Glucagon-like Peptide-1 Receptor Agonists Associated Gastrointestinal Adverse Events: A Cross-Sectional Analysis of the National Institutes of Health All of Us Cohort
Abstract
:1. Introduction
2. Results
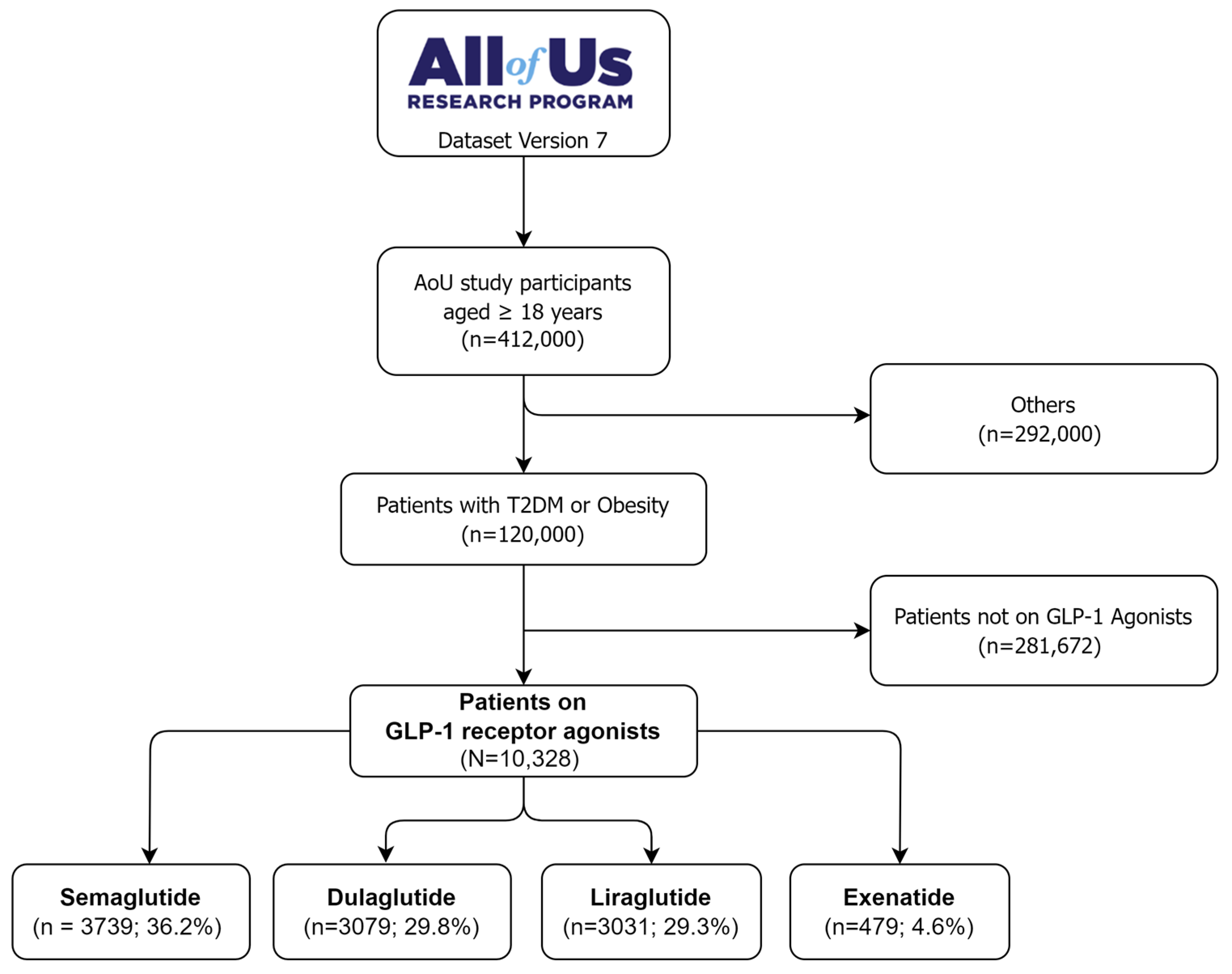
2.1. Cohort Description
2.2. Characteristics of the Study Population
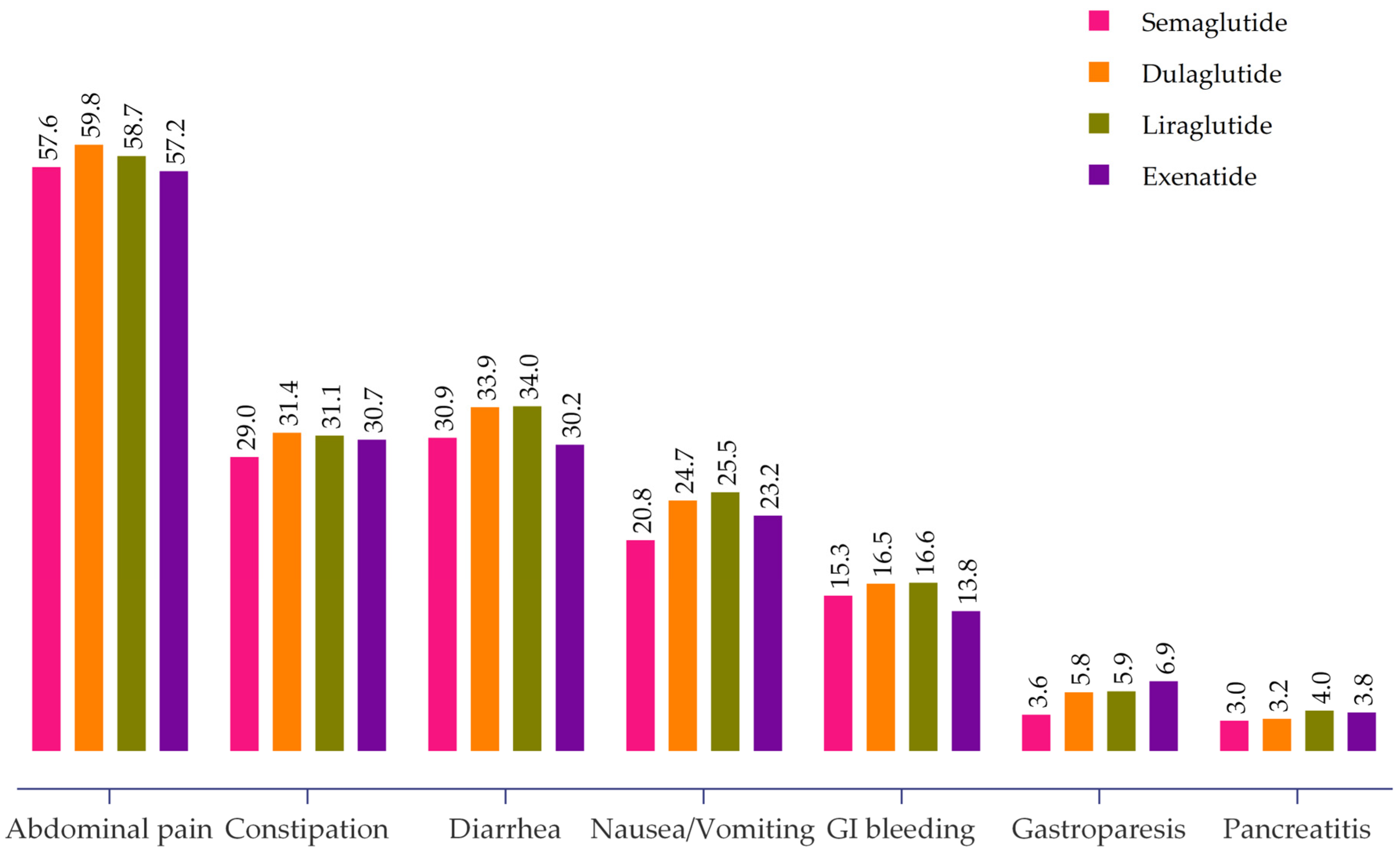
2.3. GLP-1 RAs Associated GI Adverse Events
2.4. Factors Associated with GI Adverse Events among GLP-1 RAs Users
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. NIH All of Us Research Program
4.3. Study Population
4.4. Cohort Construction
4.5. Cohort Selection
4.5.1. Inclusion Criteria
- Aged 18 years or older.
