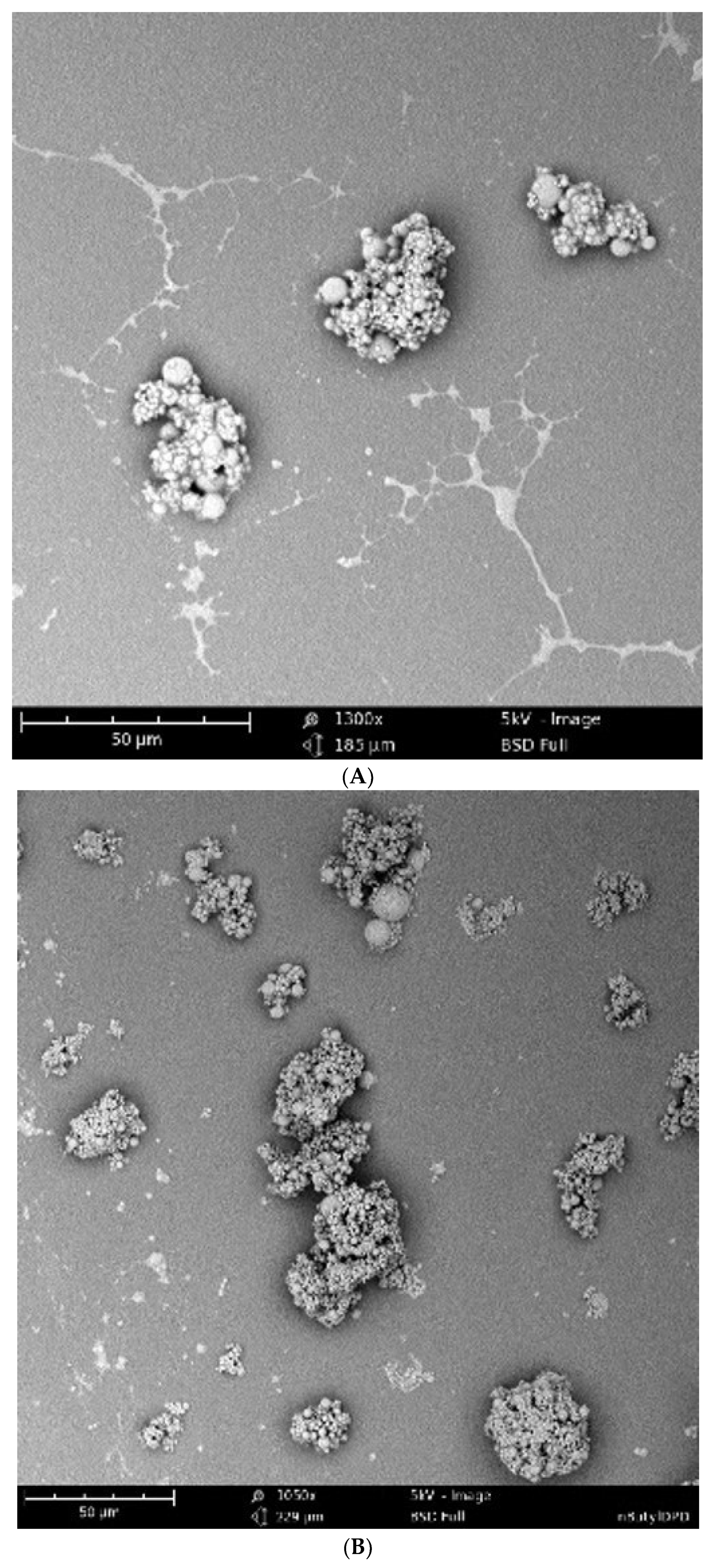
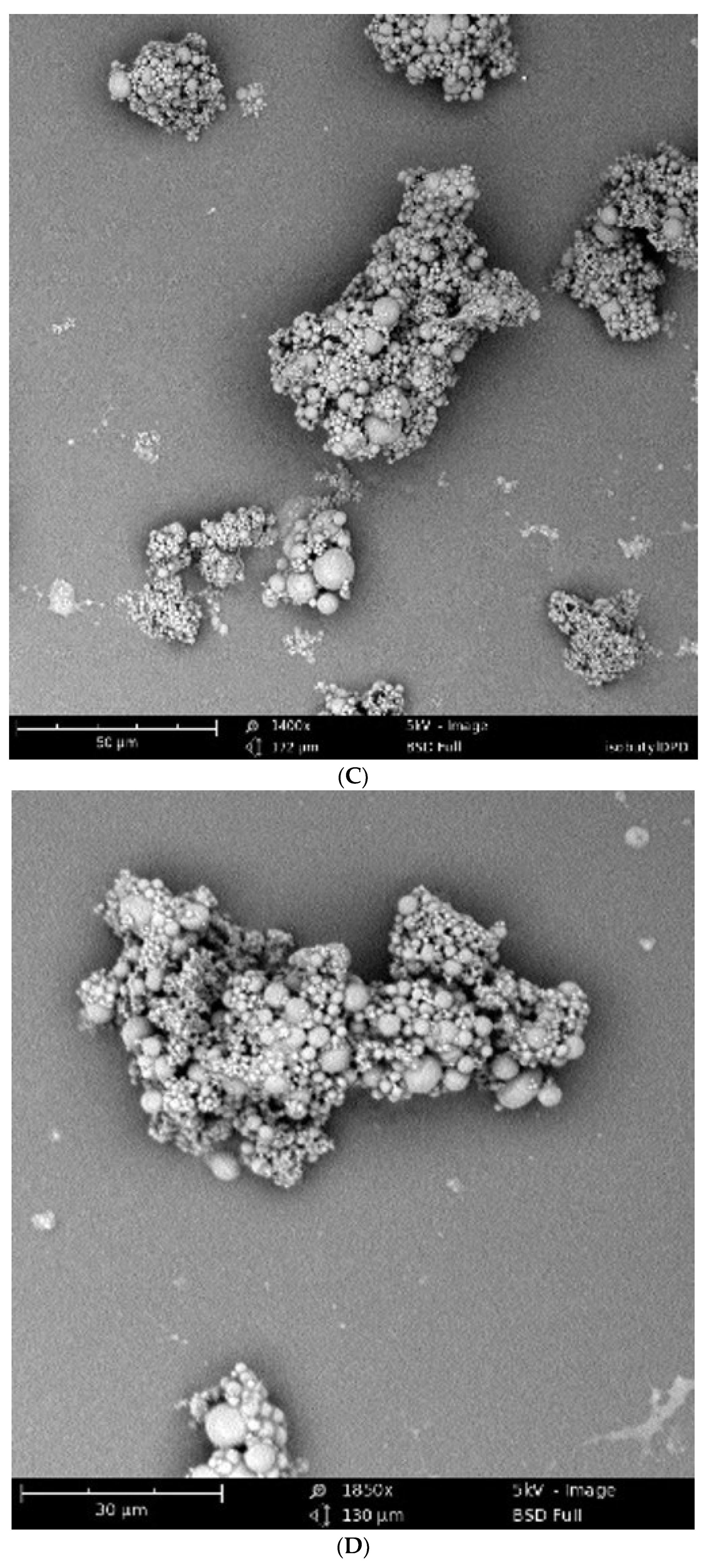
2.4. Scanning Electron Microscopy (SEM) Analysis
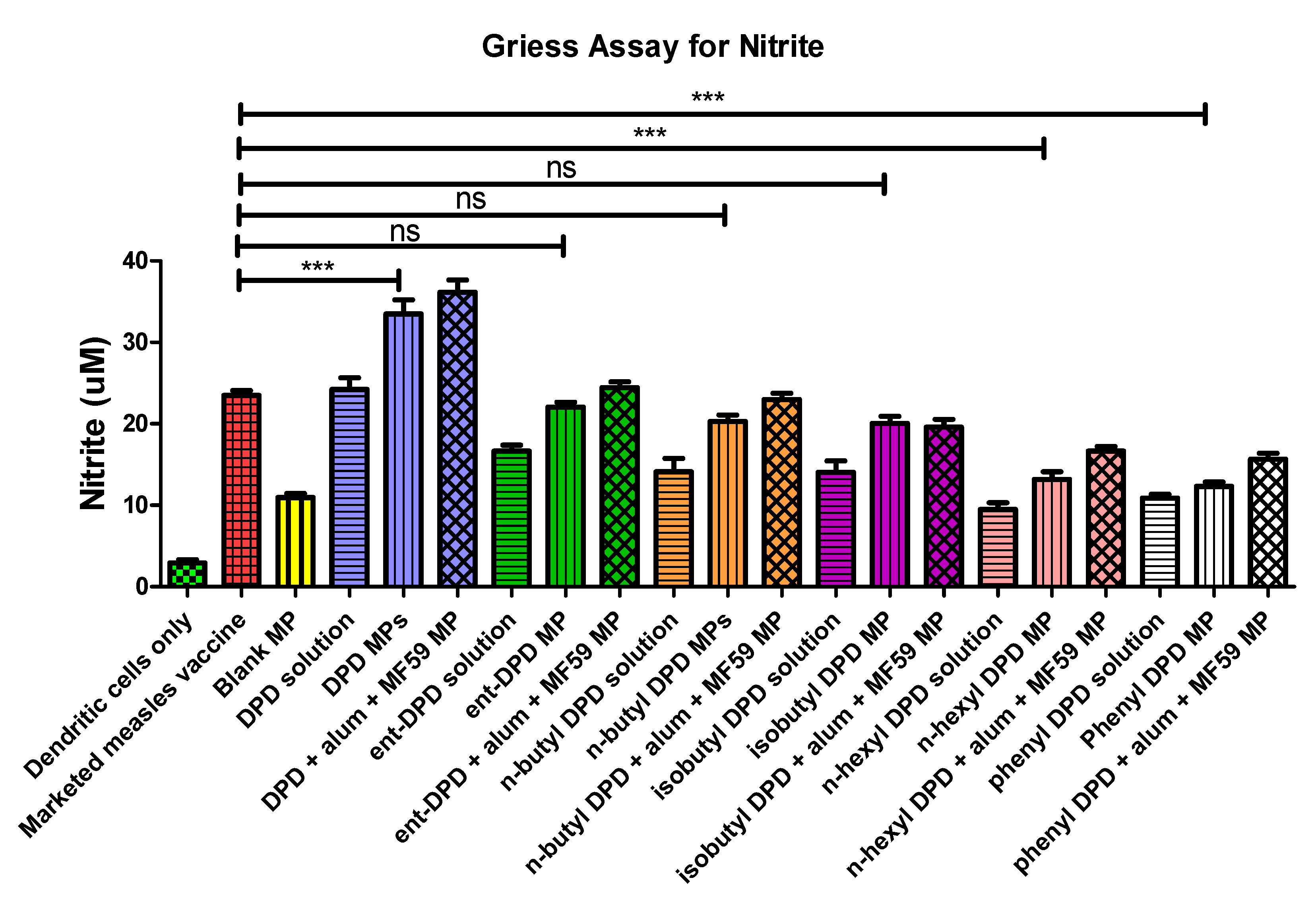
2.5. Griess’s Assay Analysis
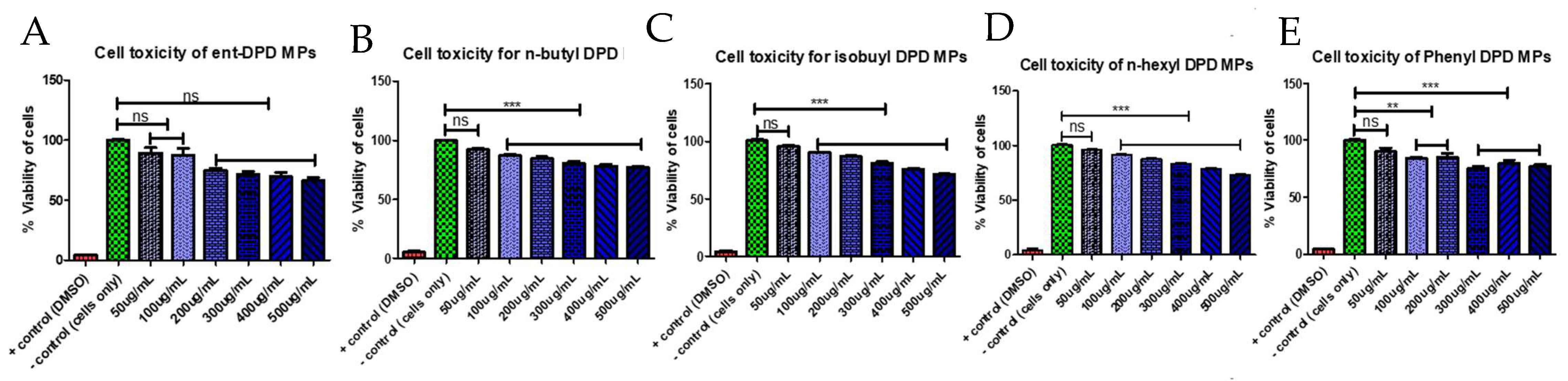
2.6. Cytotoxicity Study
2.7. In Vitro Release Study of Analogs of DPD
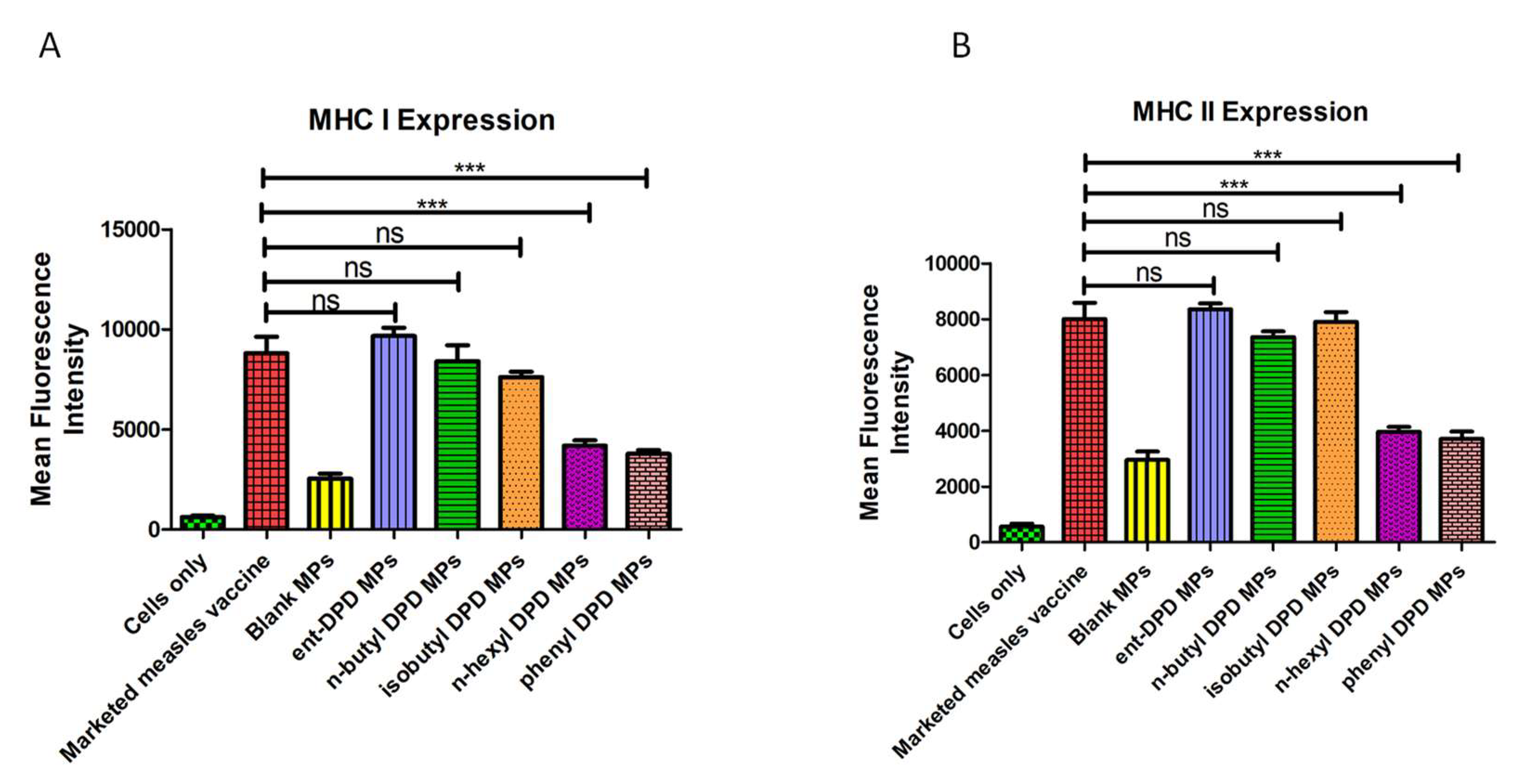
2.8. Expression of Antigen-Presenting Molecules
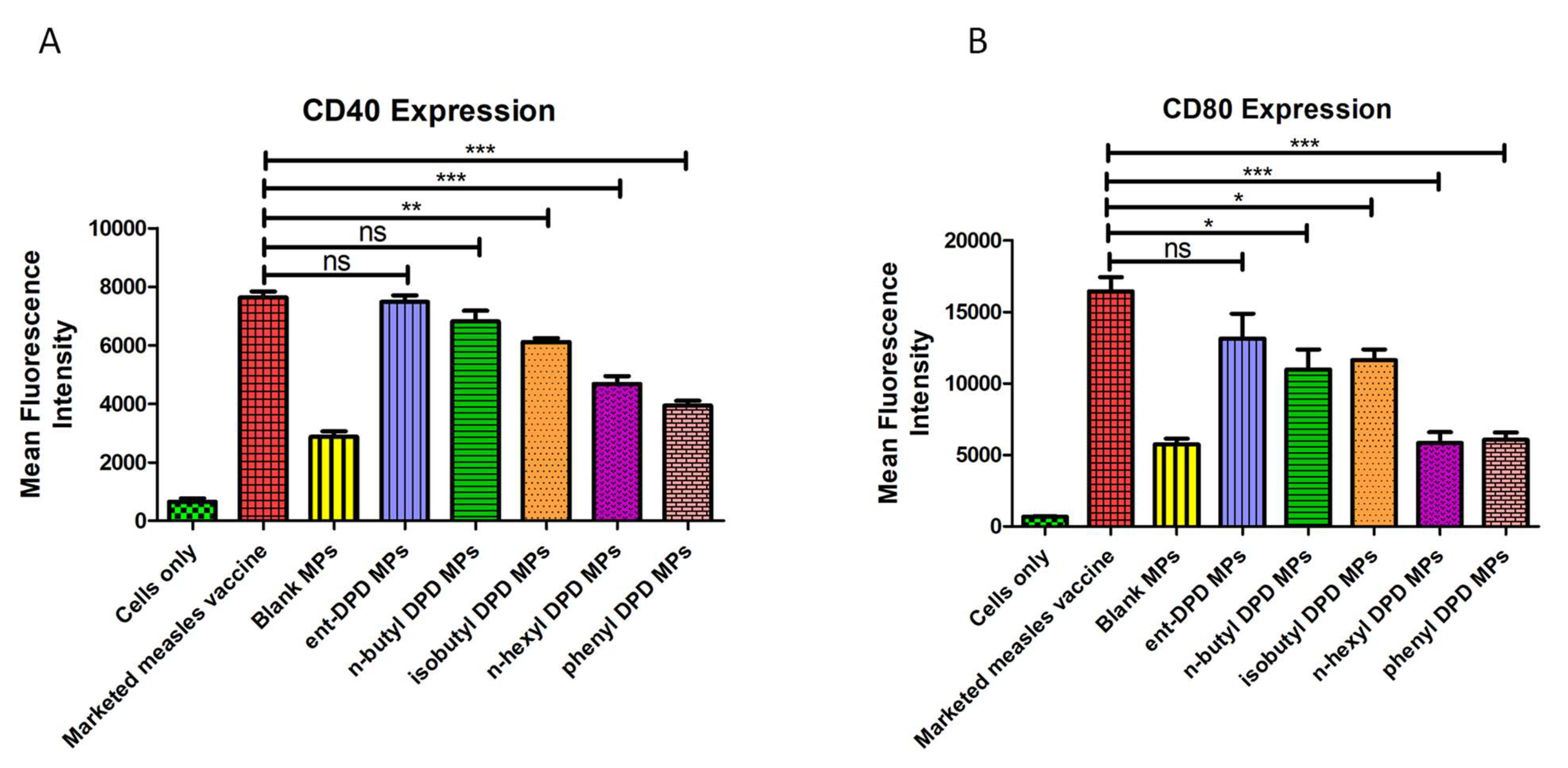
2.9. Expression of Costimulatory Molecules
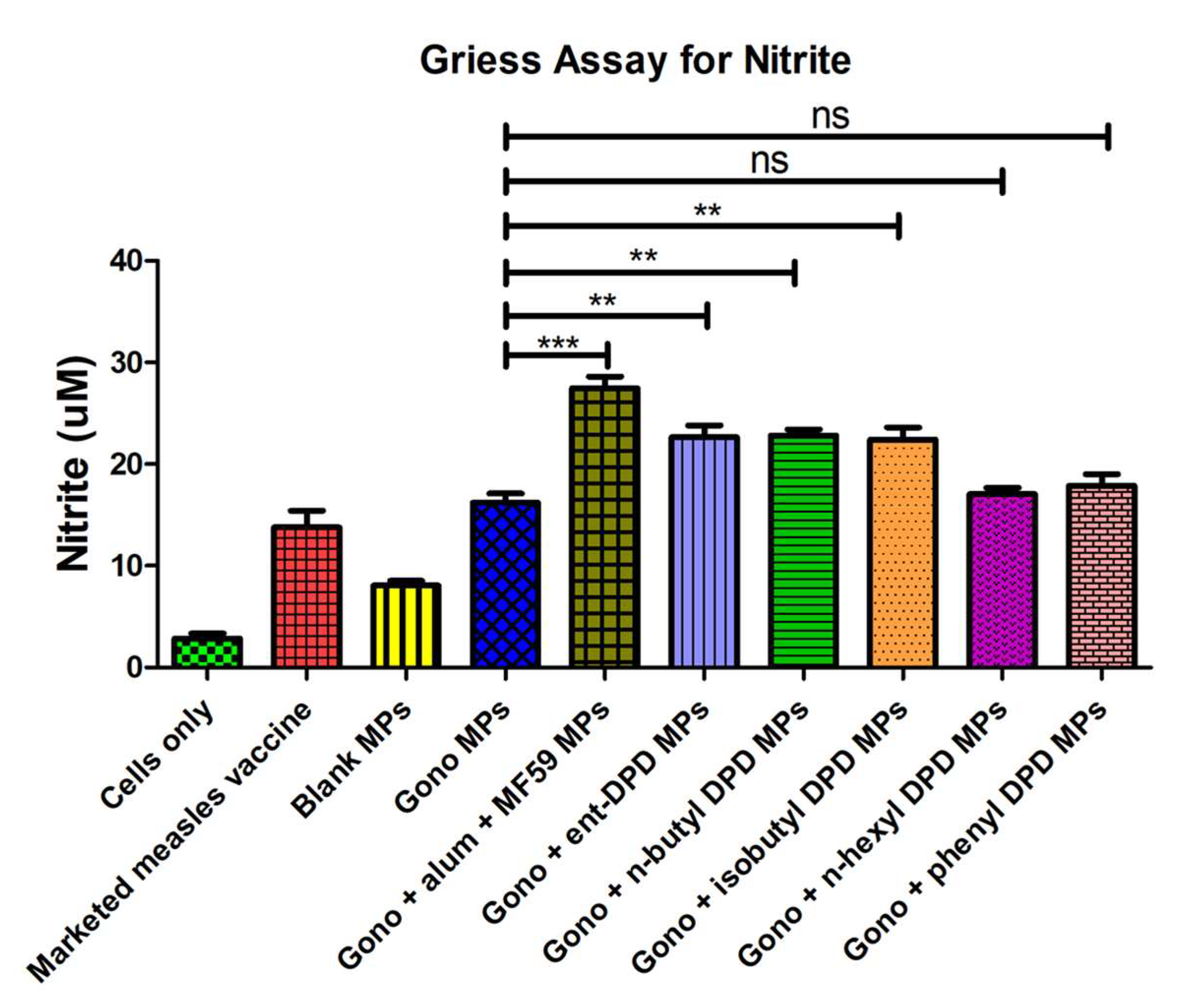
2.10. Evaluation of Adjuvant Effect: Griess’s Assay for Nitrite
2.11. Evaluation of Adjuvant Effect with Bacterial and Viral Vaccines: Griess’s Assay for Nitrite
2.11.1. Evaluation of Adjuvant Effect with MenAfriVac (MAV), a Meningococcal A Conjugate Vaccine Microparticle
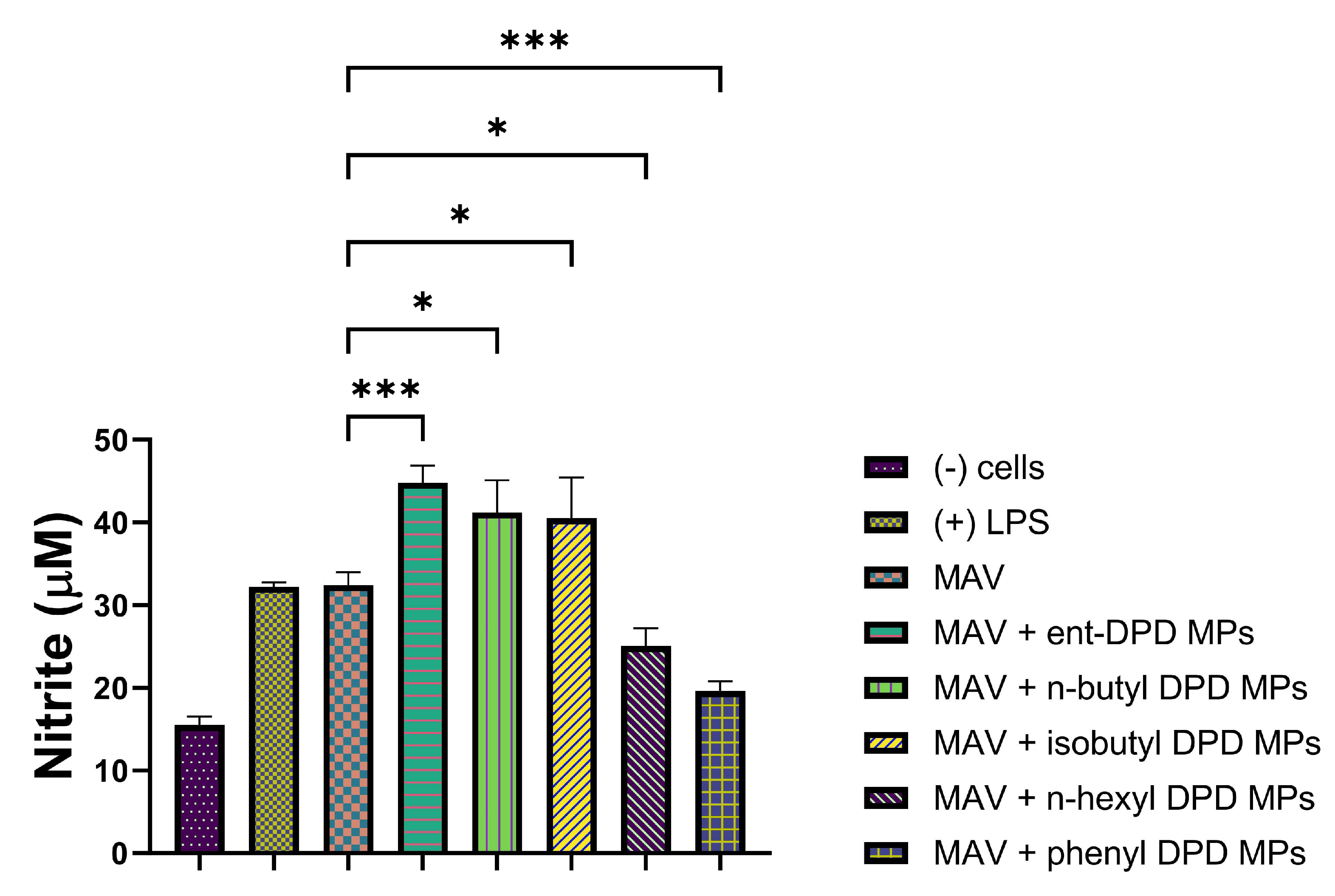
2.11.2. Evaluation of Adjuvant Effect with Hemophilus Influenzae Type B (HIB) Conjugate Vaccine Microparticles
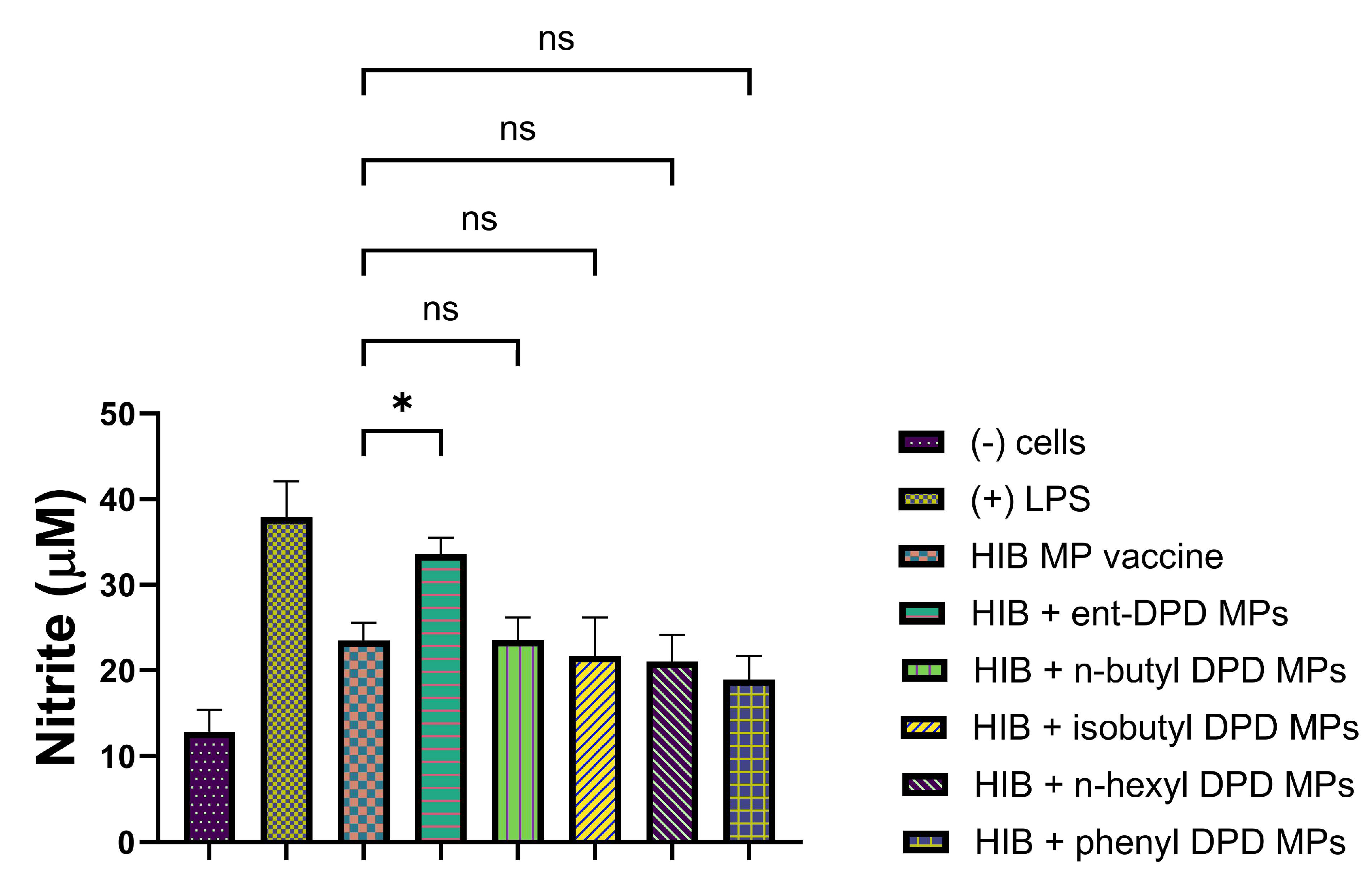
2.11.3. Evaluation of Adjuvant Effect with Measles Vaccine Microparticles
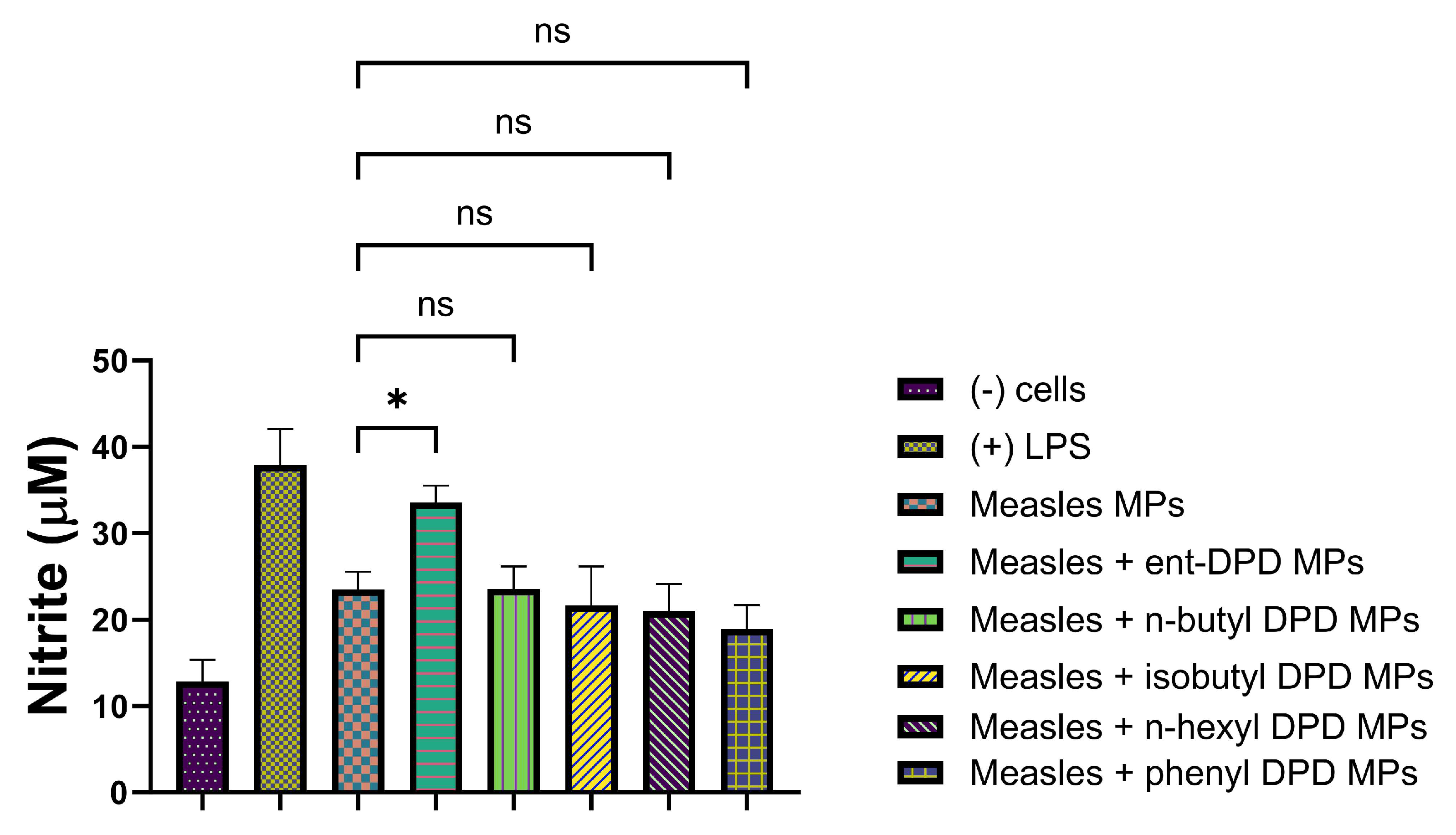
2.11.4. Evaluation of Adjuvant Effect with Influenza (S) Vaccine

2.11.5. Evaluation of Adjuvant Effect with Inactivated Zika Vaccine Microparticles

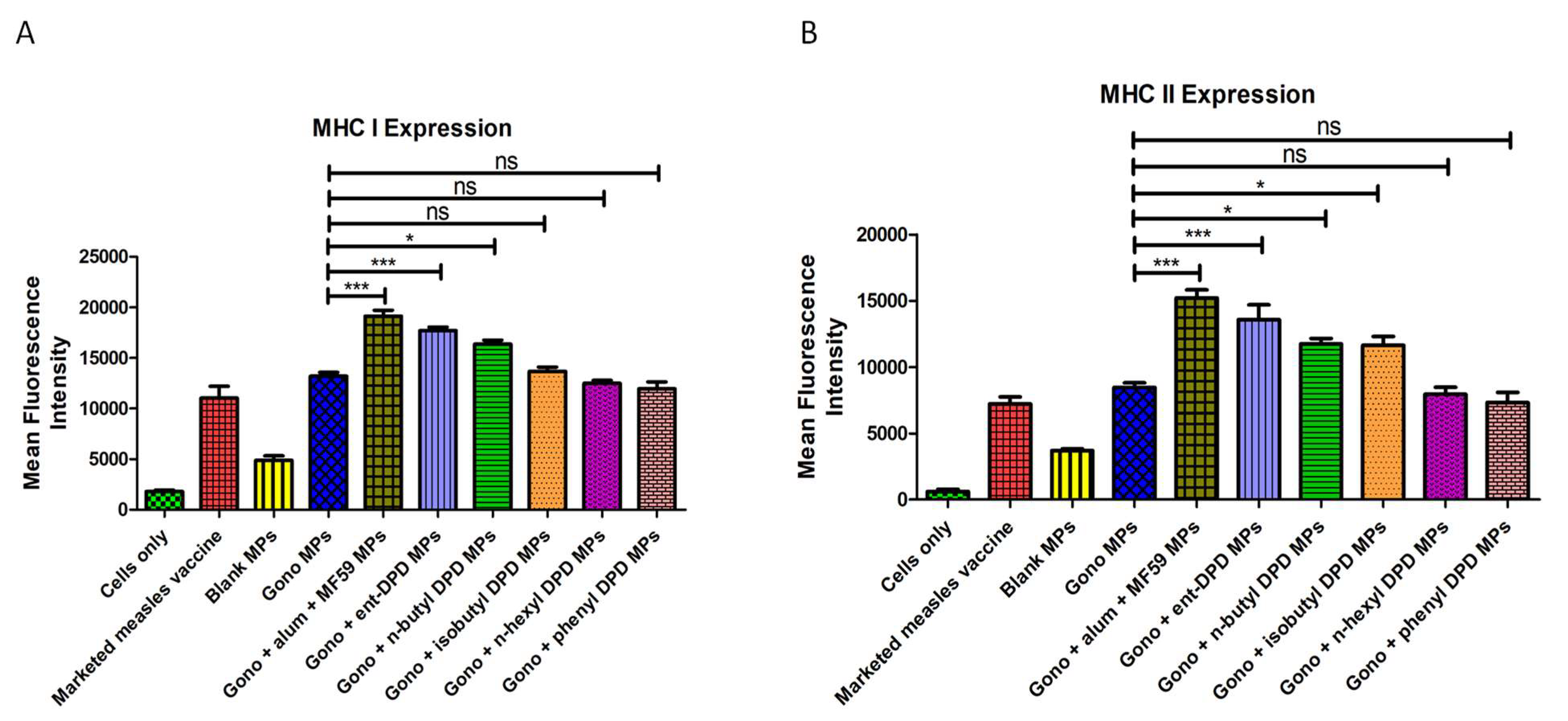
2.12. Evaluation of Adjuvant Effect: Expression of Antigen-Presenting Molecules
2.13. Evaluation of Adjuvant Effect: Expression of Costimulatory Molecules
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Formulation of Microparticles