- Incident T2DM or obesity as a primary diagnosis.
- Used any GLP-1 Ras for at least one month.
- Had complete medical data.
4.5.2. Exclusion Criteria
- Type 1 diabetes mellitus.
- Gestational diabetes.
- Not on GLP-1 RAs therapy.
- Patients with missing data in their treatment records.
- Patients with the outcome of interest before treatment initiation.
4.6. Study Variables
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef]
- Ard, J.; Fitch, A.; Fruh, S.; Herman, L. Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-Like Peptide 1 Receptor Agonists. Adv. Ther. 2021, 38, 2821–2839. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wang, Q.W.; Yang, X.Y.; Yang, W.; Li, D.R.; Jin, J.Y.; Zhang, H.C.; Zhang, X.F. GLP-1 receptor agonists for the treatment of obesity: Role as a promising approach. Front. Endocrinol. 2023, 14, 1085799. [Google Scholar] [CrossRef]
- Michos, E.D.; Lopez-Jimenez, F.; Gulati, M. Role of Glucagon-Like Peptide-1 Receptor Agonists in Achieving Weight Loss and Improving Cardiovascular Outcomes in People with Overweight and Obesity. J. Am. Heart Assoc. 2023, 12, e029282. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Rizzo, M.; Haluzík, M.; Janež, A. Efficacy of GLP-1 RA Approved for Weight Management in Patients with or Without Diabetes: A Narrative Review. Adv. Ther. 2022, 39, 2452–2467. [Google Scholar] [CrossRef] [PubMed]
- Popoviciu, M.S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.B.; Yahya, T.; Satish, P.; Laird, R.; Agatston, A.S.; Cainzos-Achirica, M.; Patel, K.V.; Nasir, K. Glucagon-Like Peptide 1 Receptor Agonists: A Medication for Obesity Management. Curr. Atheroscler. Rep. 2022, 24, 643–654. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Lehmann, E.W.; Torekov, S.S. Glucagon-like peptide-1 receptor agonists: The key to healthy weight loss maintenance? Cardiovasc. Res. 2021, 117, e120–e122. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Wang, L.; Chen, C.; Chen, L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database. Front. Endocrinol. 2022, 13, 1043789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, M.; Liu, L.; Chen, Z. Difference in Gastrointestinal Risk Associated with Use of GLP-1 Receptor Agonists: A Real-World Pharmacovigilance Study. Diabetes Metab. Syndr. Obes. 2022, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Basheer, F.T.; Poojari, P.G.; Thunga, G.; Chandran, V.P.; Acharya, L.D. Adverse drug reactions of GLP-1 agonists: A systematic review of case reports. Diabetes Metab. Syndr. 2022, 16, 102427. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, M.; Rezaeianzadeh, R.; Kezouh, A.; Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 2023, 330, 1795–1797. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse effects of GLP-1 receptor agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef]