4.2.2. Microparticle Recovery Yield
4.2.3. Particle Size and Zeta Potential
4.2.4. Scanning Electron Microscopy
4.2.5. Griess’s Assay for Nitrite
4.2.6. Cytotoxicity Measurement
4.2.7. In Vitro Release Study
4.2.8. Expression of Antigen-Presenting Molecules
4.2.9. Expression of Costimulatory Molecules
4.2.10. Griess’s Assay
4.2.11. Adjuvant Effect: Expression of Antigen-Presenting Molecules
4.2.12. Adjuvant Effect: Expression of Costimulatory Molecules
4.2.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilar, J.C.; Rodríguez, E.G. Vaccine adjuvants revisited. Vaccine 2007, 25, 3752–3762. [Google Scholar] [CrossRef]
- Baba, T.A.M.; Akashi, M. Regulation of Immune Responses by Nanoparticle-Based Vaccine. In Biodegradable Nanoparticles as Vaccine Adjuvants and Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2011; p. 31. [Google Scholar]
- Singh, M.; O’Hagan, D.T. Recent advances in vaccine adjuvants. Pharm. Res. 2002, 19, 715–728. [Google Scholar] [CrossRef]
- Gupta, R.K. Aluminum compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 1998, 32, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-X.; Xie, Y.; Ye, Y.-P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, C.; Fenizia, C.; Carnovale, C.; Pozzi, M.; Trabattoni, D.; Cervia, D.; Clementi, E. Updated Considerations for the Immunopharmacological Aspects of the “Talented mRNA Vaccines”. Vaccines 2023, 11, 1481. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Jailani, A.A.K.; Mandal, B. Exigency of Plant-Based Vaccine against COVID-19 Emergence as Pandemic Preparedness. Vaccines 2023, 11, 1347. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Alsaab, H.O.; Rawas-Qalaji, M.M.; Uddin, M.N. A Review on Current COVID-19 Vaccines and Evaluation of Particulate Vaccine Delivery Systems. Vaccines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Singh, M.; O’Hagan, D. Advances in vaccine adjuvants. Nat. Biotechnol. 1999, 17, 1075–1081. [Google Scholar] [CrossRef]
- Surette, M.G.; Miller, M.B.; Bassler, B.L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: A new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 1999, 96, 1639–1644. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. QUORUM SENSING: Cell-to-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Bassler, B.L.; Losick, R. Bacterially Speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Lowery, C.A.; Dickerson, T.J.; Janda, K.D. Interspecies and interkingdom communication mediated by bacterial quorum sensing. Chem. Soc. Rev. 2008, 37, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.-L.; Bassler, B.L. Bacterial Quorum-Sensing Network Architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The Involvement of Cell-to-Cell Signals in the Development of a Bacterial Biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.M.; Ferreira, R.B.R.; Buckner, M.M.C.; Finlay, B.B. Quorum sensing in bacterial virulence. Microbiology 2010, 156, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.A.A.M.; Petersen, F.C.; Scheie, A.A. AI-2 quorum sensing affects antibiotic susceptibility in Streptococcus anginosus. J. Antimicrob. Chemother. 