- Horowitz, M.; Aroda, V.R.; Han, J.; Hardy, E.; Rayner, C.K. Upper and/or lower gastrointestinal adverse events with glucagon-like peptide-1 receptor agonists: Incidence and consequences. Diabetes Obes. Metab. 2017, 19, 672–681. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, J.; Gu, H.; Li, M.; Chen, J. Comparison of the efficacy and safety of 10 glucagon-like peptide-1 receptor agonists as add-on to metformin in patients with type 2 diabetes: A systematic review. Front. Endocrinol. 2023, 14, 1244432. [Google Scholar] [CrossRef]
- Wharton, S.; Calanna, S.; Davies, M.; Dicker, B.; Goldman, B.; Lingvay, I.; Mosenzo, O.; Rubino, D.M.; Thomsen, M.; Wadden, T.A.; et al. Gastrointestinal tolerability of once-weekly semaglutide 2.4 mg in adults with overweight or obesity, and the relationship between gastrointestinal adverse events and weight loss. Diabetes Obes. Metab. 2022, 24, 94–105. [Google Scholar] [CrossRef]
- Ahrén, B.; Atkin, S.L.; Charpentier, G.; Warren, M.L.; Wilding, J.P.H.; Birch, J.; Holst, A.G.; Leiter, L.A. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes Metab. 2018, 20, 2210–2219. [Google Scholar] [CrossRef]
- Qin, W.; Yang, J.; Deng, C.; Ruan, Q.; Duan, K. Efficacy and safety of semaglutide 2.4 mg for weight loss in overweight or obese adults without diabetes: An updated systematic review and meta-analysis including the 2-year STEP 5 trial. Diabetes Obes. Metab. 2023, 15. [Google Scholar] [CrossRef]
- Shu, Y.; He, X.; Wu, P.; Liu, Y.; Ding, Y.; Zhang, Q. Gastrointestinal adverse events associated with semaglutide: A pharmacovigilance study based on FDA adverse event reporting system. Front Public Health 2022, 10, 996179. [Google Scholar] [CrossRef] [PubMed]
- All of Us Research Program Investigators. The “All of Us” research program. N. Engl. J. Med. 2019, 381, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Mapes, B.M.; Foster, C.S.; Kusnoor, S.V.; Epelbaum, M.I.; AuYoung, M.; Jenkins, G.; Lopez-Class, M.; Richardson-Heron, D.; Elmi, A.; Surkan, K.; et al. Diversity and inclusion for the All of Us research program: A scoping review. PLoS ONE 2020, 15, e0234962. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, N.; Cho, S.M.J.; Bhattacharya, R.; Truong, B.; Hornsby, W.; Natarajan, P. Representation of race and ethnicity in the contemporary US health cohort all of US research program. JAMA Cardiol. 2023, 8, 859–864. [Google Scholar] [CrossRef]
- Ramirez, A.H.; Sulieman, L.; Schlueter, D.J.; Halvorson, A.; Qian, J.; Ratsimbazafy, F.; Loperena, R.; Mayo, K.; Basford, M.; Deflaux, N.; et al. The All of Us Research Program: Data quality, utility, and diversity. Patterns 2022, 3, 100570. [Google Scholar] [CrossRef]
- Ramirez, A.H.; Kelly, A.G.; Paul, A.H. Progress with the All of Us research program: Opening access for researchers. JAMA 2022, 325, 2441–2442. [Google Scholar] [CrossRef]
- FDA. Ozempic (Semaglutide) Injection, Subcutaneous Use. 20 September 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf (accessed on 14 December 2023).