2007, 60, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Stotani, S.; Gatta, V.; Medda, F.; Padmanaban, M.; Karawajczyk, A.; Tammela, P.; Giordanetto, F.; Tzalis, D.; Collina, S. A Versatile Strategy for the Synthesis of 4,5-Dihydroxy-2,3-Pentanedione (DPD) and Related Compounds as Potential Modulators of Bacterial Quorum Sensing. Molecules 2018, 23, 2545. [Google Scholar] [CrossRef]
- Lowery, C.A.; Park, J.; Kaufmann, G.F.; Janda, K.D. An Unexpected Switch in the Modulation of AI-2-Based Quorum Sensing Discovered through Synthetic 4,5-Dihydroxy-2,3-pentanedione Analogues. J. Am. Chem. Soc. 2008, 130, 9200–9201. [Google Scholar] [CrossRef]
- De Keersmaecker, S.C.J.; Varszegi, C.; van Boxel, N.; Habel, L.W.; Metzger, K.; Daniels, R.; Marchal, K.; De Vos, D.; Vanderleyden, J. Chemical Synthesis of (S)-4,5-Dihydroxy-2,3-pentanedione, a Bacterial Signal Molecule Precursor, and Validation of Its Activity in Salmonella typhimurium. J. Biol. Chem. 2005, 280, 19563–19568. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory Mechanisms and Promising Applications of Quorum Sensing-Inhibiting Agents in Control of Bacterial Biofilm Formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Chbib, C.; Uddin, M.N.; D’souza, M.J. Evaluation of Microparticulate (S)-4,5-Dihydroxy-2,3-pentanedione (DPD) as a Potential Vaccine Adjuvant. AAPS J. 2021, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Quillin, S.J.; Seifert, H.S. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, J.; Jean, R.S.; Khaliq, Y. Gonorrhea. Can. Pharm. J. Rev. Pharm. Can. 2015, 148, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Gala, R.P.; Zaman, R.U.; D’souza, M.J.; Zughaier, S.M. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Bagwe, P.; Bajaj, L.; Gala, R.P.; D‘souza, M.J.; Zughaier, S.M. Assessment of In Vitro Immunostimulatory Activity of an Adjuvanted Whole-Cell Inactivated Neisseria gonorrhoeae Microparticle Vaccine Formulation. Vaccines 2022, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S. Biomaterials as vaccine adjuvants. Biotechnol. Prog. 2008, 24, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Mallapragada, S.K.; Narasimhan, B. Immunomodulatory biomaterials. Int. J. Pharm. 2008, 364, 265–271. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Bungener, L.; Geeraedts, F.; ter Veer, W.; Medema, J.; Wilschut, J.; Huckriede, A. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine 2008, 26, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Jarolim, E. Aluminium in Allergies and Allergen immunotherapy. World Allergy Organ. J. 2015, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Haensler, J.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol. Sci. 2009, 30, 287–295. [Google Scholar] [CrossRef] [PubMed]
- O’hagan, D.T.; Singh, M. Microparticles as vaccine adjuvants and delivery systems. Expert Rev. Vaccines 2003, 2, 269–283. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Rahman, D.; McGee, J.P.; Jeffery, H.; Davies, M.C.; Williams, P.; Davis, S.S.; Challacombe, S.J. Biodegradable microparticles as controlled release antigen delivery systems. Immunology 1991, 73, 239–242. [Google Scholar] [PubMed]
- O’Hagan, D.; Jeffery, H.; Roberts, M.; McGee, J.; Davis, S. Controlled release microparticles for vaccine development. Vaccine 1991, 9, 768–771. [Google Scholar] [CrossRef]
- Joshi, V.B.; Adamcakova-Dodd, A.; Jing, X.; Wongrakpanich, A.; Gibson-Corley, K.N.; Thorne, P.S.; Salem, A.K. Development of a Poly (lactic-co-glycolic acid) Particle Vaccine to Protect Against House Dust Mite Induced Allergy. AAPS J. 2014, 16, 975–985. [Google Scholar] [CrossRef]
- Silva, A.L.; Soema, P.C.; Slütter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccines Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Koerner, J.; Horvath, D.; Groettrup, M. Harnessing Dendritic Cells for Poly (D,L-lactide-co-glycolide) Microspheres (PLGA MS)—Mediated Anti-tumor Therapy. Front. Immunol. 2019, 10, 707. [Google Scholar] [CrossRef]
- Saha, D.; Kumar, S.; Ray, D.; Mata, J.; Aswal, V.K. Structure and stability of biodegradable polymer nanoparticles in electrolyte solution. Mater. Lett. X 2021, 10, 100066. [Google Scholar] [CrossRef]
- Jiang, W.; Gupta, R.K.; Deshpande, M.C.; Schwendeman, S.P. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv. Drug Deliv. Rev. 2005, 57, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Wusiman, A.; Zhang, Y.; Liu, Z.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Rational Design of PLGA Nanoparticle Vaccine Delivery Systems to Improve Immune Responses. Mol. Pharm. 2019, 16, 5000–5012. [Google Scholar] [CrossRef]