| Characteristics | N = 10,328 | Semaglutide (n = 3739) | Dulaglutide (n = 3079) | Liraglutide (n = 3031) | Exenatide (n = 479) |
|---|---|---|---|---|---|
| T2DM, % | 8489 (82.2) | 2904 (77.6) | 2793 (90.7) | 2343 (77.3) | 30 (6.2) |
| Obesity, % | 7886 (76.4) | 2929 (78.3) | 2183 (70.9) | 2404 (79.3) | 370 (77.2) |
| Sex, % | |||||
| Female | 6785 (65.7) | 2423 (64.8) | 1937 (62.9) | 2113 (69.7) | 312 (65.1) |
| Male | 3543 (34.3) | 1316 (35.2) | 1142 (37.1) | 918 (30.3) | 167 (34.9) |
| Age (Mean ± SD) | 61.4 ± 12.6 | 61.7 ± 12.1 | 60.5 ± 12.3 | 60.9 ± 11.8 | 61.8 ± 11.9 |
| Race, % | |||||
| White | 5296 (51.3) | 2058 (55.0) | 1415 (45.9) | 1590 (52.4) | 233 (48.6) |
| Black | 2414 (23.4) | 846 (22.6) | 790 (25.6) | 662 (21.8) | 116 (24.2) |
| Asian | 185 (1.8) | 80 (2.1) | 54 (1.7) | 47 (1.5) | 4 (0.8) |
| Others | 2433 (23.5) | 755 (20.2) | 820 (26.6) | 732 (23.1) | 126 (26.3) |
| Lipase levels (Mean ± SD) | 97.4 ± 2.9 | 95.1 ± 3.0 | 100 ± 2.3 | 97 ± 2.5 | 94 ± 2.6 |
| Amylase (Mean ± SD) | 67.4 ± 5.6 | 68 ± 5.4 | 66 ± 5.3 | 66 ± 5.6 | 66.2 ± 5.1 |
| Comorbidities, % | |||||
| CKD | 2641 (25.6) | 2947 (78.8) | 2191 (71.1) | 2222 (73.3) | 327 (68.2) |
| HF | 1982 (19.2) | 620 (16.6) | 609 (19.7) | 643 (21.2) | 119 (24.8) |
| Abdominal Pain (n = 5949) | Constipation (n = 3144) | Diarrhea (n = 3374) | Nausea and Vomiting (n = 2421) | GI Bleeding (n = 1648) | Gastroparesis (n = 524) | Pancreatitis (n = 348) | |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Female | 4256 (71.5) | 2274 (72.3) | 2395 (71.0) | 1857 (76.7) | 1027 (62.3) | 389 (74.2) | 244 (70.1) |
| Male | 1663 (27.9) | 870 (27.6) | 979 (29.0) | 564 (23.3) | 621 (37.7) | 135 (25.7) | 104 (29.8) |
| Race | |||||||
| White | 2810 (47.2) | 1314 (41.8) | 1761 (52.2) | 1110 (45.8) | 815 (49.5) | 241 (46.0) | 161 (46.2) |
| Black | 1433 (24.1) | 896 (28.5) | 728 (21.6) | 598 (24.7) | 383 (23.2) | 136 (25.9) | 74 (21.2) |
| Asian | 82 (1.3) | 37 (1.2) | 34 (1.0) | 20 (0.8) | 19 (1.1) | 5 (0.9) | 5 (1.4) |
| Others | 1624 (27.3) | 897 (28.5) | 851 (25.2) | 693 (24.6) | 431 (26.1) | 142 (27.1) | 108 (31.0) |
| Comorbidities | |||||||
| CKD | 1737 (29.2) | 1112 (35.3) | 1153(34.2) | 818 (33.7) | 601 (36.4) | 232 (44.3) | 144 (41.4) |
| HF | 1374 (23.1) | 905 (28.8) | 899 (26.6) | 638 (26.3) | 522 (31.6) | 188 (35.8) | 114 (32.7) |
| Adjusted Odds Ratio † (95% Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|
| Abdominal Pain | Constipation | Diarrhea | Nausea and Vomiting | GI Bleeding | Gastroparesis | Pancreatitis | |
| T2DM | 1.91 | 1.53 | 1.81 | 2.31 | 1.34 | 4.81 | 2.45 |
| [1.7–2.1] ** | [1.32–1.78] * | [1.61–1.91] * | [1.82–2.41] ** | [1.13–1.60] ** | [3.41–7.82] ** | [1.59–3.98] ** | |
| Obesity | 2.50 | 1.97 | 2.11 | 2.04 | 2.50 | 1.31 | 1.45 |
| [2.1–2.6] ** | [1.72–2.31] * | [1.59–2.29] ** | [1.41–2.71] * | [1.98–2.74] ** | [1.01–1.71] * | [1.05–2.03] * | |
| Sex | |||||||