- Uddin, M.N.; Patel, N.J.; Bhowmik, T.; D’souza, B.; Akalkotkar, A.; Etzlar, F.; Oettinger, C.W.; D’souza, M. Enhanced bioavailability of orally administered antisense oligonucleotide to nuclear factor kappa B mRNA after microencapsulation with albumin. J. Drug Target. 2013, 21, 450–457. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines on the Nonclinical Evaluation of Vaccine Adjuvants and Adjuvanted Vaccines; WHO Press: Geneva, Switzerland, 2013; p. 56. [Google Scholar]
- Gu, P.; Liu, Z.; Sun, Y.; Ou, N.; Hu, Y.; Liu, J.; Wu, Y.; Wang, D. Angelica sinensis polysaccharide encapsulated into PLGA nanoparticles as a vaccine delivery and adjuvant system for ovalbumin to promote immune responses. Int. J. Pharm. 2019, 554, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Demento, S.L.; Cui, W.; Criscione, J.M.; Stern, E.; Tulipan, J.; Kaech, S.M.; Fahmy, T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33, 4957–4964. [Google Scholar] [CrossRef]
- Gala, R.P.; D’Souza, M.; Zughaier, S.M. Evaluation of various adjuvant nanoparticulate formulations for meningococcal capsular polysaccharide-based vaccine. Vaccine 2016, 34, 3260–3267. [Google Scholar] [CrossRef]
- Joshi, D.J.; Chitre, N.M.; Bansal, A.; Murnane, K.S.; D’souza, M.J. Formulation and Characterization of Microcapsules Encapsulating PC12 Cells as a Prospective Treatment Approach for Parkinson’s Disease. Aaps. Pharmscitech. 2021, 22, 1–11. [Google Scholar] [CrossRef]





| Vaccine Technologies | Advantages | Limitations |
|---|---|---|
| Live-attenuated |
|
|
| Inactivated |
|
|