| Female | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Male | 0.50 | 0.57 | 0.59 | 0.47 | 1.10 | 0.53 | 0.69 |
| [0.46–0.55] ** | [0.51–0.67] ** | [0.53–0.65] ** | [0.40–0.51] ** | [0.97–1.23] ** | [0.43–0.66] ** | [0.53–0.89] ** | |
| Age | 0.98 | 1.00 | 0.99 | 0.98 | 1.00 | 0.97 | 0.99 |
| [0.97–0.99] ** | [0.93–1.2] | [0.97–1.2] | [0.96–0.99] ** | [0.97–1.1] | [0.96–0.99] ** | [0.98–1.00] | |
| Race | |||||||
| Asian | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| White | 1.15 | 1.01 | 1.71 | 2.11 | 1.17 | 1.61 | 1.82 |
| [0.84–1.45] | [0.73–1.49] | [1.23–2.54] ** | [1.33–3.51] ** | [0.73–1.97] | [0.71–4.51] | [0.65–7.38] | |
| Black | 1.20 | 1.58 | 0.96 | 1.89 | 1.14 | 1.41 | 1.40 |
| [0.86–1.63] | [1.09–2.35] ** | [0.79–1.12] | [1.21–3.22] * | [0.71–1.97] | [0.62–4.21] | [0.49–5.75] | |
| Others | 1.75 | 1.64 | 1.80 | 2.48 | 1.34 | 0.69 | 2.38 |
| [1.27–2.41] ** | [1.11–2.43] ** | [1.31–2.81] ** | [1.51–4.52] * | [0.83–2.21] | [0.23–4.52] | [0.85–9.69] | |
| Comorbidities | |||||||
| CKD | 1.44 | 1.63 | 1.71 | 1.78 | 1.38 | 1.99 | 1.59 |
| [1.3–1.6] ** | [1.46–1.83] ** | [1.54–1.96] ** | [1.54–2.41] ** | [1.22–1.57] ** | [1.63–4.23] ** | [1.22–2.07] ** | |
| HF | 1.55 | 1.74 | 1.51 | 1.54 | 1.73 | 1.90 | 1.63 |
| [1.4–1.8] ** | [1.4–1.91] * | [1.34–1.93] ** | [1.36–1.78] ** | [1.5–1.97] ** | [1.5–2.35] ** | [1.24–2.14] ** | |
| Drugs | |||||||
| Semaglutide | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Dulaglutide | 1.19 | 1.10 | 0.97 | 1.17 | 0.91 | 1.43 | 0.91 |
| [1.05–1.23] * | [0.99–1.96] | [0.71–1.95] | [1.03–1.34] * | [0.90–1.25] | [1.21–1.95] * | [0.68–1.23] | |
| Liraglutide | 1.12 | 0.99 | 0.89 | 1.19 | 1.03 | 1.51 | 1.21 |
| [1.01–1.24] * | [0.85–1.23] | [0.79–1.12] | [1.01–1.45] * | [0.92–1.2] | [1.21–1.91] * | [0.92–1.61] | |
| Exenatide | 0.75 | 0.88 | 0.91 | 1.10 | 1.04 | 1.65 | 1.04 |
| [0.59–1.73] | [0.71–1.89] | [0.79–1.73] | [0.79–2.14] | [0.57–1.73] | [1.10–2.41] * | [0.59–1.73] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldhaleei, W.A.; Abegaz, T.M.; Bhagavathula, A.S. Glucagon-like Peptide-1 Receptor Agonists Associated Gastrointestinal Adverse Events: A Cross-Sectional Analysis of the National Institutes of Health All of Us Cohort. Pharmaceuticals 2024, 17, 199. https://doi.org/10.3390/ph17020199
Aldhaleei WA, Abegaz TM, Bhagavathula AS. Glucagon-like Peptide-1 Receptor Agonists Associated Gastrointestinal Adverse Events: A Cross-Sectional Analysis of the National Institutes of Health All of Us Cohort. Pharmaceuticals. 2024; 17(2):199. https://doi.org/10.3390/ph17020199
Chicago/Turabian StyleAldhaleei, Wafa Ali, Tadesse M. Abegaz, and Akshaya Srikanth Bhagavathula. 2024. "Glucagon-like Peptide-1 Receptor Agonists Associated Gastrointestinal Adverse Events: A Cross-Sectional Analysis of the National Institutes of Health All of Us Cohort" Pharmaceuticals 17, no. 2: 199. https://doi.org/10.3390/ph17020199