| Subunit |
|
|
| Viral Vector |
|
|
| Nucleic acid DNA |
|
|
| Messenger RNA (mRNA) |
|
|
| Parameter | Mean ± SEM | ||||
|---|---|---|---|---|---|
| ent DPD | n-butyl—DPD | Isobutyl—DPD | n-hexyl—DPD | Phenyl—DPD | |
| Recovery Yield (%) | 90.12 ± 2.1 | 87.87 ± 2.3 | 89.94 ± 1.7 | 90.61 ± 2.4 | 91.49 ± 1.9 |
| Particle size (nm) | 462.85 ± 5.85 (351.63 ± 4.49) | 368.2 ± 10.46 (273.34 ± 6.17) | 380.2 ± 16.26 (247.38 ± 13.11) | 357.47 ± 4.99 (286.46 ± 5.16) | 468.26 ± 16.41 (402.61 ± 14.69) |
| Polydispersity index (PDI) | 0.74 (0.52) | 0.49 (0.31) | 0.71 (0.63) | 0.82 (0.68) | 0.83 (0.62) |
| Zeta potential (mV) | −33.3 ± 7.12 (−31.7 ± 4.63) | −29.9 ± 8.22 (−30.1 ± 7.39) | −30.6 ± 6.57 (−28.5 ± 3.61) | −32.9 ± 4.78 (−30.7 ± 6.14) | −35.0 ± 7.76 (−32.8 ± 4.37) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, D.; Shah, S.; Chbib, C.; Uddin, M.N. Potential of DPD ((S)-4,5-dihydroxy-2,3-pentanedione) Analogs in Microparticulate Formulation as Vaccine Adjuvants. Pharmaceuticals 2024, 17, 184. https://doi.org/10.3390/ph17020184
Joshi D, Shah S, Chbib C, Uddin MN. Potential of DPD ((S)-4,5-dihydroxy-2,3-pentanedione) Analogs in Microparticulate Formulation as Vaccine Adjuvants. Pharmaceuticals. 2024; 17(2):184. https://doi.org/10.3390/ph17020184
Chicago/Turabian StyleJoshi, Devyani, Sarthak Shah, Christiane Chbib, and Mohammad N. Uddin. 2024. "Potential of DPD ((S)-4,5-dihydroxy-2,3-pentanedione) Analogs in Microparticulate Formulation as Vaccine Adjuvants" Pharmaceuticals 17, no. 2: 184. https://doi.org/10.3390/ph17020184




